Supplemental Digital Content is Available in the Text.
Key Words: inflammatory bowel disease, ulcerative colitis, Crohn's disease, early-onset inflammatory bowel disease, Saudi Arabia
Abstract
Background:
The objectives of this multicenter national study were to compare the clinical phenotype of early-onset inflammatory bowel disease (IBD) (EO-IBD) with IBD in older children and to examine whether there is any variability in consanguinity rate and familial aggregation in EO-IBD compared with later onset IBD.
Methods:
A retrospective analysis was performed on children aged 0 to 14 years with IBD in 17 centers located in geographically distinct regions in Saudi Arabia, from 2003 to 2012. Data of patients with EO-IBD (0 to <6 yrs) were compared with those with later onset IBD (6–14 yrs). Moreover, we evaluated differences in clinical pattern of infantile or toddler onset IBD subgroup (0–3 yr) as compared with those presenting in older children.
Results:
Of 352 IBD patients identified during the 10-year study period, 76 children (21.6%) younger than 6 years were diagnosed with IBD. Among the Crohn's disease (CD) group, infantile or toddler onset CD subgroup showed a more frequent isolated colonic involvement (L2) than later-onset group (57% versus 20%; P = 0.002). Positive family history was significantly more common in the infantile or toddler onset ulcerative colitis subgroup (29.4% versus 4.2% in later onset ulcerative colitis; P < 0.0001). The consanguinity rate was significantly higher in the infantile or toddler onset CD subgroup as compared with later onset CD group (57.1% versus 25.3%; P = 0.04).
Conclusions:
In conclusion, EO-IBD exhibits a unique clinical phenotype with a strikingly higher familial aggregation in early-onset ulcerative colitis. Our data suggest a significant genetic impact on the onset of CD in the very young children.
The epidemiology of early-onset (<6 yr old) (EO) pediatric inflammatory bowel disease (IBD) has been described in population-based studies from European and North American countries.1–7 The prevalence of EO-IBD reported by these studies ranges from 4% to 15% of pediatric IBD cases. Data from these studies suggest that EO-IBD differs epidemiologically, and it is distinguished by a predominant colonic involvement and a high positive family history, compared with older age of onset. In contrast, previous studies from developing countries, and in particular the Middle East, originated from single centers and did not examine age-related differences in pediatric IBD or had insufficient sample size to identify such differences.8–14 In particular, the clinical phenotype of IBD and the disease distribution in different age groups have been poorly defined. Moreover, it remains unknown whether family history of IBD and consanguinity (as a surrogate marker for genetic susceptibility) differs among age groups according to the type of IBD.
The objectives of this multicenter national study were to compare the clinical phenotype of EO-IBD to IBD in older children and to examine whether there is any variability in consanguinity rate and familial aggregation in EO-IBD compared with later onset (LO) IBD (LO-IBD). Moreover, we evaluated potential differences in the clinical pattern of the infantile or toddler onset IBD (0–3 yr) compared with those presenting in older children.
MATERIALS AND METHODS
Patient Population
A retrospective, comparative, descriptive analysis was performed on children aged 0 to 14 years with confirmed diagnosis of IBD—both ulcerative colitis (UC) and Crohn's disease (CD)—in 17 centers located in geographically distinct regions in Saudi Arabia from January 2003 to December 2012. Children with suspected IBD in other regions are referred to the 17 sites participating in this study because these centers are staffed with certified pediatric gastroenterologists and equipped with adequate facilities for the investigation and management of IBD. For the purpose of this study, data were analyzed and compared in all patient populations. As a primary outcome of the study, data of patients with EO-IBD (0 to <6 yrs) were compared with those with a later onset (6–14 yrs). As a secondary outcome, we evaluated the clinical phenotype of the infantile or toddler onset IBD (0 to <3 yrs) compared with IBD diagnosed in children aged 6 to 14 years. We found no significant differences in variables between the 6- to 10-year strata and 10- to 14-year strata; therefore, we combined them for subsequent analyses. The study was approved by local institutional review board at the participating centers.
Diagnosis of IBD
Diagnosis of IBD was based on the clinical history, physical examination, endoscopic appearance, histologic findings, and radiologic studies, according to Porto criteria,15 which implied that ileocolonoscopy and upper endoscopy were performed in all patients (including at least 2 biopsies from each segment of the examined gastrointestinal tract), and imaging of the small bowel (abdominal ultrasound, small bowel follow-through, CT enterography, or magnetic resonance enterography). It is a standard protocol in the participating centers to exclude allergic disorders and infectious enteritis or colitis during the initial workup for any suspected IBD case. Our initial immunologic workup in all very young children with suspected IBD includes testing for immunoglobulin levels, lymphocyte markers, and neutrophil oxidative burst assay. However, if the disease is associated with severely ulcerating perianal disease or shows a high rate of resistance to conventional therapy, our practice is to investigate further for underlying immunodeficiency by testing for blastogenesis and genetic screen by whole-exome sequence.
Description of Variables
The information retrieved for the purpose of this study included demographic features, age at diagnosis, clinical symptoms at presentation, growth parameters, complications of the disease including surgical procedures required, family history of IBD, disease distribution, and laboratory tests (including full blood count, C-reactive protein, erythrocyte sedimentation rate, perinuclear antineutrophil cytoplasmic antibodies, anti–Saccharomyces cerevisiae antibodies, and serum albumin).
Family History of IBD
“First-degree relatives” defines biological parents or full siblings. “Farther family history” defines grandparents and cousins. First-degree cousins define first cousins, and farther relations define more distant relations.
Disease Location
Anatomical location at diagnosis and at follow-up was defined according to the Paris classification.16 Ulcerative proctitis (E1) was defined as an involvement limited to the rectum, left-sided UC (E2) as an involvement limited to the portion of the colorectum distal to the splenic flexure, extensive UC (E3) as a disease extending proximally to the splenic flexure but distally to the hepatic flexure, and pancolitis (E4) defined a colitis that is extended proximally to the hepatic flexure. For CD, L1 was defined as an involvement of the terminal ileum and limited cecal disease, L2 an isolated colitis, L3 an ileocolonic disease, L4a an upper disease proximal to ligament of Treitz, and L4b distal to ligament of Treitz and proximal to distal one third of ileum.
Statistical Analysis
Data were analyzed using SPSS PC+ version 21.0 statistical software (SPSS Inc., Chicago, IL). Descriptive statistics (mean, SD and percentages) were used to describe the quantitative and categorical study and outcome variables. Karl Pearson chi-square test was used to observe the association between the categorical study and outcome variables. Student's t test was used to compare the mean values of quantitative variables in relation to the 2 categories of onset of UC and CD diseases. One-way of analysis of variance was used to compare the mean values of quantitative variables in relation to 3 categories of onset of UC and CD diseases. Nonparametric statistical tests (Mann–Whitney U test and Kruskal–Wallis test) were used to compare the mean ranks of skewed data in relation to 2 and 3 categories of UC and CD diseases. A P value of <0.05 was used to report the statistical significance of results. The association between presenting clinical characteristics and laboratory findings among the EO-IBD patients and the type of IBD disease (UC versus CD) was evaluated using univariate analysis with the Pearson chi square.
RESULTS
Study Population and Demographics
From 2003 to 2012, we identified 352 patients with a diagnosis of IBD from 0 to 14 years of age (males, 186; 52.8%); of them, 143 (40.5%) were UC cases (mean age at diagnosis, 8.12 ± 3.77 yr), 193 (55%) were CD cases (mean age at diagnosis, 12 ± 3.6 yr), and 16 (4.5%) were unclassified IBD (U-IBD). Seventy-six children (21.6%) younger than 6 years were diagnosed with IBD during the 10-year study period. UC was the most frequent diagnosis in EO-IBD (n = 45; 59.2% versus 35.5% in LO-IBD; P = 0009), whereas CD contributed to 38.2% (n = 29) of EO-IBD versus 59.5% (n = 164) in LO-IBD (P = 0.006) and 2 cases of U-IBD (Fig. 1). Two hundred seventy-six children of 6 years and older were diagnosed with IBD during the study period. CD was predominant in LO-IBD (n = 164, 59.5% versus 98 cases of UC, 35.5%, and 14 cases of U-IBD). Family history of IBD was present in 17.6% of CD cases and 7.7% of UC cases. Positive family history of IBD was significantly associated with early-onset UC (16.3% versus 4.2% in late onset of UC; P = 0.015). Consanguinity did not differ between early-onset and late-onset IBD groups (Tables 1 and 2).
FIGURE 1.
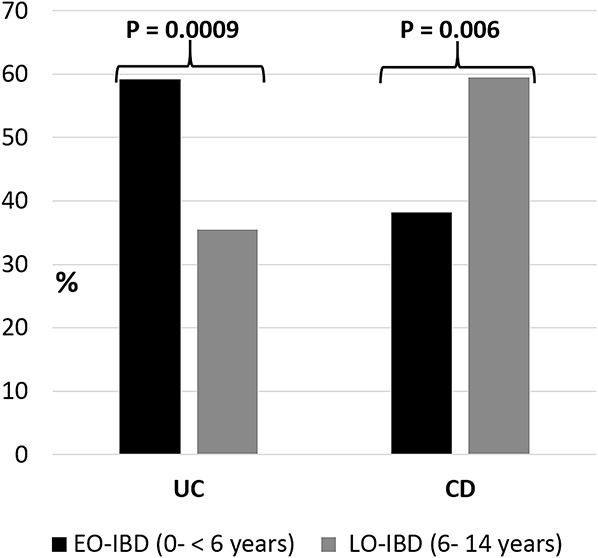
Distribution of UC and CD cases based on the age of onset.
TABLE 1.
Comparison of EO-UC and LO-UC
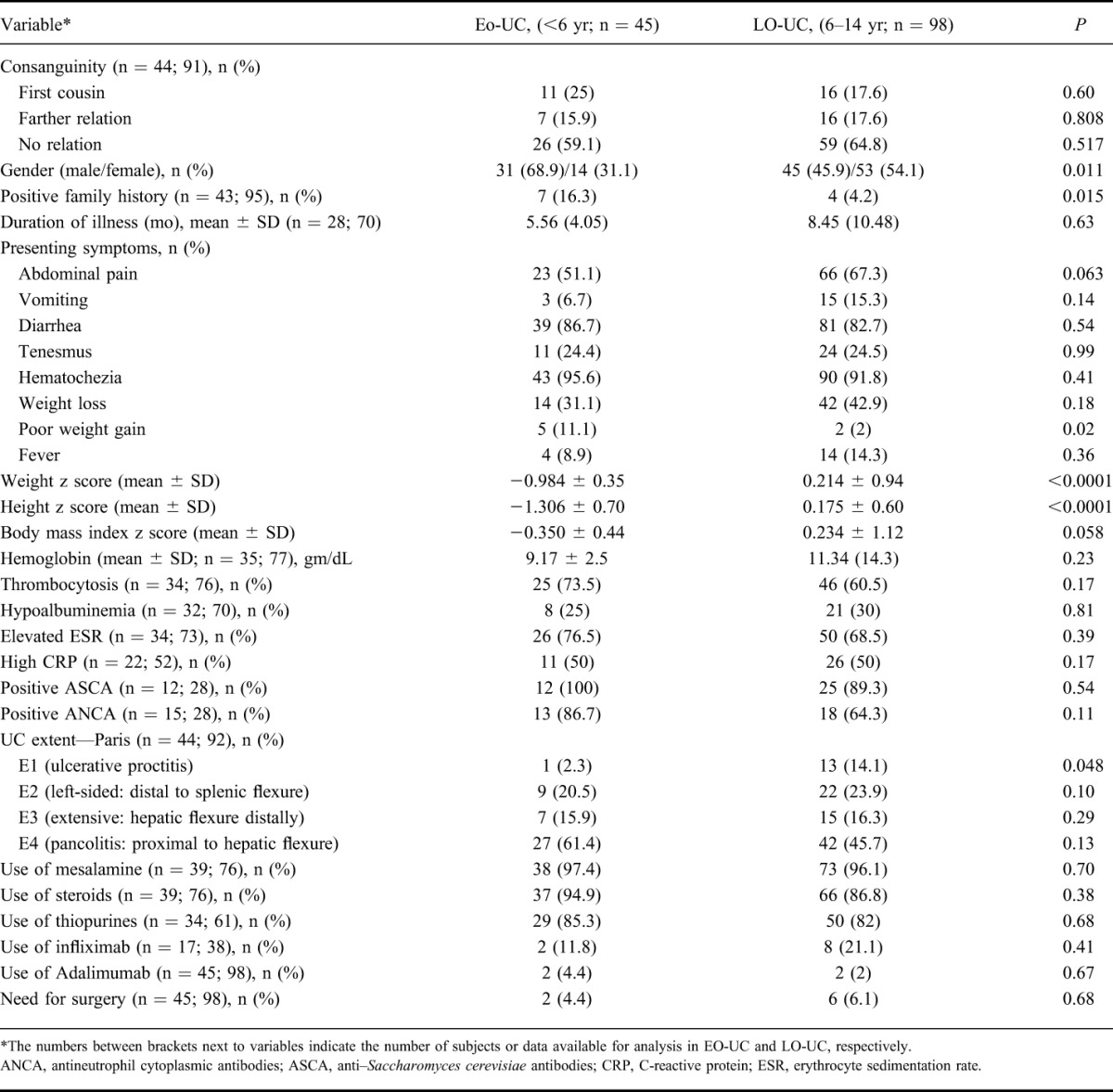
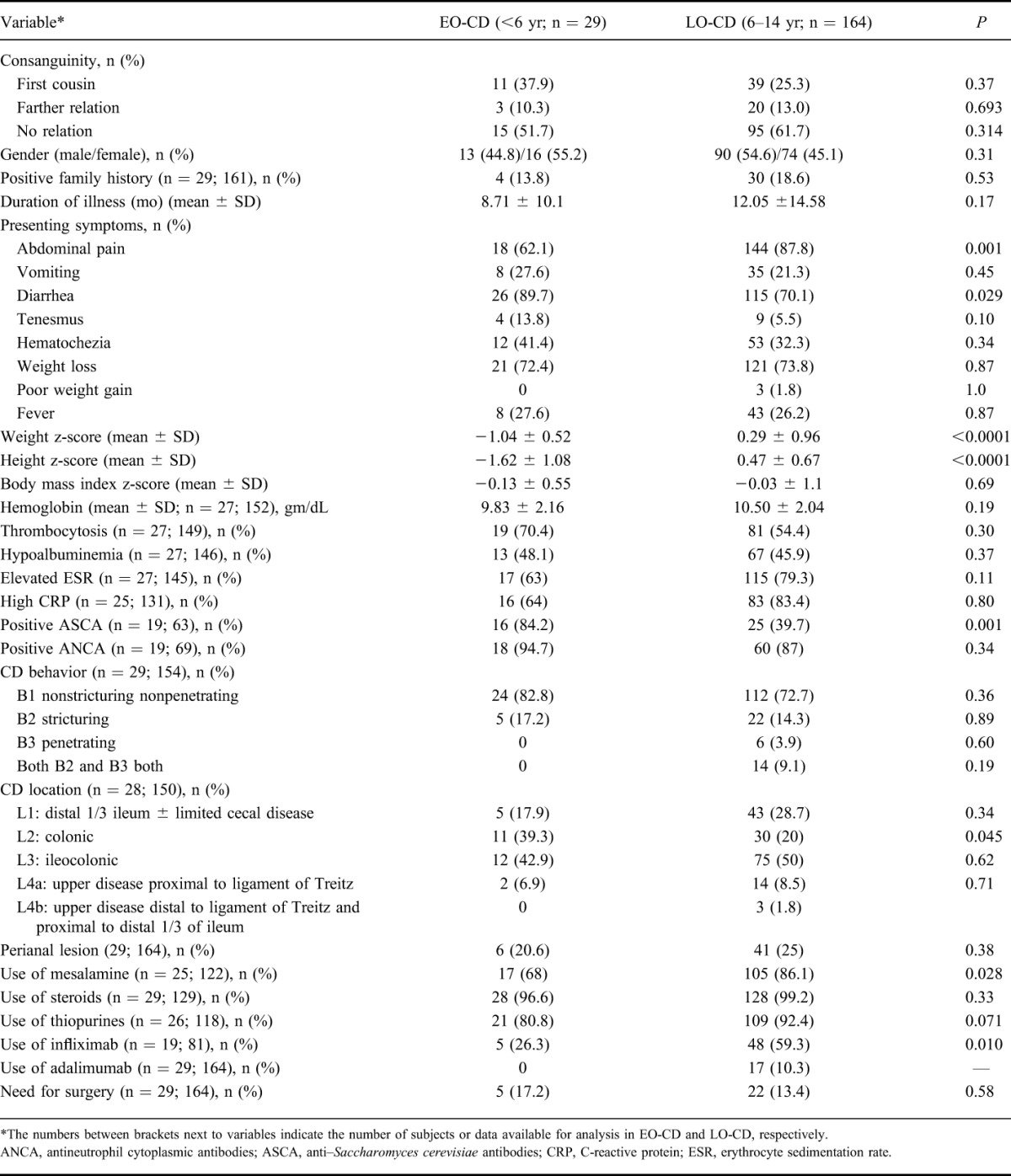
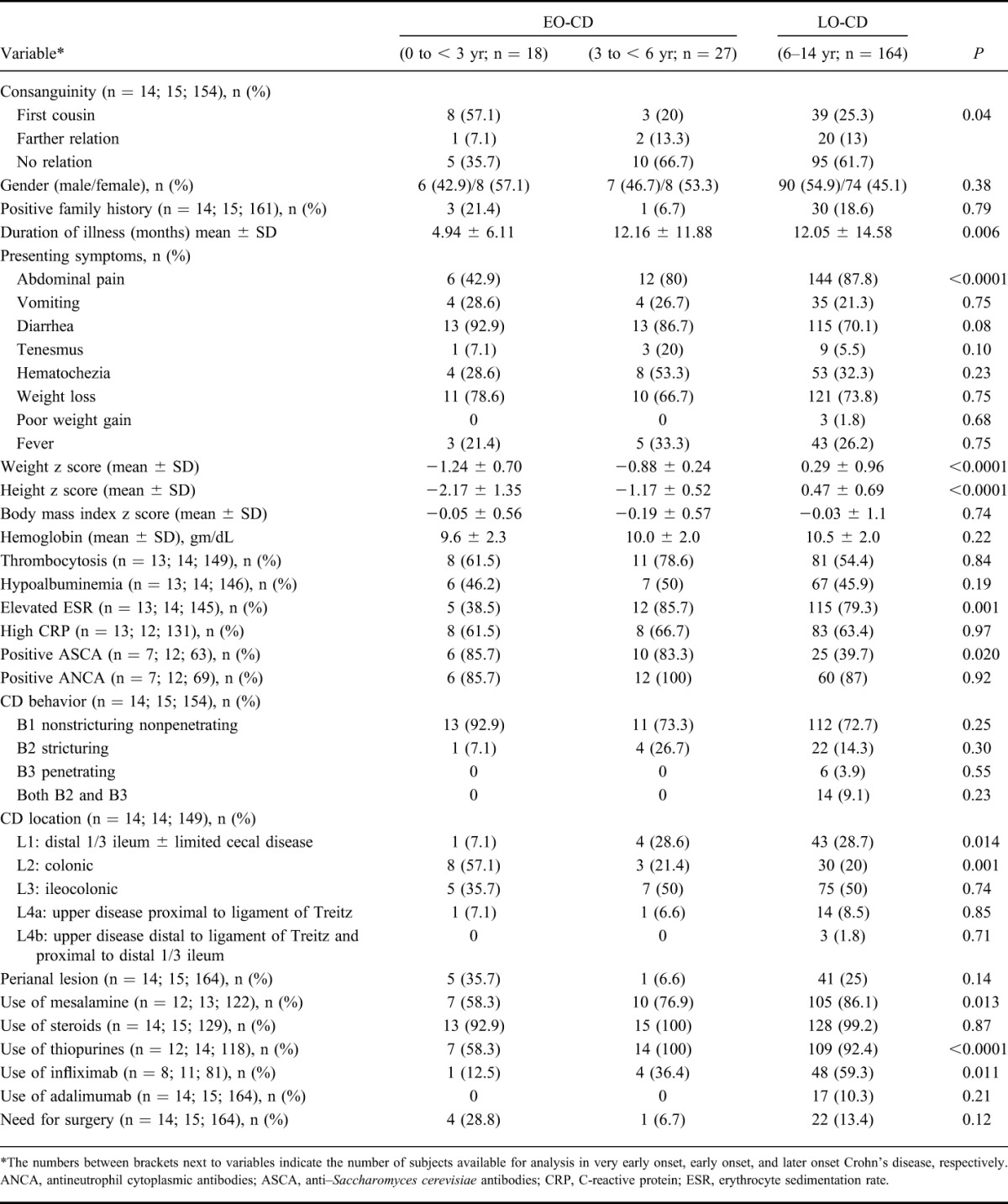
TABLE 2.
Comparison of EO-CD and LO-CD
Clinical and Laboratory Characteristics
Ulcerative Colitis
Table 1 highlights the main clinical and laboratory characteristics of early-onset and late-onset UC. There was statistically significant association between gender and onset of UC; higher proportion of male children (68.9%) had early onset of UC when compared with 45.9% of male children who had late onset of UC (P = 0.011). Growth failure was an important feature of early-onset UC; the age and sex-adjusted mean weight and height z scores were significantly lower in early-onset UC (P < 0.0001). No significant differences were found in the prevalence of extraintestinal manifestations between the 2 age groups. The mean follow-up period for EO-UC group was 95 ± 41 months.
Crohn's Disease
Table 2 highlights the main clinical and laboratory characteristics of early-onset and late-onset CD. Early-onset CD was significantly more likely to be associated with a history of diarrhea (90% versus 70%; P = 0.001) but less frequently abdominal pain than later onset disease (62% versus 88%; P = 0.029). Similar to EO-UC, growth parameters were significantly lower in EO-CD as compared with LO-CD (P < 0.0001). The mean follow-up period for EO-CD was 81.5 ± 44 months.
Location and Classification of Disease
Figure 2 shows the disease location and classification at the time of diagnosis in UC and CD. Among the UC group, children with EO-UC were significantly less likely to have isolated proctitis as compared with LO-UC group (2.3% versus 14.1%; P = 0.048; Fig. 2A). No other significant differences were found for the other UC locations between the 2 groups.
FIGURE 2.
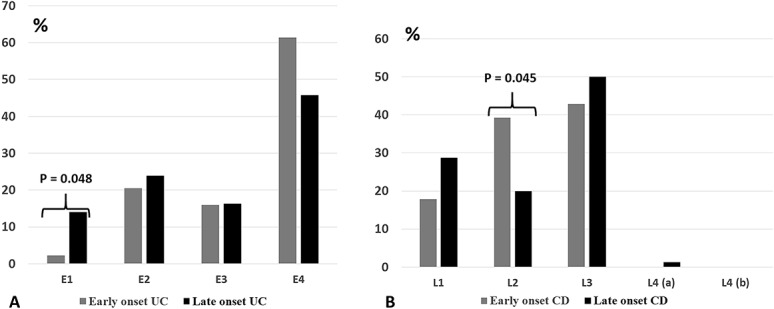
A, Disease location at the diagnosis according to Paris classification in EO-UC (0 to <6 yr) and LO-UC (6–14 yr). E1, proctitis; E2, left-sided colitis; E3, extensive colitis; E4, pancolitis. B, Disease location at the diagnosis according to Paris classification in EO-CD (0 to <6 yr) and LO-CD (6–14 yr). L1, ileum; L2, colon; L3, ileocolon; L4, upper gastrointestinal.
Among the CD group, EO-CD showed a more frequent isolated colonic involvement (L2) than later-onset disease (P = 0.045; Fig. 2B). No significant differences were found for the other CD locations in the 2 groups, although an isolated upper gastrointestinal disease (L4) was not observed in the young age group.
Subgroup Analysis of Infantile or Toddler Onset IBD
A total of 32 cases in the infantile or toddler onset IBD subgroup were diagnosed during the study period. Eighteen cases were diagnosed with UC (0 to <1 yr, 1 case; 1 to <2 yr, 11 cases; 2 to <3 yr, 6 cases) and 14 cases were diagnosed with CD (0 to <1 yr, 8 cases; 1 to <2 yr, 4 cases; 2 to <3 yr, 2 cases). We made a subgroup analysis evaluating potential peculiar differences in clinical characteristics and disease pattern of very early forms (0 to <3 yr) compared with IBD presenting in older children (Tables 3 and 4). The difference in familial occurrence of IBD between EO-UC and LO-UC became more pronounced when infantile or toddler onset UC subgroup was compared with LO-UC group (29.4% versus 4.2%; P < 0.0001). The influence of EO-IBD on growth parameters was even greater when infantile or toddler onset IBD subgroup (in both UC and CD groups) was compared with LO-IBD. The consanguinity rate was significantly higher in the infantile or toddler onset CD subgroup as compared with LO-CD group (57.1% versus 25.3%; P = 0.04). Among the CD group, the infantile or toddler onset cases showed a more frequent isolated colonic involvement (L2) than later onset cases (57% versus 20%; P = 0.002). On the other hand, ileal involvement was significantly greater in LO-CD group as compared with the infantile or toddler onset CD subgroup (28.7% versus 7%; P = 0.014).
TABLE 3.
Comparison of Very EO-UC and LO-UC
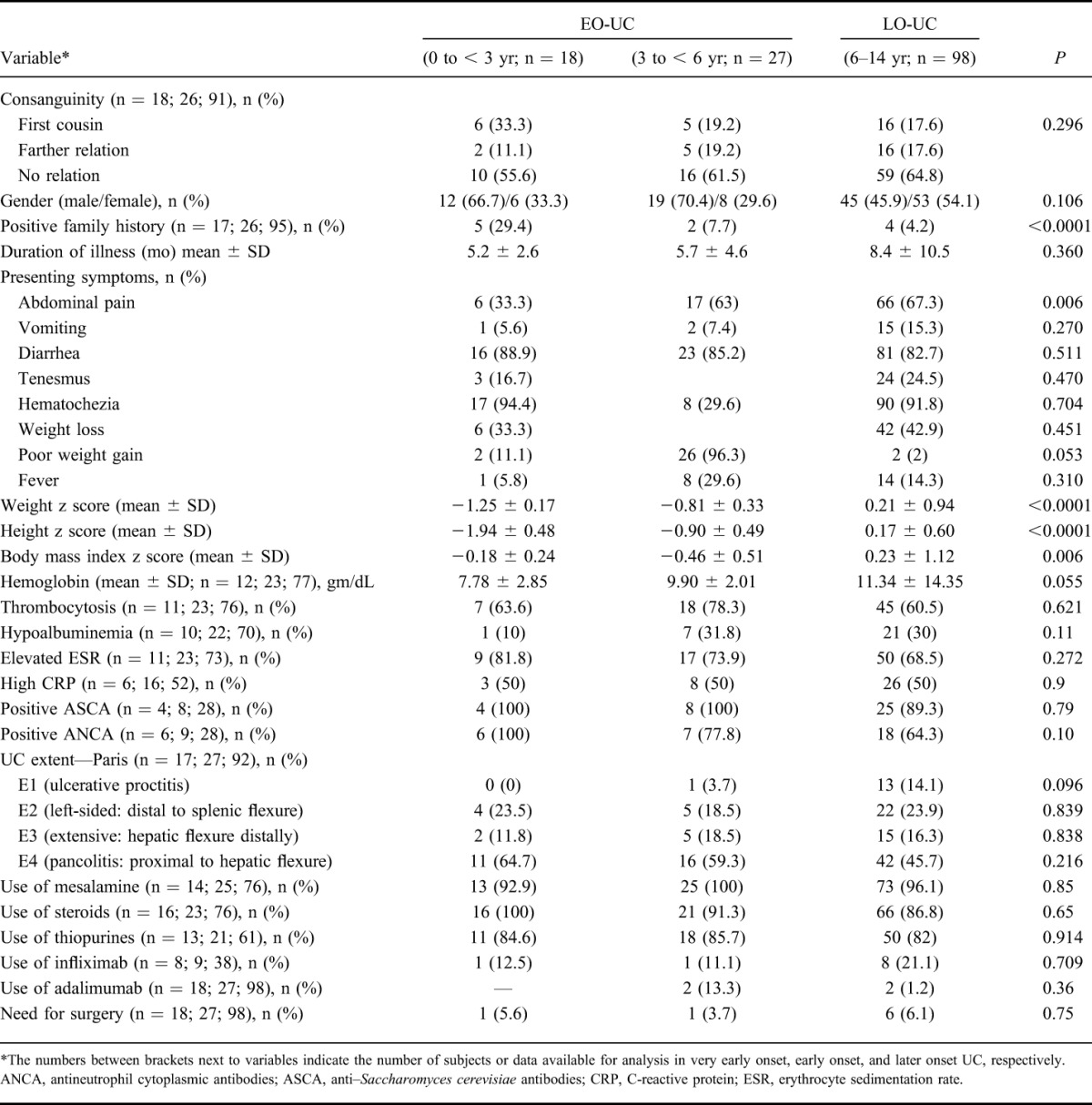
TABLE 4.
Comparison of Very EO-CD and LO-CD
Comparison of EO-UC to EO-CD
The association between presenting clinical characteristics and laboratory findings among the EO-IBD patients and the type of IBD disease (UC versus CD) was evaluated. Sixty-nine percent of the EO-UC were males, whereas 45% of the EO-CD were males (P = 0.04). Hematochezia was more commonly associated with EO-UC than with EO-CD (95.6% versus 41.4%; P < 0.001). Fever was associated with EO-CD and not with EO-UC (27.6% versus 9%; P = 0.03). Vomiting was more common in patients with EO-CD than in those with EO-UC (27.6% versus 6.7%; P = 0.014). Serum albumin level was significantly lower in EO-CD (mean, 29.4 ± 10 g/dL) than in those with EO-UC (mean, 35 ± 6 g/dL; P = 0.02). Other clinical and laboratory findings did not differ between the 2 groups. On subgroup analysis evaluating differences in clinical and laboratory findings among the infantile or toddler onset UC group compared with the infantile or toddler onset CD group, only hematochezia was significantly associated with infantile or toddler onset UC (94.4% versus 28.6% in infantile or toddler onset CD; P < 0.0001).
Comparison of Consanguineous and Non-consanguineous IBD Cases
Further subgroup analysis evaluating the consanguineous and nonconsanguineous IBD cases revealed few differences between the 2 groups (see Tables 1–4, Supplemental Digital Content 1, http://links.lww.com/IBD/B266). The most important difference is that consanguineous cases in the EO-CD group showed a more frequent isolated colonic involvement (L2) than nonconsanguineous cases in the same group (45.5% versus 0%; P = 0.011). Tendency for familial occurrence of IBD and isolated colonic involvement was greater among consanguineous versus nonconsanguineous CD (all children 0–14 yrs; 24% versus 13.6%; P = 0.054 and 30.4% versus 13.8%; P = 0.05, respectively).
DISCUSSION
We report the epidemiologic characteristics of the single largest cohort of pediatric patients with confirmed EO-IBD in the Middle East. We describe a distinct clinical phenotype of EO-IBD that is characterized by the predominance of UC over CD (59.2% versus 38.2%) and the predominance of isolated Crohn's colitis among children with very EO-CD (57%) as compared with LO-CD (20%). Another important finding in our study is the strikingly high familial aggregation in EO-UC (29.4% versus 4.2% in LO-UC). Previous studies from Western countries have reported an increased rate of familial aggregation in EO-UC, ranging from 26% to 44%,5,6 suggesting that this subgroup may represent a more highly penetrant and genetically distinct form of UC. Furthermore, an important novel observation from our data was the significantly higher consanguinity rate in the infantile or toddler onset CD subgroup (57%) as compared with LO-CD group (25%). These findings suggest that the EO-IBD present a stronger genetic impact than the later-onset disease.
An increased interest for early forms of IBD came from the discovery of aggressive forms of Crohn's-like colitis, not responding to conventional therapies of IBD, developing before 5 years of age.17 Clinical data on EO-IBD are scarce because this age group represents only a small portion of the pediatric IBD population. Studies from North America, Europe, and Australia reported a prevalence rate of EO-IBD that ranges from 4% to 15% of pediatric IBD.1–7,18 To the best of our knowledge, we report the highest prevalence of EO-IBD (76 of 352; ≈21.6%) and infantile or toddler onset IBD (32 of 352; ≈9%). Such a high prevalence could be related to the high consanguinity rate (up to 60%) in the Saudi community, which might confer genetic susceptibility to early development of IBD.19 The relation between consanguinity and IBD has been questioned in a local study by El-mouzan et al,20 who did not find significant association between consanguinity and IBD in 138 pediatric patients. In addition to the relatively small sample size, another limiting factor was that the authors did not perform a subgroup analysis based on the age of onset to evaluate differences in consanguinity rate. The increasing understanding of age-specific characteristics (site of disease, progression, and response to therapy) has led to changes in the classification of pediatric IBD.16 Our proposition of an age group <3 years makes sense when taking into account the literature supporting higher rates of affected first-degree family relatives, severe disease course, and high rate of resistance to immunosuppressive treatment in young children with IBD.17 Furthermore, the age of onset is often younger than 3 years in a subgroup of patients with monogenic IBD-like immunopathologic findings (such as those with defects in interleukin-10 signaling, XIAP deficiency, chronic granulomatous disease, or other neutrophil defects).17 The latest disorders manifest similar clinical picture to the conventional polygenic forms of EO-IBD but require a different treatment strategy and therefore warrant special consideration in the diagnostic approach to young children with suspected IBD. Therefore, prospective multicenter studies are needed in this population to investigate potential combinations of genetic and environmental factors in disease onset. Understanding these factors could facilitate intervention strategy development, thereby influencing disease outcome.
The incidence of pediatric IBD is increasing internationally21 and locally.22 Some studies have reported that the incidence of IBD is increasing rapidly in young children.23 In contrast, we have recently observed a significantly decreased trend in the incidence of CD over time in the 0- to 4-year age group in the present study cohort22 that has not been reported previously. This finding could be related to the fact that the awareness and recognition of monogenic disorders with underlying immunodeficiency by pediatric gastroenterologists in Saudi Arabia has improved over the past decade, thus avoiding misdiagnosis of these monogenic disorders as conventional IBD. Very young children with monogenic disorders and underlying immunodeficiency are more likely to develop severely ulcerating perianal disease and have a high rate of resistance to conventional therapy and increased death rate.17 These features were not present in our cohort of infantile or toddler onset IBD, which, together with the negative immunologic workup, indicate that the infantile or toddler cases in our study cohort are of conventional polygenic forms of EO-IBD.
The clinical phenotype of EO-IBD in our cohort manifests many similarities to data from North America and Europe1–7: UC was twice more frequently diagnosed than CD in younger children with reversal of the ratio in older children, significantly higher rates of isolated colonic CD compared with older children, rare involvement of upper gastrointestinal tract in EO-CD, and rare occurrence of isolated proctitis in EO-UC. In our cohort, children with EO-UC showed a high prevalence of extensive colitis (77.3%), although not significantly different from older children (62%) and that is also consistent with data from Western countries.2,4,5,7,18 Malnutrition and linear growth failure were significant features of EO-IBD, both UC and CD, which were probably attributed to the extensive disease and limited nutritional reserve in young children when compared with older children. This result is remarkable because it is generally accepted that failure to thrive and linear growth impairment at presentation is a feature of CD but not UC.7 Therefore, young children with IBD require special attention because of the large impact of the disease on their growth and development. In view of the high prevalence of extensive colitis and failure to thrive in both EO-UC and EO-CD and the limited clinical use of anti–Saccharomyces cerevisiae antibodies and antineutrophil cytoplasmic antibodies, differentiation between CD and UC in this young age group can be very difficult. We noted that male gender and hematochezia in young children were predictive of a diagnosis of UC. Propensity of EO-UC to affect males has been previously reported.5 In contrast, fever, vomiting, and hypoalbuminemia were predictive of a diagnosis of CD. Mamula et al7 have similarly reported frequent occurrence of fever and vomiting among young children with CD as compared with young children with UC.
Although it is generally thought that EO-IBD is more severe disease than LO-IBD, in terms of behavior and disease course, and generally more challenging to manage,18,24 there is a paucity of well-designed studies to support this hypothesis, and some existing literature debate this thought.3–5,18 Although we have not used the pediatric activity indices in our study, we evaluated the need of medications and surgery in an attempt to identify differences in severity according to age. Therapies at diagnosis were similar except for significantly greater use of infliximab and thiopurines in older children with CD as compared with young children with CD, and the surgical risk did not differ according to age. However, the difference in the use of medications may reflect variations in individual treatment approaches between pediatric gastroenterologists in our country. In addition, we did not find significant differences in albumin and hemoglobin levels, which are considered proxy for a more severe disease. Prospective studies using pediatric activity indices of IBD would be helpful to evaluate the differences in disease severity by age group.
This study has some limitations. Our registry is based on a retrospectively collected data with its inherent limitations. As a result, we had not been able to monitor and document changes in the extent of inflammation over the course of illness. Also, we have not used the pediatric activity indices to properly assess disease activity at presentation; instead, we have used indirect measures (hemoglobin, serum albumin, and need for biologics or surgery) to compare the severity of disease between EO-IBD and LO-IBD.
In conclusion, this first study in Arabs adds to the growing body of data that EO-IBD exhibits a unique clinical phenotype with a strikingly higher familial aggregation in EO-UC and significantly higher consanguinity rate among very young children with CD. These data suggest a significant genetic impact on the onset of IBD in the very young children.
ACKNOWLEDGMENTS
The authors extend their appreciations to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RG-1436-007.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.ibdjournal.org).
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:597–605. [DOI] [PubMed] [Google Scholar]
- 2.Aloi M, D'Arcangelo G, Pofi F, et al. Presenting features and disease course of pediatric ulcerative colitis. J Crohn's Colitis. 2013;7:e509–e515. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early- compared to later-onset pediatric Crohn's disease. Am J Gastroenterol. 2008;103:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul T, Birnbaum A, Pal DK, et al. Distinct phenotype of early childhood inflammatory bowel disease. J Clin Gastroenterol. 2006;40:583–586. [DOI] [PubMed] [Google Scholar]
- 5.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. [DOI] [PubMed] [Google Scholar]
- 6.Sawczenko A, Sandhu B. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamula P, Telega GW, Markowitz JE, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–2010. [DOI] [PubMed] [Google Scholar]
- 8.Al-Qabandi WA, Buhamrah EK, Harandi KA, et al. Inflammatory bowel disease in children, an evolving problem in Kuwait. Saudi J Gastroenterol. 2011;17:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmaida AI, Al-Shaikhi SA. Childhood inflammatory bowel disease in Libya: epidemiological and clinical features. Libyan J Med. 2009;4:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallahi GH, Moazzami K, Tabatabaeiyan M, et al. Clinical characteristics of Iranian pediatric patients with inflammatory bowel disease. Acta Gastroenterol Belg. 2009;72:230–234. [PubMed] [Google Scholar]
- 11.Saadah O. Ulcerative colitis in children and adolescents from the western region of Saudi Arabia. Saudi Med J. 2011;32:943–947. [PubMed] [Google Scholar]
- 12.Saadah OI. Childhood onset Crohn's disease; experience from a university teaching hospital in Saudi Arabia. Ann Saudi Med. 2012;32:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Mouzan MI, Al Mofarreh MA, Assiri AM, et al. Presenting features of childhood-onset inflammatory bowel disease in the central region of Saudi Arabia. Saudi Med J. 2012;33:423–428. [PubMed] [Google Scholar]
- 14.Hasosah MY, Sukkar GA, Alsahafi AF, et al. Pediatric inflammatory bowel disease in the western region of Saudi Arabia. A retrospective analysis. Saudi Med J. 2013;34:651–653. [PubMed] [Google Scholar]
- 15.IBD Working Group of the European Society for Pediatric Gastroenterology Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis—the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. [DOI] [PubMed] [Google Scholar]
- 17.Uhlig H, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledder O, Catto-Smith AG, Oliver MR, et al. Clinical patterns and outcome of early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59:562–564. [DOI] [PubMed] [Google Scholar]
- 19.El-Hazmi M, Al-Swailem AR, Warsy AS, et al. Consanguinity among the Saudi Arabian population. J Med Genet. 1995;32:623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Mouzan M, Al-Mofarreh M, Assiri A, et al. Consanguinity and Inflammatory Bowel Diseases: Is There a Relation? J Pediatr Gastroenterol Nutr. 2013;56:182–185. [DOI] [PubMed] [Google Scholar]
- 21.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. [DOI] [PubMed] [Google Scholar]
- 22.El Mouzan MI, Saadah O, Al-Saleem K, et al. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: a multicenter national study. Inflamm Bowel Dis. 2014;20:1085–1090. [DOI] [PubMed] [Google Scholar]
- 23.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. [DOI] [PubMed] [Google Scholar]
- 24.Benchimol EI, Mack DR, Nguyen GC, et al. Outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803–813. [DOI] [PubMed] [Google Scholar]


