Abstract
Purpose
To assess the biologic basis of massage therapies, we developed an experimental approach to mimic Swedish massage and evaluate this approach on recovery from eccentric exercise-induced muscle damage using a well-controlled animal model.
Methods
Tibialis anterior muscles of six New Zealand White rabbits were subjected to one bout of damaging, eccentric contractions. One muscle was immediately subjected to cyclic compressive loads, and the contralateral served as the exercised control.
Results
We found that commencing 30 min of cyclic compressive loading to the muscle, immediately after a bout of eccentric exercise, facilitated recovery of function and attenuated leukocyte infiltration. In addition, fiber necrosis and wet weight of the tissue were also reduced by compressive loading.
Conclusion
We conclude that subjecting muscle to compressive loads immediately after exercise leads to an enhanced recovery of muscle function and attenuation of the damaging effects of inflammation in the rabbit model. Although these observations suggest that skeletal muscle responds to cyclic compressive forces similar to those generated by clinical approaches, such as therapeutic massage, further research is needed to assess the translational efficacy of these findings.
Keywords: SKELETAL MUSCLE, MASSAGE, INFLAMMATION, TORQUE
Muscle is a mechanically responsive tissue capable of generating distinct intracellular signals that lead to specific downstream adaptations. For example, chronic stretch increases sarcomere number in series and therefore muscle length (16). Conversely, functional overload (pressure) results in an increase in sarcomere number, resulting in a concomitant increase in muscle cross-sectional area (26). Furthermore, evidence suggests that muscle cells themselves are highly sensitive to mechanical signals and can distinguish between loading patterns to produce various downstream effects (18).
The in vitro and in vivo responses of skeletal muscle to controlled loading have illustrated that biomechanical signals can be reparative (11) or damaging (31) and that the optimal tissue adaptation depends on the magnitude, frequency, and duration of loading. This has been further supported by studies using cultured myoblasts and myocytes, wherein magnitude, frequency, and type of mechanical forces are all shown to influence the gene expression (4). Although these investigations have primarily focused on the mechanosensitivity of muscle cells in vitro, the biologic responses of skeletal muscle tissue to tensile and compressive loads in vivo still remain unclear.
Intense exercise, particularly repetitive eccentric exercise (EEX), has shown to be associated with muscle damage, soreness, and inflammation (13,21,23). These signs and symptoms typically peak within 48 h of exercise and can persist for up to 1 wk, depending on the duration of the exercise bout and the subject’s level of conditioning (12,21). Not surprisingly, optimal clinical strategies to treat and prevent these signs and symptoms, and therefore reduce associated loss of time from sport and physical activity, remain a topic of debate. Clinical interventions include, but are not limited to the following: therapeutic ultrasound (30), electrical stimulation (34), ice immersion (28), static and ballistic stretching (29), therapeutic massage (15), and nonsteroidal anti-inflammatory medications (24). Unfortunately, there remains little consensus in the literature regarding the efficacy of these treatments. However, anecdotal evidence gleaned from clinical practice does suggest that cyclic compressive loading (in the form of therapeutic massage) may improve functional recovery of skeletal muscle after a bout of intense EEX.
In support of this evidence, a recent Cochrane systematic review concluded that therapeutic massage is beneficial for improving both symptoms and level of function for individuals with both subacute and chronic low back pain (14). Along with many studies included in the Cochrane review, massage has been shown to reduce pain and improve range of motion in a randomized controlled trial of 29 patients with shoulder pain (35), relieve muscular pain in patients with fibromyalgia (27), and improve performance and recovery from postexercise injury (10,15). Of particular interest to us is Swedish (muscular) massage, which incorporates an assortment of component techniques including slow rhythmic stroking (effleurage), circular compression (kneading), forceful skin rolling (pétrissage), and penetrating pressure from fingertips with circular or transverse movement (friction) (22).
Swedish massage and similar approaches are used by athletes for a variety of musculoskeletal disorders, despite the lack of high-quality clinical or basic science studies to support or refute their efficacy. We hypothesized that (i) cyclic compressive loading during massage improves recovery of muscle function after a bout of intense EEX and (ii) cyclic compression of the exercised muscle will decrease leukocyte infiltration and myofiber damage. Therefore, to assess the biologic basis of massage therapies, the primary goals of this study were to 1) develop an experimental approach to mimic Swedish massage and 2) evaluate this approach to improve recovery from EEX-induced muscle damage using a well-controlled animal model that mimics EEX-induced muscle damage in humans. Such findings are important to form a theoretical basis for the efficacy of therapeutic massage, a popular clinical strategy in the treatment of EEX-associated muscle pain and loss of function (33,36).
METHODS
The rabbit model for EEX-induced muscle damage
Six skeletally mature New Zealand White (NZW) female rabbits (3.9 ± 0.51 kg; Harlan Sprague Dawley, Indianapolis, IN) were obtained for this study after approval by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University. Animals were tranquilized with 0.18 mL of acepromazine (10 mg·mL−1; Vedco, Inc, St. Joseph, MO) and held under anesthesia with 1.5% isoflurane. Rabbits were surgically instrumented with bilateral peroneal nerve cuffs and subcutaneous interfaces for control of both groups of dorsiflexor muscles of the hindlimbs (9).
Seven days after surgery to implant the nerve cuffs, rabbits were sedated and anesthetized as outlined previously and were secured supine in a sling. One foot was randomly selected and strapped to a footplate connected to a torque sensor on the cam of a servomotor. Immediately preceding the exercise bout, an isometric torque–joint angle (T–Θ) relationship was determined by supramaximally stimulating (three times the α-motoneuron threshold voltage, pulse duration = 0.1 ms, frequency = 150 Hz, train duration = 1000 ms) the dorsiflexor muscles, beginning at a tibiotarsal angle of 55° and progressing in 5° increments to 155° (i.e., 21 measurements). The foot was returned to a dorsiflexed position (55° tibiotarsal angle) for 2 min of rest between contractions to minimize muscle fatigue. Once the preexercise T–Θ relationship had been obtained, one bout of intense EEX was performed. The protocol consisted of seven sets of 10 cyclic lengthening contractions, with 2-min rest between sets. Cyclic lengthening contractions were performed from a tibiotarsal angle of 95° to 145° of plantarflexion at 150°·s−1 for the EEX groups, with the activation preceding the muscle–tendon unit stretch by 100 ms (total stimulus train duration = 433 ms). Pilot studies showed that this set of parameters (range of motion, timing of activation, and speed of stretch) resulted in a reproducible magnitude of muscle damage (70% loss of peak torque production in all hindlimbs tested).
After the EEX, a second T–Θ relationship was obtained to assess the magnitude of muscle damage or alterations in functional mechanical measures (5). The exercised limb (the lower extremity dorsiflexor group) was immediately subjected to 30 min of cyclic compression (EEXC limbs; see protocol below). The 30 min of compressive loading was carried out on four consecutive days (each session approximately 24 h apart). The first session was initiated within 30 min of completion of the EEX protocol. Immediately after the cyclic loading, the exercise protocol was repeated on the contralateral limb, but the cyclic compression was not performed as the contralateral limb served as the exercised and rested control (EEXR limbs). Muscle function (T–Θ) was evaluated one final time on the day after the fourth (and final) bout of compressive loading.
Device to subject muscles to cyclic compressive forces in vivo
A computer-driven device was designed to apply cyclic compressive forces at a frequency of 0.5 Hz to the rabbit lower extremity dorsiflexor muscles of the EEXC hindlimbs (Fig. 1A). The device consisted of pneumatically driven wheel (0.625 inches in diameter and 0.75 inches in width; Solid Polypropylene, Durometer 83D) that was operated in displacement feedback to apply a compressive force to the anterior portion of the tibialis anterior (TA). The contact pressure applied by the polypropylene wheel was measured in situ using a 50-μm film piezoelectric polymer sensor (Measurement Specialties, Inc). The sensors were carefully secured using double-sided tape to the midbelly of the front surface of the TA to ensure the film was essentially flat without substantial surface bending. The contact pressure was measured from the electric voltage change at the two ends of the film using a data acquisition system at 100 samples per second (National Instrumentation). The voltage change during mechanical compression of the muscle depends on the geometries and material properties of the film sensors, the material properties of the muscle, and the applied pressure. Accordingly, the forces can be calculated from voltage changes read from the sensor by providing material properties of the subject muscle and using a computational biomechanical model, which incorporates the influence from the compliant backing and the transverse stress, on the basis of equation 1
| [1] |
where Vo is the out circuit voltage, g33 and g31 are the piezo stress constants (−330 and −216 for the applied thin film), X0 is normal stress, t is the film thickness, t′ is the muscle thickness, and X(X0,t,t′,E,E′) and X′(X0,t,t′,E,E′) are functions of the elastic modulus (E and E′) and the thickness of the thin film and the muscle.
FIGURE 1.
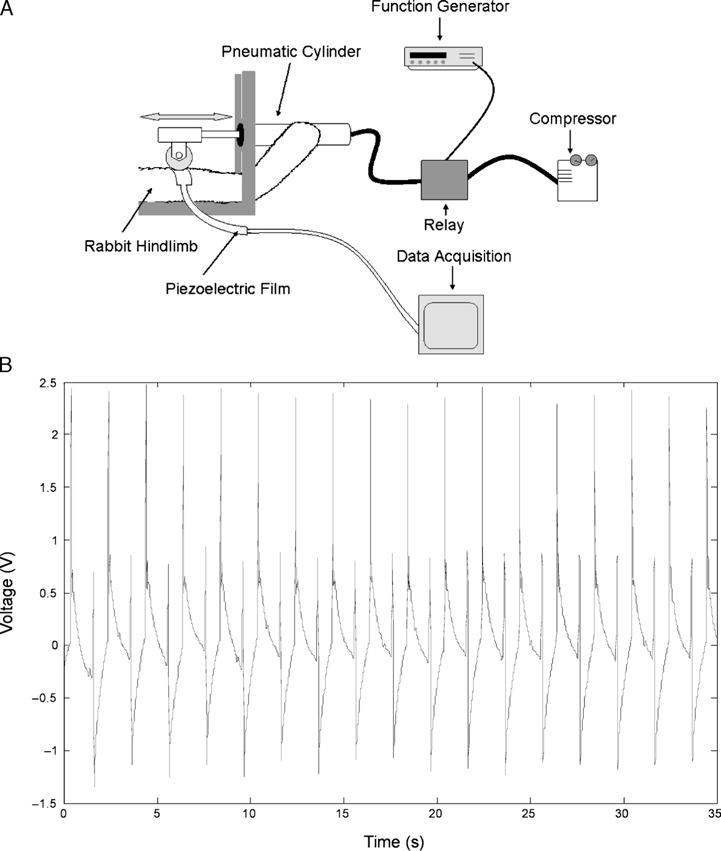
A. Schematic representation of rabbit TA receiving cyclic compressions with the computer-driven device. A closed loop system operates through both distance (position) and pressure feedback sensors. Tissue responses during compression are quantified in real time with a series of piezoelectric thin film sensors applied to the muscle’s surface. B. Voltage output versus time. Data are band pass-filtered in Matlab. Muscle compressions were performed at a frequency of 0.5 Hz. Note the reasonable consistency of measured voltage for both magnitudes from cycle to cycle. In this particular case, the calculated average normal force was approximately 11.4 N. Note that the primary peak is approximately 11.4 N, the secondary is approximately 6 N. The difference is believed to be due to bending of the film.
It was assumed that the wheel produced uniform normal stress across the muscle. It was also assumed that both bending and in-plane stretching of the film sensor were negligible. Therefore, the normal stress to compress the film’s thickness was derived by simplifying equation 1 and using the piezo stress constant g33 and the voltage output (Vo) of the sensing film; where NS is the normal stress, g33 = 330 × 10−3 V·m·N−1, and t = 50 × 10−6 m is the film thickness (equation 2).
| [2] |
Solving equation 2 with Vo = 2.5 V, NS = 2.5 V/(330 × 10−3 V·m·N−1 × 50 × 10−6 m) = 151,515 N·m−2.
To derive the contact force applied by the polyethylene wheel, the contact area was estimated during muscle compression. The length of contact was observed as 0.009525 m. Because the width of the wheel is 0.01905 m, greater than the width of the piezo film (0.015748 m), the contact area for the film is A = 0.0047625 m × 0.015748 m = 0.000075 m2. Therefore, given the calculated normal stress (NS) above is 151,515 N·m−2, the contact force is 11.36 N.
Pilot testing revealed that compressive loads could be applied in a repeatable and quantifiable manner at a frequency of 0.5 Hz to the rabbit lower extremity dorsiflexors (Fig. 1B). We therefore used compressive loading at 0.5 Hz for 30 min for each of the four sessions in this study.
Biomechanical measures of muscle function
A T–Θ relationship was obtained for 21 tibiotarsal joint angles (55° to 155° in 5° increments) for both the exercised–cyclically loaded (EEXC) and the exercised–rested (EEXR) limbs at three time points; immediately before EEX, immediately after EEX, and just before sacrifice on day 5. This strategy allowed us to estimate the degree of injury caused by the EEX (pre- and postexercise values) and to estimate the functional recovery in both hindlimbs (after exercise and after 4 d of massage). Immediately after the final T–Θ measurement in both hindlimbs, the rabbit was killed, and both TAs were excised, weighed, and immediately sectioned. Multiple sections from various regions of the TA were fixed in neutral buffered formalin and stored for future histologic analysis.
Theoretical estimation of forces applied to rabbit TA
Typical massage consists of two major force components in a three-dimensional field, shear forces in the Fz direction and compressive forces in the Fx direction (2). To our knowledge, these forces have not been previously quantified in the rabbit TA. However, an estimation of these forces can be derived through extrapolation of data from massage forces measured during spine manipulative therapy in humans (SMT). The compression and shear forces acting in humans during SMT are 35 and 160 N, respectively, over a spine area of 78.1 mm2 (20). A rabbit has a spine area of 22 mm2 (20). Comparatively, human and rabbit TA muscles have cross-sectional areas of 905 (19) and 86 mm2 (1), respectively. Therefore, the spine to TA area ratio increase is 2.96 (equation 3).
| [3] |
| [4] |
| [5] |
Corresponding shear and compressive forces on rabbit TA would therefore be approximately 45 and 9.9 N, respectively (equations 4 and 5).
Histological analysis
Immediately after sacrifice, the rabbit TA muscles (control and cyclically loaded) were harvested, sectioned, and fixed in neutral formalin. Samples were later embedded in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin and eosin to quantify myofiber damage and cellular infiltration. Cross-sections were viewed with magnifications of ×200 and ×400 (Nikon light microscope; Fryer Company, Inc) for torn fiber and leukocyte infiltration analysis. Fibers were counted as damaged if there was any evidence of membrane discontinuity. Infiltrating leukocytes were counted as those cells that completely infiltrated the fiber. Quantitative analysis was performed by an individual blinded to the treatment condition. Counts were recorded in duplicate to test for repeatability. Three randomly selected equal areas of the specimen were assessed for leukocyte infiltration of myofibers and each area was 0.23 mm2 (0.48 mm × 0.48 mm). A characteristic polymorphonuclear and multilobulated shaped infiltrating leukocytes were observed. The leukocytes were counted, and a mean value and SD was calculated for each condition.
Statistical analysis
The effects of cyclic compressive loading on functional, muscle recovery after EEX indicated that a sample size of 5 was required to achieve a statistical power of 0.80. Functional data (peak torque, T–Θ relationship) were described with a sample mean and SD and were compared between contralateral limbs using paired Student’s t-tests to assess the effects of compressive loading on muscle function. After cyclic compressive loading, Student’s paired t-tests were used to compare the effects of cyclic compressive loading on muscle function (EEXC and EEXR) and histology between each experimental hindlimbs. ANOVA was used to analyze the effect of compressive loading on recruitment of leukocytes to the interstitial space, infiltrating cells within the myofibers, and the number of torn myofibers. The Tukey’s HSD post hoc test was used to differentiate significant effects, and the level of significance was set at P ≤ 0.05. SPSS version 15.0 was used for all analyses.
RESULTS
Biomechanical evaluation
The EEX protocol resulted in remarkably similar reductions in peak isometric torque for the left and the right hindlimbs for all six rabbits (left −70.5 ± 0.55% vs right −69.8 ± 0.39%, P = 0.624), verifying the effectiveness of our exercise protocol to produce a consistent loss of muscle function. For all six animals, recovery of muscle function after the EEX protocol was positively influenced by the application of cyclic compressive loading (EEXC) over the 4-d period, when compared with contralateral limb (EEXR) function (Fig. 2). Although the damaging effects of the EEX regimen were similar across all muscles, all muscles receiving daily bouts of cyclic compression (EEXC) experienced a greater recovery of function as measured by peak torque production compared with the muscles of the EEXR group.
FIGURE 2.
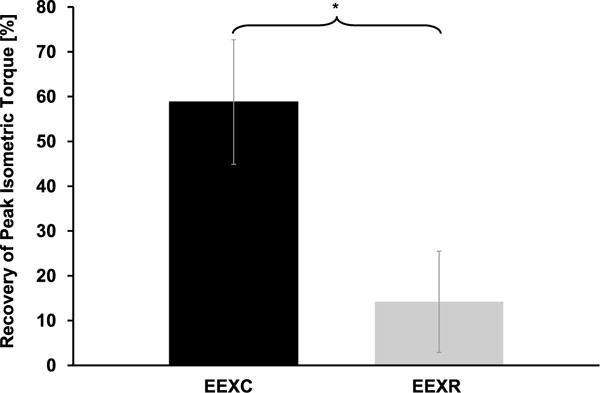
Recovery of peak torque after damaging eccentric exercise and 4 d of cyclic compressive loading (EEXC) and 4 d of rest (EEXR). (*) indicates significantly different values.
On average, muscles subjected to cyclic compressive loads (EEXC) recovered 58.8 ± 34.1% of peak torque produced immediately after the EEX bout compared with the exercised and rested muscles (EEXR) that only produced an additional +14.2 ± 27.8% torque after 4 d, P = 0.008 (Fig. 2). In addition, we also observed a significant difference in the mass of the excised TA muscles that were subjected to 4 d of cyclic compressive loading (EEXC; 3.79 ± 0.30 g) when compared with the mass of the exercised muscles that were subjected to rest only (EEXR; 4.05 ± 0.32 g, P = 0.014; Fig. 3).
FIGURE 3.
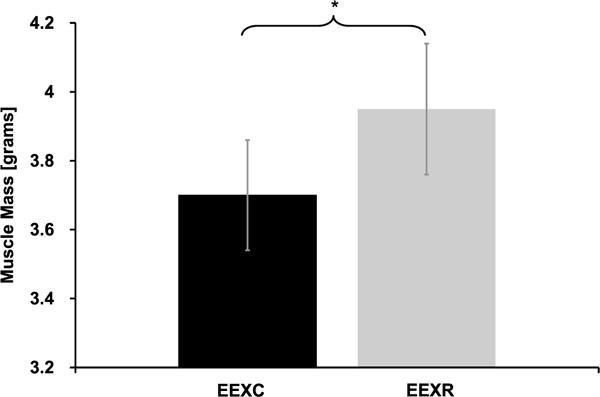
TA muscle wet weight after eccentric exercise and 4 d of cyclic compressive loading (EEXC) or eccentric exercise and rest (EEXR). Four days of cyclic compressive loading significantly reduced (*) TA mass when compared with the mass of the exercised and rested TA.
Validation of muscle compressive forces
Equations 3–5 provide an estimation of the theoretical compressive and shear forces that could be applied during compressive loading of the rabbit TA. Using our cyclic compression device and piezo transducer, we estimated a consistent compressive force application to the rabbit TA of 11.4 N. These measured forces, therefore, compare well to the theoretical estimates obtained in equations 3–5.
Histological analysis
Striking visual differences were always noted between the muscles of the EEXC and EEXR hindlimbs (Fig. 4). Typical cross-sections revealed that most fibers of the EEXC limbs had a polygonal profile, although fibers in the EEXR limbs were more rounded in appearance (Fig. 4). There was an average of 0.60 ± 0.31·0.23 mm−2 torn fibers in the EEXR muscles compared with a striking absence of torn fibers (similar to the healthy muscle) for the EEXC muscles (P = 0.0005). We noted an average of 7.4 ± 3.43 leukocytes·0.23 mm−2 infiltrating leukocytes·cross-section−1 for the EEXR muscles. In no case were infiltrating leukocytes observed in the EEXC muscles, similar to those in EEXR muscles (Fig. 4). Finally, 39.13 ± 4.93 leukocytes were noted in the interstitial space of the EEXR muscles, with 18.89 ± 2.62 leukocytes in the EEXC muscles (healthy controls = 27.83 ± 3.43 leukocytes) per 0.23-mm2 sample area observed. The number of leukocyte recruitment in the interstitial space of EEXC muscles was significantly lower (P < 0.05) than in the EEXR muscles and control. Furthermore, control had significantly fewer leukocytes recruitment in the interstitial space than in the EEXR muscles (Table 1).
FIGURE 4.
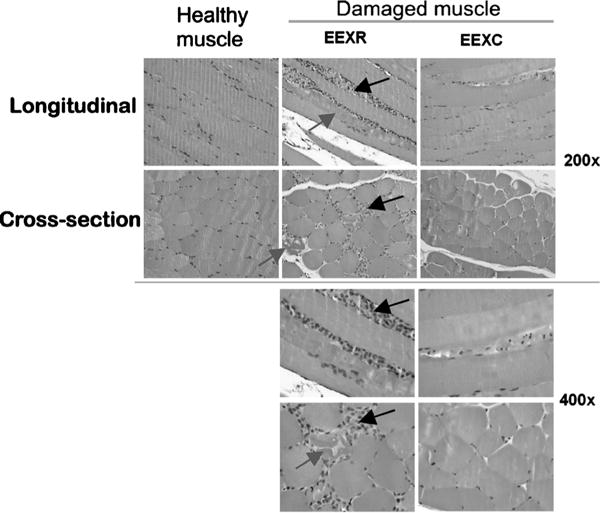
The left panel shows control, healthy TA muscle with negligible neutrophil infiltration or muscle damage. The middle panel shows ×200 and ×400 magnifications of cross-sectional and longitudinal sections of TA muscle from one representative hindlimb exposed to eccentric exercise and rest (EEXR). Muscle damage within the fibers was frequently observed (gray arrows). The tissue was also extensively infiltrated with leukocytes comprised mainly of polymorphonuclear cells and some mononuclear cells between muscle fibers (black arrows). In these sections, blood vessels draining leukocytes in the muscle tissue were also prominent. The right panel shows muscle from one representative hindlimb exposed to eccentric exercise and treated with cyclic compressive loading (EEXC) for four consecutive days, 30 min·d−1. The EEXC tissue demonstrates a striking histology as shown by minimal muscle fiber damage and infiltrating leukocytes.
TABLE 1.
Number of torn fibers and infiltrating leukocytes after eccentric exercise and rest (EEXR), eccentric exercise and cyclic compressive loading (EEXC), and in control muscle (mean ± SD).
| EEX Protocol | Torn Fibers (no. per 0.23-mm2 Sample) |
Infiltrating Leukocytes (no. per 0.23-mm2 Sample) |
Leukocytes in the Interstitial Space (no. per 0.23-mm2 Sample) |
|---|---|---|---|
| EEXR | 0.60 ± 0.31 | 7.4 ± 3.43 | 39.13 ± 4.93 |
| EEXC | 0 | 0 | 18.89 ± 2.62 |
| Healthy controls | 0 | 0 | 27.83 ± 3.43 |
DISCUSSION
Muscle cells respond to biomechanical signals and lead to qualitative and quantitative changes in gene expression that can initiate both muscle damage and repair (11,17,18). The results of the current study demonstrate in a well-validated in vivo model (5,7,8) that cyclic compression of skeletal muscle after an intense bout of EEX enhances recovery of muscle and joint function. Moreover, 4 d of cyclic compression reduced muscle edema and decreased leukocyte infiltration and muscle damage.
To our knowledge, this is the first study to systematically examine the effects of cyclic compressive loading and improvement in function after exercise-induced skeletal muscle damage. From a theoretical standpoint, this is perhaps due to challenges in formulating constitutive equations that can completely describe the viscoelastic response of the tissue to both tension and compression (2). Nevertheless, understanding the tolerance of skeletal muscle to compressive forces is particularly important to the pathophysiology of problems such as pressure (decubitus) ulcers. In addition, from a therapeutic standpoint, compressive forces are applied to skeletal muscle with the use of massage therapies. Despite the frequency of their use and self-reported positive effects, there is a paucity of evidence for the efficacy of massage therapies to overcome soft tissue pain and weakness (10,15). This discrepancy between our current scientific understanding of the benefits of massage and its application to practice is noteworthy. Accordingly, a more complete understanding of the response of skeletal muscle to compressive loading may help in establishing a scientifically sound rationale for this therapy.
Massage therapies have been used to treat a wide variety of musculoskeletal conditions. Massage has been defined as “a mechanical manipulation of body tissues with rhythmical pressure and stroking for the purpose of promoting health and well-being” (10). According to the American Massage Therapy Association (AMTA; http://www.amtamassage.org), the physical benefits of therapeutic massage include relief of muscle tension and stiffness, faster healing of strained muscles and sprained ligaments, reduction in muscle pain and swelling, greater joint flexibility and range of motion, and even enhanced athletic performance. Despite the lack of conclusive scientific evidence, providers spend a large portion of their treatment time providing massage to athletes during major athletic events. In fact, up to 45% of total time in physiotherapy for sport-related injury and performance consists of massage treatments (15). For the most part, clinical studies to date have not provided conclusive evidence that massage can facilitate recovery of muscle function that occurs with delayed-onset muscle soreness and intense EEX.
We acknowledge that our device does not directly mimic therapeutic Swedish massage, the most common clinical strategy purported for treatment of soft tissue inflammation and pain (22). It is also important to note that three assumptions were made in calculating the average normal stress resulting from muscle compression. First, the force was uniformly applied on the piezo sensor. Second, we assumed no bending and torsion occurred. Finally, all areas of the piezo film were subjected to the applied forces. Because a cylindrical wheel was used to apply the compressive forces, assumptions 1 and 3 cannot be completely satisfied. Moreover, because the muscle is deformed as the force is applied and is not perfectly flat, bending and torsion will be induced in the sensor and, therefore, contribute to the observed voltage measurements. Accordingly, equation 2 cannot be completely satisfied but does provide a method of computing normal stress that was compared from animal to animal. In future studies, these assumptions will be more accurately validated with microfabricated electrostatic tactile sensors that will estimate the bulk forces applied to the muscle and the force distribution over the contact area. Nevertheless, the compressive forces applied to the rabbit TA do seem to be physiologically relevant to the forces applied during spinal manipulative therapy in humans. We also acknowledge that the 70% functional deficit produced by our bout of EEX may not necessarily replicate what occurs in humans after intense EEX.
We realize that our well-controlled in vivo model and device to apply a cyclic compressive load to the rabbit TA muscle approximate the human condition of EEX and the use of Swedish massage. For example, anatomic differences exist between humans and rabbits (amount of subcutaneous adipose tissue, muscle depth, skin thickness, etc.) that may limit the extrapolation of our findings. However, it is notable that the compressive loads measured (average 11.4 N) with our device are a reasonable approximation to humans when the data are scaled for anatomic differences (9.9 N). Given these differences and similarities, we believe that future studies that can better address some of the differences between humans and rabbits to perhaps make these results more directly translatable are warranted.
Interestingly, pilot work (data not shown) revealed that delaying the application of cyclic compressive loading for 24 h after the bout of EEX did not result in the same improvement of function as noted here, where the compressive loading was initiated within 30 min of the EEX. We speculate that this difference may be related to the attenuation of swelling and damage that resulted when cyclic compressive loading was initiated immediately after the EEX (see Fig. 4). We have observed that the exercised and cyclically compressed (EEXC) muscles weighed approximately 8% less than the exercised and rested (EEXR) muscles. Although plausible mechanisms have not been established, it is likely that proinflammatory mediators such as interleukin 1, tumor necrosis factor α, and inducible nitric oxide synthase may be involved in the regulation of muscle mass during both damage and repair (32). Alternatively, early intervention by cyclic compression may reduce the infiltration and thus the damage induced by neutrophils and macrophages via production of free radicals (3,6). Through this mechanism, early cyclic compressive loading may serve to limit edema formation, thus reducing the mass of the tissue. However, this hypothesis requires further testing.
Taken together, we have demonstrated that the rabbit EEX model generates a reproducible functional deficit to assess different interventions and permits an acceptable way of comparing muscle function both within and between-groups. Using this approach, we have demonstrated that rabbit skeletal muscle exhibits improved muscle function together with decreased damage and leukocyte infiltration after cyclic compressive loading. Furthermore, these observations suggest that skeletal muscle responds to cyclic compressive forces similar to those generated by clinical approaches such as therapeutic massage (25). However, direct translation of these results to human clinical models must be hypothesized at this time. Future studies are essential to examine the magnitude-, frequency-, and duration-dependent effects of cyclic compressive loading using a modified device that overcomes some of the limitations of the current apparatus.
Acknowledgments
The authors thank Thomas J. Knobloch and Priyangi Perera. This work was funded by the Ohio State University Pomerene Chair in Family Medicine and National Institutes of Health grant no. AT00646. The results of the present study do not constitute endorsement by the ACSM.
References
- 1.Ashley Z, Salmons S, Boncompagni S, et al. Effects of chronic electrical stimulation on long-term denervated muscles of the rabbit hind limb. J Muscle Res Cell Motil. 2007;28(4–5):203–17. doi: 10.1007/s10974-007-9119-4. [DOI] [PubMed] [Google Scholar]
- 2.Bosboom EM, Hesselink MK, Oomens CW, Bouten CV, Drost MR, Baaijens FP. Passive transverse mechanical properties of skeletal muscle under in vivo compression. J Biomech. 2001;34(10):1365–8. doi: 10.1016/s0021-9290(01)00083-5. [DOI] [PubMed] [Google Scholar]
- 3.Brickson S, Ji LL, Schell K, Olabisi R, St Pierre SB, Best TM. M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J Appl Physiol. 2003;95(3):969–76. doi: 10.1152/japplphysiol.00005.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bryer SC, Koh TJ. Mechanical strain increases gene transfer to skeletal muscle cells. J Biomech. 2006;40(9):1995–2001. doi: 10.1016/j.jbiomech.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield TA, Herzog W. Effect of altering starting length and activation timing of muscle on fiber strain and muscle damage. J Appl Physiol. 2005;100(5):1489–98. doi: 10.1152/japplphysiol.00524.2005. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457–65. [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield TA, Herzog W. Is the force–length relationship a useful indicator of contractile element damage following eccentric exercise? J Biomech. 2005;38(9):1932–7. doi: 10.1016/j.jbiomech.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield TA, Herzog W. Quantification of muscle fiber strain during in-vivo repetitive stretch-shortening cycles. J Appl Physiol. 2005;99:593–602. doi: 10.1152/japplphysiol.01128.2004. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield TA, Herzog W. The magnitude of muscle strain does not influence serial sarcomere number adaptations following eccentric exercise. Pflugers Arch. 2006;451(5):688–700. doi: 10.1007/s00424-005-1503-6. [DOI] [PubMed] [Google Scholar]
- 10.Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise. An overview. Sports Med. 1992;14(1):1–9. doi: 10.2165/00007256-199214010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Chandran R, Knobloch TJ, Anghelina M, Agarwal S. Biomechanical signals upregulate myogenic gene induction in the presence or absence of inflammation. Am J Physiol Cell Physiol. 2007;293(1):C267–76. doi: 10.1152/ajpcell.00594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friden J, Sjostrom M, Ekblom B. A morphological study of delayed muscle soreness. Experientia. 1981;37(5):506–7. doi: 10.1007/BF01986165. [DOI] [PubMed] [Google Scholar]
- 13.Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170–6. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 14.Furlan AD, Brosseau L, Imamura M, Irvin E. Massage for low-back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2002;27(17):1896–910. doi: 10.1097/00007632-200209010-00017. [DOI] [PubMed] [Google Scholar]
- 15.Galloway SD, Watt JM. Massage provision by physiotherapists at major athletics events between 1987 and 1998. Br J Sports Med. 2004;38(2):235–6. doi: 10.1136/bjsm.2002.003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldspink G, Williams P, Simpson H. Gene expression in response to muscle stretch. Clin Orthop. 2002;403(Suppl):S146–52. doi: 10.1097/00003086-200210001-00017. [DOI] [PubMed] [Google Scholar]
- 17.Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(4):443–50. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- 18.Hornberger TA, Armstrong DD, Koh TJ, Burkholder TJ, Esser KA. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am J Physiol Cell Physiol. 2005;288(1):C185–94. doi: 10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- 19.Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand. 2002;176(3):215–25. doi: 10.1046/j.1365-201X.2002.t01-2-01004.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanchiku T, Taguchi T, Kaneko K, Yonemura H, Kawai S, Gondo T. A new rabbit model for the study on cervical compressive myelopathy. J Orthop Res. 2001;19(4):605–13. doi: 10.1016/S0736-0266(00)00058-9. [DOI] [PubMed] [Google Scholar]
- 21.Lieber RL, Friden J. Morphologic and mechanical basis of delayed-onset muscle soreness. J Am Acad Orthop Surg. 2002;10(1):67–73. [PubMed] [Google Scholar]
- 22.Lund I. Massage as a pain relieving method. Physiotherapy. 2007;86(12):638–54. [Google Scholar]
- 23.MacIntyre DL, Sorichter S, Mair J, Berg A, McKenzie DC. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol. 2001;84(3):180–6. doi: 10.1007/s004210170002. [DOI] [PubMed] [Google Scholar]
- 24.McAnulty S, McAnulty L, Nieman D, Morrow J, Dumke C, Henson D. Effect of NSAID on muscle injury and oxidative stress. Int J Sports Med. 2007;28(11):909–15. doi: 10.1055/s-2007-964966. [DOI] [PubMed] [Google Scholar]
- 25.Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol. 2001;90(5):1936–42. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 26.Russell B, Motlagh D, Ashley WW. Form follows function: how muscle shape is regulated by work. J Appl Physiol. 2000;88(3):1127–32. doi: 10.1152/jappl.2000.88.3.1127. [DOI] [PubMed] [Google Scholar]
- 27.Sarac AJ, Gur A. Complementary and alternative medical therapies in fibromyalgia. Curr Pharm Des. 2006;12(1):47–57. [PubMed] [Google Scholar]
- 28.Sellwood KL, Brukner P, Williams D, Nicol A, Hinman R. Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial. Br J Sports Med. 2007;41(6):392–7. doi: 10.1136/bjsm.2006.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LL, Brunetz MH, Chenier TC, et al. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res Q Exerc Sport. 1993;64(1):103–7. doi: 10.1080/02701367.1993.10608784. [DOI] [PubMed] [Google Scholar]
- 30.Stay JC, Richard MD, Draper DO, Schulthies SS, Durrant E. Pulsed ultrasound fails to diminish delayed-onset muscle soreness symptoms. J Athl Train. 1998;33(4):341–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Stekelenburg A, Strijkers GJ, Parusel H, Bader D, Nicolay K, Oomens C. The role of ischemia and deformation in the onset of compression-induced deep tissue injury: MRI-based studies in a rat model. J Appl Physiol. 2007;102(5):2002–11. doi: 10.1152/japplphysiol.01115.2006. [DOI] [PubMed] [Google Scholar]
- 32.Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98(5):1900–8. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- 33.Tiidus PM, Shoemaker JK. Effleurage massage, muscle blood flow and long-term post-exercise strength recovery. Int J Sports Med. 1995;16(7):478–83. doi: 10.1055/s-2007-973041. [DOI] [PubMed] [Google Scholar]
- 34.Tourville TW, Connolly DA, Reed BV. Effects of sensory-level high-volt pulsed electrical current on delayed-onset muscle soreness. J Sports Sci. 2006;24(9):941–9. doi: 10.1080/02640410500357226. [DOI] [PubMed] [Google Scholar]
- 35.van den Dolder PA, Roberts DL. A trial into the effectiveness of soft tissue massage in the treatment of shoulder pain. Aust J Physiother. 2003;49(3):183–8. doi: 10.1016/s0004-9514(14)60238-5. [DOI] [PubMed] [Google Scholar]
- 36.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–56. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]


