Abstract
Objective:
Candida esophagitis belongs to the most common AIDS-defining diseases; however, a comprehensive immune pathogenic concept is lacking.
Design:
We investigated the immune status of 37 HIV-1-infected patients from the Swiss HIV cohort study at diagnosis of Candida esophagitis, 1 year before, 1 year later and after 2 years of suppressed HIV RNA. We compared these patients with three groups: 37 HIV-1-infected patients without Candida esophagitis but similar CD4+ cell counts as the patients at diagnosis (advanced HIV group), 15 HIV-1-infected patients with CD4+ cell counts higher than 500 cells/μl, CD4+ cell nadirs higher than 350 cells/μl and suppressed HIV RNA under combination antiretroviral therapy (cART) (early cART group) and 20 healthy individuals.
Methods:
We investigated phenotype, cytokine production and proliferative capacity of different immune cells by flow cytometry and enzyme-linked immunosorbent spot.
Results:
We found that patients with Candida esophagitis had nearly abolished CD4+ cell proliferation in response to Candida albicans, significantly increased percentages of dysfunctional CD4+ cells, significantly decreased cytotoxic natural killer cell counts and peripheral innate lymphoid cell counts and significantly reduced IFN-γ and IL-17 production compared with the early cART group and healthy individuals. Most of these defects remained for more than 2 years despite viral suppression. The advanced HIV group without opportunistic infection showed partly improved immune recovery.
Conclusion:
Our data indicate that Candida esophagitis in HIV-1-infected patients is caused by an accumulation of multiple, partly Candida-specific immunological defects. Long-term immune recovery is impaired, illustrating that specific immunological gaps persist despite cART. These data also support the rationale for early cART initiation to prevent irreversible immune defects.
Keywords: Candida esophagitis, early combination antiretroviral therapy, HIV, IL-17 response, long-term immune recovery, proliferative impairment
Introduction
The risk of opportunistic infections in patients with HIV infection has markedly declined since 1996 because of the widespread use of combination antiretroviral therapy (cART) [1]. Nevertheless, opportunistic infections still remain a leading complication with an incidence of 16% in late presenting patients [2]. Absolute CD4+ cell counts less than 200 cells/μl and uncontrolled HIV RNA replication are well described major risk factors for the development of opportunistic infection, yet they also occur in patients with CD4+ cell counts higher than 200 cells/μl with an incidence of 10.5 per 1000 patient-years follow-up, highlighting that apart from the absolute CD4+ cell counts, additional risk factors for opportunistic infection must be present [3]. This is further supported by recent studies documenting that early initiation of cART at CD4+ cell counts higher than 500 cells/μl is beneficial as it significantly reduces the risk for opportunistic infection and malignancies [4,5], yet opportunistic infections are not completely eliminated. It remains uncertain why certain HIV-infected patients are susceptible to specific opportunistic infections and how the infection influences long-term immune recovery.
Candida esophagitis is one of the most common AIDS-defining diseases, occurring in up to 10–15% of HIV-infected patients before introduction of cART [1,6,7]. Importantly, Candida esophagitis is often the first opportunistic infection and also develops in patients with rather high CD4+ cell counts suggesting that the functionality of immune responses is diminished [7].
Earlier studies considered that susceptibility to Candida esophagitis is enhanced by a lack of protective Th1 responses and/or a shift to Th2 responses [8]. However, recent studies show that individuals with impaired IL-17 responses exhibit enhanced susceptibility to chronic mucocutaneous candidiasis [9]. In the context of HIV, progressive infection is accompanied by continuous loss of Th17 cells [10] and a decrease in the ratio of Th17 to Th1 cells in peripheral blood [11]. Recently, it has been demonstrated in a mouse model of oropharyngeal candidiasis that IL-17 secreting RORγt+ type 3 innate lymphoid cells (ILCs) also contribute to fungal clearance [12]. Moreover, natural killer (NK) cells are increasingly considered as part of the host defense against fungi [13], and their function was shown to be impaired against Cryptococcus neoformans in HIV-infected patients [14].
In this study, we took the advantage of prospectively stored patient samples within the Swiss HIV Cohort Study (SHCS) and investigated the numbers and functions of different immune cell subsets in patients with Candida esophagitis over a longitudinal follow-up, including samples before disease development and after long-term suppression of HIV RNA and compared them with three groups of individuals, including HIV-infected patients with similarly advanced HIV infection without opportunistic infection, HIV-infected patients that initiated cART at CD4+ cell nadirs higher than 350 cells/μl and were HIV RNA suppressed and healthy individuals.
Methods
Patients and healthy blood donors
The Swiss HIV Cohort Study is a large prospective observational cohort study with continuous enrolment of adult HIV-infected individuals initiated in 1988 and approved by the local institutional review boards [15]. Basic socio-demographic characteristics, data on clinical course, antiretroviral therapy, immunologic and virologic parameters are collected at enrolment and every 6 months thereafter. Viable peripheral blood mononuclear cells (PBMC) and plasma are stored every 6–12 months. Ethical approval and written informed consent from all patients enrolled in the SHCS have been obtained.
The diagnosis Candida esophagitis was based on clinical findings defined according to Centers for Disease Control and Prevention (CDC) criteria [16]. From January 2000 until December 2013, 465 HIV-1 infected patients were diagnosed with Candida esophagitis. Of these, 277 patients had Candida esophagitis as first and only AIDS-defining disease. Of these, 37 patients with available longitudinal PBMC were included. We analyzed cryopreserved PBMC from three time points: 6–18 months before diagnosis, at diagnosis (±6 months) and 6–18 months after diagnosis. For patients with suppressed HIV RNA (<50 copies/ml) over 2 years, an additional time point was included. These patients were compared with three groups. First, HIV-1-infected patients with similarly advanced disease but without opportunistic infection. Patients were matched to Candida esophagitis patients according to CD4+ cell counts (±25 cells/μl), date of diagnosis of Candida esophagitis, use of cART, sex, age and absence of other opportunistic infection within 6 months prior to sample collection [17]. As for the Candida esophagitis patients, four time points were analyzed. Second, 15 SHCS patients with well controlled HIV-1 infection from outpatients of the HIV clinic at the University Hospital of Basel. Patients had the following criteria: HIV CDC A1 or A2 classification with suppressed HIV RNA (<50 copies/ml) and stable cART therapy for at least 6 months, CD4+ cell counts higher than 500 cells/μl and CD4+ cell nadirs higher than 350 cells/μl. Third, 20 healthy individuals after receipt of informed consent according to the ethic approval from the Ethikkommission Nordwest und Zentralschweiz (EKBB 242/11). For both latter groups, only one time point was analyzed. Baseline characteristics of patients and healthy individuals are included in the Supplementary table.
Generation of heat-inactivated C. albicans
A mixture of Candida albicans strain SC5314 yeast and hyphae was cultured and heat-inactivated as previously described [18,19].
Phenotypic characterization
T-cell activation/exhaustion was analyzed by staining with anti-CD3-PerCP, anti-CD4-PacificBlue, anti-CD8-APC, anti-CD25-PE/Cy7 and anti-CD279-PE (PD-1); ILCs by staining with anti-Lineage-Cocktail-APC (anti-CD3/CD14/CD16/CD19/CD20/CD56) and anti-CD127-PE; NK-cell subsets by staining with anti-CD3-PerCP, anti-CD14-FITC, anti-CD19-PE/Cy7, anti-CD56-APC/Cy7, anti-CD16-PacificBlue (all BioLegend, Fell, Germany), anti-NKG2A-APC (clone 131411) and anti-NKG2C-PE (both R&D Systems, Abingdon, UK) [20]. Samples were acquired on a CyAn ADP Analyzer (Beckman Coulter, Nyon, Switzerland) and data analyzed with FlowJo software vX.0.7 (FlowJo, Ashland, Oregon, USA).
Enzyme-linked immunosorbent spot assay
IFN-γ and IL-17 enzyme-linked immunosorbent spot (ELISPOT; Mabtech, Nacka Strand, Sweden) were performed according to manufacturer's instructions as previously published [21]. Briefly, 3–5 × 105 cells/well were stimulated in duplicates with C. albicans [multiplicity of infection (MOI) 0.05], 0.05 μg/ml cytomegalovirus (CMV) pp65 (JPT Peptide Technologies, Berlin, Germany) or 0.5 μg/ml staphylococcal enterotoxin B (SEB; Sigma-Aldrich, Buchs, Switzerland) for 72 h. The number of spot forming counts per well was counted by ELISPOT reader (Cellular Technologies Ltd., Bonn, Germany). Data are shown after subtraction of unstimulated controls.
Proliferation assay
PBMC were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Fisher Scientific, Reinach, Switzerland) according to manufacturer's instructions and stimulated with C. albicans (MOI 0.05) or 0.5 μg/ml SEB for 7 days in RPMI 1640 (Gibco, Fisher Scientific, Reinach, Germany) with 5% pooled human serum. Medium was replenished as needed. Cells were stained with anti-CD3-PerCP, anti-CD4-PacificBlue, anti-CD8-APC and anti-CD56-APC/Cy7 (all Biolegend) and acquired on a CyAn ADP Analyzer (Beckman Coulter) and data analyzed with FlowJo software vX.0.7.
Statistical analysis
Comparisons between two groups were performed with the two-sided Mann–Whitney U test. P values of 0.05 or less were considered statistically significant. Statistical analyses were done using GraphPad Prism 6.0f and Stata 13.1 software (StataCorp LP, College Station, Texas, USA). Shown are median values + interquartile ranges (Tukey plots).
Results
Patients with Candida esophagitis have low and dysfunctional CD4+ cell counts and decreased Candida-specific cytokine responses and proliferative capacity
We first analyzed T-cell phenotype and function of patients diagnosed with Candida esophagitis and compared them with HIV-1-infected individuals with early initiation of cART and sustained viral suppression (<50 copies/ml) and healthy individuals (for baseline characteristics of patients and healthy individuals see the Supplementary table).
As expected from earlier studies, absolute CD4+ cell counts were significantly lower in Candida esophagitis patients compared with patients with early initiated cART and healthy individuals (Fig. 1a) and the frequencies of exhausted PD-1+ and activated/regulatory CD25+ CD4+ cell counts were significantly increased compared with healthy individuals (Fig. 1b). The median percentage of CD25+ CD4+ cell counts was 6-fold higher in patients with Candida esophagitis compared with healthy individuals. In accordance with the significantly reduced number of functional CD4+ cell counts, the IFN-γ and IL-17 responses of PBMC to the superantigen SEB or C. albicans were significantly lower in Candida esophagitis patients compared with patients with early initiated cART and healthy individuals. The IFN-γ response to CMV pp65 in CMV seropositive donors was not affected, showing that viral reactivation was still able to trigger functional immune responses independent of the CD4+ cell counts (Fig. 1c and d).
Fig. 1.
Patients with Candida esophagitis have low and dysfunctional CD4+ cell counts with decreased Candida-specific cytokine responses and proliferative capacity.
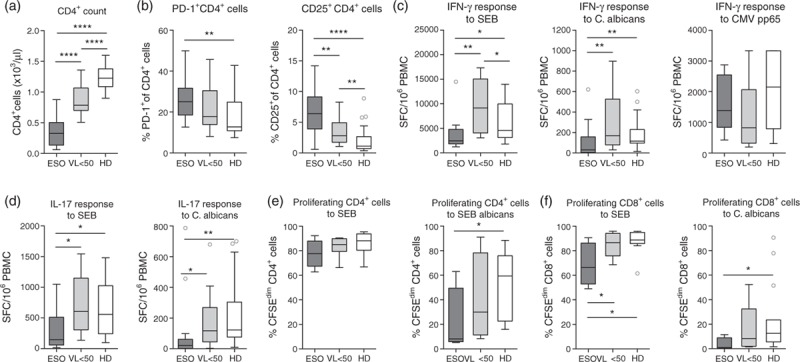
(a) Absolute CD4+ T-cell counts, (b) percentage of PD1+ and CD25+ CD4+ T cells, (c) IFN-γ response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated Candida albicans, (d) IL-17 response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated C. albicans, (e) percentage of proliferating (CFSEdim) cells in the CD4+ T-cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans and (f) percentage of proliferating (CFSEdim) cells in the CD8+ T-cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans of patients at diagnosis of Candida esophagitis (ESO), patients with early initiated combination antiretroviral therapy and a viral load less than 50 copies/ml (viral load <50) and healthy donors. Shown are median values + interquartile ranges (Tukey plot). Data (c)–(f) are shown after subtraction of unstimulated controls. Number of ESO/viral load less than 50/healthy donor were n = 37/15/20 (a), n = 33/15/20 (b), n = 18/11/19 (c), n = 12/14/20 (d) and n = 4/8/14 (e and f). ∗P ≤ 0.05, ∗∗P ≤ 0.01 and ∗∗∗∗P ≤ 0.0001 (Mann–Whitney test).
In line, the proliferative capacity of CD4+ and CD8+ cells to C. albicans was significantly lower in patients with Candida esophagitis compared with healthy individuals (Fig. 1e and f). The median percentage of proliferating CD4+ cell counts was 7-fold and of proliferating CD8+ cell counts 12-fold decreased compared with healthy individuals. Interestingly, CD4+ cell proliferation to SEB was comparable to healthy individuals. To examine whether the inability to proliferate was due to a lack of IL-2 production, we supplemented some cultures with 50 U/ml recombinant IL-2 on day 2. However, T-cell proliferation was not improved (Supplemental Fig. 1).
Thus, development of Candida esophagitis was associated with reduced and dysfunctional CD4+ cell counts that showed significant impairments in cytokine production and proliferation to specific antigens.
Patients with Candida esophagitis have decreased peripheral natural killer cells and innate lymphoid cells
NK cells and ILC are increasingly considered as part of the host defense against fungi. We therefore investigated whether these cells and their functionality are also impaired in patients with Candida esophagitis.
Absolute NK-cell counts (CD3−CD56+) and especially the cytotoxic CD16+ NK-cell subset were significantly lower in patients with Candida esophagitis compared with patients with early initiated cART and healthy individuals (Fig. 2a). The absolute number of CD16+ NK cells was with a median of 42 cells/μl nearly 5-fold lower than in healthy controls (201 cells/μl). The percentage of NK cells expressing the inhibitory receptor NKG2A was higher in cases with Candida esophagitis than in the other groups, whereas the percentage of cells expressing the activating receptor NKG2C was higher in Candida esophagitis cases and in HIV-1-infected virologically suppressed patients than in healthy individuals (Fig. 2b). The proliferative capacity of NK cells to C. albicans was not significantly affected (Fig. 2c).
Fig. 2.
Patients with Candida esophagitis have decreased peripheral natural killer cell counts and innate lymphoid cells.
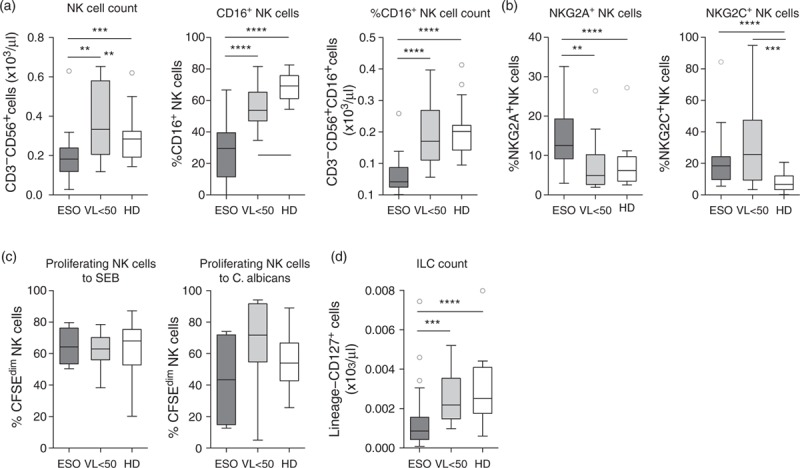
(a) Absolute CD3−CD56+ natural killer cell counts, percentage of CD16+ natural killer cell counts and absolute CD16+CD3−CD56+ natural killer cell counts, (b) percentages of NKG2A+ and NKG2C+ natural killer cell counts, (c) percentage of proliferating (CFSEdim) cells in the natural killer cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans and (d) absolute lineage−CD127+ innate lymphoid cell-count of patients at diagnosis of Candida esophagitis (ESO), patients with early initiated combination antiretroviral therapy and a viral load less than 50 copies/ml (viral load <50) and healthy donors. Shown are median values + interquartile ranges (Tukey plot). Data in (b) are shown after subtraction of unstimulated controls. Number of ESO/viral load less than 50/healthy donor were n = 31/15/20 (a, b and d) and n = 4/8/14 (c). ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 and ∗∗∗∗P ≤ 0.0001 (Mann–Whitney test).
Similar to NK cells, also peripheral ILC counts (lineage−CD127+) were significantly reduced in patients with Candida esophagitis compared with patients with early initiated cART and healthy individuals (Fig. 2d).
In conclusion, additionally to defective CD4+ cell responses, patients with Candida esophagitis had significantly reduced numbers of NK cells and ILC.
The proliferative responses to C. albicans and NK-cell counts and function are impaired despite higher CD4+ cell counts at diagnosis
Next, we examined whether higher CD4+ cell counts at diagnosis of Candida esophagitis are associated with better functionality of the different cell subsets (Fig. 3).
Fig. 3.
Proliferative response to C. albicans and natural killer cell counts and function are impaired despite higher CD4+ cell counts at diagnosis.
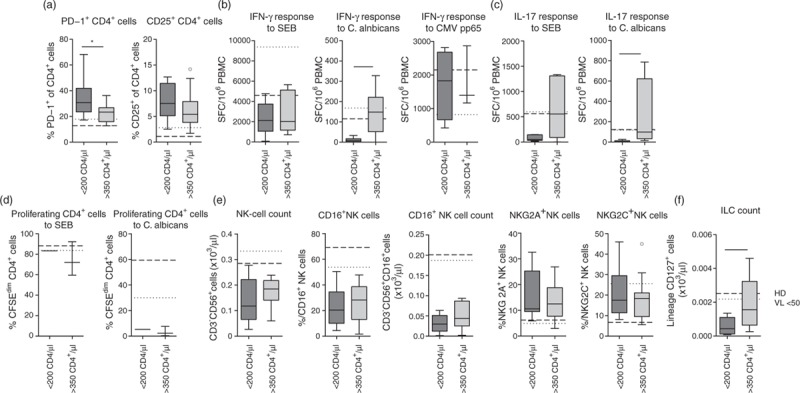
(a) Percentage of PD1+ and CD25+ CD4+ T cells, (b) IFN-γ response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated C. albicans, (c) IL-17 response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated C. albicans, (d) percentage of proliferating (CFSEdim) cells in the CD4+ T-cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans, (e) absolute CD3−CD56+ natural killer cell counts, percentage of CD16+ natural killer cell counts, absolute CD16+CD3−CD56+ natural killer cell counts, percentages of NKG2A+ and NKG2C+ natural killer cell counts and (f) absolute lineage−CD127+ innate lymphoid cell counts in patients with Candida ESO and CD4+ T-cell counts less than 200 cells/μl or more than 350 cells/μl. Shown are median values + interquartile ranges (Tukey plot). Broken and dotted lines represent medians of healthy individuals (healthy donor) and HIV-1-infected patients with early initiated combination antiretroviral therapy and a viral load less than 50 copies/ml (viral load <50), respectively. Data (b)–(d) are shown after subtraction of unstimulated controls. Number of patients less than 200/more than 350 CD4+ cell counts were n = 10/16 (a), n = 8/7 (b), n = 5/5 (c), n = 1/3 (d), n = 9/13 (e) and n = 11/13 (f). ∗P ≤ 0.05 and ∗∗P ≤ 0.01 (Mann–Whitney test).
Patients with CD4+ cell counts higher than 350 cells/μl (18 of 37 patients) showed decreased percentages of dysfunctional PD-1+ and CD25+ CD4+ cells and increased cytokine responses to SEB and C. albicans compared with patients with CD4+ cell counts less than 200 cells/μl (12 of 37 patients; Fig. 3a–c; the Supplementary table). By contrast, CMV-specific responses were comparable regardless of the absolute CD4+ cell counts. The proliferative response to C. albicans was reduced even in patients with CD4+ cell counts higher than 350 cells/μl (Fig. 3d).
Although NK-cell counts and the percentage of cytotoxic CD16+ NK cells increased with higher CD4+ cell counts, the absolute numbers of cytotoxic NK cells in patients with CD4+ cell counts higher than 350 cells/μl still remained more than 4-fold reduced compared with healthy individuals (Fig. 3e). The number of ILC in peripheral blood also significantly increased with higher CD4+ cell counts (Fig. 3f).
These data highlight that CD4+ cell phenotype and cytokine production and ILC counts normalized with increasing CD4+ cell counts whereas the proliferative capacity of CD4+ cells specifically to C. albicans and the number of cytotoxic NK cells were strongly impaired in all patients with Candida esophagitis irrespective of the CD4+ cell count.
Patients with Candida esophagitis show a significant drop in CD4+ cell counts and nearly abolished T-cell proliferation to C. albicans at diagnosis and retain immunological impairments even after disease resolution and successful combination antiretroviral therapy
We further analyzed the immune status of patients with Candida esophagitis prior to disease development to identify immunological changes associated with disease development and after disease resolution and successful cART to identify possible long-term defects. We therefore additionally examined PBMC 6–18 months before diagnosis, after disease resolution (6–18 months after diagnosis) and after successful cART with stably suppressed HIV RNA (<50 copies/ml) for more than 2 years (Fig. 4).
Fig. 4.
Patients with Candida esophagitis retain immunological impairments after disease resolution and successful combination antiretroviral therapy.
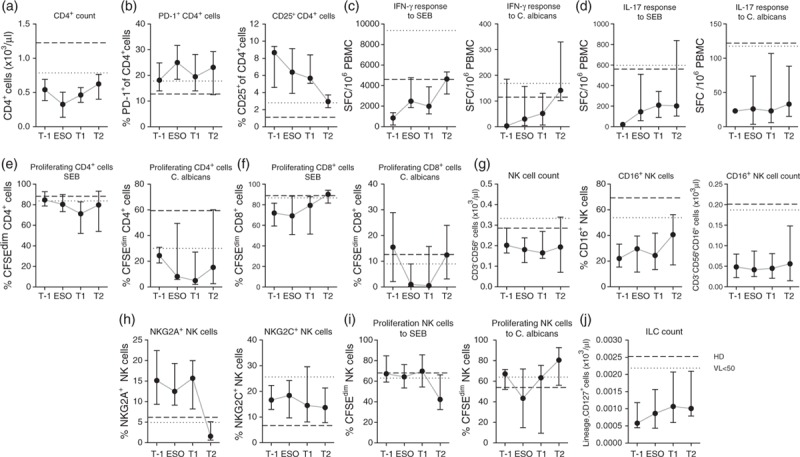
Immune cell numbers and function of patients with Candida esophagitis 6–18 months before diagnosis (T-1), at the time of diagnosis (ESO), 6–18 months after disease resolution (T1) and after suppression of HIV-1 RNA for at least 2 years (T2). (a) Absolute CD4+ T-cell counts, (b) percentage of PD1+ and CD25+ CD4+ T-cell counts, (c) IFN-γ response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated C. albicans, (d) IL-17 response of peripheral blood mononuclear cell to staphylococcal enterotoxin B or heat-inactivated C. albicans, (e) percentage of proliferating (CFSEdim) cells in the CD4+ T-cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans, (f) percentage of proliferating (CFSEdim) cells in the CD8+ T-cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans, (g) absolute CD3−CD56+ natural killer cell counts, percentage of CD16+ natural killer cell counts, absolute CD16+CD3−CD56+ natural killer cell counts, (h) percentages of NKG2A+ and NKG2C+ natural killer cell counts, (i) percentage of proliferating (CFSEdim) cells in the natural killer cell population after 7 days stimulation with staphylococcal enterotoxin B or heat-inactivated C. albicans and (j) absolute lineage−CD127+ innate lymphoid cell-count. Shown are median values + interquartile ranges. Broken and dotted lines represent medians of healthy individuals (healthy donor) and HIV-1-infected patients with early initiated combination antiretroviral therapy and a viral load less than 50 copies/ml (viral load <50), respectively. Data (c)–(f) and (i) are shown after subtraction of unstimulated controls. Number of patients T-1/ESO/T1/T2 were n = 12/37/37/8 (a), n = 10/33/34/8 (b), n = 3/18/27/6 (c), n = 1/12/22/6 (d), n = 3/4/6/6 (e and f), n = 10/31/34/8 (g and h), n = 3/4/6/5 (i) and n = 10/31/36/8 (j).
Interestingly, the patients with Candida esophagitis showed a significant drop in absolute CD4+ cell counts at diagnosis and nearly abolished proliferation of CD4+ and CD8+ cells in response to stimulation with C. albicans. Proliferation to SEB was not significantly affected. Also NK-cell proliferation in response to C. albicans dropped during development of Candida esophagitis (Fig. 4a,e,f,i).
In line with previous studies, Candida esophagitis patients with low CD4+ cell nadirs (<320 cells/μl, median 87 cells/μl) restored CD4+ cell counts after viral suppression to a lower level compared with patients with early cART and higher CD4+ cell nadirs (>350 cells/μl, median 397 cells/μl) (Fig. 4a). The percentage of CD25+ CD4+ cell counts and the IFN-γ responses to SEB and C. albicans normalized after suppression of viral replication, whereas the IL-17 response remained impaired despite long-term viral suppression under cART (Fig. 4b–d). The proliferative capacity of CD4+ cells to C. albicans after suppression of HIV replication recovered but remained in median lower than in patients with early initiated cART and healthy individuals (Fig. 4e and f).
Median NK-cell counts remained below 200 cells/μl despite viral suppression. Although the percentage of CD16+ cells increased, the absolute number of cytotoxic CD16+ NK-cell counts remained more than 3.5-fold lower compared with healthy individuals (Fig. 4g). By contrast, the percentages of NKG2A+ and NKG2C+ NK cells and the proliferative responses were comparable with healthy controls (Fig. 4h and i). Similarly to NK cells, also ILC counts remained 2.5-fold reduced for at least 2 years after viral suppression (Fig. 4j). NK-cell and ILC reconstitution seemed to correlate directly with CD4+ cell reconstitution (Supplemental Fig. 2).
The data show that development of Candida esophagitis is associated with a drop in absolute CD4+ cell counts and proliferative capacity of Candida-specific T cells and that despite successful cART, patients with previous Candida esophagitis have prolonged immune defects of CD4+ cells, NK cells and ILC.
Patients with similarly advanced HIV infection but without an opportunistic infection show overall better immune recovery after successful combination antiretroviral therapy
The last comparator group consisted of patients with similarly advanced HIV infection but without opportunistic infection. These patients were matched to Candida esophagitis patients according to CD4+ cell counts, use of cART, sex, age and absence of other opportunistic infection within 6 months prior to sample collection. Also the viral load was comparable between these two patient groups. Notably, patients with similarly advanced HIV infection but without opportunistic infection showed overall very similar immune cell impairments and reconstitution as patients with Candida esophagitis (Supplemental Fig. 3). However, they differed by a 2-fold lower percentage of CD25+ CD4+ cells prior to disease development and consistently higher CD4+ cell proliferation to C. albicans. In addition, long-term recovery of the IL-17 response, total NK cells, CD16+ cytotoxic NK cells and ILCs was better in these patients without Candida esophagitis.
Discussion
The detailed pathogenesis of opportunistic infections is still unknown for many pathogens. Candida esophagitis is one of the most frequent opportunistic diseases in untreated HIV-infected individuals but also occurs in patients with other underlying conditions.
In this study, we investigated in depth 37 HIV-1-infected Candida esophagitis patients and compared them with advanced HIV-1-infected patients without opportunistic infection, HIV-1-infected patients with early initiation of cART and healthy individuals. We found that patients with Candida esophagitis showed a significant drop in CD4+ cell counts at diagnosis, a nearly abolished proliferative capacity to C. albicans, an impaired IFN-γ and IL-17 production to C. albicans and a dysfunction of CD4+ cells with increased percentages of CD25+ and PD-1+ cells. In addition, these patients had significantly decreased peripheral ILC and cytotoxic NK-cell counts. Recovery of the proliferative capacity of CD4+ ce and IL-17 production to C. albicans and of ILCs and cytotoxic NK cells was impaired for years despite effective cART.
HIV infection is commonly associated with an inability to proliferate and produce IL-2. Even patients with normal IFN-γ production often have a proliferative defect to different antigens, especially patients with previously low CD4+ cell counts and persistent HIV replication [22–24]. In line with these data, we observed an overall decreased proliferative capacity to Candida in patients with Candida esophagitis at diagnosis and even after recovery of the IFN-γ response. These findings suggest that the proliferative defect could contribute to disease development and that these patients might remain vulnerable despite effective cART.
CD4+ T cells from chronically infected HIV patients show diminished IFN-γ and IL-17 production [25], but data on cytokine production in response to different fungi in HIV-infected patients are scarce. This study demonstrates that all patients with advanced HIV infection including patients with and without Candida esophagitis had an overall impaired IFN-γ and IL-17 production to C. albicans, which probably is partly due to the significantly reduced CD4+ cell count. However, HIV-infected patients with early initiation of cART had comparable or even higher Candida-specific cytokine responses compared with healthy individuals despite significantly reduced CD4+ cell counts, showing that low CD4+ cell counts do not necessarily lead to reduced antigen-specific responses. Similarly, CMV pp65-specific IFN-γ production by CMV-seropositive patients was comparable in all groups, independent of CD4+ cell counts. It is however possible that patients with acute Candida esophagitis have additional impairments in antigen-presenting cells leading to reduced T-cell responses or that Candida-specific T cells in these patients are recruited to sites of infection and therefore disappear from the blood. Interestingly, the recovery of IL-17-producing cells was slower in patients with Candida esophagitis compared with patients with advanced HIV infection without opportunistic infection. These data fortify previous findings in chronic mucocutaneous candidiasis in humans and oropharyngeal candidiasis in mice [9–11,25,26]. Interestingly, CMV pp65-specific IFN-g production by CMV-seropositive patients was comparable to healthy controls, showing that not all antigen-specific responses are impaired in these patients. [[NB change g in IFN-g to Greek gamma]].
We further found that patients with Candida esophagitis had a significantly higher percentage of CD25+ CD4+ cells before disease development compared with healthy individuals. The patients with advanced HIV without Candida esophagitis showed a 2-fold lower percentage compared with the patients with Candida esophagitis. We cannot state if these cells were activated or regulatory T cells, as we did not include additional markers. However, previous studies showed that a higher percentage of regulatory T cells was associated with lower HIV-specific and Candida-specific responses [27]. Furthermore, in-vitro depletion of the Treg-containing CD25+ T-cell population greatly enhanced the response to HIV and CMV antigens [28–30]. Moreover, PD-1+ CD4+ cells significantly increased at diagnosis of Candida esophagitis, which further supports the assumption that a dysfunction of CD4+ cells might be one factor leading to susceptibility to Candida esophagitis.
Healthy individuals also show proliferation of CD8+ cells in response to C. albicans. Previous work in mouse models has shown that in the absence of CD4+ cells, CD8+ cells were able to confer protection to the fungal pathogens Blastomyces dermatitidis and Histoplasma capsulatum[31]. Interestingly, in our study we did not observe compensatory proliferation of CD8+ cells in patients with low CD4+ cell responses after stimulation with C. albicans. It is possible that the CD8+ cells proliferating to C. albicans in healthy individuals are mucosal-associated invariant T cells that respond to C. albicans and are depleted in the course of HIV infection [32–34].
Recently, it has been shown that not only Th17 cells, but also other cells such as ILCs can be a source of IL-17 and are involved in the host defense against fungal infections [12]. We found that the ILC counts in peripheral blood of Candida esophagitis patients were unable to recover even with suppressed viral replication. Remarkably, patients with CD4+ cell counts higher than 350 cells/μl had significantly higher ILC counts in the peripheral blood compared with patients with CD4+ cell counts less than 200 cells/μl, suggesting that the loss of ILCs occurs in parallel with the loss of CD4+ cells over time and may additionally increase the risk of developing Candida esophagitis. Furthermore, recovery of ILCs after suppressed viral replication seems to be correlated with CD4+ cell recovery. Nevertheless, these data should be interpreted with caution, as the number of patients is low, and we did not investigate the different subsets of ILCs and their involvement in the mucosa.
Also NK cells are increasingly considered as part of the host defense against fungi [13,14,35–37]. Impaired NK-cell activity was observed in patients with chronic mucocutaneous candidiasis [38]. Also HIV-infected individuals show quantitative and functional NK-cell impairments that continue during disease progression, such as a decrease of the CD3−CD56+ cell subset, a decreased cytotoxic capacity and aberrant expression of several surface receptors [39–42]. In fact, we found significantly lower NK-cell counts and a significantly lower percentage of CD16+ cytotoxic NK cells in patients with Candida esophagitis compared with healthy individuals and patients with early initiated cART. They also did not recover under stable, virologically successful cART. Furthermore, similar to ILCs, NK-cell recovery seemed to correlate with CD4+ cell recovery.
Recently, evidence has accumulated that early initiation of cART is beneficial for virological as well as immunological parameters. Early treatment decreases cell-associated HIV RNA and DNA and limits the HIV reservoir [43–47], maintains numbers and function of the CD4+ cell compartment [4,48–50] and reduces the risk for disease transmission and the development of opportunistic viral and fungal infections and malignancies [4,51,52]. However, it was not clear, how early or late treatment affects T-cell responses to opportunistic pathogens and how other immune cell subsets such as NK cells or ILC are affected. In this study, we could confirm improved overall and Candida-specific CD4+ cell recovery in patients with early cART. We could further show that ILC and NK-cell reconstitution, immune cells with a likely role in antifungal defense, correlated with CD4+ cell recovery and was therefore superior in HIV-1-infected patients with early treatment, further arguing for early initiation of cART in HIV-1-infected individuals.
The strength of this study is the comprehensive longitudinal analysis of quantitative and qualitative immune responses in a large number of HIV-infected patients with Candida esophagitis. This allowed identifying significant immunological impairments compared with healthy individuals and HIV-infected patients with early initiation of cART. However, due to the limited availability of PBMC, we did not further characterize different cell subsets such as Treg or TH17 cells, and functional analysis could not be performed in every sample.
In conclusion, this study demonstrates that HIV-1-infected patients with Candida esophagitis not only have deficient T-cell responses, but an accumulation of multiple, partly Candida-specific immunological defects. This may explain the fact that despite the high frequency of Candida esophagitis, only a part of AIDS patients develop this opportunistic infection. These defects are only partially reversible under cART, and long-term immune impairments remain. This is particularly apparent in patients with low CD4+ cell counts at initiation of cART showing greater general and Candida-specific immune impairments initially and under stable cART. Nevertheless, certain individuals even experienced Candida esophagitis at higher CD4+ cell counts. These patients showed similar immune defects as patients with low CD4+ cell counts highlighting that the presence of specific immunological gaps is relevant. We hypothesize that specific gaps due to underlying genetic and/or immunological predisposition may explain why certain individuals also develop opportunistic infection at higher CD4+ cell counts. In line with other current studies, our study similarly supports the rationale for early initiation of cART.
Acknowledgements
The authors thank Fabrizia Ferracin for excellent technical assistance. N.K. was supported by the Swiss National Foundation grant PZ00P3_142403I, and the OPO Stiftung and C.B. was supported by Societa Italiana Malattie Infettive. This study was financed in the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (SNF grant no. 33CS30–148522) and SHCS project 699. The members of the Swiss HIV Cohort Study are Aubert V., Battegay M., Bernasconi E., Böni J., Braun D.L., Bucher H.C., Burton-Jeangros C., Calmy A., Cavassini M., Dollenmaier G., Egger M., Elzi L., Fehr J., Fellay J., Furrer H. (Chairman of the Clinical and Laboratory Committee), Fux C.A., Gorgievski M., Günthard H. (President of the SHCS), Haerry D. (deputy of ‘Positive Council’), Hasse B., Hirsch H.H., Hoffmann M., Hösli I., Kahlert C., Kaiser L., Keiser O., Klimkait T., Kouyos R., Kovari H., Ledergerber B., Martinetti G., Martinez de Tejada B., Marzolini C., Metzner K., Müller N., Nadal D., Nicca D., Pantaleo G., Rauch A. (Chairman of the Scientific Board), Regenass S., Rudin C. (Chairman of the Mother & Child Substudy), Schöni-Affolter F. (Head of Data Centre), Schmid P., Speck R., Stöckle M., Tarr P., Trkola A., Vernazza P., Weber R. and Yerly S.
Authors’ contributions: The study was conceived and designed by N.K., C.S., M.B. and C.B. Data acquisition and analysis was performed by C.B. and C.S. Sample recruitment and acquisition of clinical data was performed by C.B., M.S., S.Z., H.F. and H.F.G. Statistical analysis was done by L.E. The article was written by N.K., C.S., M.B. and S.L., and reviewed by all coauthors.
Financial support. N.K. was supported by the Swiss National Foundation grant no. PZ00P3_142403I and the OPO Stiftung, and C.B. was supported by Societa Italiana Malattie Infettive. This study has been financed in the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (SNF grant no. 33CS30-134277) and SHCS project 699. The funding institutions had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interests.
Supplementary Material
Supplementary Material
Contributor Information
Collaborators: the Swiss HIV Cohort Study
References
- 1.Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet 2000; 356:291–296. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Lundgren JD, Sabin ML, Monforte A, Brockmeyer N, Casabona J, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med 2013; 10:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥200 cells/μL in the postcombination antiretroviral therapy era. Clin Infect Dis 2013; 57:1038–1047. [DOI] [PubMed] [Google Scholar]
- 4.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group TAS, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez JA. Invasive oesophageal candidiasis: current and developing treatment options. Drugs 2003; 63:971–989. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Oancea C, van Lunzen J, Vanhems P, Banhegyi D, Chiesi A, et al. Decline in esophageal candidiasis and use of antimycotics in European patients with HIV. Am J Gastroenterol 2005; 100:1446–1454. [DOI] [PubMed] [Google Scholar]
- 8.Pirofski LA, Casadevall A. Rethinking T cell immunity in oropharyngeal candidiasis. J Exp Med 2009; 206:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 2012; 12:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Chen J, Jia M, Hong K, Ruan Y, Liang H, et al. Loss of balance between T helper type 17 and regulatory T cells in chronic human immunodeficiency virus infection. Clin Exp Immunol 2011; 165:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008; 112:2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 2013; 190:521–525. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt S, Zimmermann SY, Tramsen L, Koehl U, Lehrnbecher T. Natural killer cells and antifungal host response. Clin Vaccine Immunol 2013; 20:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe 2013; 14:387–397. [DOI] [PubMed] [Google Scholar]
- 15.Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Gunthard HF, Telenti A, et al. Swiss HIVCS. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39:1179–1189. [DOI] [PubMed] [Google Scholar]
- 16.Ledergerber B, von Overbeck J, Egger M, Luthy R. The Swiss HIV Cohort study: rationale, organization and selected baseline characteristics. Soz Praventivmed 1994; 39:387–394. [DOI] [PubMed] [Google Scholar]
- 17.Khanna N, Wolbers M, Mueller NJ, Garzoni C, Du Pasquier RA, Fux CA, et al. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J Virol 2009; 83:4404–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737–748. [DOI] [PubMed] [Google Scholar]
- 19.Stuehler C, Khanna N, Bozza S, Zelante T, Moretti S, Kruhm M, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood 2011; 117:5881–5891. [DOI] [PubMed] [Google Scholar]
- 20.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol 2010; 88:1119–1130. [DOI] [PubMed] [Google Scholar]
- 21.Khanna N, Stuehler C, Conrad B, Lurati S, Krappmann S, Einsele H, et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood 2011; 118:1121–1131. [DOI] [PubMed] [Google Scholar]
- 22.Sieg SF, Mitchem JB, Bazdar DA, Lederman MM. Close link between CD4+ and CD8+ T cell proliferation defects in patients with human immunodeficiency virus disease and relationship to extended periods of CD4+ lymphopenia. J Infect Dis 2002; 185:1401–1416. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JD, Imami N, Watkins A, Gill J, Hay P, Gazzard B, et al. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J Infect Dis 2000; 182:792–798. [DOI] [PubMed] [Google Scholar]
- 24.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J Virol 2002; 76:5925–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J Virol 2008; 82:6767–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011; 365:54–61. [DOI] [PubMed] [Google Scholar]
- 27.Tenorio AR, Martinson J, Pollard D, Baum L, Landay A. The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J Acquir Immune Defic Syndr 2008; 48:577–580. [DOI] [PubMed] [Google Scholar]
- 28.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol 2004; 78:2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol 2005; 174:4407–4414. [DOI] [PubMed] [Google Scholar]
- 30.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA 2007; 104:3390–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wuthrich M, Filutowicz HI, Warner T, Deepe GS, Jr, Klein BS. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J Exp Med 2003; 197:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 2013; 121:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010; 11:701–708. [DOI] [PubMed] [Google Scholar]
- 34.Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013; 121:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voigt J, Hunniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis 2014; 209:616–626. [DOI] [PubMed] [Google Scholar]
- 36.Quintin J, Levitz SM. NKp30 enables NK cells to act naturally with fungi. Cell Host Microbe 2013; 14:369–371. [DOI] [PubMed] [Google Scholar]
- 37.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014; 40:117–127. [DOI] [PubMed] [Google Scholar]
- 38.de Moraes-Vasconcelos D, Orii NM, Romano CC, Iqueoka RY, Duarte AJ. Characterization of the cellular immune function of patients with chronic mucocutaneous candidiasis. Clin Exp Immunol 2001; 123:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 2011; 11:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol 2004; 34:2313–2321. [DOI] [PubMed] [Google Scholar]
- 41.Portales P, Reynes J, Pinet V, Rouzier-Panis R, Baillat V, Clot J, et al. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS 2003; 17:495–504. [DOI] [PubMed] [Google Scholar]
- 42.Ansari AW, Ahmad F, Meyer-Olson D, Kamarulzaman A, Jacobs R, Schmidt RE. Natural killer cell heterogeneity: cellular dysfunction and significance in HIV-1 immuno-pathogenesis. Cell Mol Life Sci 2015; 72:3037–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ananworanich J, Dube K, Chomont N. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs?. Curr Opin HIV AIDS 2015; 10:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M, et al. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–1721. [DOI] [PubMed] [Google Scholar]
- 45.Gianella S, von Wyl V, Fischer M, Niederoest B, Battegay M, Bernasconi E, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther 2011; 16:535–545. [DOI] [PubMed] [Google Scholar]
- 46.Schmid A, Gianella S, von Wyl V, Metzner KJ, Scherrer AU, Niederost B, et al. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PLoS One 2010; 5:e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–1418. [DOI] [PubMed] [Google Scholar]
- 48.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macatangay BJ, Rinaldo CR. Preserving HIV-specific T cell responses: does timing of antiretroviral therapy help?. Curr Opin HIV AIDS 2015; 10:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okulicz JF, Le TD, Agan BK, Camargo JF, Landrum ML, Wright E, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015; 175:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014; 14:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.When To Start C, Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009; 373:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


