Abstract
Background
A nutrient-rich maternal diet before and during pregnancy is associated with improved fetal health, more appropriate birth weight, and increased rates of maternal and infant survival. Physicians need a better understanding of the role of diet in shaping fetal outcomes. Given this background, we reviewed and summarized articles on maternal nutrition found in MEDLINE since 1981, written in English, and limited to human subjects.
For the Offspring
Maternal diets high in sugar and fat lead to an increased incidence of metabolic syndrome, diabetes, and cardiovascular disease later in life. Folic acid should be supplemented prior to conception and continued through at least the first 28 days of fetal life to prevent neural tube defects, and vitamin C should be given to women who smoke to lower the incidence of asthma and wheezing in the children. Iodine deficiency is increasing, and iodine should be included in prenatal supplements. If the maternal hemoglobin is 7 g/dL or more, there is no evidence that iron supplementation is needed. Fish intake during pregnancy is protective against atopic outcomes, whereas high-meat diets contribute to elevated adult blood pressure and hypersecretion of cortisol.
For the Mother
Calcium supplementation lowers the risk of preeclampsia and hypertensive disease in pregnancy.
Conclusions
Given the limits of our current knowledge, a diet rich in whole grains, fruits, vegetables, and selected fish is desirable for the best outcomes. Diets high in sugar and fat lead to higher rates of diabetes, metabolic syndrome, and cardiovascular disease. Folic acid, iodine, and calcium in all pregnant women and vitamin C in smokers are the only supplements so far shown to be of value for routine use. The physician treating a pregnant woman should be ready to advise a healthy diet for the benefit of the fetus.
Target Audience
Obstetricians and gynecologists, family physicians
Learning Objectives
After participating in this activity, the obstetricians, gynecologists, and family physicians should be better able to discuss the role of various diets in helping with healthy fetal development; understand the role of diet prior to conception and throughout the pregnancy; and develop knowledge of the role of the maternal diet in affecting the risk for development of diabetes, cardiac disease, cancer, and allergic disease in the offspring.
Maternal nutritional requirements for optimal fetal development are difficult to discern in a human pregnancy. The ethical constraints regarding what can reasonably be put to experimental study, coupled with the length of gestation and our differing genetic strains, limit what can be stated with confidence for any given woman in any given location. Furthermore, we now know that our diet, our environment, and our responses to each in combination can alter the expression of our genes. This adds another layer of complexity to our findings. The purposes of this article are to summarize what appears to be known at this time about maternal nutritional requirements and to discuss what is not yet established.
Fetal programming, or maternal epigenetic influence, occurs not by changing the genes themselves, but by altering how they are expressed. Methylation of histones, one example of epigenetic change, can influence gene expression. This epigenetic influence is transgenerational and long lasting.1
The risk for the developing fetus of developing adult-onset disease is determined, at least in part, by maternal nutritional status at conception, during pregnancy, and in early infancy. The fetal strategy of limiting growth in the absence of adequate nutrition creates an infant with a higher insulin response to food and less growth of muscle (including heart), nephrons, and bone. If the diet improves in infancy or childhood, this infant will gain weight at a higher-than-normal rate and will be at higher risk of type 2 diabetes and the metabolic syndrome.2 As an adult, the lower number of nephrons and cardiac cells will set the stage for hypertension and cardiac failure.
After fertilization, the fertilized ovum and early blastocyst appear to detect and respond to the nutritional quality of the fallopian tube environment, even before implantation. During this time, and throughout the first 10 weeks of gestation, nourishment of the developing fetus seems to be provided solely by the fluid produced by the glands of the endometrium. Studies of mice and other animal species show that there is no maternal bloodstream access to the embryo until 10 weeks of gestation, and the fluid found in the fallopian tubes matches that found in the endometrial glands.3 This is significant as all organ differentiation takes place by 11 weeks of gestation.4 Maternal nutrition must be optimized before conception, so that the preimplantation and early differentiation environment is ready to support early fetal and initial placental development. Maternal nutritional status influences nutrient partitioning to the placenta or fetus, which subsequently affects disease risk.5
Overlying all of this is the current phenomenon of high-calorie malnutrition brought on by the increased reliance on processed foods and the drop in vegetable and fruit consumption around the world. The higher rate of maternal diabetes brought on by a high-calorie diet and the strong effect of diabetes on pregnancy outcome make this phenomenon particularly important.
There is a growing appreciation of the chemical communication between the mother and the fetus and the competing interests of the mother versus those of the fetus. It appears that the developing fetus, from conception onward, evaluates the nutritional environment available to it and adjusts its rate of growth accordingly. At the same time, while the goal of the fetus is to maximize its chances of successful development and reproduction, the goal of the mother’s body is to maximize her long-term reproductive potential, even if it means sacrificing the current fetus to do so. This means that in the face of limited nutrition the placenta may limit what is available to the fetus, even if the mother receives supplementation.
The following is a brief summary of what is known and what is not known about the impact of the maternal diet on fetal development and pregnancy outcomes organized by gestational weight gain goals, specific nutrient recommendations, and general dietary needs.
General Overview
Pregnancy and lactation are associated with major metabolic and physiologic changes in the mother.
Nutritional requirements increase to optimize both maternal adaptation and fetal development.
Improving the mother’s diet before and during pregnancy reduces the risk of medical problems for her and her infant.
Gestational Weight Gain Recommendations
The Institute of Medicine of the National Academy of Sciences provided gestational weight gain recommendations based on maternal prepregnancy body mass index (BMI) (Table 1; Fig. 1). These recommendations, however, specifically excluded women with diabetes, who now make up a significant portion of the US population. For diabetic women, there is evidence that weight gain at the lower edge of the recommended range or even below this range leads to better maternal and fetal outcomes.6,7 The additional energy increment needed to support appropriate weight gain during pregnancy is 90 to 125 kcal/d in the first trimester, 286 to 350 kcal/d in the second trimester, and 466 to 500 kcal/d in the third trimester.8,9 Thus, the old adage “eating for two” is not mathematically representational of the 10% to 25% increase in caloric intake actually needed to support a healthy pregnancy. Furthermore, given the average caloric intake of many individuals, there is no need to increase caloric intake in pregnancy, but rather to shift low-nutritional calories into more nutrient-dense calories. The protein requirement during pregnancy is not much higher than that needed by a nonpregnant woman, coming to 0.45 g/lb of maternal body weight, or an average of 71 g/d. High protein supplementation or balanced protein supplementation is not helpful and may be harmful to the pregnancy,10 whereas balanced energy/protein supplementation appears to lead to a lower risk of small-for-gestational-age (SGA) births, as well as a small increase in mean birth weight and maternal weight gain.10–13
TABLE 1.
Recommended Weight Gain in Pregnancy
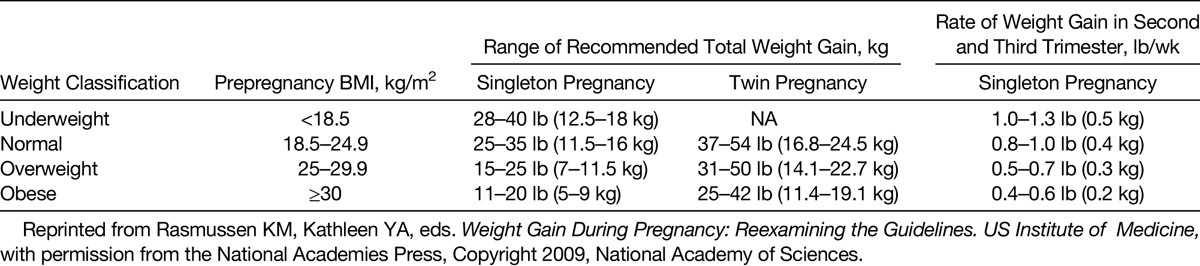
FIG. 1.

Metabolic changes in pregnancy. Maternal metabolism is altered to redirect nutrients to the placenta and fetus.
40% of weight gain is associated with the fetus and placenta
60% of weight gain is associated with maternal changes
Women with a high prepregnancy BMI have infants with higher birth weights, and heavier babies have a higher body fat mass. Except for the very overweight group, birth weight is strongly correlated to maternal weight gain.5,14
An elevated energy intake is associated with the following:
increased maternal weight gain
increased risk of hypertension
gestational diabetes (GDM)
cesarean deliveries, macrosomia (birth weight >4500 g)
childhood obesity in the offspring
An inadequate energy intake is associated with the following:
SGA or low birth weight (LBW; <2500 g)
SGA is associated with an increased risk of adult metabolic diseases including type 2 diabetes
The risk ratio for developing impaired glucose tolerance or type 2 diabetes mellitus is 4 times higher among babies with a birth weight of 5.5 lb or less than those with a birth weight of 7.5 to 8.5 lb.15 Intrauterine growth restriction is associated with an increased risk of adult onset of hypertension and stroke. Large-for-gestational-age infants are at increased risk of infant and adult obesity and other obesity-related comorbidities.15
Episodes of famine in otherwise well-nourished populations (the Netherlands in World War II and rural China in the 1950’s Great Leap Forward) provide valuable data to evaluate the impact of maternal diet on short- and long-term health outcomes in the offspring. Exposure to famine during gestation resulted in higher rates of adult-onset disease in the offspring including impaired glucose tolerance,16–18 obesity,19 coronary heart disease,19–23 atherogenic lipid profiles,19,22 hypertension,24,25 microalbuminuria,19,26 schizophrenia,19,22,27,28 antisocial personality and affective disorders,19,29–31 and a higher rate of addictive disorder in men.32 The offspring also tend to eat a higher fat diet.33 Women exposed to famine in utero tend to have more offspring, more twin gestations, and younger age at first pregnancy34 and show a higher incidence of metabolic syndrome.35 There are also differing effects of famine depending on when it occurs during the pregnancy. As organogenesis takes place along a rigid timeline, nutrient deficits early in the pregnancy may lead to compromised organ development, whereas deficits later in pregnancy may lead to an LBW infant with normal organ function.
Carbohydrates
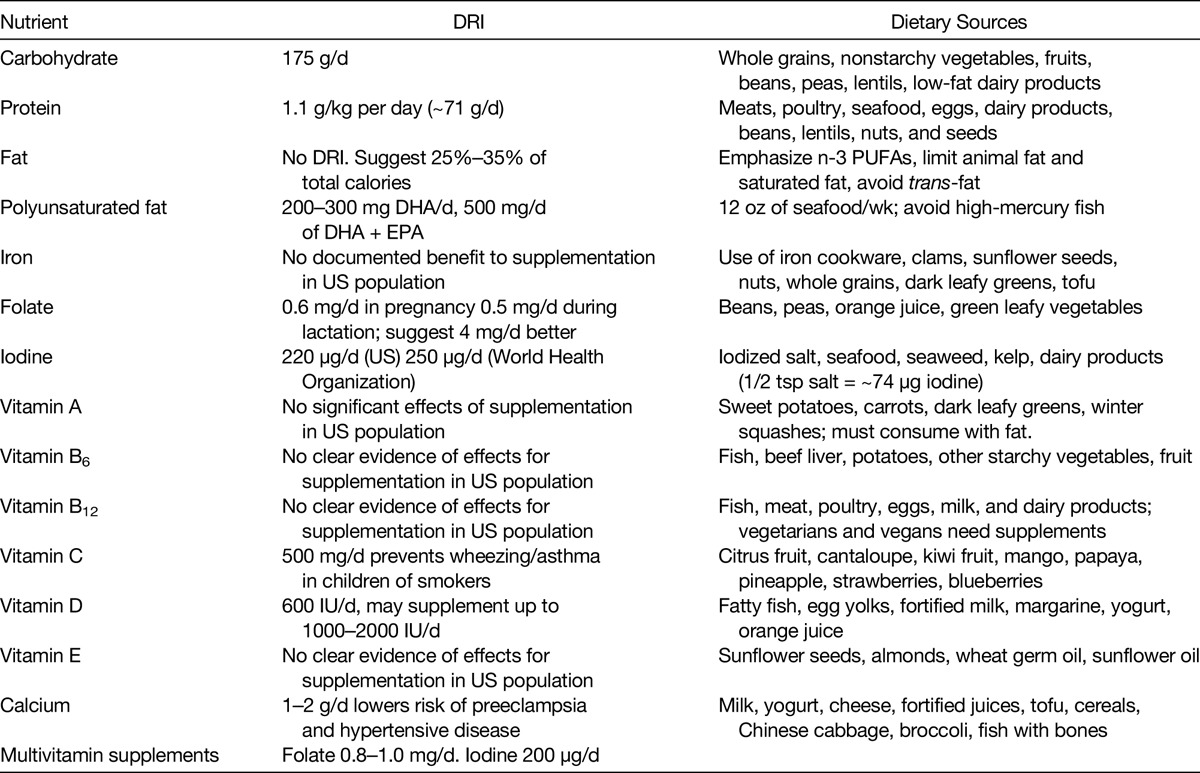
Carbohydrates are the body’s, and importantly the brain’s, main source of energy. Pregnant women need the energy provided by carbohydrates to grow a healthy baby, as glucose, derived from carbohydrate, is the main fuel used for intrauterine growth.36 The Daily Recommended Intake (DRI) for carbohydrates during pregnancy is 175 g/d. It is important that pregnant women choose high-quality carbohydrates with a low glycemic index (GI), found naturally in whole foods such as whole grains, nonstarchy vegetables, fruits, beans, peas, lentils, and low-fat dairy.36 Carbohydrates may also take the form of added sugars such as table sugar, honey, syrup, cane sugar, agave, high-fructose corn syrup, and fruit juice concentrate. All women, including pregnant women, should limit their intake of these sugars, as well as foods that have high amounts of added sugar such as candy, desserts, and sugar-sweetened beverages (soda, juice, lemonade, sweetened teas, and other fruit-flavored drinks). A study by Loy et al37 in Malaysia showed that increased consumption of fruits and vegetables was associated with an increase in birth weight, birth length, and head circumference, but was not associated with any specific measured micronutrient intake (Table 2).
TABLE 2.
Recommended Daily Intake During Pregnancy and Dietary Sources of Macronutrients and Micronutrients
Protein
Dietary protein provides important building blocks during times of growth, development, or repair and also provides structural components for human cells and for the synthesis of enzymes, which help to ensure proper function of these cells. A diet low in protein during pregnancy decreases insulin secretion in rats, whereas a diet high in protein induces changes in genes associated with energy metabolism in the liver in rats, mice, and pigs.38,39
In some cases, there appear to be important interrelated nutritional effects, such that supplementation with one nutrient has differing effects based on the availability of other nutrients. A good example of this would be providing only protein supplementation to a population that is both protein and energy deficient (dangerous) versus the effect of supplementing both protein and energy in a balanced manner (promotes growth) or by providing folate with or without iron supplementation. While the primary intent here is to discuss each nutrient separately, some discussion of their interactions is necessary. The DRI for protein during pregnancy is 1.1 g/kg per day or approximately 71 g protein per day starting in the second trimester, which is approximately 25 g more than what is recommended for nonpregnant women. Meats, poultry, seafood, eggs, milk and dairy products, beans, lentils, nuts, and seeds are rich sources of protein. Protein, in smaller amounts, is also found in vegetables and grains.
Fat
Fats are part of a healthy diet during pregnancy. Fat is primarily used as an energy source by the body, but is also used to transport the fat-soluble vitamins A, D, E, and K and to provide essential fatty acids that cannot be synthesized by the body.
Although there is no DRI for fat specific to pregnancy, it seems reasonable for pregnant women to follow the current guidelines for Americans, which suggest keeping total dietary fat intake to between 25% and 35% of total calories.40,41 The quality of the fat is also an important consideration when looking at overall fat intake. Recent scientific investigation allows for greater understanding of specific fatty acid functions and is helpful to clarify the fatty acids best for health. Based on recent research and consensus, increased intake of the omega-3 (n-3) polyunsaturated fatty acids (PUFAs) should be emphasized, and intake of saturated fatty acids should be limited to no more than 7% to 10% of total calorie intake; trans-fatty acid intake should be avoided whenever possible.42
High-fat diets in pregnancy have been found to increase insulin resistance. A high saturated fat intake is associated with development of glucose abnormalities in pregnancy and an increased risk of GDM. Higher intakes of animal fat and cholesterol before pregnancy are also associated with an increased risk of GDM, as is a higher intake of saturated fat during pregnancy.43–45
Docosahexaenoic acid (DHA) and n-3 fatty acids are needed for brain and retinal development throughout the third trimester of gestation and the first year of life. The fetus needs 200 to 300 mg/d of DHA and 500 mg of DHA plus eicosapentaenoic acid (EPA) per day. Good sources of n-3 fatty acids include fatty fish and seafood (recommendation of 12 oz per week), walnuts, and dietary supplements.
Polyunsaturated Fatty Acids
For women who do not eat fish, n-3 PUFA supplementation appears to be associated with a small decrease in preterm birth and LBW infants,46,47 and fish consumption is associated with increased birth weight and gestation length.48 Docosahexaenoic acid and EPA are important in visual and cognitive development.49 Some studies in women who do eat some fish fail to show effects,50 however, and other studies both support and refute a correlation between the ratio of n-6:n-3 fatty acids, where a ratio of less than 9 is considered healthful.51,52 However, there is also concern about consuming too much fish during pregnancy as some fish contain significant amounts of organic mercury compounds relative to the amount of DHA they provide. Studies done in the Faroe Islands53,54 showed an effect of fish consumption on measures of neurologic function at ages 7 and 14 years that were correlated with cord blood mercury concentrations. The population living on these islands eats both fish and whale blubber, and maternal blood levels of mercury have been shown to peak at 10 times higher levels after eating blubber than after eating fish. In addition, there is a higher intake of polychlorinated biphenylbromine compounds in the Faroe Island diet, although controlling for this contaminant did not affect the relationship with mercury.
A study done in the Seychelles islands, where fish consumption is high (with the offspring now 14 years old), did not show these findings,55–58 but a recent study out of Hong Kong59 confirmed the Faroe Island findings in a population that did not consume whale blubber. These results appear to show that a moderate amount of fish consumption in pregnant women is beneficial, but that fish with a low ratio of DHA to mercury (albacore, halibut, red snapper, for example) should be eaten in limited quantities and that shark, swordfish, king mackerel, and tilefish, fish known to have higher mercury concentrations, should be avoided. Also of note are several studies showing a decrease in atopic syndrome, eczema, and wheezing in the face of small-particle air pollution in infants born after prenatal supplementation with DHA in the third trimester60–62 and studies showing that the incidence of eczema and allergies can be increased by n-6 fatty acid intake in the last trimester and decreased by n-3 fatty acid intake in the same period.63–67 A recent report shows that pregnant women have avoided fish consumption altogether as a result of concerns about mercury, while it is clear that moderate consumption (8–12 oz/wk) of fish with low levels of mercury is beneficial for all pregnant women and needs to be reinforced.68
Iron
Maternal iron stores at conception are a strong predictor of maternal iron status and risk of iron deficiency and anemia in later pregnancy. There are some data that show that in populations with a high prevalence of anemia there is an increase in birth weight in infants following in utero iron supplementation (usually provided along with folate).69 The incidence of anemia and iron deficiency in the United States is about 12% in nonpregnant women and 18% in pregnant women. These conditions are found in 43% and up to 75% of nonpregnant and pregnant women, respectively, in developing countries. This is due to poor diet, limited consumption of iron-rich foods, intestinal parasites, and chronic blood loss.
There is no doubt that adequate iron stores are crucial for maternal safety. While there is no evidence of fetal growth restriction with a hemoglobin level greater than 6 g/dL, hemorrhage in the presence of anemia is the cause of at least 20% of maternal deaths worldwide.70 Mild anemia reflected by a hemoglobin concentration of 8 to 10.9 g/dL may be physiologic. There is a normal drop in hemoglobin concentration during pregnancy because of the increase in the circulating blood volume, and this may actually improve blood flow across the placental bed by decreasing viscosity,71 thus providing more efficient oxygen transfer.
A recent review of current literature by the US Preventive Services Task Force72 failed to show any benefit to routine iron supplementation in pregnancy for women in the developed world. However, as noted previously, any woman with an existing iron deficiency or a major risk of severe bleeding (placenta previa, abruption, clotting disorder, etc) should of course receive treatment.
Folate
Folate, derived from food, and folic acid, the synthetic form of folate found in vitamin supplements, is a water-soluble B vitamin. It is a methyl donor that is required for DNA synthesis and cell division. Folate/folic acid is required for neural tube development occurring within 28 days of conception. The value of folate in the prevention of neural tube defects (NTDs) is well established,73,74 and recent studies showing hypomethylation of neural tissue in cases of NTD support this observation.75
Folate is needed for the formation of the brain and spinal cord. The neural tube closes by day 28 of gestation. If it does not close completely, an opening at the lower end of the spine causes spina bifida, whereas a larger defect can lead to anencephaly (complete failure of the brain to develop).
Since 1998, the US Food and Drug Administration mandated increased fortification of cereal grains to 140 μg (0.14 mg) folic acid/100 g grain. Since then, the incidence of NTDs has decreased by approximately 30% to 40% (rates of spina bifida declined ~31%; anencephaly declined 16%) in the United States, but this differs by racial/ethnic group. It has been proposed that only folate in food, rather than more in supplements, will lead to further improvement.76 Data have shown, however, that the current supplementation policy has only dropped the incidence of NTDs by 20%, and calculations predict that if supplementation were increased to 4 mg/d, the incidence of NTDs would drop by 82%. Observational studies also suggest that folate given in pregnancy may drop the incidence of preterm labor between 20 and 32 weeks,77–80 while controlled trials do not.81 If the rest of the world adopted similar food fortification and folate supplementation policies, the worldwide prevalence of 300,000 NTDs could drop by 150,000 to 210,000 cases per year.
The US recommendations are for women of childbearing age to consume 0.4 mg of folate in vitamin form or fortified foods daily, in addition to a diet high in folate-rich foods, because of the lower bioavailability in foods. This amount should increase to 0.6 mg/d in pregnancy and 0.5 mg/d during lactation. Women with a history of pregnancy affected by a neural tube disorder (eg, spina bifida) should consume 4 mg/d of supplemental folic acid (a 10-fold increase, which requires a prescription), starting at least 1 month before conception. Foods rich in folate include beans, peas, orange juice, and green leafy vegetables. Prenatal vitamins contain folic acid, with up to 0.8 to 1 mg/tablet depending on the brand. Low folate intake (<.15 mg/d) has been associated with an increased risk of cancer, and very high intakes of folic acid (>4 times the normal dose) have been possibly associated with an increased risk of cancer in animal studies.82 Unfortunately, while benefit of folic acid supplementation during pregnancy shows up quickly, cancer risks must be studied over decades, so a small amount of uncertainty concerning high doses of folic acid remains.74 There is a biological basis for the concern, as folic acid is metabolized by a liver enzyme that may be unable to handle the higher amounts of this vitamin, and circulating folic acid is associated with reduced natural killer cell cytotoxicity.83
Hispanic women have higher rates of NTDs than non-Hispanic women: 10.34 versus 7.9 per 10,000 live births; this shifted to 7.92 versus 5.35 after the folic acid fortification program was established. Hispanic women consume less folate from food and less from supplements, 20% versus 37%, and 60% consume corn masa flour, which is not fortified with folic acid. Fortification of corn masa flour in the United States would increase folate intake by approximately 20% among 15- to 44-year-old Mexican American women and possibly lead to fewer NTD-affected pregnancies in this population.84
High supplement levels of folic acid after the first trimester have also been associated with an increase in childhood asthma and eczema,85 whereas low folate levels have been linked to language delay, emotional problems, and schizophrenia in the offspring.85–87 An epidemiological study in Pune, India, found that a combination of low blood B12 levels and high folate levels in women (measured in late pregnancy) are associated with small, relatively obese offspring with a high level of insulin resistance.88 Vitamin B12 deficiency blocks the metabolism of folate and leads to a buildup of 5-methyltetrahydrofolate, which may be the source of the problem. This population differs from a US cohort, as it had almost no women with folate deficiency, and a large number of the pregnant women were prescribed a dose of 5 mg folate daily from 12 weeks’ gestation or later until term.
Iodine
Iodine requirements during pregnancy are increased because of a 50% increase in maternal thyroid (T4) hormone production. Fetal thyroid-stimulating hormone is not synthesized until the 10th to 12th week of gestation, approximately the same time that the fetal thyroid is capable of concentrating iodine and synthesizing iodothyronine. Little fetal hormone synthesis occurs, however, until the 18th to 20th week of gestation. There is also an increased loss of iodine in the urine during pregnancy.
Therefore, through the first half of pregnancy, the fetus relies on the mother for thyroid hormone. When maternal iodine intake is low, maternal thyroid hormone production is low. Thyroid hormone is required for normal neuronal migration, myelination, and synaptic transmission and plasticity during fetal and early postnatal life. Poor neuronal development in the fetus, due to iodine deficiency during critical time points, causes irreversible brain damage, is the leading cause of preventable mental retardation worldwide, and can result in up to a 20-point drop in IQ. Iodine deficiency during fetal development is also associated with fetal goiter (potentially causing obstruction at delivery), hypothyroidism, and cretinism.
Major sources of iodine include iodized salt, seafood, seaweed, kelp, and dairy products. Iodized salt provides 77 μg iodine/g of salt (~220 μg iodine in ½ teaspoon of salt). The US Recommended Daily Allowance for iodine is 220 μg/d. The World Health Organization/International Council for Control of Iodine Deficiency Disorders recommends 250 μg/d.
Iodine intake was not felt to be a problem in the United States for many decades but is again an issue. The push to limit salt intake, coupled with the rise in popularity of sea salt and kosher salt, a fall in the number of bakeries using iodized dough, the popularity of organic milk (which has approximately 40% less iodine than regular commercial milk), soy and other “nonmilk” milks, and the rapid loss of iodine from iodinated salt in warm humid climates, has led to iodine intake dropping 50% between 1970 and 1990.89 Soy intake inhibits iodine absorption and interferes with thyroid hormone production, but can be circumvented by adequate iodine intake. Currently, as many as 30% of pregnant women in this country are estimated to have low levels (<100 μg/L), and as recently as 2012, just under 50% of prenatal vitamins contained no iodine. At least 1 study90 showed that supplementation given after 6 to 8 weeks of gestation was ineffective at preventing a drop in IQ levels (8–12 points) in the offspring. The current recommendation is for all pregnant women to take prenatal vitamins containing 150 to 250 μg daily.91 Older women and multiparas should be taking the higher doses. The iodine should come from potassium iodide (KI), not from kelp, as kelp has been shown to contain highly variable amounts of iodine, as well as (in some cases) large concentrations of arsenic and other heavy metals.
In addition, Connelly et al92 recently described 3 cases of congenital hypothyroidism caused by excess maternal iodine ingestion (12.5 mg/d from nutritional supplements, Iodoral [Optimox Corp, Torrance, CA]) Infants were identified through the Oregon newborn screening program. Concentrations of whole-blood iodine were 10 times above mean control levels. One infant required chronic levothyroxine treatment. Maternal breast milk iodine content was also significantly elevated in the mother. The other 2 infants were dizygotic twins who were treated with levothyroxine for 3 weeks, which was then discontinued. None of the infants presented with neonatal goiter; the long-term consequences are not known. These cases emphasize the need for health care providers to evaluate prenatal vitamin/mineral use among pregnant women and to recommend appropriate supplements to prevent unintentional toxicity.
Vitamin A
Deficiency as a cause of night blindness is a major problem worldwide, but not a significant one in the United States. Supplementation does prevent and cure this condition.93–95 All studies to date fail to show any significant effects of vitamin A supplementation on pregnancy outcomes for mother or fetus.96 More than 10,000 IU of vitamin A supplementation per day (4 times the Recommended Daily Allowance) increases the risk of cleft lip or palate, hydrocephalus, and heart defects. β-Carotene, found in food, does not pose a risk.
Vitamins B6, B12, and C
A low level of B12 has been associated with high levels of homocysteine, which has been associated with preeclampsia97 and LBW.98 Deficiency may occur in vegans who do not take supplements. By serum testing, 17% to 39% of pregnant women are deficient in B12, but this was not shown to correlate with pregnancy complications or outcome. Vitamin B6 supplementation has been shown in 3 small studies to give a modest gain in birth weight.99 Vitamin C supplementation has not been shown to be of value in improving pregnancy outcomes,99 but has shown value for prevention of wheezing and asthma in the children of smokers.100
Vitamin E
One study correlated risk of wheeze inversely to the level of vitamin E as well as a linear relationship between the level and the forced expiratory volume at 1 minute.101 No other studies appear to support or refute this. Deficiency during pregnancy may cause miscarriage, preterm birth, preeclampsia, and intrauterine growth retardation.102 There does not seem to be a need for supplements.
Vitamin D
Adequate vitamin D status during pregnancy is necessary to ensure appropriate maternal responses to the calcium demands of the fetus for bone mineral accretion. Approximately 25 to 30 g of calcium are transferred to the fetal skeleton during pregnancy—approximately 250 mg/d in the third trimester. Major dietary sources of vitamin D are fatty fish (salmon), egg yolks, fortified milk, margarine, yogurt, and orange juice.
Vitamin D screening and supplementation during pregnancy is a topic of great interest and controversy. American College of Obstetricians and Gynecologists103 suggests that current evidence does not support routine screening of all pregnant women for vitamin D deficiency. Serum 25-OH vitamin D concentrations of 20 ng/mL (50 nmol/L) or greater are associated with bone health; values of 32 ng/mL (80 nmol/L) or greater are associated with appropriate biomarker concentrations. The recommended intake of Vitamin D set by the Institute of Medicine in 2010 is 600 IU/d. However, when vitamin D deficiency is identified during pregnancy, supplementation with up to 1000 to 2000 IU/d is considered safe by most experts. Others feel that supplementation in doses up to 4000 IU/d is safe during pregnancy and lactation. It has been identified as a nutrient of public health concern by the US Department of Health and Human Services.104
Numerous studies have shown a prevalence of vitamin D levels of less than 50 nmol/L in 30% to 96% of pregnant women, increasing with latitude and with nonwhite race.105–111 A study done using archived samples of cord blood in Denmark showed an increased risk of developing schizophrenia with either elevated or low levels of Vitamin D. It was hypothesized that if all the infants could have been born with normal levels there would have been a reduction of 43% in the incidence of schizophrenia among the adults.112 There is also an association of vitamin D deficiency and insulin resistance,113 which was reversed with a single injection of the vitamin114 (not approved in the United States). Some studies report higher long-bone density in fetuses of women with adequate amounts of vitamin D,115,116 but the numbers are small.117 The general recommendation is 1000 to 2000 mg/d of supplementation, but there are no agreed-upon levels for sufficiency or insufficiency in pregnancy. There are no studies clearly showing differences in maternal outcomes or fetal survival, birth weight, or gestational length relative to vitamin D,118 but studies on bone health (and lung function and asthma at age 6 years119) support a level of at least 50 nmol/L, which implies a need for some supplementation for all pregnant women.120–123
Calcium
Women lose 3% to 5% of their bone mass while lactating but rapidly regain it within 6 months after weaning. Calcium and/or vitamin D deficiency leads to porous, weak bones and to rickets. A Cochrane Review has concluded that there is sufficient evidence to show that calcium supplementation lowers the risk of preeclampsia and other hypertensive disease of pregnancy, especially in the face of a low-calcium diet.124
Food sources of calcium are milk, yogurt, cheese, fortified juices, tofu, cereals, Chinese cabbage, kale, broccoli, and fish with bones. Multivitamin supplements, including prenatal supplements, contain little calcium.
Multiple Micronutrients (Multivitamins)
When compared with iron and folate alone, multiple micronutrients can significantly lower the incidence of SGA infants, but in 5 studies, they increased the risk of neonatal death when started after the first trimester.125 Regular consumption of fortified cereal grains appears to be an effective source of all but B vitamins, iron, and folate.
In the United States, prenatal vitamins should all contain 2 essential elements:
0.8 to 1.0 mg folate
120 to 150 μg iodine
Calcium, in the amount of 1000 to 2000 mg, should be provided as a supplement. It is not clear that any other specific components are necessary at this time. The introduction of a nutrient known to be lacking in an isolated population can highlight the effects of a deficiency over a short time, but the recognition of epigenetic mechanisms means that patience is required to evaluate the full effect on subsequent generations.
High Calorie Malnutrition
Lack of adequate protein in a prenatal diet has been shown to cause lifelong damage to the developing fetus in humans and numerous animal studies. The goal should be to have a diet of 20% protein or more throughout the pregnancy. Specific damage has been shown at levels of 7.2% or less, but an extremely high protein intake has also been shown to be damaging.88,126 Damage from a low-protein diet includes decreased brain size,127 altered fat distribution,128 increased obesity,129 shorter gestation and decreased birth weight,130 increased stress sensitivity,131 decreased sperm quality,132 altered cardiac energy metabolism,133 and changes in muscular tone.134 A diet filled with carbohydrates and fats (soft drinks, chips, etc) can easily lead to satiation before an adequate amount of protein and other nutrients has been consumed. A diet skewed toward a high-meat, low-carbohydrate intake126 leads to a higher incidence of hypertension in the offspring as well as high cortisol levels.
DIETARY PATTERNS
Evidence from international scientific research has identified various eating patterns that may provide short- and long-term health benefits, including a reduced risk of chronic disease.135 Analysis of overall food patterns takes into account the complex interactions and cumulative effects of multiple nutrients in the entire diet, therefore offering a more comprehensive and complementary approach to public health.
The “Western” diet is a pattern of eating that is associated with adverse health outcomes. The typical Western diet is one low in fruits, vegetables, whole grains, fish/seafood, and low-fat dairy. It is often called the meat-sweet diet because it is high in refined sugars, refined grains (baked goods, desserts, chips), red meat, and saturated fat. It also typically contains high-sugar drinks, high-fat dairy, and higher intakes of processed meats.
Other traditional eating patterns alternatively can provide health benefits. Their variety demonstrates that people can eat healthfully in a number of ways, which also likely applies to pregnancy.
Several healthful dietary patterns have been inversely associated with the risk of type 2 diabetes mellitus, cardiovascular disease, and total mortality135 Examples of healthy dietary patterns include the aMED (alternative Mediterranean diet), DASH (Dietary Approaches to Stop Hypertension), and aHEI (alternative Healthy Eating Index). These healthy dietary patterns share common components, namely, emphasizing a high intake of vegetables and fruits, high-quality carbohydrates including whole grains, protein from beans and smaller amounts from lean meats, healthy fats from nuts and seeds, fish and seafood and liquid oils, high in fiber, low in added sugar, and low intake of red meat and processed meats.
The types of vegetarian diets consumed in the United States vary widely.135 Vegans do not consume any animal products, whereas lacto-ovo vegetarians consume milk and eggs. Vegan diets can be low in B12, riboflavin, vitamin D, calcium, and long-chain n-3 fats if not properly supplemented. Vegetarian diets can also potentially be low in certain nutrients depending on which food groups might be avoided such as dairy, eggs, and/or fish and seafood, so supplement recommendations should be individualized.
Prepregnancy adherence to dietary patterns is now being investigated, with a few studies showing adherence to healthful dietary patterns being significantly associated with a lower risk of GDM,136 and a recent study showed that adherence to a Mediterranean diet pattern of eating during pregnancy was associated with lower incidence of GDM and better degree of glucose tolerance even in women without GDM.137 It has been speculated that these healthy dietary patterns may minimize the susceptibilities a pregnant woman has to β-cell dysfunction and insulin resistance.
These data suggest that efforts to encourage dietary patterns similar to the aMED, DASH, or aHEI might yield benefits for women of reproductive age and for pregnant women as well. My Pregnancy Plate is an education tool created using this emerging evidence of healthy eating patterns that can be advocated for in this population (Fig. 2).
FIG. 2.
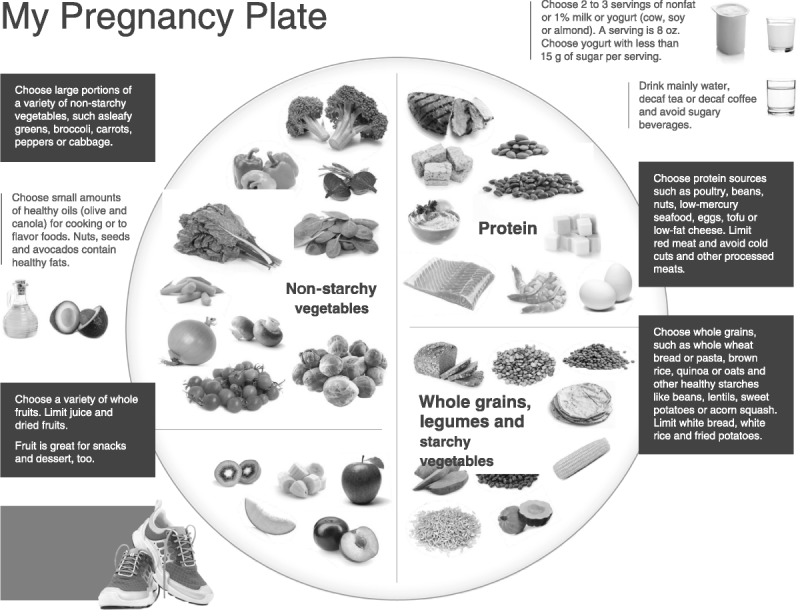
My Pregnancy Plate. Dietary suggestions for healthy nutrition during pregnancy. © 2014 Oregon Health & Science University. All rights reserved. Reproduction in whole or in part without expressed written consent from OHSU is prohibited.
Glycemic Index
The GI was proposed over 30 years ago as a classification of the blood glucose–raising potential of carbohydrate-containing foods,138 and the concept has stirred up controversy ever since. The GI uses a 100-point scale to measure how rapidly the carbohydrates in food cause blood sugars to rise. Foods with a high-GI value cause a big rise in blood glucose, and foods with a low-GI value cause a smaller rise. A competing measure, the glycemic load, adjusts this value by taking into account serving sizes and the amount of carbohydrates per serving. The glycemic load may be more practical than the GI because it accounts for quantity in addition to carbohydrate quality.139 There is evidence that low-GI diets improve glycemic control in people with diabetes140,141 and reduce risk of type 2 diabetes in men and women.142,143
The application of the GI to pregnancy outcomes is more recent. Pregnancy is a condition in which the GI may be of particular relevance because maternal glucose is the main energy substrate for intrauterine growth, and elevated maternal blood glucose levels are well recognized to contribute to excessive fetal growth. A review article144 evaluating the evidence regarding the effect of GI on maternal and fetal nutrition concluded that there was insufficient evidence to recommend a low-GI diet during normal pregnancy, as 1 of 8 studies showed an increase in SGA babies. That study, however, had very small numbers. There is probably some benefit of low-GI diet advice in reducing a woman’s risk of having a large-for-gestational age infant.145 For pregnancy complicated by GDM, however, a low-GI diet may confer benefits. Current evidence, although limited, consistently supports the advantages of, and has demonstrated no disadvantages of, a low-GI diet.146 Pregnant women with GDM are likely to benefit from following a low-GI meal pattern, with no significant adverse effects, and consideration of the GI should be given when formulating a diet for GDM.146 Using a low-GI diet for women with GDM has been shown to halve the number needing to use insulin, with no compromise of obstetric or fetal outcomes.147
Glycemic index is only one tool that can be used to determine carbohydrate quality. In another clinical trial, in intensively monitored women with GDM, a low-GI diet and a conventional high-fiber diet produced similar pregnancy outcomes.148 Excess glucose is also not the only fuel that can contribute to fetal overgrowth (see section on fat). Until further larger-scale intervention trials, preferably randomized controlled trials, are completed, a low-GI diet should not replace the current pregnancy recommendations from government and health agencies.144,145
CONCLUSIONS
There is good evidence to support a need for supplementation with folate, iodine, and calcium for all pregnancies. There is good evidence for supplementation with vitamin C in pregnant women who smoke. There is no good current evidence to show value in supplementation for iron and vitamins A, B6, B12, A, E, or D at this time, although there are many suggestive studies for vitamin D. All pregnant women should be encouraged to eat a balanced diet rich in fresh or frozen fruits and vegetables, high-quality carbohydrates including whole grains, and with a good mix of proteins from beans, lean meats, fish, and seafood. Their diet should be low in added sugar, red meat, and processed foods. Information beyond this simple prescription is simply not yet available for pregnant women or their offspring.
Footnotes
All authors and staff in a position to control the content of this CME activity and their spouses/life partners (if any) have disclosed that they have no financial relationships with, or financial interests in, any commercial organizations pertaining to this educational activity.
REFERENCES
- 1.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34:841–845. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Human growth and cardiovascular disease. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:21–38. [DOI] [PubMed] [Google Scholar]
- 3.Burton GJ, Jauniaux E, Charnock-Jones DS. Human early placental development: potential roles of the endometrial glands. Placenta. 2007;28(suppl A):S64–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streeter G. Developmental horizons in human embryos: age groups XI to XXIII. In: Contrib Embryol. Washington, DC: Carnegie Institution; 1951. [Google Scholar]
- 5.Kunz LH, King JC. Impact of maternal nutrition and metabolism on health of the offspring. Semin Fetal Neonatal Med. 2007;12:71–77. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YW, Chung JH, Kurbisch-Block I, et al. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008;112:1015–1022. [DOI] [PubMed] [Google Scholar]
- 7.Yee LM, Cheng YW, Inturrisi M, et al. Effect of gestational weight gain on perinatal outcomes in women with type 2 diabetes mellitus using the 2009 Institute of Medicine guidelines. Am J Obstet Gynecol. 2011;205:257.e1–257.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8(7A):1010–1027. [DOI] [PubMed] [Google Scholar]
- 9.Forsum E, Lof M. Energy metabolism during human pregnancy. Annu Rev Nutr. 2007;27:277–292. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003:CD000032. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS. Maternal nutrition and adverse pregnancy outcomes: lessons from epidemiology. Nestle Nutr Workshop Ser Pediatr Program. 2005;55:1–10; discussion 1–5. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS. Balanced protein/energy supplementation in pregnancy. Cochrane Database Syst Rev. 2000:CD000032. [DOI] [PubMed] [Google Scholar]
- 13.Ota E, Tobe-Gai R, Mori R, et al. Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2012;9:CD000032. [DOI] [PubMed] [Google Scholar]
- 14.Abrams BF, Laros RK. Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol. 1986;154:503–509. [DOI] [PubMed] [Google Scholar]
- 15.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 16.de Rooij SR, Painter RC, Phillips DI, et al. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–1901. [DOI] [PubMed] [Google Scholar]
- 17.de Rooij SR, Painter RC, Roseboom TJ, et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006;49:637–643. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, He Y, Qi L, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388–394. [DOI] [PubMed] [Google Scholar]
- 20.Painter RC, de Rooij SR, Bossuyt PM, et al. Maternal nutrition during gestation and carotid arterial compliance in the adult offspring: the Dutch famine birth cohort. J Hypertens. 2007;25:533–540. [DOI] [PubMed] [Google Scholar]
- 21.Painter RC, de Rooij SR, Bossuyt PM, et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84:322–327; quiz 466–7. [DOI] [PubMed] [Google Scholar]
- 22.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491. [DOI] [PubMed] [Google Scholar]
- 23.Roseboom TJ, van der Meulen JH, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Li Z, Wang M, et al. Early life exposure to the 1959–1961 Chinese famine has long-term health consequences. J Nutr. 2010;140:1874–1878. [DOI] [PubMed] [Google Scholar]
- 25.Stein AD, Zybert PA, van der Pal-de Bruin K, et al. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–765. [DOI] [PubMed] [Google Scholar]
- 26.Painter RC, Roseboom TJ, van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16:189–194. [DOI] [PubMed] [Google Scholar]
- 27.Hulshoff Pol HE, Hoek HW, Susser E, et al. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. [DOI] [PubMed] [Google Scholar]
- 28.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. [DOI] [PubMed] [Google Scholar]
- 29.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AS, van Os J, Driessens C, et al. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry. 2000;157:190–195. [DOI] [PubMed] [Google Scholar]
- 31.Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. [DOI] [PubMed] [Google Scholar]
- 32.Franzek EJ, Sprangers N, Janssens AC, et al. Prenatal exposure to the 1944-45 Dutch ‘hunger winter’ and addiction later in life. Addiction. 2008;103:433–438. [DOI] [PubMed] [Google Scholar]
- 33.Lussana F, Painter RC, Ocke MC, et al. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88:1648–1652. [DOI] [PubMed] [Google Scholar]
- 34.Painter RC, Westendorp RG, de Rooij SR, et al. Increased reproductive success of women after prenatal undernutrition. Hum Reprod. 2008;23:2591–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng X, Wang Y, Ren W, et al. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur J Clin Nutr. 2012;66:231–236. [DOI] [PubMed] [Google Scholar]
- 36.Clapp JF., 3rd Maternal carbohydrate intake and pregnancy outcome. Proc Nutr Soc. 2002;61:45–50. [DOI] [PubMed] [Google Scholar]
- 37.Loy SL, Marhazlina M, Azwany YN, et al. Higher intake of fruits and vegetables in pregnancy is associated with birth size. Southeast Asian J Trop Med Public Health. 2011;42:1214–1223. [PubMed] [Google Scholar]
- 38.Simmons RA. Developmental origins of beta-cell failure in type 2 diabetes: the role of epigenetic mechanisms. Pediatr Res. 2007;61(5 pt 2):64R–67R. [DOI] [PubMed] [Google Scholar]
- 39.Oster M, Murani E, Metges CC, et al. A high protein diet during pregnancy affects hepatic gene expression of energy sensing pathways along ontogenesis in a porcine model. PLoS One. 2011;6:e21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013. [Google Scholar]
- 41. National Heart, Lung, Blood Institute in cooperation with the National Institute of Diabetes and Digestive and Kidney Diseases. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Washington, DC: National Institutes of Health; 1998. [Google Scholar]
- 42.Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114:136–153. [DOI] [PubMed] [Google Scholar]
- 43.Bowers K, Tobias DK, Yeung E, et al. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Scholl TO, Leskiw M, et al. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care. 2010;33:2049–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S, Kim MY, Baik SH, et al. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur J Clin Nutr. 2013;67:196–201. [DOI] [PubMed] [Google Scholar]
- 46.Jensen CL. Effects of n-3 fatty acids during pregnancy and lactation. Am J Clin Nutr. 2006;83(suppl 6):1452S–1457S. [DOI] [PubMed] [Google Scholar]
- 47.Imhoff-Kunsch B, Briggs V, Goldenberg T, et al. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(suppl 1):91–107. [DOI] [PubMed] [Google Scholar]
- 48.Guldner L, Monfort C, Rouget F, et al. Maternal fish and shellfish intake and pregnancy outcomes: a prospective cohort study in Brittany, France. Environ Health. 2007;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Decsi T, Koletzko B. N-3 fatty acids and pregnancy outcomes. Curr Opin Clin Nutr Metab Care. 2005;8:161–166. [DOI] [PubMed] [Google Scholar]
- 50.Meldrum S, Dunstan JA, Foster JK, et al. Maternal fish oil supplementation in pregnancy: a 12 year follow-up of a randomised controlled trial. Nutrients. 2015;7:2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med. 2008;36:101–109. [DOI] [PubMed] [Google Scholar]
- 52.da Rocha CM, Kac G. High dietary ratio of omega-6 to omega-3 polyunsaturated acids during pregnancy and prevalence of post-partum depression. Matern Child Nutr. 2012;8:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budtz-Jørgensen E, Keiding N, Grandjean P, et al. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Debes F, Budtz-Jorgensen E, Weihe P, et al. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years [reprint in Neurotoxicol Teratol. 2006;28(5):536–547]. Neurotoxicol Teratol. 2006;28:363–375. [DOI] [PubMed] [Google Scholar]
- 55.Strain JJ, Davidson PW, Thurston SW, et al. Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J Nutr. 2012;142:1943–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson PW, Cory-Slechta DA, Thurston SW, et al. Fish consumption and prenatal methylmercury exposure: cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology. 2011;32:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson PW, Strain JJ, Myers GJ, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J Nutr. 2007;137:2805–2808. [DOI] [PubMed] [Google Scholar]
- 59.Lam HS, Kwok KM, Chan PH, et al. Long term neurocognitive impact of low dose prenatal methylmercury exposure in Hong Kong. Environ Int. 2013;54:59–64. [DOI] [PubMed] [Google Scholar]
- 60.Jedrychowski W, Flak E, Mroz E, et al. Modulating effects of maternal fish consumption on the occurrence of respiratory symptoms in early infancy attributed to prenatal exposure to fine particles. Ann Nutr Metab. 2008;52:8–16. [DOI] [PubMed] [Google Scholar]
- 61.Jedrychowski W, Perera F, Maugeri U, et al. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masters ET, Jedrychowski W, Schleicher RL, et al. Relation between prenatal lipid-soluble micronutrient status, environmental pollutant exposure, and birth outcomes. Am J Clin Nutr. 2007;86:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sausenthaler S, Heinrich J, Koletzko S. Early diet and the risk of allergy: what can we learn from the prospective birth cohort studies GINIplus and LISAplus? Am J Clin Nutr. 2011;94(suppl 6):2012S–2017S. [DOI] [PubMed] [Google Scholar]
- 64.Sausenthaler S, Koletzko S, Schaaf B, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530–537. [DOI] [PubMed] [Google Scholar]
- 65.Calder PC, Kremmyda LS, Vlachava M, et al. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc. 2010;69:373–380. [DOI] [PubMed] [Google Scholar]
- 66.Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94–102. [DOI] [PubMed] [Google Scholar]
- 67.Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507–513. [DOI] [PubMed] [Google Scholar]
- 68.Razzaghi H, Tinker SC, Crider K. Blood mercury concentrations in pregnant and nonpregnant women in the United States: National Health and Nutrition Examination Survey 1999–2006. Am J Obstet Gynecol. 2014;210:357.e1–357.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishra V, Thapa S, Retherford RD, et al. Effect of iron supplementation during pregnancy on birthweight: evidence from Zimbabwe. Food Nutr Bull. 2005;26:338–347. [DOI] [PubMed] [Google Scholar]
- 70.Bhutta ZA, Cabral S, Chan CW, et al. Reducing maternal, newborn, and infant mortality globally: an integrated action agenda. Int J Gynaecol Obstet. 2012;119(suppl 1):S13–S17. [DOI] [PubMed] [Google Scholar]
- 71.Reveiz L, Gyte MLG, Cuervo GL, et al. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst Rev. 2012. [DOI] [PubMed] [Google Scholar]
- 72.Cantor AG, Bougatsos C, Dana T, et al. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:566–576. [DOI] [PubMed] [Google Scholar]
- 73.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3:370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagerstad M. Folic acid fortification prevents neural tube defects and may also reduce cancer risks. Acta Paediatr. 2012;101:1007–1012. [DOI] [PubMed] [Google Scholar]
- 75.Chang H, Zhang T, Zhang Z, et al. Tissue-specific distribution of aberrant DNA methylation associated with maternal low-folate status in human neural tube defects. J Nutr Biochem. 2011;22:1172–1177. [DOI] [PubMed] [Google Scholar]
- 76.Ahrens K, Yazdy MM, Mitchell AA, et al. Folic acid intake and spina bifida in the era of dietary folic acid fortification. Epidemiology. 2011;22:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bukowski R, Malone FD, Porter FT, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw GM, Carmichael SL, Yang W, et al. Periconceptional intake of folic acid and food folate and risks of preterm delivery. Am J Perinatol. 2011;28:747–752. [DOI] [PubMed] [Google Scholar]
- 79.Siega-Riz AM, Savitz DA, Zeisel SH, et al. Second trimester folate status and preterm birth. Am J Obstet Gynecol. 2004;191:1851–1857. [DOI] [PubMed] [Google Scholar]
- 80.Martí-Carvajal A, Peña-Martí G, Comunián-Carrasco G, et al. Prematurity and maternal folate deficiency: anemia during pregnancy study group results in Valencia, Venezuela. Arch Latinoam Nutr. 2004;54:45–49. [PubMed] [Google Scholar]
- 81.Chiaffarino F, Ascone GB, Bortolus R, et al. Effects of folic acid supplementation on pregnancy outcomes: a review of randomized clinical trials [in Italian]. Minerva Ginecol. 2010;62:293–301. [PubMed] [Google Scholar]
- 82.Kloosterman J, de Jong N, Rompelberg CJ, et al. Folic acid fortification: prevention as well as promotion of cancer [in Dutch]. Ned Tijdschr Geneeskd. 2006;150:1443–1448. [PubMed] [Google Scholar]
- 83.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–194. [DOI] [PubMed] [Google Scholar]
- 84.Hamner HC, Mulinare J, Cogswell ME, et al. Predicted contribution of folic acid fortification of corn masa flour to the usual folic acid intake for the US population: National Health and Nutrition Examination Survey 2001–2004. Am J Clin Nutr. 2009;89:305–315. [DOI] [PubMed] [Google Scholar]
- 85.Whitrow MJ, Moore VM, Rumbold AR, et al. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–1493. [DOI] [PubMed] [Google Scholar]
- 86.Steenweg-de Graaff J, Roza SJ, Steegers EA, et al. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr. 2012;95:1413–1421. [DOI] [PubMed] [Google Scholar]
- 87.Roth C, Magnus P, Schjolberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrick K, Phillips DIW, Haselden S, et al. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: relation to adult cortisol concentrations in the offspring. J Clin Endocrinol Metab. 2003;88:3554–3560. [DOI] [PubMed] [Google Scholar]
- 89.Stagnaro-Green A, Sullivan S, Pearce EN. Iodine supplementation during pregnancy and lactation. JAMA. 2012;308:2463–2464. [DOI] [PubMed] [Google Scholar]
- 90.Qian M, Wang D, Watkins WE, et al. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr. 2005;14:32–42. [PubMed] [Google Scholar]
- 91.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Connelly KJ, Boston BA, Pearce EN, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161:760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haskell MJ, Pandey P, Graham JM, et al. Recovery from impaired dark adaptation in nightblind pregnant Nepali women who receive small daily doses of vitamin A as amaranth leaves, carrots, goat liver, vitamin A–fortified rice, or retinyl palmitate. Am J Clin Nutr. 2005;81:461–471. [DOI] [PubMed] [Google Scholar]
- 94.Tielsch JM, Rahmathullah L, Katz J, et al. Maternal night blindness during pregnancy is associated with low birthweight, morbidity, and poor growth in South India. J Nutr. 2008;138:787–792. [DOI] [PubMed] [Google Scholar]
- 95.van den BN, Dou L, Othman M, et al. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2010:CD008666. [DOI] [PubMed] [Google Scholar]
- 96.Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26(suppl 1):36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vollset SE, Refsum H, Irgens LM, et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71:962–968. [DOI] [PubMed] [Google Scholar]
- 98.Takimoto H, Mito N, Umegaki K, et al. Relationship between dietary folate intakes, maternal plasma total homocysteine and B-vitamins during pregnancy and fetal growth in Japan. Eur J Nutr. 2007;46:300–306. [DOI] [PubMed] [Google Scholar]
- 99.Dror DK, Allen LH. Interventions with vitamins B6, B12 and C in pregnancy. Paediatr Perinat Epidemiol. 2012;26(suppl 1):55–74. [DOI] [PubMed] [Google Scholar]
- 100.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014:311:2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Devereux G, Turner SW, Craig LC, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507. [DOI] [PubMed] [Google Scholar]
- 102.Gagne A, Wei SQ, Fraser WD, et al. Absorption, transport, and bioavailability of vitamin e and its role in pregnant women. J Obstet Gynaecol Can. 2009;31:210–217. [DOI] [PubMed] [Google Scholar]
- 103. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 495: vitamin D: screening and supplementation during pregnancy. Obstet Gynecol. 2011;118:197–198. [DOI] [PubMed] [Google Scholar]
- 104. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans. 8th ed 2015. Published on health.gov/dietaryguidelines/2015/guidelines. [Google Scholar]
- 105.Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr. 2011;31:89–115. [DOI] [PubMed] [Google Scholar]
- 106.Cavalier E, Delanaye P, Morreale A, et al. Vitamin D deficiency in recently pregnant women [in French]. Rev Med Liege. 2008;63:87–91. [PubMed] [Google Scholar]
- 107.Crozier SR, Harvey NC, Inskip HM, et al. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holmes VA, Barnes MS, Alexander HD, et al. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr. 2009;102:876–881. [DOI] [PubMed] [Google Scholar]
- 109.Johnson DD, Wagner CL, Hulsey TC, et al. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28:7–12. [DOI] [PubMed] [Google Scholar]
- 110.Marwaha RK, Tandon N, Chopra S, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–1389. [DOI] [PubMed] [Google Scholar]
- 111.Sahu M, Bhatia V, Aggarwal A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf). 2009;70:680–684. [DOI] [PubMed] [Google Scholar]
- 112.McGrath JJ, Burne TH, Feron F, et al. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull. 2010;36:1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maghbooli Z, Hossein-Nezhad A, Karimi F, et al. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008;24:27–32. [DOI] [PubMed] [Google Scholar]
- 114.Mozaffari-Khosravi H, Hosseinzadeh-Shamsi-Anar M, Salami MA, et al. Effects of a single post-partum injection of a high dose of vitamin D on glucose tolerance and insulin resistance in mothers with first-time gestational diabetes mellitus. Diabetic Med. 2012;29:36–42. [DOI] [PubMed] [Google Scholar]
- 115.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study [published correction appears in Lancet 2006;367(9521):1486]. Lancet. 2006;367:36–43. [DOI] [PubMed] [Google Scholar]
- 116.Viljakainen HT, Korhonen T, Hytinantti T, et al. Maternal vitamin D status affects bone growth in early childhood—a prospective cohort study. Osteoporos Int. 2011;22:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Specker BL. Does vitamin D during pregnancy impact offspring growth and bone? Proc Nutr Soc. 2012;71:38–45. [DOI] [PubMed] [Google Scholar]
- 118.De-Regil LM, Palacios C, Ansary A, et al. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zosky GR, Hart PH, Whitehouse AJ, et al. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11:571–577. [DOI] [PubMed] [Google Scholar]
- 120.Aghajafari F, Nagulesapillai T, Ronksley PE, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. [DOI] [PubMed] [Google Scholar]
- 121.Lucas R, Xiang F, Ponsonby AL. Vitamin D sufficiency in pregnancy. BMJ. 2013;346:f1675. [DOI] [PubMed] [Google Scholar]
- 122.Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abrams SA. Vitamin D supplementation during pregnancy. J Bone Miner Res. 2011;26:2338–2340. [DOI] [PubMed] [Google Scholar]
- 124.Hofmeyr GJ, Lawrie TA, Atallah ÁN, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014;6:CD001059. [DOI] [PubMed] [Google Scholar]
- 125.Ramakrishnan U, Grant FK, Goldenberg T, et al. Effect of multiple micronutrient supplementation on pregnancy and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(suppl 1):153–167. [DOI] [PubMed] [Google Scholar]
- 126.Shiell AW, Campbell-Brown M, Haselden S, et al. High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspring. Hypertension. 2001;38:1282–1288. [DOI] [PubMed] [Google Scholar]
- 127.Portman OW, Neuringer M, Alexander M. Effects of maternal and long-term postnatal protein malnutrition on brain size and composition in rhesus monkeys. J Nutr. 1987;117:1844–1851. [DOI] [PubMed] [Google Scholar]
- 128.Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes (Lond). 2006;30:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sutton GM, Centanni AV, Butler AA. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology. 2010;151:1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rasmussen KM, Habicht J-P. Maternal supplementation differentially affects the mother and newborn. J Nutr. 2010;140:402–406. [DOI] [PubMed] [Google Scholar]
- 131.Augustyniak RA, Singh K, Zeldes D, et al. Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1375–R1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toledo FC, Perobelli JE, Pedrosa FP, et al. In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod Biol Endocrinol. 2011;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Slater-Jefferies JL, Lillycrop KA, Townsend PA, et al. Feeding a protein-restricted diet during pregnancy induces altered epigenetic regulation of peroxisomal proliferator-activated receptor-α in the heart of the offspring. J Dev Orig Health Dis. 2011;2:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toscano AE, Ferraz KM, Castro RM, et al. Passive stiffness of rat skeletal muscle undernourished during fetal development. Clinics (Sao Paulo). 2010;65:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans. 7th ed Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 136.Tobias DK, Zhang C, Chavarro J, et al. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karamanos B, Thanopoulou A, Anastasiou E, et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur J Clin Nutr. 2014;68:8–13. [DOI] [PubMed] [Google Scholar]
- 138.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. [DOI] [PubMed] [Google Scholar]
- 139.Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care. 2004;27:2266–2271. [DOI] [PubMed] [Google Scholar]
- 140.Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. [DOI] [PubMed] [Google Scholar]
- 141.Jenkins DA, Kendall CC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753. [DOI] [PubMed] [Google Scholar]
- 142.Salmerón J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. [DOI] [PubMed] [Google Scholar]
- 143.Salmerón J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non–insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. [DOI] [PubMed] [Google Scholar]
- 144.Louie JCY, Brand-Miller JC, Markovic TP, et al. Glycemic index and pregnancy: a systematic literature review. J Nutr Metab. 2010;2010:282464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oostdam N, van Poppel MN, Wouters MG, et al. Interventions for preventing gestational diabetes mellitus: a systemic review and meta-analysis. J Womens Health (Larchmt). 2011;20:1551–1563. [DOI] [PubMed] [Google Scholar]
- 146.Louie J, Brand-Miller J, Moses R. Carbohydrates, glycemic index, and pregnancy outcomes in gestational diabetes. Curr Diab Rep. 2013;13:6–11. [DOI] [PubMed] [Google Scholar]
- 147.Moses RG, Barker M, Winter M, et al. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care. 2009;32:996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Louie JCY, Markovic TP, Perera N, et al. A randomized controlled trial investigating the effects of a low–glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care. 2011;34:2341–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]


