Abstract
Background:
Patients with Crohn's disease in whom tumor necrosis factor antagonist therapy fails have limited treatment options, and the benefit of reintroducing the same therapy remains unclear. Here, we report results from PRECiSE 4 (NCT00160706), an open-label extension study of certolizumab pegol in patients who withdrew from the placebo-controlled studies PRECiSE 1 or 2.
Methods:
Patients eligible for PRECiSE 4 had Crohn's disease exacerbation on placebo or primary or secondary failure to certolizumab pegol in PRECiSE 1 or 2, and received 400 mg certolizumab pegol subcutaneously at weeks 0, 2, and 4 and every 4 weeks thereafter up to 360 weeks. We assessed safety (adverse events) and efficacy (clinical remission) of extended certolizumab pegol therapy.
Results:
Patients enrolled in PRECiSE 4 (N = 310; mean age, 37 yr; 58% female; 95% white) had a mean Crohn's disease duration of 8.5 years before entering the qualifying studies. At weeks 52, 104, and 156, remission rates were 28.5%, 17.5%, and 12.6% by nonremitter imputation, and 63.8%, 60.0%, and 63.5% by observed cases, with 47.4%, 31.9%, and 23.2% of patients, respectively, remaining on therapy. By study end (7.5 yr), 92.3% of patients discontinued therapy, 49% on account of adverse events. No new safety signals emerged. Incidence rate (new cases)/100 patient-years was 6.11 for serious infections and 1.29 for malignancies.
Conclusions:
Certolizumab pegol was effective in many patients who previously discontinued certolizumab pegol for lack or loss of response. Thus, discontinuation of therapy may not always be necessary. Safety was consistent with previous findings.
Key Words: Crohn's disease, certolizumab pegol, efficacy
Crohn's disease is a lifelong, incurable inflammatory disease of the gastrointestinal tract that often arises in the earlier decades of life and is characterized by periods of exacerbation and remission. Tumor necrosis factor (TNF) α has a central role in the pathogenesis of inflammatory bowel diseases, and specific inhibition of this pleotropic cytokine with biological anti-TNF agents has been a major advancement in the treatment of these diseases including Crohn's disease.1,2 Anti-TNF therapy is generally well tolerated.3–5 However, because these agents have immunosuppressive properties, long-term use may increase the risk of serious infections,6 and careful consideration is given to the initiation of therapy. Only 3 anti-TNF therapies, including infliximab, adalimumab, and certolizumab pegol, are currently approved for the treatment of moderate to severe Crohn's disease in the United States.
In randomized controlled trials, approximately 40% to 60% of patients with Crohn's disease had an initial response to induction therapy with anti-TNF therapy during the first 2 to 6 weeks, and of those approximately 40% to 60% were in remission after 6 months of therapy.3–5,7 However, the duration of clinical response varies widely among patients. Results of PRECiSE 2, a randomized, placebo-controlled study of certolizumab pegol maintenance therapy in patients with moderate to severe Crohn's disease, showed that approximately 40% of initial responders lost response within 6 months after treatment initiation.5 In clinical practice, 30% to 40% of patients treated with adalimumab required dose escalation; however, approximately one-third of those who received increased doses failed to maintain responses longer than 6 months.8 These examples illustrate that both primary and secondary failure to anti-TNF therapy remain significant challenges in the treatment of patients with Crohn's disease. Given the need for long-term maintenance therapy and the small number of available anti-TNF therapies, identifying strategies to maximize the benefit that a patient can derive from each agent is important. Recent studies have shown that reinduction therapy with another9 or the same10 anti-TNF agent in patients with secondary treatment failure provides clinically important short-term benefits. However, the longer-term benefit of reintroducing the same therapy to patients with primary and secondary treatment failure, including patients who previously received as little as 1 dose of induction treatment, has not been explored.
Certolizumab pegol is a recombinant, humanized, polyethylene glycol–conjugated antigen-binding antibody fragment (Fab') with specificity for human TNFα. Two randomized, double-blind, placebo-controlled phase 3 studies, PRECiSE 17 and PRECiSE 2,5 demonstrated that certolizumab pegol is effective in the induction and maintenance of clinical response and remission in patients with moderate to severe Crohn's disease. Unlike patients in PRECiSE 2, who were randomized to maintenance treatment with placebo or certolizumab pegol after responding to 6 weeks of induction therapy with certolizumab pegol, patients in PRECiSE 1 were randomized to consecutive induction and maintenance treatment with either placebo or certolizumab pegol at the start of the trial. Patients who completed PRECiSE 1 or PRECiSE 2 were eligible for inclusion in PRECiSE 3, an open-label, 7-year extension study.11 In contrast, PRECiSE 4 (NCT00160706) was an open-label extension study for patients who withdrew from either treatment arm of PRECiSE 1 or PRECiSE 2 owing to exacerbation of Crohn's disease and was designed to assess the safety and efficacy of chronic certolizumab pegol therapy for up to 7 years. Here, we report efficacy and safety outcomes from the PRECiSE 4 study, which included patients with no previous exposure to certolizumab pegol, as well as patients with primary or secondary certolizumab pegol treatment failure.
MATERALS AND METHODS
The PRECiSE studies were conducted in compliance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. In addition, all local regulatory requirements were followed. The protocol was approved by the Institutional Review Board or Ethics Committee at each center. All patients signed an informed consent form.
Study Design
PRECiSE 4 (clinicaltrials.gov identifier: NCT00160706) enrolled patients from 141 centers in 24 countries, with initial enrollment in February 2004 and completion in May 2012. Patients with active Crohn's disease who withdrew from PRECiSE 1 or 2 owing to the exacerbation of Crohn's disease (determined by the investigator and defined as an increase of ≥70 points in the Crohn's Disease Activity Index score above baseline or an absolute score ≥350 points) were eligible to enroll in PRECiSE 4.
The enrollment criteria and study design for PRECiSE 1 and 2 have been described.5,7 In brief, both studies enrolled patients ≥18 years of age with moderate to severe Crohn's disease (Crohn's Disease Activity Index score of 220–450 points). Patients in PRECiSE 1 were randomized to receive placebo or certolizumab pegol 400 mg for induction at weeks 0, 2, and 4 and subsequent maintenance treatment until week 26. In PRECiSE 2, all enrolled patients received certolizumab pegol 400 mg induction treatment at weeks 0, 2, and 4, and patients with response at week 6 were randomized to receive placebo or certolizumab pegol 400 mg every 4 weeks until week 26. Patients were eligible for entry into PRECiSE 4 at any time after completing the week 2 assessment of PRECiSE 1 or week 6 randomization in PRECiSE 2, if exacerbation of Crohn's disease occurred.
Patients enrolled in PRECiSE 4 were stratified in 3 separate analysis groups according to their treatment in the qualifying studies: patients who received placebo in PRECiSE 1 study were classified as First Exposure group, patients who received placebo after induction therapy with certolizumab pegol in PRECiSE 2 were classified as Reinduction/First Maintenance group, and patients who received both induction and maintenance therapy with certolizumab pegol 400 mg in PRECiSE 1 or PRECiSE 2 were classified as Reinduction/Remaintenance group.
Baseline was defined as the week 0 assessment in each of the qualifying studies (PRECiSE 1 and 2). For PRECiSE 4, patients were treated with a 3-dose induction regimen of certolizumab pegol 400 mg subcutaneously at weeks 0, 2, and 4 followed by maintenance therapy of 400 mg every 4 weeks until week 360 (up to 92 doses).
Patients who required rescue therapy for exacerbation of their disease were considered to have treatment failure and were withdrawn from the study. These patients were considered to be nonresponders/nonremitters from the time of discontinuation. In addition, patients who received rescue therapy but were not withdrawn from the study (in deviation from the study protocol) were classified as nonresponders/nonremitters from the time of receiving rescue therapy. Rescue therapies included anti-TNF therapy other than certolizumab pegol, natalizumab, corticosteroids at a dose >15 mg of prednisolone or equivalent above the dose at study entry, introduction of immunomodulator therapy or any increase in immunomodulator dosage above that at study entry, and surgery or in-patient hospitalization for exacerbation of Crohn's disease.
Assessments
Efficacy was assessed by the Harvey Bradshaw Index (HBI) in the intent-to-treat population, which included all patients who received at least 1 injection of certolizumab pegol and provided at least 1 postbaseline efficacy measurement. HBI scores were recorded at weeks 2 and 4, every 4 weeks until week 104, every 8 weeks until week 256, every 12 weeks until week 352, at week 362/withdrawal visit, and at week 372 (safety follow-up or 12 wk after the last dose). Efficacy evaluation was based on the proportion of patients who achieved remission (HBI total score ≤4) at various time points. Confirmed loss of remission was defined as HBI >4 at 2 consecutive visits.
Safety was assessed in all patients who received at least 1 injection of certolizumab pegol based on adverse event (AE) reports, laboratory analyses, physical examination, and tuberculosis (TB) evaluation. AE data were obtained by observation and direct questioning at each visit. All AEs were classified by System Organ Class and Preferred Term according to the Medical Dictionary for Regulatory Activities (version 6.1). Any infection that required treatment with parenteral antibiotics was considered a serious infection.
Concentrations of certolizumab pegol and anti–certolizumab pegol antibodies (ADAbs) were measured during PRECiSE 1 and 2 at weeks 0, 2, 4, 6, 8, 12, 16, 20, 24, and 26, and during PRECiSE 4 at weeks 2 and 4, every 4 weeks until week 104, and at weeks 128, 156, 180, 208, 232, 256, 316, 362/withdrawal visit, and the safety follow-up visit (week 372 or 12 weeks after the last dose). The concentration of certolizumab pegol or certolizumab pegol ADAbs were determined by enzyme-linked immunosorbent assay as previously described.12 Patients were considered certolizumab pegol ADAb-positive if the certolizumab pegol ADAb concentration was >2.4 U/mL at any point during the treatment period.
Statistical Methods
No formal statistical hypothesis testing was performed, as this was an open-label extension study with safety as the primary objective. Remission rates were analyzed based on observed cases (with patients who discontinued excluded from the analysis) and separately by nonremitter imputation (NRI; with patients who discontinued or received rescue therapy considered in the analysis as nonremitters). Kaplan–Meier analysis was performed to estimate times to confirmed loss of remission for each certolizumab pegol exposure group, with differences between groups assessed by log-rank test. Incidence rates (IRs; cases per 100 patient-years) with 95% CIs were calculated for serious AEs. Multiple reasons for discontinuation were considered for the calculation of discontinuation rates.
RESULTS
Patients
Disposition
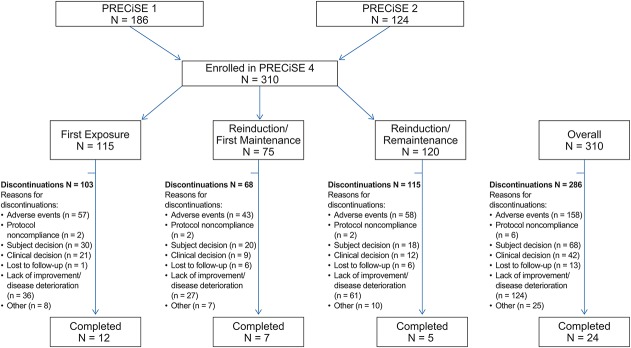
A total of 322 patients who withdrew from PRECiSE 1 and 2 were eligible for inclusion in PRECiSE 4 and 310 patients were enrolled in the study (Fig. 1). All 310 patients were included in the safety population and 309 patients were included in the intent-to-treat population. The median number of certolizumab pegol doses in the First Exposure (n = 115), Reinduction/First Maintenance (n = 75), and Reinduction/Remaintenance (n = 120) groups was 14, 21, and 7, respectively. Patients in the First Exposure group stayed in PRECiSE 1 for a mean period of 61 days before withdrawing and entering PRECiSE 4. Patients in the Reinduction/First Maintenance group stayed in PRECiSE 2 for a mean period of 111 days and subsequently were certolizumab pegol–free for a mean period of 82.3 days, with most (53.3%) starting to receive certolizumab pegol 400 mg in PRECiSE 4 after a drug-free interval of 12 weeks. Patients in the Reinduction/Remaintenance group stayed in the qualifying studies for a mean period of 81 days and were certolizumab pegol–free for a mean period of 21.7 days (slightly less than the expected interval of 28 d between scheduled maintenance doses of 400 mg certolizumab pegol) before starting reinduction therapy in PRECiSE 4; all patients in this group received certolizumab pegol 400 mg within <8 weeks of withdrawing from the qualifying studies. Overall, 286 patients discontinued therapy over the 7-year study period for 1 or more reasons, with the most common being AEs (n = 158), lack of improvement/disease deterioration (n = 124), and subject decision (n = 68) (Fig. 1). Throughout the study, 53.5% of the safety population received 1 or multiple rescue therapies (corticosteroids, 33.2%; immunomodulators, 16.5%; biological therapies, 8.1%; hospitalization/surgery, 24.8%; cyclosporine, 0.6%).
FIGURE 1.
Flow of patients through the PRECiSE 4 trial. More than 1 reason for discontinuation may have been recorded for a single subject.
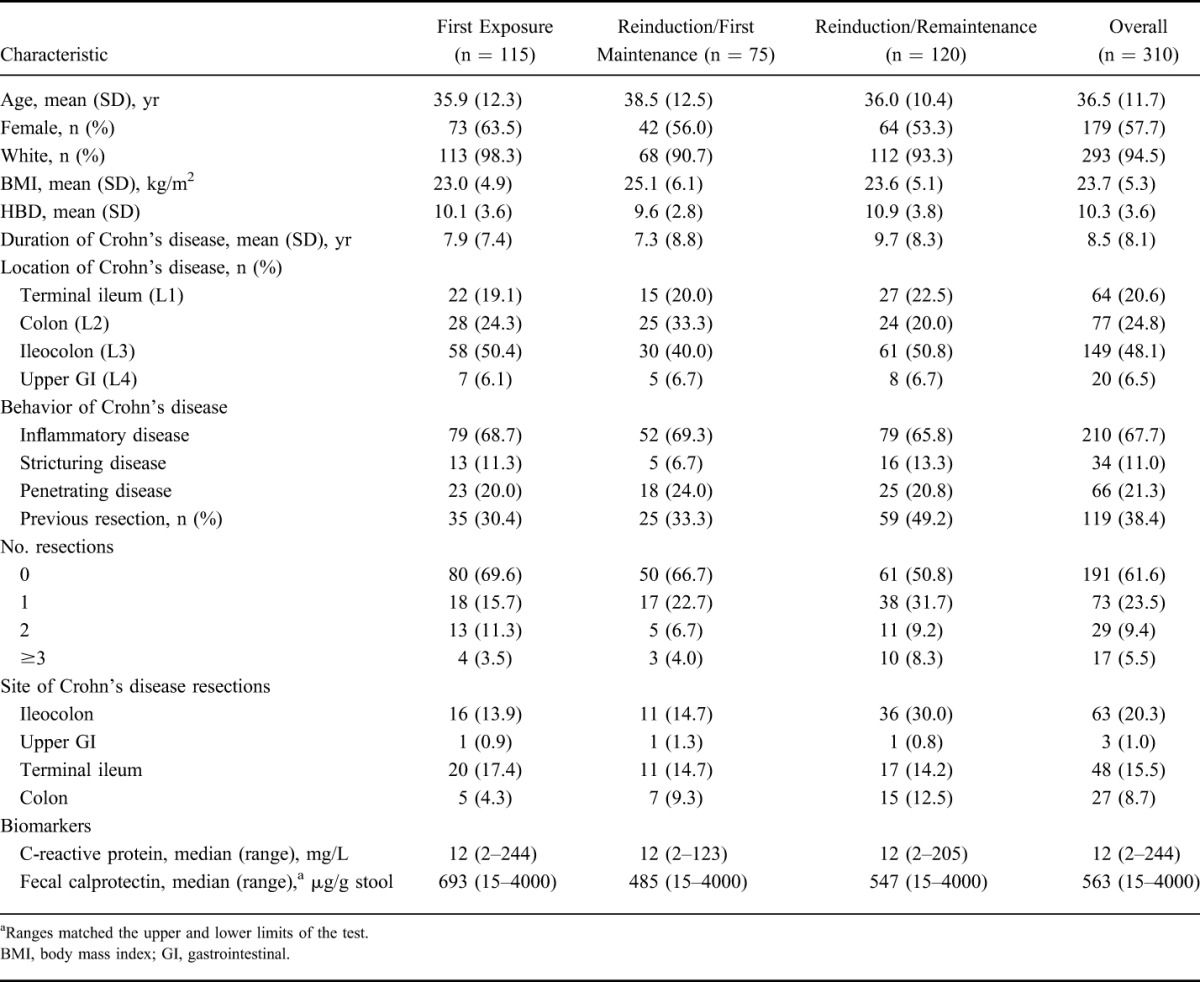
Demographics and Baseline Characteristics
At screening of PRECiSE 1 or 2, patients enrolled in PRECiSE 4 had a mean (SD) age of 36.5 (11.7) years; most were female (57.7%) and most were white (94.5%). The mean (SD) duration of Crohn's disease was 8.5 (8.1) years. Ileocolonic disease location (48.1%) and inflammatory disease behavior (67.7%) were prevalent, and 38.4% of patients had had a previous resection (Table 1). Demographic and patient baseline characteristics were generally similar across all analysis groups. Overall, 36.1% of patients had a history of infliximab therapy, with a higher percentage of patients in the Reinduction/Remaintenance group having previously received infliximab (44.2%) compared with the Reinduction/First Maintenance and First Exposure groups (28.0% and 33.0%, respectively). At entry to PRECiSE 4, 15.2% of all patients were using both corticosteroids and immunomodulators, 39.0% were using corticosteroids only, and 36.5% were using immunomodulators only.
TABLE 1.
Summary of Demographic and Baseline Characteristics at the Screening Visit of the Qualifying Studies PRECiSE 1 and PRECiSE 2 (PRECiSE 4 Safety Population)
Efficacy
The mean (SD) HBI score at study entry was 12.1 (4.7) in the overall intent-to-treat population, 13.3 (4.2) in the First Exposure group, 9.5 (3.8) in the Reinduction/First Maintenance group, and 12.6 (5.0) in the Reinduction/Remaintenance group. Remission was reached by 63.8% of patients remaining on therapy (observed cases) at week 52 (n = 138) and by 60.0% at week 104 (n = 90). Similar remission rates were observed at weeks 156 to 316. The time course of remission rates based on observed cases was overall similar in each of the 3 certolizumab pegol exposure groups (classified by treatment history in PRECiSE 1 or 2) (Fig. 2A). Remission rates in the First Exposure, Reinduction/First Maintenance, and Reinduction/Remaintenance groups were 72.5% (n = 51), 60.5% (n = 43), and 56.8% (n = 44), respectively, at week 52, and 57.1% (n = 35), 74.1% (n = 27), and 50.0% (n = 28), respectively, at week 104 (Fig. 2A).
FIGURE 2.
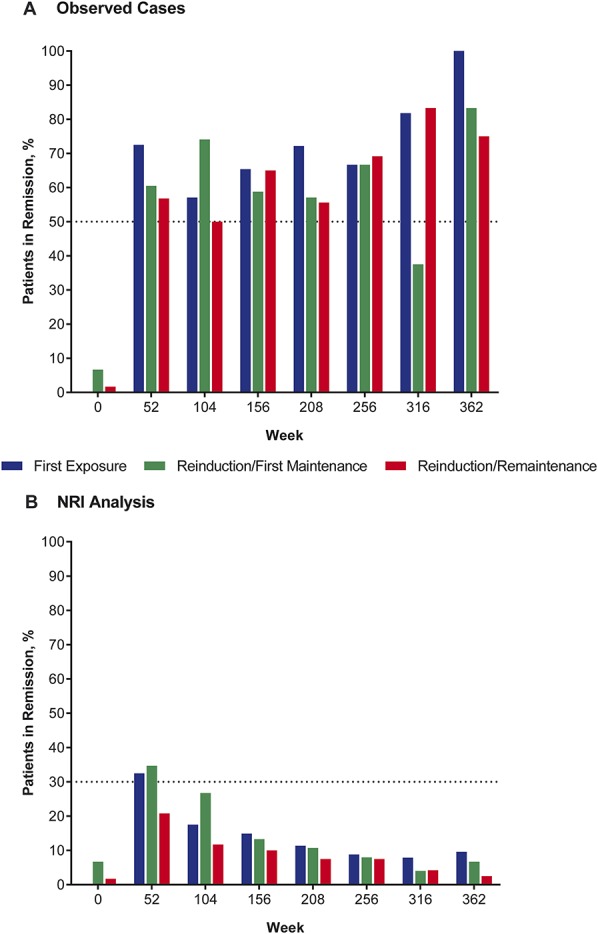
Proportion of patients in remission (HBI score of ≤4 points) over the course of PRECiSE 4 analysis groups defined by previous exposure to certolizumab pegol in the qualifying studies for observed cases (A) and using the NRI analysis (B).
Based on an NRI analysis, which considered patients who had discontinued or received rescue therapy as nonremitters, the proportion of patients in remission decreased from 28.5% at week 52 (with 47.4% of patients on therapy) to 9.7% at week 208 (with 18.1% of patients remaining on therapy). Remission rates at week 52 were lower among patients in the Reinduction/Remaintenance group (20.8%) than they were among patients in the First Induction (32.5%) and Reinduction/First Maintenance (34.7%) groups (Fig. 2B). However, remission rates by NRI analysis were consistently higher among patients who received uninterrupted concomitant therapy with corticosteroids or immunomodulators for 6 months than among patients who did not receive these agents concurrently with certolizumab pegol. For patients with and without concomitant immunomodulator treatment, remission rates at week 52 were 50.6% and 20.9%, respectively, in the overall study population, and 55.2% and 9.9% in the Reinduction/Remaintenance group.
Among the different certolizumab pegol exposure groups, patients in the First Exposure group had the longest mean time to loss of remission (1.43 [SE, 0.218] yr), whereas progressively shorter mean times to loss of remission were determined for the Reinduction/First Maintenance group (1.31 [SE, 0.252]) and Reinduction/Remaintenance group (0.73 [SE, 0.156]) (Fig. 3). Differences between Kaplan–Meier estimates were statistically significant (P = 0.0072, log-rank test).
FIGURE 3.
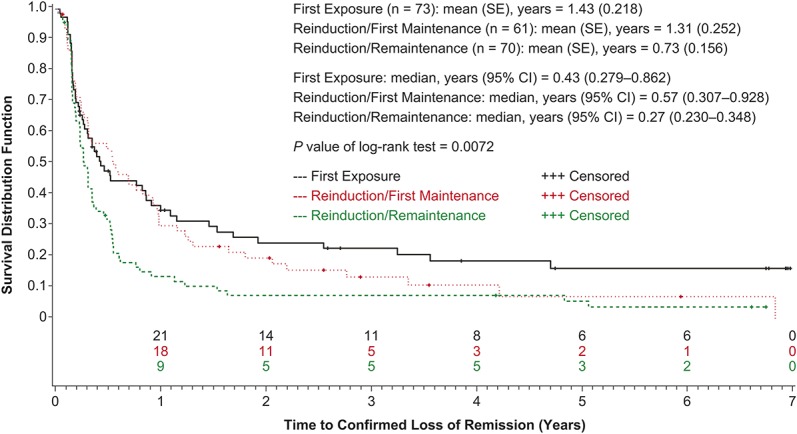
Time to confirmed loss of remission by qualifying study treatment categories (intent-to-treat population).
Immunologic Findings
Overall, 73 (23.6%) patients in the safety population tested positive for certolizumab pegol ADAbs in at least 1 assessment during PRECiSE 4 and the qualifying studies. However, 7 patients tested positive only at the safety follow-up visit and 8 patients had transient certolizumab pegol ADAb-positive tests that were not associated with a persistent effect on certolizumab pegol plasma concentrations, whereas 58 (17.8%) patients had persistent certolizumab pegol ADAbs. Cumulative proportions of patients who tested positive for certolizumab pegol ADAbs at least on 1 occasion were greater in the Reinduction/First Maintenance group than in the Reinduction/Remaintenance and First Exposure groups, both after 26 weeks of certolizumab pegol exposure (22.7% versus 14.2% and 9.6%, respectively) and at the end of PRECiSE 4 (30.7% versus 20.8% and 21.9%, respectively). Cumulative proportions of certolizumab pegol ADAb-positive patients also were much higher among patients who were not taking immunomodulators at baseline than those who were taking immunomodulators (20.4% versus 7.7% at week 26, and 30.5% versus 15.5% at study end) (Fig. 4A).
FIGURE 4.
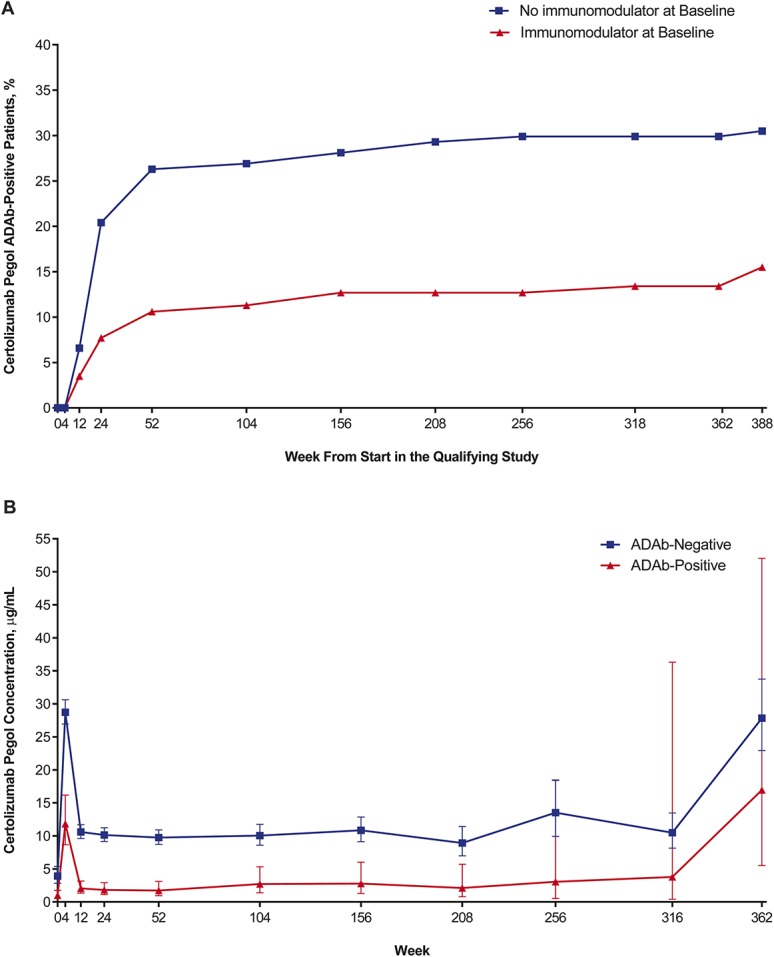
Relationship of certolizumab pegol ADAb status with certolizumab pegol concentrations and immunomodulator use. A, Cumulative proportion of certolizumab pegol ADAb-positive patients by immunomodulator use at baseline. B, Geometric mean concentrations of certolizumab pegol over time by ADAb status. Error bars indicate 95% CIs.
Geometric mean certolizumab pegol plasma concentrations in ADAb-negative patients were generally 3 to 5 times those in ADAb-positive patients (Fig. 4B). However, ADAb-positive and ADAb-negative patients showed no major differences in the incidence of AEs (94.5% versus 94.1%), serious AEs (SAEs) (43.8% versus 44.7%), AEs possibly, probably, or definitely related to certolizumab pegol (46.4% versus 46.6%), and AEs leading to discontinuation (52.1% versus 48.1%). In addition, certolizumab pegol ADAb status had no significant effect on discontinuation rates or time to discontinuation. The respective discontinuation rates for certolizumab pegol ADAb-positive versus certolizumab pegol ADAb-negative patients were 90.4% versus 92.8% overall, with mean (SE) times to discontinuation of 1.81 (0.24) years versus 1.88 (1.4) years (P = 0.8444, log-rank test). There was no consistent correlation between certolizumab pegol concentrations and HBI scores.
Safety
Over the study period of 7 years, 94.2% of patients reported at least 1 AE and 44.5% reported an SAE (Table 2). The most common AEs (percent of patients) were exacerbation of Crohn's disease (46.8%), abdominal pain (22.3%), arthralgia (16.1%), and headache (15.5%). The most common SAEs were CD exacerbation (15.8%), perianal abscess (2.9%), abdominal pain (2.6%), and small intestinal obstruction (2.3%). Patients in the Reinduction/First Maintenance group had a numerically higher incidence of AEs leading to discontinuation (56.0%) than patients in the First Exposure groups and Reinduction/Remaintenance (48.7% and 45.0%, respectively) (Table 2).
TABLE 2.
Summary of TEAEs in PRECiSE 4
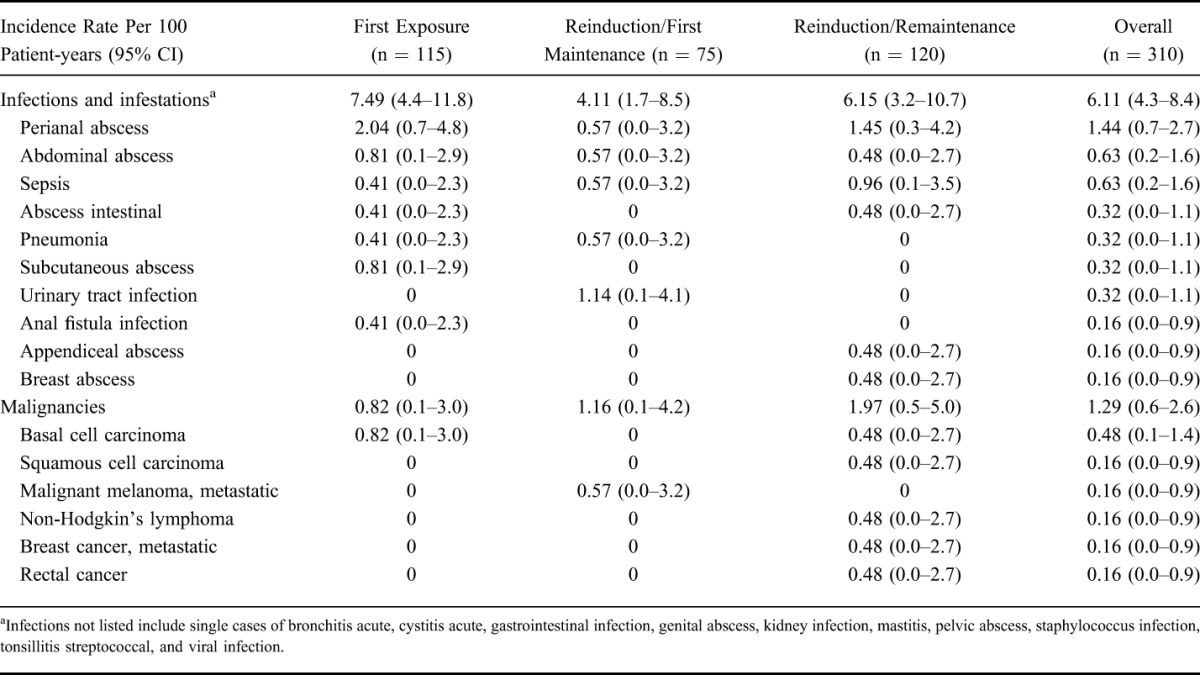
Serious infections occurred in 11.9% of patients, including perianal abscess in 2.9% and abdominal abscess and sepsis each in 1.3% of patients. The IRs for serious infections were similar across the different certolizumab pegol exposure groups (Table 3), with no reports of TB over the course of the trial. The overall incidence of malignancies was low (Table 3). Four patients, of whom 3 had previous exposure and all had concurrent exposure to immunomodulators, exhibited nonmelanoma skin cancer, including 3 patients with basal cell carcinoma and 1 patient with squamous cell carcinoma. In addition, 1 patient each had metastatic malignant melanoma, non-Hodgkin's lymphoma, metastatic breast cancer, and rectal cancer (Table 3).
TABLE 3.
Incidence Rates for Serious Infections and Malignancies in PRECiSE 4 (Safety Population)
The IR of serious cardiac disorders was 0.32/100 patient-years, including a 55-year-old man with acute coronary syndrome and myocardial infarction, and a 64-year-old woman with myocardial ischemia. Serious skin and subcutaneous tissue disorders had an IR of 0.32 cases/100 patient-years, including 1 patient (ADAb-negative) with acute febrile neutrophilic dermatosis and 1 patient (ADAb-positive) with generalized urticaria. Psoriasis occurred in 3 (1.0%) patients, all of whom were ADAb-negative; psoriasis in 1 patient was considered treatment related. There were no reports of lupus, Stevens–Johnson's reaction, or toxic epidermal necrosis during the trial. AEs occurring within 30 minutes of a certolizumab pegol injection included injection site reactions (1.6%), injection site burning (0.3%), and injection site erythema (0.3%). There were no reports of demyelinating disorders during the trial.
Two patients died during the study. A 51-year-old white man in the Reinduction/First Maintenance group experienced SAEs of chest pain, pneumonia, acute renal failure, and myocardial infarction that led to his death 7.4 years after the diagnosis of Crohn's disease and 5 years after starting certolizumab pegol treatment. The SAE of chest pain was deemed by the investigator as possibly related to certolizumab pegol treatment, whereas the other SAEs were considered unlikely related to certolizumab pegol. The second patient who died during the study was a 45-year-old white woman in the Reinduction/First Maintenance group who had an SAE of exacerbation of Crohn's disease. She subsequently developed psychologic problems and refused further intervention. Her death 2.3 years after being diagnosed with Crohn's disease and 1 year after starting certolizumab pegol treatment was considered by the investigator to be unrelated to certolizumab pegol treatment.
DISCUSSION
The PRECiSE 4 study evaluated the efficacy and safety of certolizumab pegol over a treatment period of up to 7 years in patients with moderate to severe Crohn's disease who previously withdrew from 2 randomized placebo-controlled studies of certolizumab pegol, PRECiSE 17 and PRECiSE 2,5 owing to the exacerbation of Crohn's disease. Thirty-seven percent of the PRECiSE 4 study population had no previous exposure to certolizumab pegol, whereas 63% experienced primary or secondary failure of previous certolizumab pegol induction or maintenance therapy. The results presented here show that 29% of all patients who entered the study were in clinical remission after 1 year of therapy, although slightly more than half of all enrolled patients withdrew from the study during the first year. The mean time to confirmed loss of remission was significantly shorter for patients who received reinduction therapy after primary or secondary failure to certolizumab pegol (Reinduction/Remaintenance group) than for patients in the First Exposure Group. However, at any given time point during the 7-year treatment period, at least 60% of all patients who remained on therapy were in remission. Overall, these findings suggest that the reintroduction of certolizumab pegol therapy after primary or secondary treatment failure with certolizumab pegol can be an effective strategy for achieving long-term remission in patients with moderate to severe Crohn's disease.
The results of PRECiSE 4 confirm the sustained efficacy of de novo certolizumab pegol therapy in patients with moderate to severe Crohn's disease previously observed in PRECiSE 3,11 as 1-year remission rates for patients with no previous exposure to certolizumab pegol were remarkably similar in the 2 trials (35% in PRECiSE 3 and 33% in PRECiSE 4). Although longer-term remission rates in clinical trials of infliximab and adalimumab are only available for patients who responded to initial induction therapy, the data overall suggest that certolizumab pegol has comparable efficacy in patients with no primary or secondary treatment failure.3,4,13
PRECiSE 4 and PRECiSE 311 were both 7-year open-label extension studies of the PRECiSE 1 and 2 trials. However, because of the differences in eligibility criteria, 55.0% of patients in PRECiSE 3, compared with only 2.3% of patients in PRECiSE 4, were in remission at study entry. The stark difference in patients' disease activity at study entry in the 2 trials may explain at least in part the differences in discontinuation rates (53% in PRECiSE 4 versus 29% in PRECiSE 3 during the first year) and overall remission rates by NRI analysis. Furthermore, the discontinuation rate in PRECiSE 4 may have been affected by “trial fatigue” among patients asked to attend a total of 92 study visits over 7 years, combined with the fact that certolizumab pegol became commercially available approximately 4 years after initiation of the trial.
Our findings suggest that lower remission rates and shorter times to loss of remission are to be expected for the reintroduction of anti-TNF therapy in patients who previously experienced treatment failure with the same agent. This is consistent with findings by others showing that previous discontinuation of adalimumab or infliximab therapy due to loss of response is associated with an increased risk of nonresponse to reinduction therapy with these agents (odds ratio, 6; 95% CI, 1.1–34; P = 0.04).14 In this context, it is noteworthy that concomitant corticosteroid or immunomodulatory use in PRECiSE 4 was associated with substantially increased remission rates among patients with previous treatment failure. In this patient group, remission rates of >50% by NRI analysis were achieved at week 52, if patients had received 6 months of uninterrupted concomitant corticosteroid or immunomodulatory therapy. The effect of these concomitant therapies on remission rates over time (assessed by the NRI analysis) may primarily reflect the ability of patients who receive them to remain longer on certolizumab pegol therapy than other patients.
Published reports from large clinical studies that investigated the benefit of anti-TNF reinduction therapy after a drug-free interval in patients with secondary treatment failure are scarce.15 As previously reported, more than 60% of all patients with secondary failure in PRECiSE 2 who entered PRECiSE 4 achieved a clinical response with certolizumab pegol induction therapy at week 4, and 35% of all patients were in remission at week 52 based on NRI analysis.10 In clinical practice, secondary failure of infliximab and adalimumab therapy is often addressed with dose escalation. However, although response rates greater than 60% can be achieved with infliximab and adalimumab dose escalation, historical data suggest that the restored responses are lost in one-third of patients treated with adalimumab within 6 months8 and in half of patients within 1 year of dose escalation.16
Slightly less than one-fifth of the PRECiSE 4 study population developed certolizumab pegol ADAbs with a persistent effect on certolizumab pegol plasma concentrations. Overall, ADAb-positive status was associated with an approximately 3- to 5-fold decrease in mean certolizumab pegol concentrations throughout the study. It is currently unclear to which extent certolizumab pegol ADAb development affected achievement and maintenance of remission in PRECiSE 4; however, certolizumab pegol ADAb status had no clinically significant effects on overall safety outcomes. Interestingly, the proportion of patients on therapy who were positive for certolizumab pegol ADAbs was greatest in the Reinduction/First Maintenance group, which also had the longest mean certolizumab pegol–free interval compared with the other certolizumab pegol exposure groups. This suggests that the more extended treatment interruptions in this group increased the risk of certolizumab pegol ADAb development.
Certolizumab pegol was generally well tolerated in the PRECiSE 4 study, and no new safety signals were detected with treatment for up to 7 years. The safety profile of certolizumab pegol in PRECiSE 4 is consistent with findings from PRECiSE 311 and with results from long-term studies of other anti-TNF therapies used to treat Crohn's disease.13,17 The most frequently reported AEs in PRECiSE 4 were related to Crohn's disease or infections and were primarily mild or moderate in intensity. The incidence of serious infection (6.11 cases/100 patient-years) was similar to that observed with certolizumab pegol in PRECiSE 3 (4.37 cases/100 patient-years)11 and adalimumab in ADHERE (6.1 events/100 patient-years).13 Of note, cases of TB, which occurred in other long-term studies,11,13 were not reported in PRECiSE 4, although PRECiSE 4 was conducted worldwide, including in countries in which TB has not been eradicated.
The incidence of malignancies in PRECiSE 4 was low (1.29 cases/100 patient-years) and similar to findings in PRECISE 3 (1.06 cases/100 patient-years)11 and ADHERE (1.6 events/100 patient-years).13 Inflammatory bowel disease, in general, and Crohn's disease, in particular, have been associated with small but significant increases in the incidence of melanoma and nonmelanoma skin cancer.18,19 Results of a recent case–control study further suggested that the increased risk of melanoma was primarily associated with the use of biologics, such as anti-TNF therapies (odds ratio, 1.88; 95% CI, 1.08–3.29), whereas the increased risk of nonmelanoma skin cancer was associated with the use of thiopurine-based immunomodulators (odds ratio, 1.85; 95% CI, 1.66–2.05).19 In addition, a recent analysis of pooled data from studies of adalimumab provided evidence that adalimumab therapy in combination with immunomodulators, but not as monotherapy, may increase the risk of nonmelanoma cancer in patients with Crohn's disease.20 In PRECiSE 4, only 1 case of melanoma and 4 cases of nonmelanoma skin cancer (3 cases of basal cell carcinoma and 1 case of squamous cell carcinoma) were reported during the 7-year study period. The IRs for basal cell carcinoma in PRECiSE 3 (0.21 cases/100 patient-years),11 PRECiSE 4 (0.48 cases/100 patient-years), and ADHERE (0.4 events/100 patient-years)13 were similar and within the range of IRs reported for the general US population (approximately 0.1–1.0 cases/100 person-years, depending on year of assessment and state).21 Of note, all patients with nonmelanoma skin cancer received concomitant immunosuppressants.
In conclusion, our findings show that for many patients with exacerbation of Crohn's disease, including those who experienced primary or secondary failure to certolizumab pegol induction (or reinduction) and subsequent maintenance treatment with certolizumab pegol, resulted in long-term clinical remission. No new safety signals were detected with exposure to certolizumab pegol for up to 7 years, and IRs for serious infections and malignancies were consistent with reports from other long-term studies of anti-TNF therapy in patients with Crohn's disease. Overall, the results suggest that many patients taking certolizumab pegol for moderate to severe Crohn's disease may still derive long-term benefit from reinduction and continuing maintenance therapy with certolizumab pegol after intermittent disease exacerbation and may not need a change to another therapy.
ACKNOWLEDGMENTS
The authors would like to acknowledge the investigators and patients participating in this study. The authors acknowledge Fiona Nitsche, PhD, CMPP and Roland Tacke, PhD, CMPP, of Evidence Scientific Solutions (Horsham, United Kingdom, and Philadelphia, PA), for writing and editorial assistance, which was funded by UCB Pharma.
This study was funded by UCB Pharma. S. D. Lee received research funding from Celgene, Genentech, Gilead, GSK, Janssen, Pfizer, Takeda, and UCB; and has served as consultant for Janssen, Takeda, and UCB. D. T. Rubin has served as a consultant for Abbvie, Amgen, Emmi, Genentech, Janssen, Pfizer, Prometheus, Shire, Takeda, and UCB Pharma; and received research grant support from Abbvie, Genentech, Janssen, Prometheus, Shire, Takeda, and UCB Pharma. W. J. Sandborn has served as a speaker, consultant, or advisory board member for Abbott, ActoGeniX NV, AGI, Alba, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys, Atlantic Healthcare, Axcan (now Aptalis), BioBalance, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Celek, Cellerix SL, Cerimon, ChemoCentryx, CoMentis, Cosmo, Coronado Biosciences, Cytokine PharmaSciences, Eagle Pharmaceuticals, Eisai, Elan, enGene, Eli Lilly, EnteroMedics, Exagen Diagnostics, Ferring, Flexion, Funxional Therapeutics, Genzyme, Genentech (Roche), Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, Janssen, KaloBios, Lexicon Pharmaceuticals, Lycera, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millennium (Takeda), Nisshin Kyorin, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen, PDL BioPharma, Pfizer, Procter & Gamble, Prometheus, ProtAb, PurGenesis, Receptos, Relypsa, Salient, Salix, Santarus, Schering Plough, Shire, Sigmoid Pharma, Sirtris, S.L.A. Pharma, Targacept, Teva, Therakos, Tillotts Pharma, TxCell SA, UCB Pharma, Viamet, Vascular Biogenics, Warner Chilcott, and Wyeth (Pfizer); received speakers' fees from Abbott, Bristol-Meyers Squibb, and Janssen; and received financial support for research from Abbott, Bristol-Meyers Squibb, Genentech (Roche), GlaxoSmithKline, Janssen, Millennium (Takeda), Novartis, Pfizer, Procter & Gamble, Shire, and UCB Pharma. C. Randall has served as a speaker, consultant, or advisory board member for Boston Scientific, Forest Labs, Ironwood Pharmaceuticals, Janssen, Prometheus, Salix Pharmaceuticals, Santarus Pharmaceuticals, Takeda, and UCB. Z. Younes served as a speaker for AbbVie, Takeda, and UCB; served as a consultant for Genentech; and received research support from AbbVie, Arena, Bristol-Myers Squibb, Celgene, Genentech, Gilead, Janssen, Takeda, and UCB. S. Schreiber has served on advisory boards for AbbVie, MSD, Pfizer, and UCB Pharma; and has received lecture fees for AbbVie and MSD. D. A. Schwartz served as a speaker, consultant, or advisory board member for AbbVie, Janssen, Takeda, Telgenix, and UCB; and received research funding from AbbVie and UCB. D. Binion was a consultant for and received honoraria from Cubist, Janssen Pharmaceuticals, Salix Pharmaceuticals, and Takeda Pharmaceuticals; and serves on an FDA safety registry for Janssen Pharmaceuticals and UCB. R. Arsenescu has served as a consultant for Abbvie, Janssen, and UCB Pharma; and received research grant support from Genentech, Prometheus, and UCB Pharma. A. Gutierrez has served as a consultant for AbbVie and Janssen and has received research grants from AbbVie and Centocor (Janssen). E. Scherl holds stocks from Gilead; has served as a consultant or advisory board member for AbbVie, Entera Health, Evidera, GI Health Foundation, Janssen, NPS Pharmaceutical, Prometheus, Protagonist, Salix, Seres Health, Shire, SUN FZE, Takeda, and UCB Pharma; served on the speaker's bureau for GI Health Foundation, Janssen, and ClearView Healthcare Partners; and received research funding from Abbott (AbbVie), AstraZeneca, Elan, Janssen, Mesoblast, Millennium, Osiris, Pfizer, Prometheus, Salix, and UCB Pharma. C. Kayhan, I. Hasan, G. Kosutic, M. Spearman, D. Sen, and J. Coarse are employees of UCB Pharma. S. Hanauer has served as a consultant to and has been involved in clinical research with Abbott Laboratories, Bristol-Myers Squibb, Centocor, Elan Pharmaceuticals, Ferring Pharmaceuticals, Genentech, Proctor and Gamble, Prometheus, Salix, Shire, and UCB Pharma; and has served as a consultant for AstraZeneca, GlaxoSmithKline, and Millennium Pharmaceuticals. The remaining authors have no conflict of interest to disclose.
Footnotes
Author disclosures are available in the Acknowledgments.
REFERENCES
- 1.Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep. 2015;3:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB, Feagan BG, Lichtenstein GR, et al. the ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. for the PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. [DOI] [PubMed] [Google Scholar]
- 6.Annesse V, Duricova D, Gower-Rousseau C, et al. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the Epidemiology Committee of ECCO. J Crohns Colitis. 2016;10:216–225. [DOI] [PubMed] [Google Scholar]
- 7.Sandborn WJ, Feagan BG, Stoinov S, et al. for the PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 8.Baert F, Glorieus E, Reenaers C, et al. Adalimumab dose escalation and dose de-escalation success rate and predictors in a large national cohort of Crohn's patients. J Crohns Colitis. 2013;7:154–160. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Abreu MT, D'Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn's disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–695.e682. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Schreiber S, Hanauer SB, et al. PRECiSE 4 Study Investigators. Reinduction with certolizumab pegol in patients with relapsed Crohn's disease: results from the PRECiSE 4 Study. Clin Gastroenterol Hepatol. 2010;8:696–702.e691. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Lee SD, Randall C, et al. Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn's disease: 7-year results from the PRECiSE 3 study. Aliment Pharmacol Ther. 2014;40:903–916. [DOI] [PubMed] [Google Scholar]
- 12.Wade JR, Parker G, Kosutic G, et al. Population pharmacokinetic analysis of certolizumab pegol in patients with Crohn's disease. J Clin Pharmacol. 2015;55:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Horin S, Mazor Y, Yanai H, et al. The decline of anti-drug antibody titres after discontinuation of anti-TNFs: implications for predicting re-induction outcome in IBD. Aliment Pharmacol Ther. 2012;35:714–722. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. [DOI] [PubMed] [Google Scholar]
- 16.Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn's disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026–2033. [DOI] [PubMed] [Google Scholar]
- 17.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. [DOI] [PubMed] [Google Scholar]
- 18.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterman MT, Sandborn WJ, Colombel JF, et al. Increased risk of malignancy with adalimumab combination therapy, compared with monotherapy, for Crohn's disease. Gastroenterology. 2014;146:941–949. [DOI] [PubMed] [Google Scholar]
- 21.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. [DOI] [PubMed] [Google Scholar]