Supplemental Digital Content is available in the text.
Keywords: arteriosclerosis, cohort studies, coronary artery bypass, coronary vessel, incidence
Abstract
Background—
Bilateral internal thoracic arteries (BITA) have demonstrated superior patency and improved survival in patients undergoing coronary artery bypass grafting. However, the optimal configuration for BITA utilization and its effect on long-term outcome remains uncertain.
Methods and Results—
We randomly assigned 304 patients undergoing coronary artery bypass grafting using BITA to either in situ or Y grafting configurations. The primary end point was 3-year angiographic patency. Secondary end points included major adverse cardiac and cerebrovascular events (ie, death from any cause, stroke, myocardial infarction, or repeat revascularization) at 7 years. More coronary targets were able to be revascularized using internal thoracic arteries in patients randomized to Y grafting versus in situ group (3.2±0.8 versus 2.4±0.5 arteries/patient; P<0.01). The primary end point did not show significant differences in graft patency between groups. Secondary end points occurred more frequently in the in situ group (P=0.03), with 7-year rates of 34±10% in the in situ and 25±12% in the Y grafting groups, driven largely by a higher incidence of repeat revascularization in the in situ group (14±4.5% versus 7.4±3.2% at 7 years; P=0.009). There were no significant differences in hospital mortality or morbidity or in late survival, myocardial infarction, or stroke between groups.
Conclusions—
Three-year systematic angiographic follow-up revealed no significant difference in graft patency between the 2 BITA configurations. However, compared with in situ configuration, the use of BITA in a Y grafting configuration results in lower rates of major adverse cardiovascular and cerebrovascular events at 7 years.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01666366.
WHAT IS KNOWN
Bilateral internal thoracic arteries grafting increases survival and decreases long-term major adverse cardiac cerebrovascular events.
WHAT THE STUDY ADDS
The use of the 2 internal thoracic arteries in a Y composite configuration decreases the long-term major adverse cardiac cerebrovascular events compared with the in situ configuration.
Multiple observational studies have demonstrated the superiority of bilateral internal thoracic arteries (BITA) compared with all other types of conduits in patients undergoing coronary artery bypass surgery.1 These benefits include increased short- and long-term patency, freedom from arteriosclerosis, and a survival benefit for revascularization of the left coronary system.2 Multiple cohort studies have demonstrated the 20-year survival benefits of bilateral versus single internal thoracic artery (ITA) grafting.3 Seven to 10 years of follow-up was required before the advantages of BITA grafting were apparent, but from 10 to 20 years, the benefits of BITA are statistically and clinically significant.4
The use of the left ITA to the left anterior descending (LAD) coronary artery has been shown to improve long-term survival in multiple studies and is the gold standard for revascularization of the LAD. However, the optimal utilization of the right ITA for the non-LAD circulation remains controversial. Two configurations of the right ITA are most frequently used (Figure 1). The first is in situ configuration. This approach allows the ITA to remain attached to its origin but only a single, more proximal branch of the circumflex can be revascularized because of limited conduit length. An alternative approach is that of Y grafting in which the free right ITA is proximally connected to the left ITA. This allows for multiple branches of the circumflex coronary artery and some branches of the right coronary artery to be revascularized with the ITA in a sequential manner.
Figure 1.
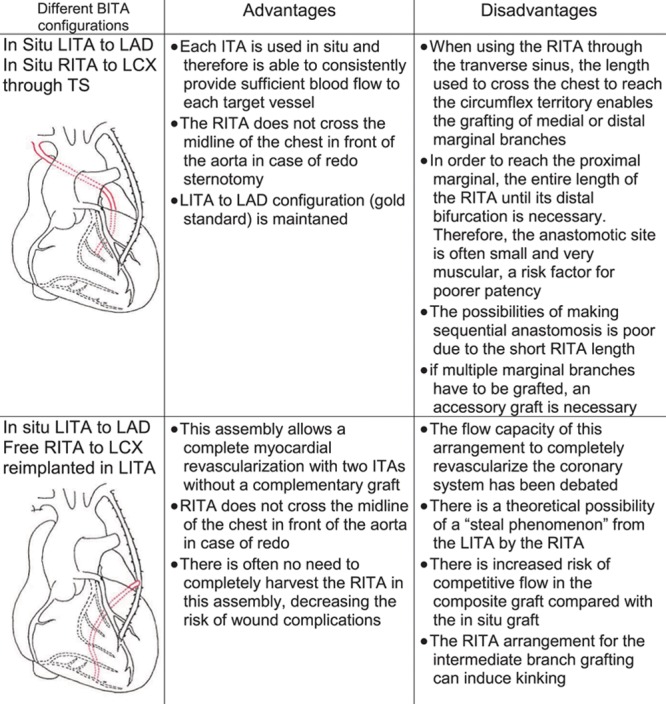
Advantages and disadvantages of the 2 bilateral internal thoracic artery (BITA) configurations. CABG indicates coronary artery bypass grafting; ITA, internal thoracic artery; LAD, left anterior descending; LCX, left circumflex artery; LITA, left internal thoracic artery; RITA, right internal thoracic artery; and TS, transverse sinus.
The purpose of this single-center, prospective, randomized trial of in situ versus Y grafting strategy for BITA utilization was to determine which strategy optimizes long-term angiographic and clinical outcomes. The primary end point was the graft patency and function at 3-year angiographic follow-up. Secondary end points included incidence of major adverse cardiac and cerebrovascular events at 7 years.
Methods
Study Design and Patients
Of 1297 patients undergoing isolated surgical coronary revascularization from April 2003 to July 2006, 481 patients (37%) met the eligibility criteria for the study. Of these, 304 patients (67%) were randomized 1:1 to 1 of the 2 surgical strategies. Reasons for nonrandomization included (1) refusal of systematic 6-month and 3-year follow-up angiography (85%) and (2) logistic incapacity to randomize patients (15%; Figure 2). In all patients, the left ITA was used to revascularize the LAD and its branches. In group IS, the right ITA was used in an in situ fashion to revascularize the obtuse marginal branches of the circumflex artery through the transverse sinus. In group Y, the right ITA was anastomosed proximally to the left internal thoracic artery in a Y configuration and distally to multiple branches of the circumflex and right coronary artery branches in a sequential fashion. Inclusion and exclusion criteria are detailed in the Materials section in the Data Supplement.
Figure 2.
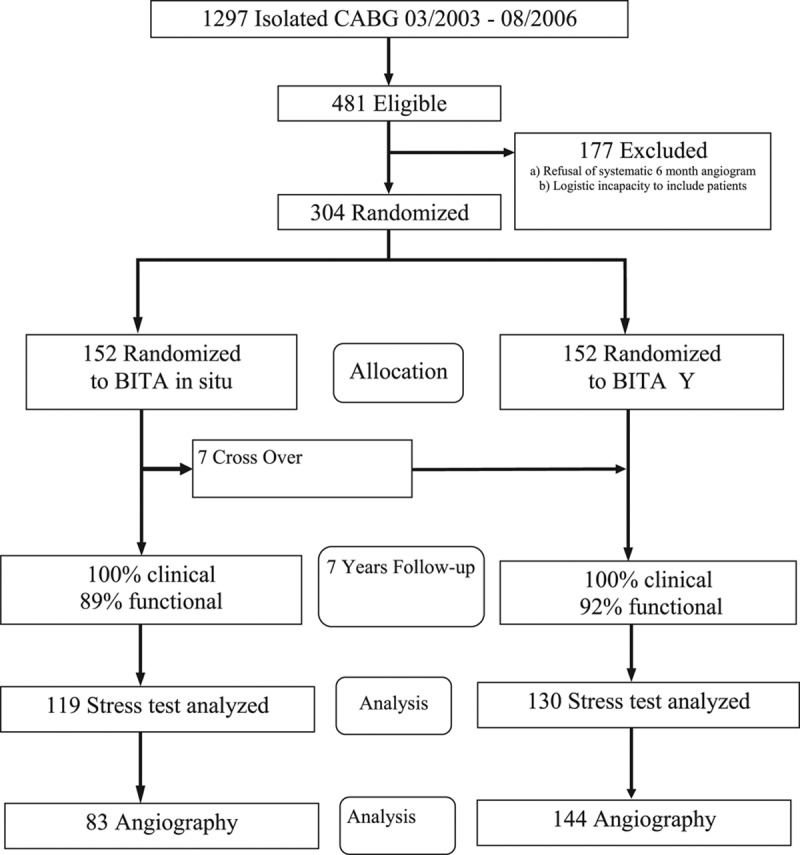
Consort flow chart of the trial. BITA indicates bilateral internal thoracic artery; and CABG, coronary artery bypass grafting.
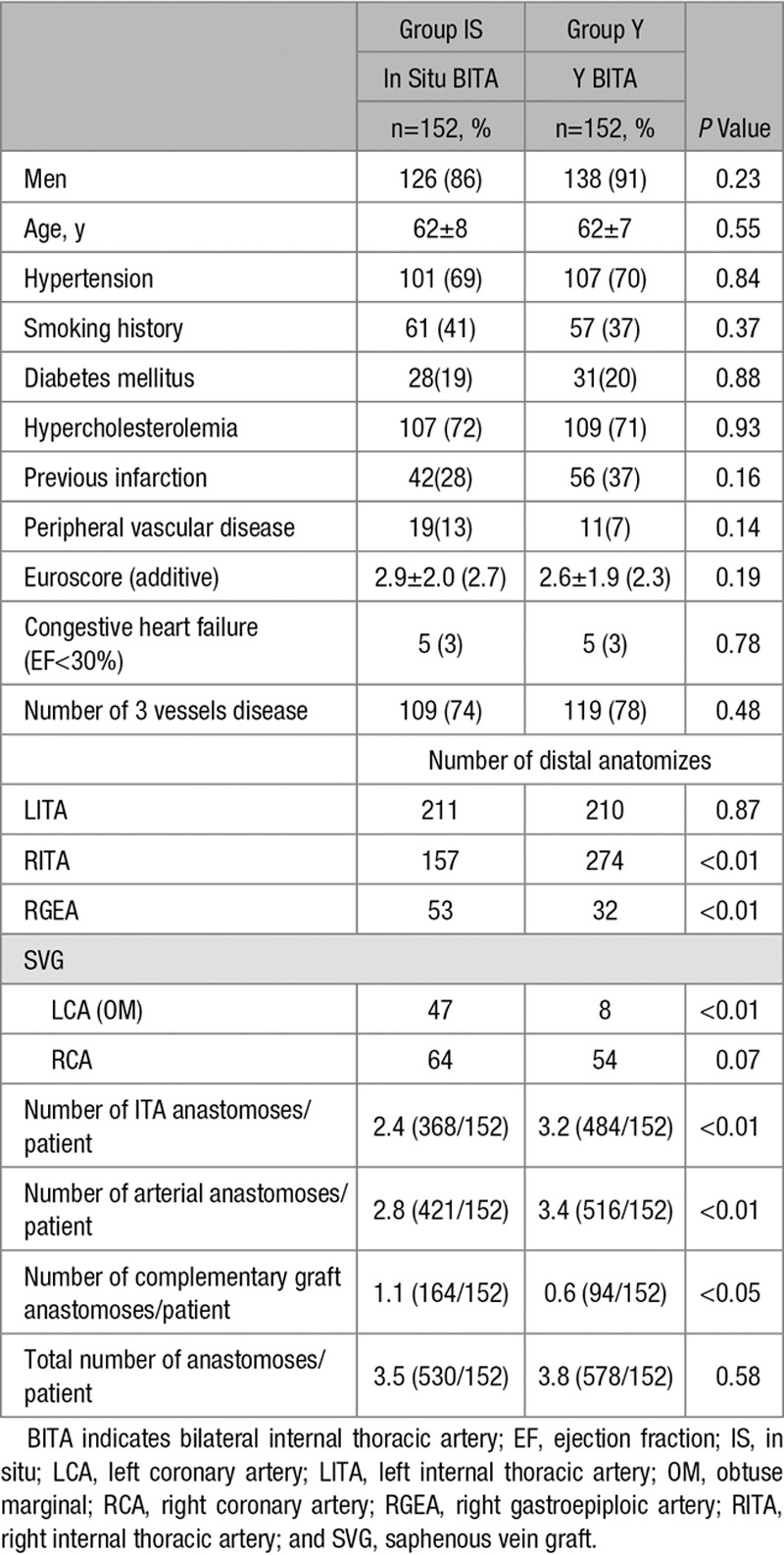
Seven patients initially allocated to group IS were crossed over to the Y grafting group. In these cases, the surgeon decided to deviate from the assigned revascularization strategy in favor of a BITA Y configuration (Figure 2). The major reason for this was insufficient length of the right internal thoracic artery (RITA) through the transverse sinus to reach the targeted marginal. Preoperative and operative patients’ characteristics are detailed in Table 1.
Table 1.
Patients and Operative Characteristics
Randomization and Study Procedures
Randomization was performed the day before the operation using randomly generated numbers contained in sealed, opaque envelopes.
All surgical procedures were performed via a median sternotomy approach using cardiopulmonary bypass. Harvest of both internal thoracic arteries was performed in a semi-pedicled fashion. Distal coronary anastomoses were performed on cardiopulmonary bypass and with cardioplegic arrest in all patients. All epicardial coronary artery branches with a stenosis >50% and a diameter >1 mm were grafted. In both groups, the aim was to use the internal thoracic arteries to preferentially revascularize the largest branches of the left coronary system. The surgical technique has been described by Dion et al.5 Complementary grafting of coronary artery branches not revascularized with the internal thoracic arteries was not specified by the study protocol and performed with either a saphenous vein graft or an in situ right gastroepiploic artery depending on the location and quality of the target coronary vessel and on the surgeon’s choice.
All patients gave written informed consent before enrollment in the study and before the angiographic investigation. The study protocol was approved by the Research Ethics Committee and by the internal review board at our institution.
All patients, referring cardiologists, and the research nurses conducting follow-up were also blinded to the treatment allocation. The surgeon and the interventional cardiologist responsible for the systematic angiographic control were not blind to the treatment assignment.
Postoperative Management and Follow-Up
Patients received prophylactic, low-dose, fractionated heparin postoperatively and 80 to 160 mg of aspirin daily starting on postoperative day 2. Study electrocardiograms were obtained preoperatively and on days 1 and 5 postoperatively. Patients were interviewed by telephone at 1 month, 3 months, 6 months, and twice yearly thereafter by a research nurse. If the patient had been hospitalized between interviews, inpatient records were obtained. Patients underwent a systematic stress test on a cycloergometer performed twice a year under the supervision of their referring cardiologist. All patients were scheduled to undergo a protocol-driven angiogram at 6 months and 3 years after surgery to assess graft patency.
Study End Points
The primary clinical end point was the proportion of ITA graft and anastomosis patent at systematic angiographic follow-up. Perfect patency was defined as a complete opacification of the graft or the anastomosis during antegrade contrast injection. A graft was defined as patent but not fully functional if one of the anastomoses in a sequential graft was found occluded or there was presence of a string sign. The secondary end point was occurrence of major adverse cardiac cerebrovascular events (MACCE) defined as a combined end point including death from any cause, perioperative myocardial infarction (occurring between 0 and 30 days), late myocardial infarction (occurring between 31 days and 7 years), additional surgical coronary revascularization, percutaneous coronary intervention (PCI), and stroke. Myocardial infarction was defined as the appearance of a new Q wave, a rise of >10 ng/mL of troponin in the early postoperative period, or any episode of chest pain with typical rise and fall of cardiac enzymes thereafter. Stroke was defined as a focal neurological deficit of central origin lasting >24 hours confirmed with brain imaging.
Statistical Analysis
We calculated that the enrollment of at least 152 patients per group would provide the study with 80% power to detect a absolute reduction of 8% in the patency rate, from an estimated 10% with BITA in situ5 to 2% with BITA Y grafting6 assuming a within-patient correlation of 0.2 for graft occlusion, a 2-tailed test, and an α value of 0.05. Continuous data are expressed as the mean±SD. The primary patency end point was analyzed using χ2 test. Each component of the MACCE end point was compared between the 2 groups using the Kaplan–Meier method. All probability values are 2-tailed. The Statistical Analysis Software (SAS, 9.1 releases; SAS Institute, Inc) was used for the statistical analysis. All patients were analyzed in an intention-to-treat analysis.
Results
Angiographic follow-up was 75% complete with a mean clinical follow-up of 3.4±2.1 years in group IS and 3±1.7 in group Y (P=0.1). We did not find a significant difference in the percentage of patent ITA graft between group IS and group Y (left internal thoracic artery: 98.2 versus 97.2%; P=0.96 and RITA: 93 versus 96.5%; P=0.1) We also found no differences in the percentage of anastomotic ITA patency between group IS and group Y (left internal thoracic artery: 98.2 versus 96.8%; P=0.96 and RITA: 93 versus 94.5%; P=0.81).
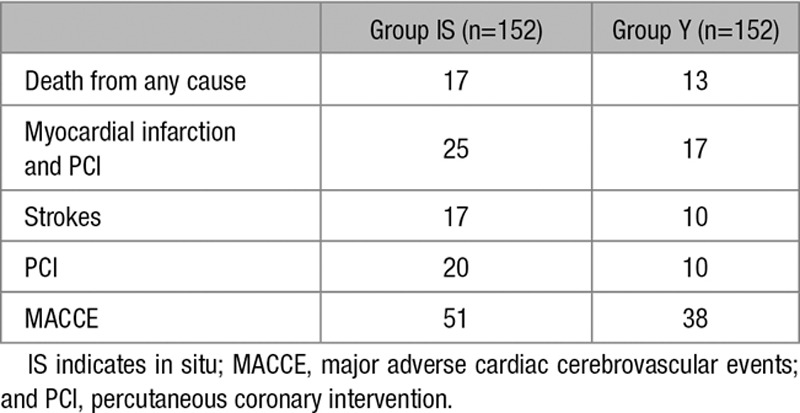
Clinical follow-up was 100% complete with a mean clinical follow-up of 7±2.6 years in group IS and 7±1.3 years in group Y (P=0.1). Seven-year mortality rate in group IS was 11.1±2.7% and 8.9±2.6% in group Y (P=0.3). The cumulative MACCE rate between the 2 groups was significantly lower in group Y (P=0.03; 7-year MACCE rate: 34±3.9% in group IS and 25±3.6% in group Y; Figure 3).
Figure 3.
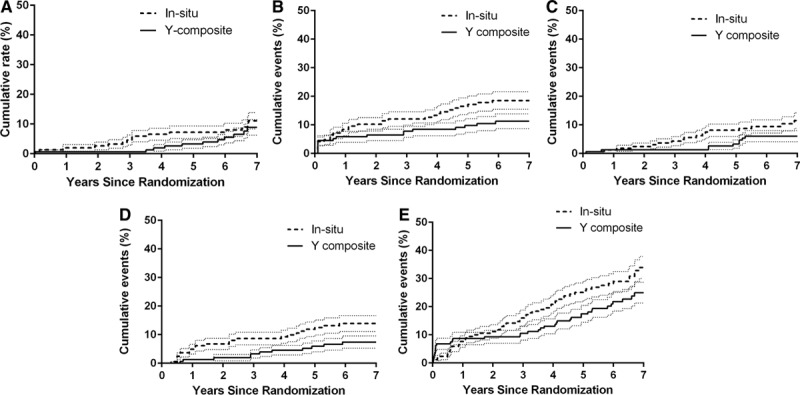
Kaplan–Meier major adverse cardiac cerebrovascular event long-term comparison. A, Overall death event rate (P=0.3). B, Myocardial infarction and percutaneous coronary intervention event rate (P=0.02). C, Stroke event rate (P=0.9). D, Revascularization event rate (P<0.01). E, Major adverse cardiac and cerebrovascular event rates (0.04). *Significant P value. The small dotted lines indicate 1.5 SE.
Combined myocardial infarction and revascularization rate during the follow-up period was higher in group IS (P=0.01; 18.5±3% in group IS versus 11.3±2.5% in group Y at 7 years). Stroke rate during the follow-up was similar between groups (P=0.2; 11.5±2.3% versus 6±1.9% in group IS versus group Y at 7 years). Re-revascularization rate during the follow-up was higher in group IS (P=0.009; 14±4.5% in group IS versus 7.4±3.2% in group Y at 7 years). There were no differences in the distribution of PCI performed on the left coronary system between the 2 groups (P=0.3), but there were a significant higher revascularization event rate for the right coronary system in the in situ group (group IS: 13 versus group Y: 3; P=0.01). There was also no significant difference in the proportion of PCI performed on coronary vessels initially revascularized by an ITA. There was a significant reduction in the proportion of PCI performed on coronary vessels not initially revascularized by an ITA in the Y group (group IS: 7 versus group Y: 0; P<0.01). The number of PCI on coronary arteries not initially revascularized was also not significantly different between the 2 groups (P=0.19; Table 2). Detailed outcomes are presented in Table 3.
Table 2.
Details of Reinterventions Events
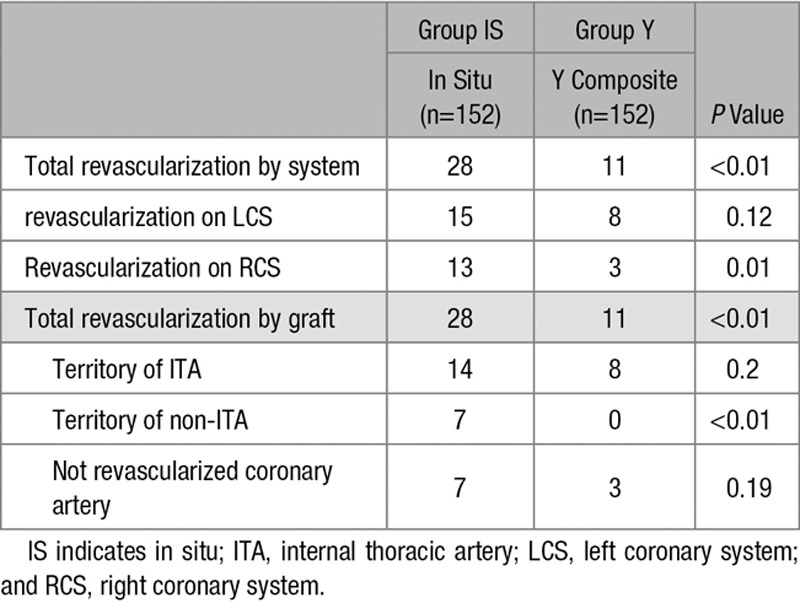
Table 3.
Outcome Detail During the Follow-Up Period
Discussion
In this large, single-center, prospective randomized clinical trial, we found a significant clinical difference in favor of the patients who underwent revascularization with BITA as a composite Y graft configuration.
Multiple observational studies have demonstrated a survival benefit 10 years postoperatively in patients with bilateral versus single ITA revascularization.1
Once a decision has been made to use both ITA, the manner in which the ITA is used is still controversial.7,8 Some propose in situ graft use, whereas others prefer free graft use. When used free, the choice must be made to reimplant the graft in the ascending aorta or in another graft in a composite fashion. Moreover, some suggest using only one graft per distal anastomosis, whereas others prefer sequential anastomoses, to revascularize multiple coronary targets with the same conduit.
Several authors have compared the in situ versus composite configuration in retrospective nonrandomized studies: Calafiore et al6 compared 1818 patients with BITA grafting used as in situ configuration to 440 patients with BITA Y composite grafting. The number of ITA anastomoses per patient was significantly higher in the composite group than in the in situ group. The authors have concluded that at 2.5 years postoperatively, the MACCE rate events was similar for BITA used either in-situ or as Y composite grafts. However, Y grafting increases the number of anastomoses per BITA and the flexibility of the right ITA. We confirmed these results at 3 years of mean follow-up in the initial results of this randomized trial.9
Hwang et al10 have also compared the 2 configurations without finding a significant MACCE difference at 5 years of follow-up. However, in their protocol, a Y composite graft was constructed when the right ITA was too short to reach the left coronary artery territory or if the left coronary artery territory could not be completely revascularized with bilateral in situ ITA grafts. Consequently, no complementary graft was needed to achieve complete revascularization of the left system, which is not the case in our study.
To the best of our knowledge, our study represents the first prospective randomized comparison of the long-term outcome of 2 BITA configurations. The clinical benefit of the composite Y configuration seems independent of the quality of revascularization provided by the ITA graft itself but seems related to the poor long-term outcome of complementary graft, more frequently needed with the in situ configuration. This suggests that the quality of surgical revascularization could be optimized either by decreasing the number of coronary segments that require a complementary third graft or by using as complementary third graft a conduit of superior quality.
In our study, the mean number of anastomoses with the RITA was 1.8 in the Y composite group versus 1 in the in situ group showing the need for a complementary graft in the in situ group. Furthermore, the number of complementary graft anastomoses needed in the Y group was significantly less than that in the IS group. The question of which complementary graft to use in this specific condition has been partially answered by Desai et al.11 in the RAPTCO trial comparing radial and saphenous vein graft. They concluded that radial graft patency at 1 year was better than saphenous vein graft patency. This advantage, however, was reliably seen only when the degree of proximal coronary artery stenosis was severe (>90%).
Another important aspect of the BITA Y configuration is the ability of this arrangement to reach the distal branches of the right coronary artery (RCA). In 40 patients, the distal end of the RITA was anastomosed to a distal branch of the RCA. The issue when anastomosing the distal branches of the RCA with the free RITA in a composite configuration is the risk of flow competition. Indeed, the longer the arterial configuration, the lower the pressure at the distal anastomosis, a phenomenon aggravated by the anatomic distal tapering of arteries. Therefore, competitive flow is more frequent at the right coronary anastomosis of composite Y grafts because of the lower pressure in the distal portion of the Y branch.12
Nakajima et al13 reviewed angiograms of 2514 bypass grafts in patients using the ITA and radial artery in a composite fashion. The rate of antegrade flow in patients with severe (76% to 100%) stenosis in both native LAD and RCA was significantly higher than that in patients with severe (76% to 100%) stenosis in LAD and moderate (51% to 75%) stenosis in RCA. To secure entire patency of the Y graft to 3-vessel territories, balanced bypass graft flow toward LAD and RCA would be crucial.
Kim et al14 have described another grafting strategy consisting of the reimplantation of a saphenous vein graft in the left internal thoracic artery as a composite configuration. They described excellent patency result at 1 year angiographic follow-up. However, these results needs to be further evaluated especially at long term considering the natural vein attrition when placed in an arterial high pressure environment. Gaudino et al15 and our group16 have studied this venoarterial configuration without finding the same excellent patency results.
According to the American Society of Thoracic Surgeons Database, in >540 000 patients undergoing coronary artery bypass in 745 institutions, only 4% received BITA grafting.17 Although there are many possible contributors to this underutilization of BITA grafting, an important factor is the uncertainty about optimal configuration of the 2 internal mammary arteries. Therefore, the result of this study provides clear information on the ideal assembly of bilateral mammary arteries and may help increasing its use.
In conclusion, 3-year systematic angiographic follow-up revealed no significant difference in graft patency between the 2 BITA configurations. However, a composite bilateral ITA grafting provides significant long-term clinical benefit compared with conventional in situ grafting. Efforts should be directed toward a more efficient use of ITA, reducing the need for a third complementary graft, and toward identification of the best alternative to saphenous vein graft as the third graft material for complete revascularization of multivessel diseases.
Study Limitation
The rate of angiographic systematic control is only of 75% with a mean of 3.4 years postop. This could lead to a misinterpretation of the patency results. However, Buxton et al18 have clearly demonstrated the significantly better patency accuracy by systematic angiographic follow-up as that in our trial compared with symptom-driven angiograms.
Sources of Funding
This study was supported by grant no. 3.4600.04 from the Fonds de la recherché scientifique médicale, Brussels, Belgium.
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available at http://circinterventions.ahajournals.org/lookup/suppl/doi:10.1161/CIRCINTERVENTIONS.115.003518/-/DC1.
References
- 1.Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–875. doi: 10.1016/S0140-6736(01)06069-X. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 2.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LA, Gill CC, Taylor PC, Sheldon WC. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 3.Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation. 2014;130:539–545. doi: 10.1161/CIRCULATIONAHA.113.004255. doi: 10.1161/CIRCULATIONAHA.113.004255. [DOI] [PubMed] [Google Scholar]
- 4.Lytle BW. Prolonging patency–choosing coronary bypass grafts. N Engl J Med. 2004;351:2262–2264. doi: 10.1056/NEJMp048238. doi: 10.1056/NEJMp048238. [DOI] [PubMed] [Google Scholar]
- 5.Dion R, Glineur D, Derouck D, Verhelst R, Noirhomme P, El Khoury G, Degrave E, Hanet C. Long-term clinical and angiographic follow-up of sequential internal thoracic artery grafting. Eur J Cardiothorac Surg. 2000;17:407–414. doi: 10.1016/s1010-7940(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 6.Calafiore AM, Contini M, Vitolla G, Di Mauro M, Mazzei V, Teodori G, Di Giammarco G. Bilateral internal thoracic artery grafting: long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg. 2000;120:990–996. doi: 10.1067/mtc.2000.110249. doi: 10.1067/mtc.2000.110249. [DOI] [PubMed] [Google Scholar]
- 7.Dorman MJ, Kurlansky PA, Traad EA, Galbut DL, Zucker M, Ebra G. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation. 2012;126:2935–2942. doi: 10.1161/CIRCULATIONAHA.112.117606. doi: 10.1161/CIRCULATIONAHA.112.117606. [DOI] [PubMed] [Google Scholar]
- 8.Sabik JF, 3rd, Stockins A, Nowicki ER, Blackstone EH, Houghtaling PL, Lytle BW, Floyd D. Does location of the second internal thoracic artery graft influence outcome of coronary artery bypass grafting? Circulation. 2008;118(suppl 14):S210–S215. doi: 10.1161/CIRCULATIONAHA.107.760827. doi: 10.1161/CIRCULATIONAHA.107.760827. [DOI] [PubMed] [Google Scholar]
- 9.Glineur D, Hanet C, Poncelet A, D’hoore W, Funken JC, Rubay J, Kefer J, Astarci P, Lacroix V, Verhelst R, Etienne PY, Noirhomme P, El Khoury G. Comparison of bilateral internal thoracic artery revascularization using in situ or Y graft configurations: a prospective randomized clinical, functional, and angiographic midterm evaluation. 2008;118(suppl 14):S216–S221. doi: 10.1161/CIRCULATIONAHA.107.751933. Circulation. doi: 10.1161/CIRCULATIONAHA.107.751933. [DOI] [PubMed] [Google Scholar]
- 10.Hwang HY, Kim JS, Cho KR, Kim KB. Bilateral internal thoracic artery in situ versus y-composite graftings: five-year angiographic patency and long-term clinical outcomes. Ann Thorac Surg. 2011;92:579–585, discussion 585. doi: 10.1016/j.athoracsur.2011.03.145. doi: 10.1016/j.athoracsur.2011.03.145. [DOI] [PubMed] [Google Scholar]
- 11.Desai ND, Cohen EA, Naylor CD, Fremes SE Radial Artery Patency Study Investigators. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302–2309. doi: 10.1056/NEJMoa040982. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 12.Glineur D, D’hoore W, El Khoury G, Sondji S, Kalscheuer G, Funken JC, Rubay J, Poncelet A, Astarci P, Verhelst R, Noirhomme P, Hanet C. Angiographic predictors of 6-month patency of bypass grafts implanted to the right coronary artery a prospective randomized comparison of gastroepiploic artery and saphenous vein grafts. J Am Coll Cardiol. 2008;51:120–125. doi: 10.1016/j.jacc.2007.09.030. doi: 10.1016/j.jacc.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima H, Kobayashi J, Toda K, Fujita T, Shimahara Y, Kasahara Y, Kitamura S. Angiographic evaluation of flow distribution in sequential and composite arterial grafts for three vessel disease. Eur J Cardiothorac Surg. 2012;41:763–769. doi: 10.1093/ejcts/ezr057. doi: 10.1093/ejcts/ezr057. [DOI] [PubMed] [Google Scholar]
- 14.Kim KB, Hwang HY, Hahn S, Kim JS, Oh SJ. A randomized comparison of the Saphenous Vein Versus Right Internal Thoracic Artery as a Y-Composite Graft (SAVE RITA) trial: one-year angiographic results and mid-term clinical outcomes. J Thorac Cardiovasc Surg. 2014;148:901–907, discussion 907. doi: 10.1016/j.jtcvs.2014.03.057. doi: 10.1016/j.jtcvs.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Gaudino M, Alessandrini F, Pragliola C, Luciani N, Trani C, Burzotta F, Girola F, Nasso G, Guarini G, Possati G. Composite Y internal thoracic artery-saphenous vein grafts: short-term angiographic results and vasoreactive profile. J Thorac Cardiovasc Surg. 2004;127:1139–1144. doi: 10.1016/j.jtcvs.2003.07.051. doi: 10.1016/j.jtcvs.2003.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Glineur D, Boodhwani M, Poncelet A, De Kerchove L, Etienne PY, Noirhomme P, Deceuninck P, Michel X, El Khoury G, Hanet C. Comparison of fractional flow reserve of composite Y-grafts with saphenous vein or right internal thoracic arteries. J Thorac Cardiovasc Surg. 2010;140:639–645. doi: 10.1016/j.jtcvs.2009.11.013. doi: 10.1016/j.jtcvs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Tabata M, Grab JD, Khalpey Z, Edwards FH, O’Brien SM, Cohn LH, Bolman RM., 3rd Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery: analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation. 2009;120:935–940. doi: 10.1161/CIRCULATIONAHA.108.832444. doi: 10.1161/CIRCULATIONAHA.108.832444. [DOI] [PubMed] [Google Scholar]
- 18.Buxton BF, Durairaj M, Hare DL, Gordon I, Moten S, Orford V, Seevanayagam S. Do angiographic results from symptom-directed studies reflect true graft patency? Ann Thorac Surg. 2005;80:896–900, discussion 900. doi: 10.1016/j.athoracsur.2005.03.097. doi: 10.1016/j.athoracsur.2005.03.097. [DOI] [PubMed] [Google Scholar]


