Restraint training caused long-lasting reductions in pain behavior, in response to the formalin test, and increases in amygdala cell activation.
Keywords: MRI habituation, Rat, Restraint, Stress, Pain, Amygdala
Abstract
With the increased interest in longitudinal brain imaging of awake rodents, it is important to understand both the short-term and long-term effects of restraint on sensory and emotional processing in the brain. To understand the effects of repeated restraint on pain behaviors and stress responses, we modeled a restraint protocol similar to those used to habituate rodents for magnetic resonance imaging scanning, and studied sensory sensitivity and stress hormone responses over 5 days. To uncover lasting effects of training, we also looked at responses to the formalin pain test 2 weeks later. We found that while restraint causes acute increases in the stress hormone corticosterone, it can also cause lasting reductions in nociceptive behavior in the formalin test, coupled with heightened corticosterone levels and increased activation of the “nociceptive” central nucleus of the amygdala, as seen by Fos protein expression. These results suggest that short-term repeated restraint, similar to that used to habituate rats for awake functional brain scanning, could potentially cause long-lasting changes in physiological and brain responses to pain stimuli that are stress-related, and therefore could potentially confound the functional activation patterns seen in awake rodents in response to pain stimuli.
1. Introduction
Magnetic resonance imaging (MRI) of rodents is a promising method for translational studies of pain processing, helping elucidate rodent brain responses to pain in noninvasive ways that no other techniques can, and potentially aiding the development of clinical treatments.66 To gather functional scan data comparable to human data, rats and mice are ideally awake in the scanner and require restraint to eliminate motion artifact. Magnetic resonance imaging environment training is often performed to habituate animals to the scanner environment and consists of exposure to restraint and scanner noise over a number of days.2,25,43,46 However, both restraint and noise are stressors for rodents29,41,56; so pain-related brain activation results could be confounded by large stress effects. Indeed, it has recently been shown that restraint stress causes large-scale alterations in rodent functional brain networks,34 suggesting that training for awake imaging could qualitatively alter the blood flow and blood oxygenation level dependent (BOLD) responses that are outcome measures of functional imaging.
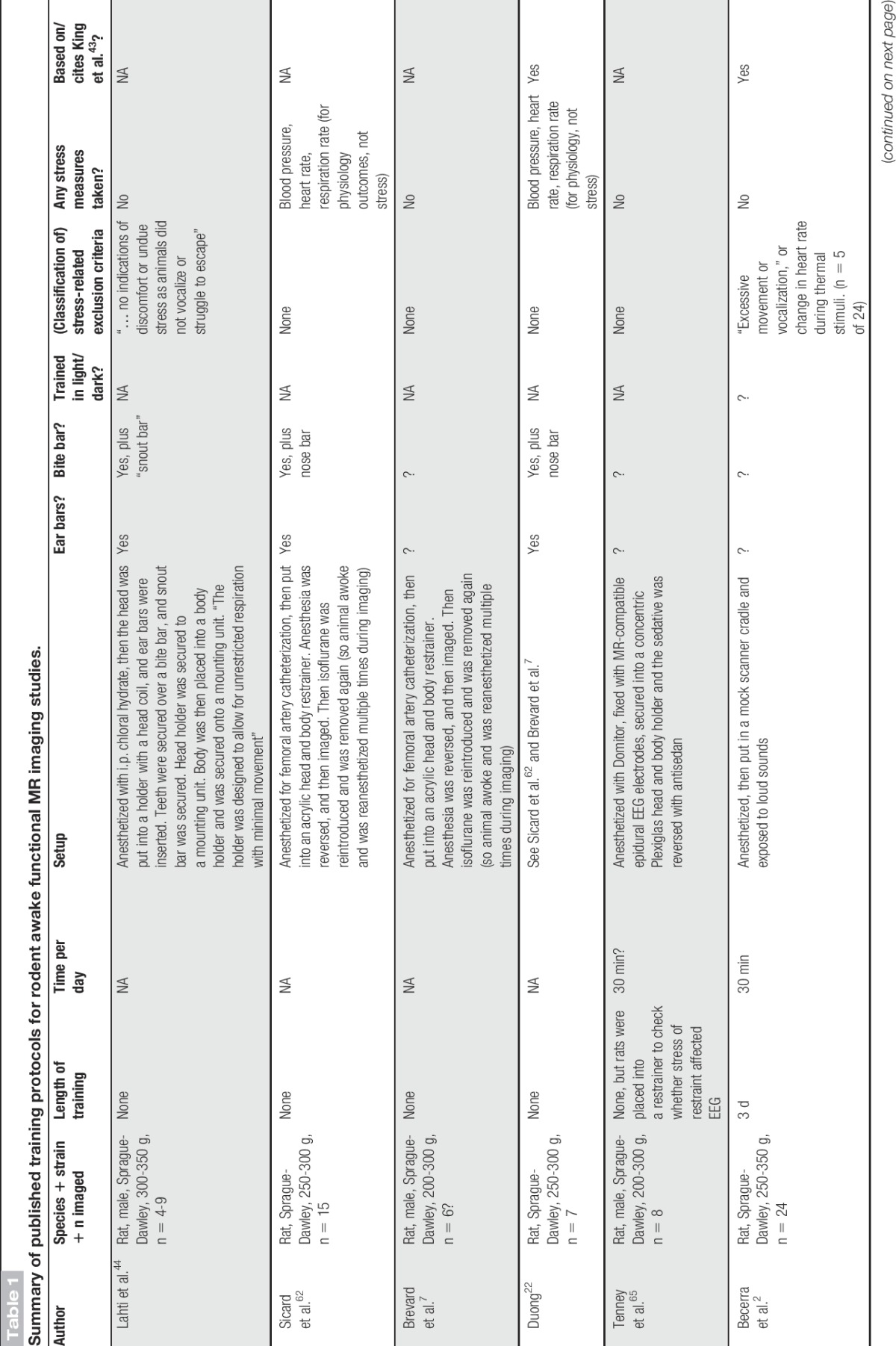
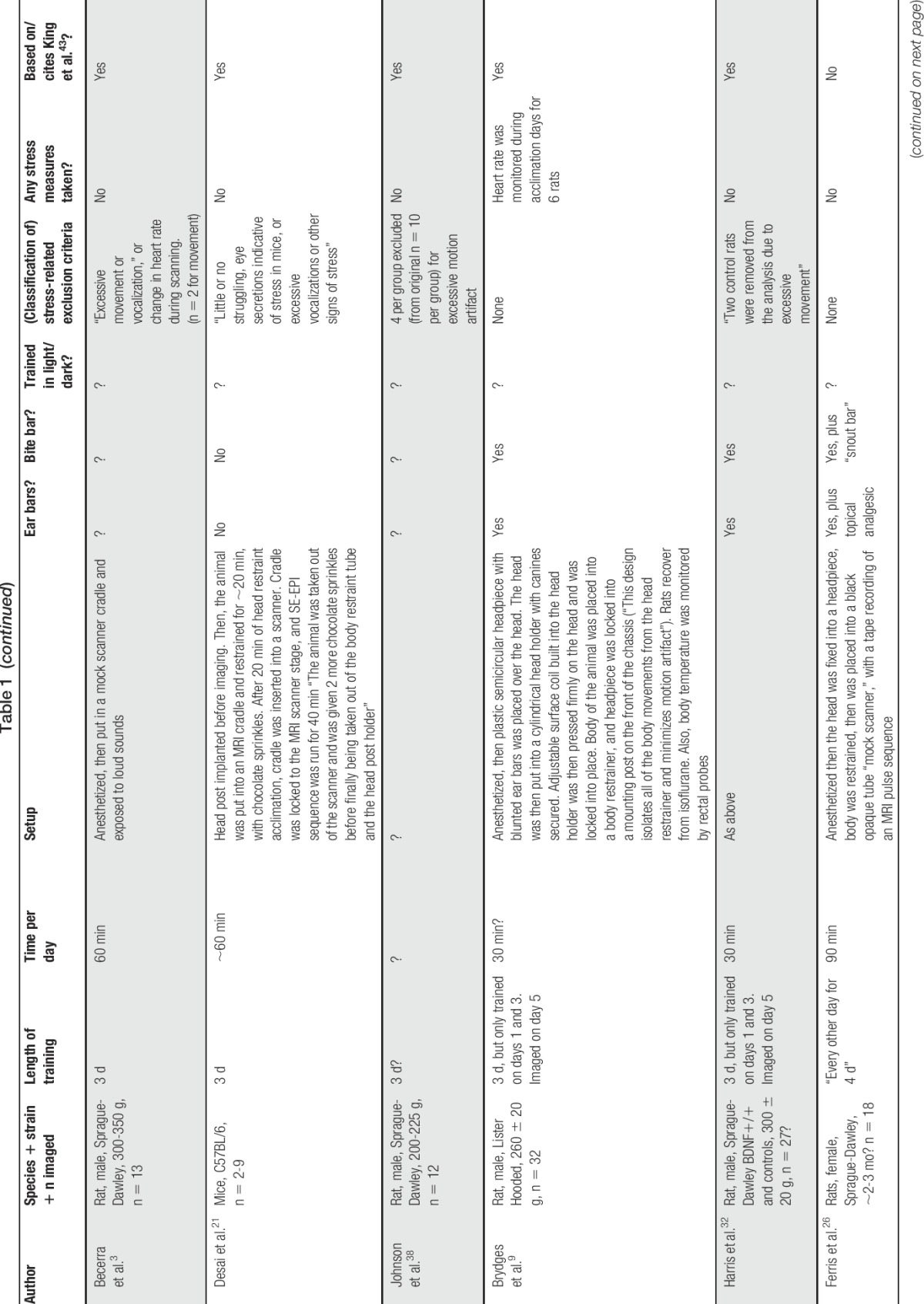
Training techniques vary among laboratories (Table 1), with some implanting cranial posts into the skulls of rodents to secure the head,31 and others using ear bars and bite bars for the front incisors to “clamp” the head into position.2,32,43,44 All techniques consist of habituating the animals to the scanner environment (or a mock one) for at least 3 days, some restraining for up to 90 minutes from the first session,2,3,21,24,26,43 and others increasing in 10- to 15-minute increments per day.31,45,46,75 King et al.43 investigated the effects of scanner acclimation on heart and breathing rate, and blood stress hormone (corticosterone) levels over 8 days and stated that as few as 3 days of acclimation could reduce physiological and hormonal stress levels enough to improve signal-to-noise ratio in functional MRI (fMRI) scans. Reed et al.58 investigated the effects of restraint training on 22 kHz ultrasonic vocalizations (a suggested index of negative effect) and behavioral outcomes and saw that vocalizations reduced by day 3, but that exploratory behaviors were decreased, and depressive-like behaviors increased after 5 days of acclimation. No study has investigated long-term effects of training on stress hormones, or on pain behaviors, which are highly influenced by stress (see reviews Refs. 10,37). These measures are important for scientists to understand, in order to improve the translational potential of rodent imaging studies.
Table 1.
Summary of published training protocols for rodent awake functional MR imaging studies.
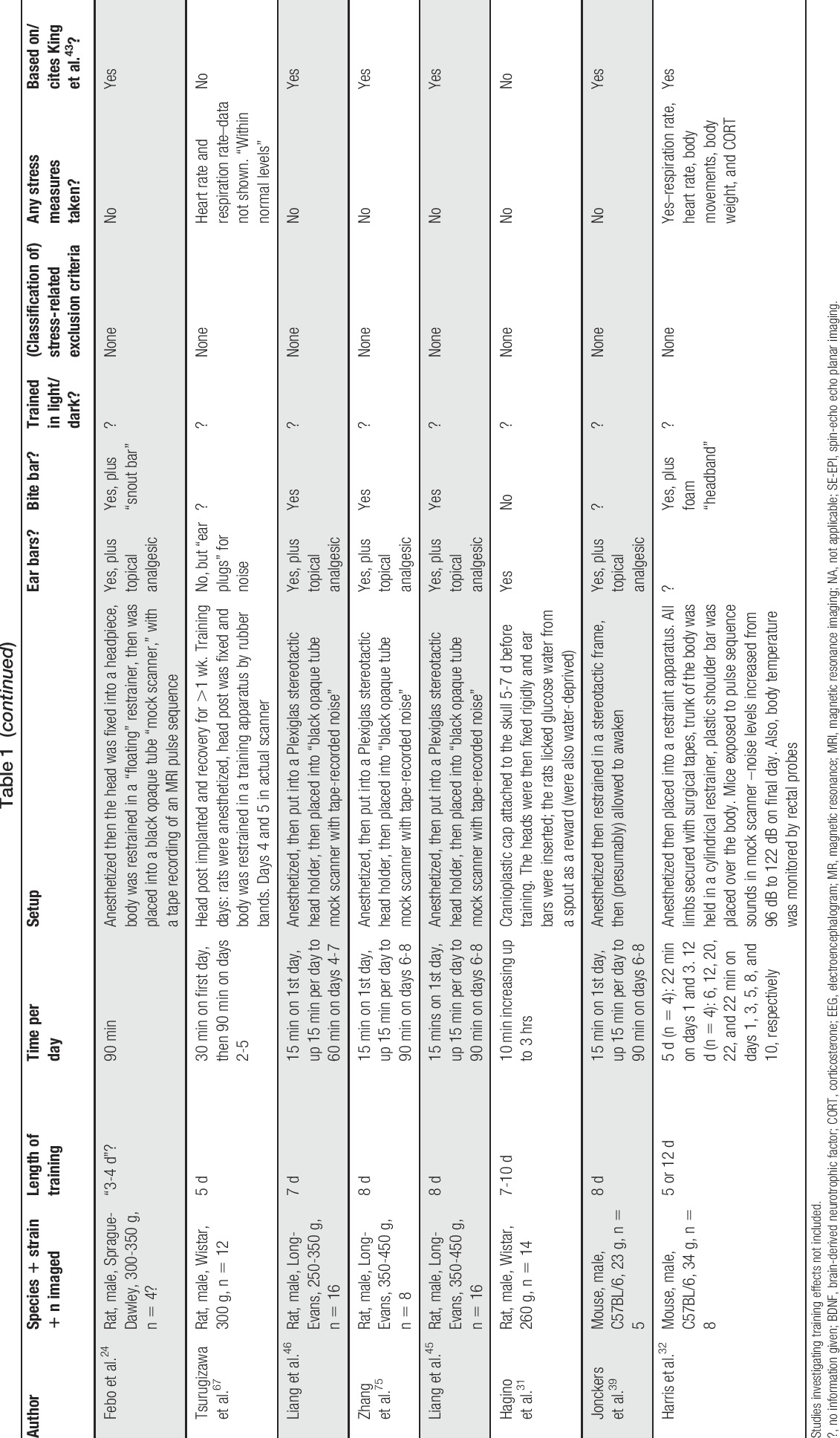
We aimed to model key features from published training protocols, all of which involve physical restraint of the head and body to some extent, and exposure to loud noise, over a number of days. We investigated the effects of 3 days of restraint training on pain behaviors and stress responses throughout the restraint-training period, and also investigated long-term effects of training by measuring pain responses to a formalin pain challenge 2 weeks later.
We suggest that repeated exposure to fMRI training may cause long-lasting alterations in pain and stress responses and may have the potential to confound the interpretation of imaging data from studies aimed at isolating pain-related brain activation in the rodent.
2. Materials and methods
2.1. Animals
Male Long Evans rats (Charles River, 8 weeks of age, 250-300 g; 10-12 per group) arrived at the animal facility and were pair-housed on a 12:12 hour light:dark cycle, with free access to food and water for 2 weeks before experiments began. All experiments were approved by the National Institute of Neurological Disorders and Stroke, Animal Care and Use Committee at the National Institutes of Health. All experiments were performed between 9 am and 1 pm, during the light phase.
Animals were assigned to either the restrained condition (REST) or control condition (CON). Treatment groups were housed in the same room within the animal facility and cohorts of 6 rats were tested at a time. Pair-housed rats received the same treatment, and restrained and control cohorts were tested separately. Although it has been suggested that acclimatizing rodent in pairs may be helpful,51 we tested the rats one at a time, as is most commonly done in studies that acclimatize the animals to the magnetic resonance (MR) environment.
2.2. Treatment groups
2.2.1. Restraint treatment
The restraint treatment was designed to replicate the main features of a number of training procedures used to train rats for awake imaging within the MRI machine,2,3,43 mainly involving restraint of the head and body, and exposure to loud scanner noise over a number of days (Table 1, pages 38-40). Magnetic resonance imaging restraint cradles, with head coil tubes, were custom made to the same specifications as the cradles used for small animal imaging, to accommodate rats from 250 to 500 g (National Institute of Neurological Disorders and Stroke/National Institute of Mental Health machine shop, National Institutes of Health).
On days 2, 3, and 4, animals arrived individually in the testing room and were briefly anesthetized (<3 minutes) with 2% isoflurane in 2 L/min O2 in an induction box while being fitted snugly into clean fabric restraint devices (Lomir Biomedical Inc, QC, Canada), which immobilized all limbs and left the head and tail protruding at each end. While still sedated, rats were placed into a clean MRI cradle mock-up (Fig. 1A) and the head placed into the head coil tube mock-up. Rats were then taped to the bench surface with duct tape to prevent self-damaging movement and escape, but not enough to restrict breathing. As animals awoke from anesthesia while restrained, a timer was started for 30 minutes and a loud recording of an MRI echo planar imaging sequence began, delivered through large over-ear headphones (Sony, model #MDR-NC8, Tokyo, Japan). Noise levels were similar to those within a 7.4T Bruker Pharmascan small animal MRI (∼90 dB) in the center of the headphones, where the rats' heads were positioned. An experimenter closely observed the animal throughout the entire testing period to monitor the animals' physical safety. After 30 minutes, tape was removed and restraint devices were undone; all animals voluntarily freed themselves immediately and were returned to holding cages before being taken through to another testing room for sensory testing.
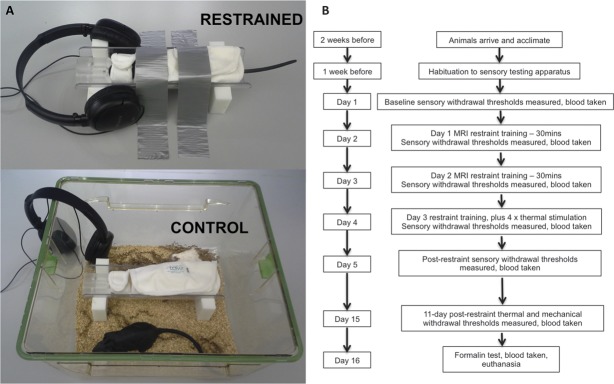
Figure 1.
(A) Images of restraint-training procedures. Restrained animals woke up from brief anesthesia in the cradle equipment, being physically firmly restrained and taped to the bench top to prevent self-injurious behavior (but not restrict breathing). An MRI EPI sequence was played through headphones for 30 minutes, at a level of 90 dB where the animal's head rested. For controls, animals awoke from brief anesthesia in a clean cage containing the same restraint cradle and jacket and heard the same EPI sequence at ∼18 dB through headphones. Black rubber rat used for illustrative purposes. (B) Experimental time line. MRI EPI, magnetic resonance imaging echo planar imaging.
To monitor distress and set ethical experimental endpoints, behavioral distress scores61 were noted every 5 minutes during the restraint period. If any animal scored at a level of 5 (“Mild agitation for about half of the restraint period”) or above for more than 5 minutes, testing was to be terminated. In addition, any animal that lost more than 10% of its body weight, or showed a decrease in body condition score36 over the course of 5 days of testing, was to be excluded. No animals were excluded from the analysis for these reasons.
After testing, all the used restraint devices were placed into a sealed bag. Restraint cradles, earphones, and bench tops were cleaned with 70% ethanol and wiped to remove traces of previous animals before the arrival of the next animal.
2.2.2. Control treatment
Control rats were tested on different days to restrained rats. Similarly, on days 2, 3, and 4, animals arrived individually in the testing room and were briefly anesthetized (<3 minutes) with 2% isoflurane in 2 L/min O2 in an induction box. However, rats were not restrained, but instead awoke from anesthesia in a clean housing cage together with clean restraint apparatus (fabric restrainer and MRI cradle, Fig. 1A). Headphones playing the same MRI echo planar imaging sequence were hung over the edge of the cage and the sequence was played at ∼18 dB. Over the 30-minute period, animals were free to explore the cage and apparatus. At the end of this period, rats were returned to holding cages before being taken through to another testing room for sensory testing.
As with the restrained group, after testing, the restraint device present in the cage was placed into a sealed bag. Restraint cradles, earphones, and bench tops were cleaned with 70% ethanol and wiped to remove traces of previous animals before the arrival of the next animal. Cages in which the animal had been exposed to equipment were removed from the room.
2.2.3. Both groups
To simulate pain testing used in previous MRI awake rodent paradigms,2 on day 4 (third restraint day), 5 minutes before the end of the exposure period, every animal was removed from the apparatus (either from the fabric restraint or from the cage containing the equipment) and restrained in a clean black towel while thermal heat stimuli were applied through a thermode (4 × 4 mm) to the plantar surface of the right hind paw (Medoc TSA II; Medoc, Ramat Yishai, Israel). All rats received four 36-second heat stimuli (48°C), with an interstimulus interval of 32 seconds. This procedure took 2 to 3 minutes. This acute pain stimulus was also used as a positive control to confirm that acute pain increases corticosterone (CORT) levels. Afterwards, as on other days, rats were returned to holding cages before being taken through to another testing room for sensory hypersensitivity testing.
2.3. Experimental timeline
See Figure 1B for the experimental time line. One week after arriving at the facility, rats were handled and acclimated to mechanical and thermal withdrawal threshold testing equipment. On 3 consecutive days before baseline testing, rats were placed into acrylic chambers, 20 × 10 × 12 cm (IITC Life Science Inc, Woodland Hills, CA) for 30 minutes and left quietly to acclimate to testing boxes.
Animals were weighed daily on testing days, except the final day 16. Baseline mechanical and thermal withdrawal thresholds were taken on day 1. Thermal thresholds were measured using the Hargreaves apparatus (Ugo Basile, Varese, Italy), where a radiant heat source shines through a thermo-neutral glass plate onto the plantar surface of the hind paw—when the rat flinches away from the heat, the heat source shuts off and the time to flinch (in seconds) is displayed. Cutoff time was set at 20 seconds to prevent tissue damage. Mechanical thresholds were measured using a dynamic plantar anesthesiometer (Ugo Basile), where a filament rises through a wire mesh floor and is applied to the plantar surface of the hind paw from underneath. When the animal flinches, the filament drops, and the force (in grams) that provoked the flinch is displayed. Maximum force was set to 50 g. Each animal was tested one at a time, in the thermal, then mechanical withdrawal equipment, and equipment was thoroughly cleaned and dried between animals (70% ethanol followed by 1:10 dilute Windex [SC Johnson, Racine, WI]). Immediately after sensory threshold testing, 70 μL of blood was taken through a ∼1 mm tail nick using an #11 scalpel blade at the tip of the tail and collected into heparin-coated capillary tubes. Tubes were immediately spun using a high-speed microcentrifuge (StatSpin CritSpin; Beckman Coulter Inc, Indianapolis, IN) for 2 minutes and blood plasma were collected and stored at −80°C.
On days 2 to 4, rats were subjected to restraint (restrained, REST, group) or exposure to restraint apparatus (control, CON, group). Testing order of animals was rotated between animals each day so that no animal was tested at the same time every day to control for the effect of time of day on nociceptive behavioral testing.14 For all animals, the order of testing was as follows:
(1) All animals in the cohort were removed from the animal facility in their home cages and placed into a quiet “holding room” for ∼30 minutes to settle.
(2) Individual rats were removed from the holding room in a clean holding cage and were taken to the testing room for treatment (REST or CON). Nontested animals remained in the holding room.
(3) Individual animals were restrained, or not, by a female experimenter in the testing room.
(4) Afterwards, each animal was placed back into their holding cage and taken to a third room where thermal and mechanical testing occurred, and blood was taken by a blinded female experimenter.
(5) After testing, rats were replaced into their holding cage and removed from the testing room, being kept separate from all other animals until all testings were complete.
(6) After the final animal was tested, all animals in the cohort were returned to their home cages and returned to the animal facility.
On day 5, all animals had sensory thresholds taken in the same manner as the baseline measurements (individually, thermal then mechanical, then finally blood was taken).
On the 14th day after baseline testing (day 15), rats were subjected to a final set of sensory threshold testing and blood collection, tested in the same manner as for baseline measurements.
On the final day, day 16, rats were brought into a testing room one at a time and received 50 μL of 1% formalin in saline injected subcutaneously into the plantar surface of the right hind paw. Animals were immediately placed into Plexiglas observation boxes (30 × 30 × 30 cm) where an angled mirror displayed the underside of the animal, for video recording over 60 minutes. Immediately after the 60-minute recording period, a final blood draw was taken and rats were anesthetized with 5% isoflurane, then transcardially perfused with 4% paraformaldehyde. Brain tissue was extracted, postfixed for 24 hours, and placed into 30% sucrose for 14 days. Tissue was then blocked and frozen into Tissue-Tek OCTcryoprotectant (Sakura Finetek USA Inc, Torrance, CA) and stored at −80°C.
2.4. Sensory sensitivity analysis
Because of large interindividual differences within groups, data were converted to percentage change from baseline for each animal. Thermal and mechanical withdrawal thresholds were analyzed using SPSS v22 (IBM, New York, NY) in a 2-way repeated-measures mixed-model analysis of variance (ANOVA) with treatment group, test day and paw as factors. Bonferroni post hoc tests were carried out as appropriate, if a main effect was seen.
2.5. Formalin test analysis
Behavioral videos of formalin responses were manually scored in 5-minute time bins up to 60 minutes, using JWatcher software (http://www.jwatcher.ucla.edu/). Outcome measures scored were time spent lifting of the injected paw; time spent licking the injected paw; number of flinches of injected paw; time spent guarding the injected paw; time spent exploring the test chamber; and number of rears per time bin. A weighted pain score was calculated, adapted from Dubuisson and Dennis (1977) according to the following equation:
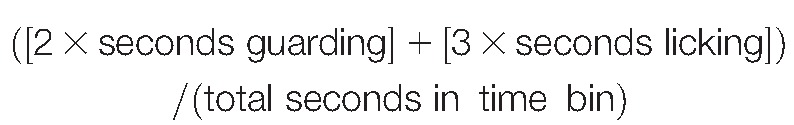 |
Data were analyzed using SPSS in a repeated-measures mixed-model 2-way ANOVA, with treatment and time bin as factors. Bonferroni post hoc tests were carried out as appropriate, if a main effect was seen.
2.6. Histology
To gain a measure of brain activation which was not MR-related and was therefore a reflection of MR training alone, brain blocks containing the amygdala (−1.50 to −3.00 mm relative to Bregma)53 were sectioned into 30-μm slices and every seventh slice (210 μm intervals) stained for Fos protein, a product of the c-fos immediate early gene and marker of cell activation. Briefly, endogenous peroxidase activity was inactivated with 0.6% hydrogen peroxidase, then free-floating sections were incubated for 48 hours at 4°C in 0.01 M phosphate-buffered saline (PBS, pH 7.4) containing 1% normal donkey serum (Jackson ImmunoResearch Labs, West Grove, PA), 0.3% Triton X-100 (Sigma, St. Louis, MO), and rabbit anti-c-fos IgG (1:3000, Cat. # SC-52; Santa Cruz Biotechnology, Santa Cruz, CA). To visualize the Fos-positive cells using the avidin–biotin complex method, sections were incubated in PBS-containing normal donkey serum, Triton-X, and biotin-SP-AffiniPure donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch Labs) for 1 hour, then in PBS-containing avidin-biotinylated horseradish peroxidase complex (Vectastain elite ABC kit; Vector Lab, Burlingame, CA) for another hour. Sections were incubated for 6 minutes in 0.05 M Tris buffer (pH 7.2) containing 0.03% 3′,3′-diaminobenzidine (Sigma) and 0.0075% hydrogen peroxide (Sigma). All steps were carried out at room temperature except when indicated, and each step was followed by PBS washes. Sections were then rinsed in distilled water, slide-mounted, dehydrated in ethanol, cleared in xylene, and finally coverslipped in Permount (Fisher Scientific, Fair Lawn, NJ).
To quantify, bright field images were taken at ×5 magnification using a Leica DM5500B microscope. Six to eight sections per animal were imaged bilaterally. Number of Fos-positive cells was quantified using NIS Elements software (Nikon Instruments Inc, Melville, NY), where a region of interest (ROI) was placed over the basolateral (BLA) (96.2 mm2) and central (CeA) (64.9 mm2) nuclei of the amygdala, bilaterally, and cell counts were automatically quantified. Average cell counts per ROI were calculated. Because of differences in staining intensity between staining batches, average cell counts per batch were calculated and each animal normalized to the batch mean. Group differences were compared by one-way ANOVA. All data were graphed in Prism 6 (GraphPad Software Inc, La Jolla, CA).
3. Results
3.1. Restrained animals gain weight more slowly than control groups
All animals gained weight over the course of the 15-day testing period (day F(5,115) = 20.01, P < 0.0001, Fig. 2). At the final time point, both groups lie within the normal weight ranges for male Long Evans rats at 12 weeks of age (350-430 g, according to http://www.criver.com/products-services/basic-research/find-a-model/long-evans-rat), but nevertheless, restrained rats gained less weight overall and weighed significantly less on day 15 than controls (P = 0.006).
Figure 2.
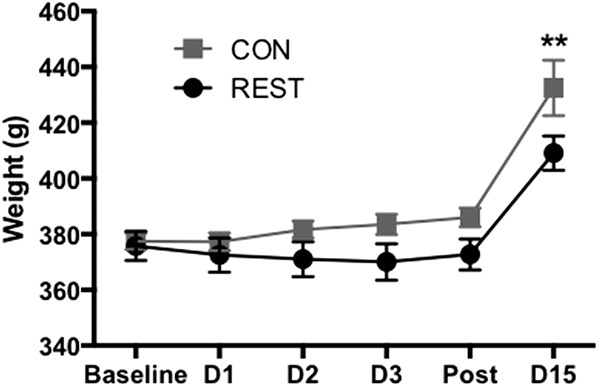
Restrained rats gained less weight over the testing period than controls (F(5, 115) = 20.01, P < 0.0001), weighing significantly less than controls on day 15 (D15, P = 0.006). **CON vs REST at day 15. n = 10 per group. CON, control; REST, restrained.
3.2. Corticosterone stress responses are greater in restrained animals
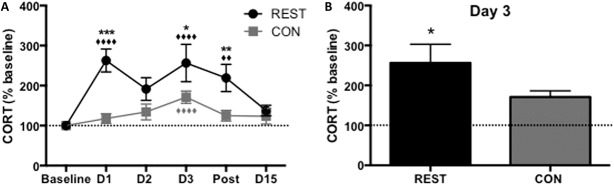
Restrained animals showed higher levels of CORT than controls overall in response to treatment (F(1,102) = 21.47, P < 0.0001, Fig. 3A), with clear peaks compared with baseline at day 1, immediately after the first restraint (P < 0.0001), and day 3, after acute thermal pain challenge (P < 0.0001). Restrained animals had significantly higher CORT levels than controls on days 1, 3, and postrestraint (P < 0.001, P = 0.011 and P = 0.005, respectively).
Figure 3.
(A) Restrained animals showed the highest levels of CORT overall in response to treatment (F(1,102) = 21.47, P < 0.0001), and there was an overall effect of treatment day on CORT levels (F(5,102) = 5.89, P < 0.0001), plus a significant treatment × day interaction (F(5,102) = 2.46, P = 0.038). On days 1, 3, and postrestraint, REST animals had significantly higher levels of CORT compared to CON animals (asterisks on graph, post hoc values: D1 P < 0.001, D3 P = 0.011, post P = 0.005). Diamonds represent each group's day vs baseline—for REST animals, D1 P < 0.0001, D3 P < 0.0001, post P = 0.007. There was a significant increase in CORT on day 3 vs baseline for both groups (P < 0.0001), after an acute thermal pain stressor. (B) After the thermal pain stressor on day 3, REST animals had higher CORT levels than CON animals (P = 0.011). n = 10 per group. CON, control; CORT, corticosterone; REST, restrained.
On day 3, while both groups showed an increase in CORT responses compared with baseline after the acute thermal pain stressor (P < 0.0001), REST animals showed the greatest response (P = 0.011 vs CON, Fig. 3B).
3.3. Thermal and mechanical withdrawal thresholds are not affected by restraint but drop after acute thermal heat challenge
Control and restrained animals did not show deviations from baseline thermal withdrawal thresholds until the third day, when both groups showed a significant drop in thermal withdrawal thresholds after noxious heat challenge (F(1,252) = 7.46, P < 0.0001, Fig. 4A). Although only the right paw received thermal stimulation on day 3, the reduced withdrawal threshold occurred bilaterally, with no difference between left and right paws on any day (F(1,252) = 1.04, P = 0.31). Similar bilateral effects were observed for mechanical withdrawal thresholds (Paw F(1, 252) = 0.00, P = 0.99), with both the restrained and control groups showing a decrease in mechanical withdrawal threshold after the noxious thermal challenge on day 3 (Treatment F(2,252) = 0.39, P = 0.53, day 3 P = 0.009, Fig. 4B).
Figure 4.
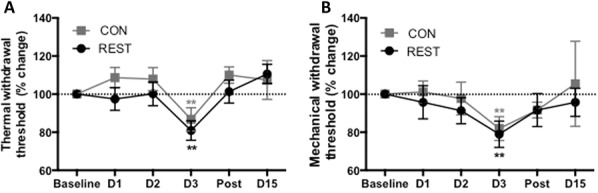
(A) Restrained and control groups showed no differences in thermal withdrawal thresholds (F(1,252) = 2.29, P = 0.13) and there were no differences between left and right hind paw thresholds (F(1,252) = 1.04, P = 0.31), so data from the right hind paw only is shown on graphs. However, there was a significant effect of day (F(5,252) = 7.46, P < 0.0001) as on day 3, both groups showed a drop in thresholds after acute thermal pain challenge (P < 0.008). **Both groups P = 0.008 day 3 vs baseline. (B) There were no group differences in mechanical paw withdrawal thresholds (F(1,252) = 0.39, P = 0.53), but there was an overall effect of time (F(5,252) = 5.45, P = 0.005) and both groups showed a drop in thresholds on day 3. **Both groups P = 0.009 day 3 vs baseline. n = 12 per group. CON, control; REST, restrained.
3.4. Restrained animals show decreased nociceptive responses to a formalin challenge 2 weeks after restraint treatment
When the formalin pain test was performed on day 16 (12 days after the final restraint), previously restrained animals showed a lower weighted pain score than CON to 1% formalin (F(1,264) = 17.69, P < 0.0001, Fig. 5A), suggesting a delayed analgesic effect of restraint training on pain behaviors.
Figure 5.
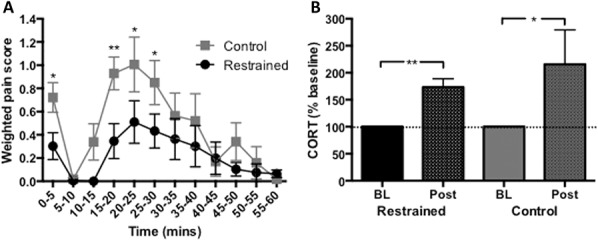
(A) Restrained animals showed decreased behavioral responses (weighted pain scores) to 1% formalin injection in the hind paw compared with control animals, when tested 2 weeks after restraint (F(1,264) = 17.69, P < 0.0001). *P < 0.05, **P < 0.01, controls vs restrained groups. n = 11 to 12 per group. (B) Both restrained and control animals showed increases in CORT responses vs baseline after the formalin test (REST P = 0.003, CON P = 0.02, 1-tailed Wilcoxon matched-pairs signed rank test). *P < 0.05 and **P < 0.01 post-restraint vs baseline, n = 11 to 12 per group. BL, baseline; CORT, corticosterone; Post, post formalin.
Corticosterone hormonal levels were evaluated immediately after formalin behavioral testing. Both restrained and control animals showed increases in stress hormone compared with their baselines (REST P = 0.003, CON P = 0.02, Fig. 5B), suggesting that formalin was stressful for both groups, despite the differences in their behavioral reactions to the formalin injection.
3.5. Restrained animals show a greater amygdalar response to formalin compared with controls
When Fos-positive cells were quantified in the BLA and CeA, there were bilateral increases in Fos immunoreactivity (P ≥ 0.63), therefore left and right hemisphere ROI counts were pooled.
In the BLA, there were no group differences in Fos immunoreactivity (P = 0.27, Fig. 6B). However, in the central, or “nociceptive” amygdala, there was significantly greater Fos immunoreactivity, and by extension cell activation, in the CeA of the restrained rats compared with controls, (P < 0.0001, Fig. 6C). These results suggest that restraint training caused lasting alterations in the processing of formalin pain in the brain.
Figure 6.
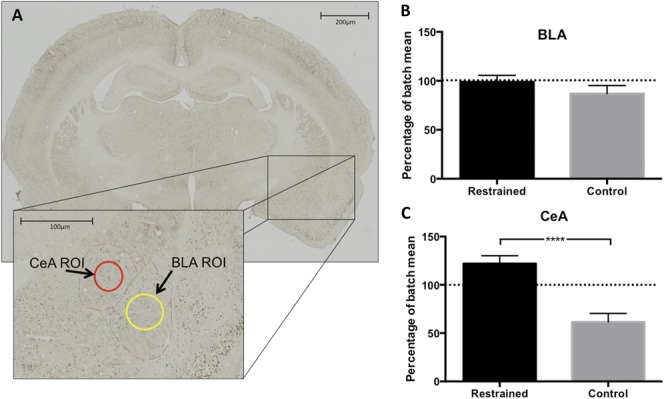
(A) Whole brain image of Fos-stained slide, plus enlargement of regions of interest (ROIs) used for cell quantification. Red dashed lines indicate the central nucleus (CeA) and basolateral nucleus of the amygdala (BLA), and red and yellow circles indicate ROIs for those regions. (B) In the BLA, there were no differences between restrained and control animals in Fos-positive cell numbers post-formalin (P = 0.27, one-tailed t test). (C) Restrained animals showed more Fos-positive cells in the CeA after the formalin test compared with controls. ****P < 0.0001, n = 11 to 12 per group.
4. Discussion
In this study, we modeled previously published training protocols for fMRI of awake rats and found both short-term and long-term effects of training. We found that although training led to similar mechanical and thermal nociceptive responses during the training days, they caused delayed effects on pain behaviors in the formalin test 2 weeks later, with associated alterations in brain processing of pain. Further, despite the absence of nociceptive behavioral differences on the training days, CORT levels were elevated, indicating that the restrained rats were physiologically more stressed than the controls. Additionally, restrained rats gained weight at a slower pace than control animals. This reduced weight gain is in line with literature showing that physical restraint (acute and chronic) prevents weight gain in rats.23,30,33,55,76 Our findings of increased CORT and reduced weight gain in response to training is consistent with studies showing that restraint reliably activates the hypothalamic-pituitary-adrenal gland axis, and as such is a stressful procedure.27,40,47,59
Behaviorally, restrained animals showed no differences to control animals in thermal and mechanical withdrawal thresholds when tested immediately after restraint. Based on the published effects of restraint, we expected to see stress-induced changes in nociceptive behavior, most likely stress-induced analgesia (SIA). Stress-induced analgesia is a phenomenon where exposure to a stressor causes a reduction in pain behavior. First described over 4 decades ago, SIA and its underlying neurobiology has been extensively studied (see Ref. 10 for a review) and has been documented in rodents and other mammals after acute restraint,11,17,42,59,71 often manifesting in longer withdrawal responses to tail flick tests (where a rodent flicks its tail to remove it from painfully hot water) and hot-plate escape latencies. As SIA has been shown in response to a thermal nociceptive stimulus, we expected restrained animals to show decreases in thermal withdrawal thresholds relative to control rats, but did not see differences. This could partly be due to the fact that MRI training and scanning not only involves physical restraint but also exposure to loud (>85 dB), unpredictable noises. Noise is a stressor for rodents and causes elevated CORT levels,1,35 increased startle responses,19 stress-related analgesia through endogenous opiate systems18 and has even been used to induce an animal model of fibromyalgia.29,41 The combination of both physical restraint and loud noise could affect the stress response in different ways to a single stressor alone. Indeed, combinations of stressors including restraint, foot shock, noise, and exposure to predator odors have been used to create rodent models of post-traumatic stress disorder, which can cause enhanced or reduced pain responses depending on the model used and nociceptive measurements employed (see Ref. 70 for a review).
An alternative explanation for the lack of expected SIA could be that, simple reflexive behavioral tests such as the Hargreaves test may not be sensitive enough to uncover the subtle and complex cognitive changes induced by fear and stress, and their interaction. The literature on the effects of physical restraint alone has documented effects on a variety of cognitive modalities including anxiety and depressive-like behaviors,16,30,49 spatial memory formation and recall,6,15 object recognition and memory,4 fear learning60 and social interaction.72
Two weeks after MRI training, rats received a formalin pain test. Previously restrained rats showed a reduction in pain behaviors relative to controls—possibly a long-lasting form of SIA. In addition, the CeA showed greater activation in the restrained group compared with controls, and vs the BLA, suggesting that MRI restraint training can qualitatively alter central pain processing. The CeA and BLA are both involved in pain processing50—the BLA acts to integrate polymodal sensory information and projects to the central nucleus, but the CeA has been called the “nociceptive” amygdala, where anxiety and fear-related inputs are integrated, and projections to the forebrain and periaqueductal gray then influence top-down and descending modulation of pain-related signals.12,13,69 The increase in cell activation of the CeA suggests that BOLD and cerebral blood flow outcome measures could also potentially be affected by changes in pain-related processing after MRI training.
Current rodent imaging protocols often anesthetize animals to eliminate movement artifact within the scanner and reduce stress effects,28,52,64 but anesthesia depresses cortical activity and can have large effects on the main outcome measures of functional imaging—blood flow and BOLD responses—in both humans and rodents.44,48,57,62 Additionally, anesthetics cause loss of consciousness, which is problematic when investigating outcome measures with a large cognitive component such as emotional processing, pain, stress, memory, and decision-making tasks. Therefore sedatives (ie, alpha-chloralose) in combination with paralytics (ie, pancuronium bromide) have been used to subdue rodents and remove the possibility of motion artifact.54,63 Although this method retains a good signal-to-noise ratio for functional imaging,68 alpha-chloralose is unsuitable for recovery and/or longitudinal studies. Furthermore, animals are still responsive to external stimuli and, coupled with paralysis, this raises the possibility that the animals are highly stressed, but cannot respond behaviorally, potentially confounding cerebral blood flow and BOLD responses.
Imaging rodents while awake is the best way to gain translational functional brain imaging data, but rats must be trained to tolerate the scanner environment—MRI experiences can be stressful in humans, but rodent imaging is obviously complicated by the fact that procedures cannot be explained to rodents, nor can they be expected to stay still. Current training models employ repeated restraint and exposure to MRI noises over a number of days,2,25,43,45 but the behavioral effects of training have not yet been fully validated—CORT levels, breathing and heart rates drop over 3 days of training but appropriate baseline comparison measures have not been reported, and the only behavioral outcome measures noted are physical struggling and audible vocalizations. These are problematic because neither outcomes are visible or audible when an animal is within a noisy scanner bore, and the majority of rodent communication is at frequencies inaudible to humans ie, alarm calls are emitted at ∼22 kHz.8 Reed et al.58 investigated the behavioral effects of restraint training and found that 22 kHz calls reduce over 5 days, but immobility in the forced swim test increases, suggesting a form of learned helplessness in MRI-trained rodents. Here, we demonstrate that repeated MR training can affect behavioral responses to the formalin pain test 2 weeks later, which is coupled with altered cell activation in the amygdala. Together with the similarity in training protocols to rodent fibromyalgia29,41 and post-traumatic stress disorder models,70 it is possible that repeated restraint training causes long-term alterations in an array of behaviors, which could potentially confound the interpretation of functional BOLD responses, particularly in the pain field.
Training protocols for awake rodent fMRI are highly variable (Table 1). However, we have incorporated the most critical aspects that are known to cause stress—namely, physical restraint and exposure to loud noise over a number of days. Training protocols also briefly anesthetize rodents for restraint. This brief (<5 minutes) repeated anesthesia rapidly influences CORT levels in female rats,5 and brief isoflurane can cause biochemical and hormonal changes in both genders.20,73,74
Some laboratories immobilize the head only to allow more free movement of the body,9,24,26 but these protocols still involve bodily restraint of some form, which is stressful for predated rodents. Other laboratories train animals for longer periods up to 10 days,31,32 but chronic exposure to restraint and noise stressors can also cause changes in pain behaviors.10 Thus, one goal of the current report is to encourage researchers to verify whether or not their training protocols affect behavioral outcome measures, despite finding near normal stress levels. Indeed, King et al.42 showed that CORT levels return to baseline levels by day 3 of training, but we have shown that despite trained rats' CORT levels being indistinguishable from control rats after a pain test 2 weeks after training, behavior and brain activation patterns were quantitatively different. This suggests that CORT levels alone may not fully describe the longer-term stress-related effects of MRI training.
In conclusion, we have investigated the long-term behavioral, hormonal, and cellular effects of MRI restraint training for imaging of awake rodents and have shown that MRI restraint training can cause increases in CORT levels and may cause long-lasting alterations in pain behavior, as shown by reduced responses to the formalin test 2 weeks later. This restraint stress also altered cellular activation of the “nociceptive” CeA, showing that restraint training for awake imaging of rodents may qualitatively alter brain activation, therefore presenting a potentially serious confound for interpretation of these studies. We encourage researchers interested in awake rodent imaging to carefully design their training protocols and verify that the protocols used cause minimal effects to behavioral outcome measures at all imaging time points, to help limit the potential stress-related effects of training.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was funded by the Intramural Research program of the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes for Health.
Acknowledgements
The authors thank Latoya Hyson, Imran Rauf, and Farid Tarum for technical expertise, and Matthew Grossman for data checking. We also thank Drs Alan Koretsky, Radi Masri, and David Seminowicz for valuable information and advice during experimental design.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Barrett AM, Stockham MA. The effect of housing conditions and simple experimental procedures upon the corticosterone level in the plasma of rats. J Endocrinol 1963;26:97–105. [DOI] [PubMed] [Google Scholar]
- [2].Becerra L, Chang PC, Bishop J, Borsook D. CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw. NeuroImage 2011;54:1355–66. [DOI] [PubMed] [Google Scholar]
- [3].Becerra L, Pendse G, Chang PC, Bishop J, Borsook D. Robust reproducible resting state networks in the awake rodent brain. PloS One 2011;6:e25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav 2002;75:661–73. [DOI] [PubMed] [Google Scholar]
- [5].Bekhbat M, Merrill L, Kelly SD, Lee VK, Neigh GN. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: implications for tissue collection methods. Behav Brain Res 2016;305:122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav 2003;43:48–59. [DOI] [PubMed] [Google Scholar]
- [7].Brevard ME, Duong TQ, King JA, Ferris CF. Changes in MRI signal intensity during hypercapnic challenge under conscious and anesthetized conditions. Magn Reson Imaging 2003;21:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobeha Rev 2001;25:611–17. [DOI] [PubMed] [Google Scholar]
- [9].Brydges NM, Whalley HC, Jansen MA, Merrifield GD, Wood ER, Lawrie SM, Wynne SM, Day M, Fleetwood-Walker S, Steele D, Marshall I, Hall J, Holmes MC. Imaging conditioned fear circuitry using awake rodent fMRI. PLoS One 2013;8:e54197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol 2009;88:184–202. [DOI] [PubMed] [Google Scholar]
- [11].Calcagnetti DJ, Stafinsky JL, Crisp T. A single restraint stress exposure potentiates analgesia induced by intrathecally administered DAGO. Brain Res 1992;592:305–9. [DOI] [PubMed] [Google Scholar]
- [12].Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci 2007;27:1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 2008;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev 2002;26:907–23. [DOI] [PubMed] [Google Scholar]
- [15].Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem 2003;79:32–40. [DOI] [PubMed] [Google Scholar]
- [16].Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 1999;113:902–13. [DOI] [PubMed] [Google Scholar]
- [17].Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett 2005;383:7–11. [DOI] [PubMed] [Google Scholar]
- [18].Cranney J. Analgesia following startle-eliciting stimuli. Psychobiol 1988;16:67–9. [Google Scholar]
- [19].Davis M. Sensitization of the rat startle response by noise. J Comp Physiol Psychol 1974;87:571–81. [DOI] [PubMed] [Google Scholar]
- [20].Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 2007;45:1709–18. [DOI] [PubMed] [Google Scholar]
- [21].Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, Kopell N, Buckner RL, Graybiel AM, Moore CI, Boyden ES. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol 2011;105:1393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res 2007;1135:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav 2002;75:507–22. [DOI] [PubMed] [Google Scholar]
- [24].Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods 2004;139:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferris CF, Febo M, Luo F, Schmidt K, Brevard M, Harder JA, Kulkarni P, Messenger T, King JA. Functional magnetic resonance imaging in conscious animals: a new tool in behavioural neuroscience research. J Neuroendocrinol 2006;18:307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferris CF, Kulkarni P, Sullivan JM, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 2005;25:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gameiro GH, Gameiro PH, da Silva Andrade A, Pereira LF, Arthuri MT, Marcondes FK, de Arruda Veiga MCF. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav 2006;87:643–9. [DOI] [PubMed] [Google Scholar]
- [28].Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. NeuroImage 2014;102:838–47. [DOI] [PubMed] [Google Scholar]
- [29].Green PG, Alvarez P, Gear RW, Mendoza D, Levine JD. Further validation of a model of fibromyalgia syndrome in the rat. J Pain 2011;12:811–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 2005;156:105–14. [DOI] [PubMed] [Google Scholar]
- [31].Hagino H, Tabuchi E, Kurachi M, Saitoh O, Sun Y, Kondoh T, Ono T, Torii K. Effects of D2 dopamine receptor agonist and antagonist on brain activity in the rat assessed by functional magnetic resonance imaging. Brain Res 1998;813:367–73. [DOI] [PubMed] [Google Scholar]
- [32].Harris AP, Lennen RJ, Marshall I, Jansen MA, Pernet CR, Brydges NM, Duguid IC, Holmes MC. Imaging learned fear circuitry in awake mice using fMRI. Eur J Neurosci 2015;42:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harris RBS, Mitchell TD, Simpson J, Redmann SM, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 2002;282:R77–88. [DOI] [PubMed] [Google Scholar]
- [34].Henckens MJAG, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, Joëls M. Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage 2015;105:312–22. [DOI] [PubMed] [Google Scholar]
- [35].Henkin RI, Knigge KM. Effect of sound on the hypothalamic-pituitary-adrenal axis. Am J Physiol 1963;204:701–4. [DOI] [PubMed] [Google Scholar]
- [36].Hickman DL, Swan M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci 2010;49:155–9. [PMC free article] [PubMed] [Google Scholar]
- [37].Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Prog Neurobiol 2014;121:1–18. [DOI] [PubMed] [Google Scholar]
- [38].Johnson TR, Smerkers B, Moulder JK, Stellar JR, Febo M. Neural processing of a cocaine-associated odor cue revealed by functional MRI in awake rats. Neurosci Lett 2013;534:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jonckers E, Delgado YPR, Shah D, Guglielmetti C, Verhoye M, Van der Linden A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn Reson Med 2013;72:1103–12. [DOI] [PubMed] [Google Scholar]
- [40].Kant GJ, Lenox RH, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Comparison of stress response in male and female rats: Pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology 1983;8:421–8. [DOI] [PubMed] [Google Scholar]
- [41].Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. PAIN 2005;116:79–86. [DOI] [PubMed] [Google Scholar]
- [42].King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res 2003;987:214–22. [DOI] [PubMed] [Google Scholar]
- [43].King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods 2005;148:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med 1999;41:412–16. [DOI] [PubMed] [Google Scholar]
- [45].Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci 2011;31:3776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liang Z, King J, Zhang N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. NeuroImage 2014;103:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Livezey GT, Miller JM, Vogel WH. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett 1985;62:51–6. [DOI] [PubMed] [Google Scholar]
- [48].Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex 2007;17:942–50. [DOI] [PubMed] [Google Scholar]
- [49].Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci 2011;46:55–66. [DOI] [PubMed] [Google Scholar]
- [50].Neugebauer V. Amygdala pain mechanisms. In: Schaible HG, editor. Pain Control, Vol. 227 Heidelberg, Germany: Springer, 2015. p. 261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Parry TJ, McElligott JG. A method for restraining awake rats using head immobilization. Physiol Behav 1993;53:1011–15. [DOI] [PubMed] [Google Scholar]
- [52].Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med 2008;59:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press, London, United Kingdom, 2005. [Google Scholar]
- [54].Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging 2001;19:821–6. [DOI] [PubMed] [Google Scholar]
- [55].Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav 1988;43:47–55. [DOI] [PubMed] [Google Scholar]
- [56].Porro CA, Carli G. Immobilization and restraint effects on pain reactions in animals. PAIN 1988;32:289–307. [DOI] [PubMed] [Google Scholar]
- [57].Qiu M, Ramani R, Swetye M, Rajeevan N, Constable RT. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: AN fMRI study in normal human subjects. Magn Reson Med 2008;60:987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reed MD, Pira AS, Febo M. Behavioral effects of acclimatization to restraint protocol used for awake animal imaging. J Neurosci Methods 2013;217:63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rushen J, Ladewig J. Stress-induced hypoalgesia and opioid inhibition of pigs' responses to restraint. Physiol Behav 1991;50:1093–6. [DOI] [PubMed] [Google Scholar]
- [60].Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 2001;102:329–39. [DOI] [PubMed] [Google Scholar]
- [61].Schultz-Darken NJ, Pape RM, Tannenbaum PL, Saltzman W, Abbott DH. Novel restraint system for neuroendocrine studies of socially living common marmoset monkeys. Lab Anim 2004;38:393–405. [DOI] [PubMed] [Google Scholar]
- [62].Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab 2003;23:472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A 2002;99:15182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sommers MG, van Egmond J, Booij LHDJ, Heerschap A. Isoflurane anesthesia is a valuable alternative for α-chloralose anesthesia in the forepaw stimulation model in rats. NMR Biomed 2009;22:414–18. [DOI] [PubMed] [Google Scholar]
- [65].Tenney JR, Duong TQ, King JA, Ludwig R, Ferris CF. Corticothalamic modulation during absence seizures in rats: a functional MRI assessment. Epilepsia 2003;44:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thompson SJ, Bushnell MC. Rodent functional and anatomical imaging of pain. Neurosci Lett 2012;520:131–9. [DOI] [PubMed] [Google Scholar]
- [67].Tsurugizawa T, Uematsu A, Uneyama H, Torii K. Functional brain mapping of conscious rats during reward anticipation. J Neurosci Methods 2012;206:132–7. [DOI] [PubMed] [Google Scholar]
- [68].Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand 1992;36:318–22. [DOI] [PubMed] [Google Scholar]
- [69].Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain 2013;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol 2014;25:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wong CL. The effect of naloxone on restraint-induced antinociception in mice. Methods Find Exp Clin Pharmacol 1992;14:695–700. [PubMed] [Google Scholar]
- [72].Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav 2003;43:205–13. [DOI] [PubMed] [Google Scholar]
- [73].Wu XY, Hu YT, Guo L, Lu J, Zhu QB, Yu E, Wu JL, Shi LG, Huang ML, Bao AM. Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav 2015;145:118–21. [DOI] [PubMed] [Google Scholar]
- [74].Zardooz H, Rostamkhani F, Zaringhalam J, Faraji Shahrivar F. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res 2010;59:973–8. [DOI] [PubMed] [Google Scholar]
- [75].Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. Mapping resting-state brain networks in conscious animals. J Neurosci Methods 2010;189:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang W, Hetzel A, Shah B, Atchley D, Blume SR, Padival MA, Rosenkranz JA. Greater physiological and behavioral effects of interrupted stress pattern compared to daily restraint stress in rats. PLoS One 2014;9:e102247. [DOI] [PMC free article] [PubMed] [Google Scholar]