Abstract
Purpose
Gallbladder diseases and cholecystectomy may play a role in the development of colorectal cancer (CRC). Our aim was to investigate the association between cholecystectomy and CRC risk overall and by sex, family history, anatomical location, and tumor mismatch repair (MMR) status.
Methods
This study comprised 5,847 incident CRC cases recruited from population cancer registries in Australia, Canada and USA into the Colon Cancer Family Registry between 1997 and 2012, and 4,970 controls with no personal history of CRC who were either randomly selected from the general population or were spouses of the cases. The association between cholecystectomy and CRC was estimated using logistic regression, after adjusting for confounding factors.
Results
Overall, there was no evidence for an association between cholecystectomy and CRC (odds ratio [OR] = 0.88, 95% confidence interval: 0.73, 1.08). In the stratified analyses, there was no evidence for a difference in the association between women and men (P=.54), between individuals with and without family history of CRC in first-degree relative (P=.64), between tumor anatomical locations (P=.45), or between MMR-proficient and MMR-deficient cases (P=.54).
Conclusion
Cholecystectomy is not a substantial risk factor for CRC, regardless of sex, family history, anatomical location, or tumor MMR status.
Keywords: gallbladder, cholecystectomy, mismatch repair, colorectal cancer
Introduction
Colorectal cancer (CRC) accounts for 10% of all cancer incidence and is the fourth ranking cause of cancer mortality worldwide [1]. In 2012, 746,000 men and 614,000 women were diagnosed with CRC globally, with highly industrialized and urbanized societies (including North America, most European countries, Japan and Australia) contributing to more than half. Identifying risk factors associated with CRC may inform strategies to prevent and detect cancers at an early stage. Certain medical conditions and interventions may play a role in the development of CRC [2].
Gallbladder diseases such as gallstones which lead to gallbladder inflammation (cholecystitis) are highly prevalent in Western populations [3,4]. The lifetime risk of developing gallstones in Western populations is between 5% and 25% and women in the US under 40 years of age are at nearly twice the risk of developing gallstones than men[5]. The common treatment for inflammatory gallstones, cholecystectomy, has the potential to lead to increased bile exposure in the colon and rectum. Bile acids cause DNA damage probably indirectly through the induction of oxidative stress and the production of reactive oxygen species. Excessive DNA damage may cause genetic instability and increase mutations of tumor suppressor genes and oncogenes, subsequently increasing the risk of CRC [6].
Previous studies have reported conflicting results on associations between gallstones and/or cholecystectomy and CRC [7,8] or colorectal adenoma [9]. Further, risks may be differentially associated with cancer at different anatomical locations within the colon [10]. No previous studies have examined the association between cholecystectomy and CRC according to their tumor mismatch repair (MMR) status. Here, we investigated associations between cholecystectomy and CRC risk by sex, family history, anatomical location and tumor MMR status.
Materials and Methods
Study Sample
This study was designed as a retrospective case-control study. Cases were individuals diagnosed with incident colon or rectal cancer who were recruited through population cancer registries from four centers of the Colon Cancer Family Registry between 1997 and 2012, Cancer Care Ontario (Toronto, Canada), Fred Hutchinson Cancer Research Center (Seattle, US), Mayo Clinic (Rochester, US), and The University of Melbourne (Melbourne, Australia) [11]. We only included cases interviewed by staff from the Colon Cancer Family Registry within 5 years of diagnosis. Controls were either individuals with no personal history of CRC who were recruited randomly from the general population through Medicare and Driver’s License files, telephone subscribers lists, electoral rolls, or the CRC cases’ spouses with no personal history of CRC. For all participants, we excluded those without information on whether or not they had cholecystectomy.
Data Collection
We collected data on demographic information, personal and familial history of CRC, medical history including surgeries, medical conditions and drug use, alcohol intake, smoking status for all participants and reproductive history for women at the time of baseline recruitment. Uniform questionnaires (http://coloncfr.org/questionnaires) were used to acquire data by telephone interviews (Fred Hutchinson Cancer Research Center, University of Melbourne and Mayo Clinic) and mails (Cancer Care Ontario and Mayo Clinic). All participants were asked whether or not they had surgical removal of their gallbladder and if so, at what age they were at the time of the removal.
Tumor molecular characterization
CRCs were characterized for MMR-deficiency by microsatellite instability (MSI) using a ten-marker panel (four mononucleotide markers (BAT25, BAT26, BAT40, and BAT34C4), five dinucleotide markers (D5S346, D17S250, ACTC, D18S55, and D10S197), and one penta-mono-tetra compound-repeat marker (MYCL)) and/or by immunohistochemistry (IHC) for the four MMR proteins. Tumors were classified as MMR-deficient if they were MSI-high (≥30% unstable markers) and/ or showed loss of expression of one or more of the MMR proteins by IHC; and MMR-proficient if they were microsatellite stable (no unstable markers) or MSI-low (<30% unstable markers) and/or showed normal expression of all four MMR proteins by IHC [11].
Statistical Analysis
Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for the association between cholecystectomy and CRC were estimated using multivariable unconditional logistic regression. We estimated the associations stratified by sex, first-degree family history, anatomical location, and tumor MMR status. We investigated potential interactions between cholecystectomy status and age at study recruitment (≤45, 45-55, 55-65 and >65 years), sex, recent body mass index (BMI) and BMI at age 20 years (underweight, normal, overweight and obese) using likelihood ratio tests comparing the results with and without the interaction terms in the logistic regression models.
To investigate whether the time since cholecystectomy was associated with CRC risk for the participants that had undergone cholecystectomy, we compared the mean of time since cholecystectomy between cases (age at CRC diagnosis minus age at cholecystectomy) and controls (age at study recruitment minus age at cholecystectomy) using a multivariable linear regression model.
Some centers of the Colon Cancer Family Registry used stratified sampling based on family history for recruitment, and we did take this into account when combining and analyzing the data. To adjust for the stratified sampling, we gave each individual a probability weight equal to the reciprocal of the family sampling fraction. All statistical analyses were conducted using STATA version 13.0 (StataCorp, College Station, TX).
Results
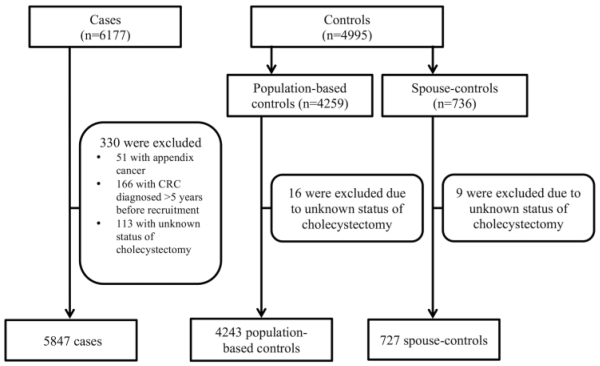
This study comprised 5,847 pathologically confirmed CRC cases, and 4,243 population-based controls and 727 spouse-controls (Figure 1). Baseline characteristics of cases and controls are presented in Table 1.
Figure 1.
Selection of colorectal cancer cases and controls from the Colon Cancer Family Registry
Table 1.
Baseline characteristics of colorectal cancer cases and controls
| Controls, N (%) |
||||
|---|---|---|---|---|
| Variables | Cases, N (%) (n = 5847) |
Total controls (n = 4970) |
Population controls (n = 4243) |
Spouse controls (n =727) |
| Age at recruitment, years | ||||
| Mean (SD) | 55.2 (11.8) | 57.5 (11.6) | 58.4 (11.7) | 52.5 (10) |
| Median (range) | 54 | 59 | 60 | 52 |
|
| ||||
| Sex | ||||
| Female | 2959 (50.6) | 2630 (52.9) | 2191 (51.6) | 439 (60.4) |
| Male | 2888 (49.4) | 2340 (47.1) | 2052 (48.4) | 288 (39.6) |
|
| ||||
| Recruitment centre | ||||
| Cancer Care Ontario | 2098 (35.9) | 1920 (38.6) | 1920 (45.2) | 0 |
| University of Melbourne | 807 (13.8) | 719 (14.5) | 270 (6.4) | 449 (61.8) |
| Mayo Clinic | 623 (10.7) | 278 (5.6) | 0 | 278 (38.2) |
| Fred Hutchinson Seattle | 2319 (39.6) | 2053 (41.3) | 2053 (48.4) | 0 |
|
| ||||
| Cholecystectomy | ||||
| Yes | 521 (8.9) | 555 (11.2) | 504 (11.9) | 51 (7) |
| No | 5326 (91.1) | 4415 (88.8) | 3739 (88.1) | 676 (93) |
|
| ||||
| Family history of CRC (first-degree relative) |
||||
| Yes (at least 1 affected) | 1248 (21.3) | 529 (10.6) | 460 (10.8) | 69 (9.5) |
| No | 4599 (78.7) | 4441 (89.4) | 3783 (89.2) | 658 (90.5) |
|
| ||||
| Body mass index, recent (kg/m2) | ||||
| Underweight (<18.5) | 86 (1.5) | 57 (1.2) | 50 (1.2) | 7 (1) |
| Normal (18.5-24.9) | 2010 (34.4) | 2020 (40.6) | 1732 (40.8) | 288 (39.6) |
| Overweight (25.0-29.9) | 2214 (37.9) | 1849 (37.2) | 1626 (38.3) | 223 (30.7) |
| Obese (≥30) | 1413 (24.2) | 922 (18.5) | 789 (18.6) | 133 (18.3) |
| Missing | 124 (2) | 122 (2.5) | 46 (1.1) | 76 (10.4) |
|
| ||||
| Body mass index at age 20 years (kg/m2) |
||||
| Underweight (<18.5) | 423 (7.2) | 439 (8.8) | 373 (8.8) | 66 (9.1) |
| Normal (18.5-24.9) | 3989 (68.2) | 3595 (72.3) | 3121 (73.6) | 474 (65.2) |
| Overweight (25.0-29.9) | 969 (16.6) | 638 (12.8) | 549 (12.9) | 89 (12.2) |
| Obese (≥30) | 230 (3.9) | 112 (2.3) | 95 (2.2) | 17 (2.3) |
| Missing | 236 (4.1) | 186 (3.8) | 105 (2.5) | 81 (11.2) |
|
| ||||
| Cigarette smoking | ||||
| Current^^ | 662 (11.3) | 638 (12.8) | 537 (12.7) | 101 (13.9) |
| Former^ | 2635 (45.1) | 2056 (41.4) | 1788 (42.1) | 268 (36.9) |
| Never | 2511 (43) | 2250 (45.3) | 1901 (44.8) | 349 (48) |
| Missing | 39 (0.6) | 256 (0.5) | 17 (0.4) | 9 (1.2) |
|
| ||||
| Education | ||||
| Primary school | 107 (1.8) | 91 (1.8) | 79 (1.9) | 12 (1.6) |
| Secondary school | 821 (14) | 658 (13.2) | 541 (12.7) | 117 (16.1) |
| High/Senior secondary school | 1310 (22.4) | 958 (19.3) | 789 (18.6) | 169 (23.3) |
| Vocational education or training | 583 (10) | 466 (9.4) | 352 (8.3) | 114 (15.7) |
| University without degree | 1330 (22.8) | 1089 (21.9) | 1025 (24.2) | 64 (8.8) |
| University graduation or higher | 1649 (28.2) | 1618 (3.6) | 1444 (34) | 174 (23.9) |
| Missing | 47 (0.8) | 90 (1.8) | 13 (0.3) | 77 (10.6) |
|
| ||||
| Annual household Income | ||||
| ≤ $15,000 | 319 (5.5) | 190 (3.8) | 186 (4.4) | 4 (0.5) |
| $15,000-30,000 | 777 (13.3) | 572 (11.5) | 538 (12.7) | 34 (4.7) |
| $30,000-45,000 | 1031 (17.6) | 925 (18.6) | 864 (20.4) | 61 (8.4) |
| $45,000-70,000 | 1068 (18.3) | 933 (18.8) | 870 (20.5) | 63 (8.6) |
| ≥ $70,000 | 1026 (17.5) | 876 (17.6) | 790 (18.6) | 86 (11.8) |
| Refused to answer | 144 (2.5) | 147 (3) | 140 (3.3) | 7 (1) |
| Missing | 1482 (25.3) | 1327 (26.7) | 855 (20.1) | 472 (64.9) |
|
| ||||
| Race | ||||
| Caucasian | 5206 89) | 4592 (92.4) | 3944 (93) | 648 (89.1) |
| Other | 514 (8.8) | 276 (5.6) | 254 (6) | 22 (3) |
| Missing | 127 (2.2) | 102 (2) | 45 (1) | 57 (7.9) |
|
| ||||
| Diabetes mellitus | ||||
| Yes | 468 (8) | 376 (7.6) | 340 (8) | 36 (5) |
| No | 5360 (91.7) | 4581 (92.2) | 3895 (91.8) | 686 (94.3) |
| Missing | 19 (0.3) | 13 (0.2) | 8 (0.2) | 5 (0.7) |
|
| ||||
| Aspirin intake** | ||||
| ≥5years | 471 (8.1) | 623 (12.5) | 577 (13.6) | 46 (6.3) |
| <5years | 884 (15.1) | 888 (17.9) | 806 (19) | 82 (11.3) |
| Never | 4231 (72.4) | 3263 (65.7) | 2705 63.8) | 558 (76.8) |
| Missing | 261 (4.4) | 196 (3.9) | 155 (3.7) | 41 (5.6) |
|
| ||||
| Ibuprofen intake** | ||||
| ≥5years | 172 (3) | 182 (3.7) | 155 (3.7) | 27 (3.7) |
| <5years | 685 (11.7) | 719 (14.5) | 642 (15.1) | 77 (10.6) |
| Never | 4756 (81.3) | 3904 (78.5) | 3332 (78.5) | 572 (78.7) |
| Missing | 234 (4) | 165 (3.3) | 114 (2.7) | 51 (7) |
|
| ||||
| Multivitamin intake** | ||||
| ≥5years | 1553 (26.6) | 1463 (29.4) | 1361 (32.1) | 102 (14) |
| <5years | 1102 18.8) | 859 (17.3) | 752 (17.7) | 107 (14.7) |
| Never | 2845 (48.7) | 2434 (49) | 1975 (46.5) | 459 (63.2) |
| Missing | 347 (5.9) | 214 (4.3) | 155 (3.7) | 59 (8.1) |
|
| ||||
| Regular physical activity* | ||||
| Yes | 5023 (85.9) | 4287 (86.3) | 3668 (86.5) | 619 (85.1) |
| No | 90 (1.5) | 108 (2.2) | 89 (2.1) | 19 (2.6) |
| Missing | 734 (12.6) | 575 (11.5) | 486 (11.4) | 89 (12.2) |
|
| ||||
| Alcohol consumption (standard drink per day) ^^^ |
||||
| Mean (SD) | 1.2 (2.3) | 1 (2.1) | 1.1 (2.1) | 0.9 (1.6) |
| Missing, N | 1684 | 1225 | 1075 | 150 |
|
| ||||
| Red meat consumption (number of serve per day) |
||||
| Mean (SD) | 0.66 (0.63) | 0.58 (0.55) | 0.54 (0.53) | 0.81 (0.66) |
| Missing, N | 187 | 222 | 149 | 73 |
|
| ||||
| Number of live birth # | ||||
| 0 | 374 (12.6) | 269 (10.2) | 230 (10.5) | 39 (8.9) |
| 1 | 349 (11.8) | 275 (10.4) | 230 (10.5) | 45 (10.3) |
| 2 | 909 (30.7) | 830 (31.6) | 656 (29.9) | 174 (39.6) |
| 3 | 605 (20.5) | 538 (20.5) | 440 (20.1) | 98 (22.3) |
| ≥4 | 516 (17.4) | 412 (15.7) | 345 (15.8) | 67 (15.3) |
| Missing | 206 (7) | 306 (11.6) | 290 (13.2) | 16 (3.6) |
|
| ||||
| Hormonal contraceptives# | ||||
| ≥5 years | 1007 (34) | 939 (35.7) | 719 (32.8) | 220 (50.1) |
| <5 years | 577 (19.5) | 545 (20.7) | 433 (19.8) | 112 (25.5) |
| Never | 1192 (40.3) | 1023 (38.9) | 935 (42.7) | 88 (20.1) |
| Missing | 183 (6.2) | 123 (4.7) | 104 (4.7) | 19 (4.3) |
|
| ||||
| Hormone replacement therapy# | ||||
| Estrogen-only users | 491 (16.6) | 473 (18) | 411 (18.8) | 62 (14.1) |
| Progesterone and estrogen users | 187 (6.3) | 214 (8.1) | 187 (8.5) | 27 (6.2) |
| Never | 1879 (63.5) | 1467 (55.8) | 1221 (55.7) | 246 (56) |
| Missing | 402 (13.6) | 476 (18.1) | 372 (17) | 104 (23.7) |
|
| ||||
| Anatomical location | ||||
| Proximal colon | 1968 (33.6) | __ | __ | __ |
| Distal colon | 1811 (31) | __ | __ | __ |
| Colon unspecified | 81 (1.4) | __ | __ | __ |
| Rectum | 1987 (34) | __ | __ | __ |
|
| ||||
| Tumor MMR status | ||||
| MMR-proficient tumor | 3790 (64.8) | __ | __ | __ |
| MMR-deficient tumor | 631 (10.8) | __ | __ | __ |
| Missing | 1426 (24.4) | __ | __ | __ |
Questions asked only for women (2959 cases and 2630 controls)
regular physical activity defined as any physical activity for at least 30 minutes per week for at least 3 months
at least twice a week for at least 1 month
former smokers defined as participants who had smoked at least 1 cigarette per day for at least 3 months and had quit more than 2 years before age at colorectal cancer or age at interview.
current smokers defined as participants who had smoked at least 1 cigarette per day for at least 3 months and continued within 2 years of age at colorectal cancer or age at interview.
4-oz. glasses of wine, or 12-oz. cans or bottles of beer or hard cider, or 1-oz. servings of sake or liquor (spirits)
MMR, mismatch repair; SD, standard deviation; N, number;__, not relevant
In total, 521 (8.9%) cases and 555 (11.2%) controls had undergone cholecystectomy. Overall, there was no evidence for an association between cholecystectomy and CRC (OR=0.88, 95% CI: 0.73, 1.08) after adjusting for confounding factors. In stratified analyses, there was no evidence for a difference in the association between women and men (P=.54), between individuals with and without a family history of CRC in first-degree relative (P=.64), between tumor anatomical locations (P=0.45), or between MMR-proficient and MMR-deficient cases (P=.54) (Table 2). There was no evidence for interactions between cholecystectomy status and age at study recruitment, sex, recent BMI and BMI at age 20 years (details not shown).
Table 2.
Association between cholecystectomy and the risk of colorectal cancer overall and stratified by sex, family history, anatomical location and tumor mismatch repair status
| Cases | Controls | Unadjusted | Adjusted** | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n/N | n/N | OR (95% CI) | P-value | OR (95% CI) | P-value | P-difference* | |
|
| |||||||
| Overall | 521/5847 | 555/4970 | 0.76 (0.66, 0.88) | .001 | 0.88 (0.73, 1.08) | .22 | |
| Sex | |||||||
| Women*** | 395/2959 | 410/2630 | 0.84 (0.72, 0.99) | .0 4 | 1.00 (0.78, 1.28) | .99 | .54 |
| Men | 126/2888 | 145/2340 | 0.74 (0.56, 0.97) | .03 | 0.78 (0.53, 1.13) | .19 | |
|
| |||||||
| Family history | 555/4970 | ||||||
| No | 399/4599 | 0.75 (0.65, 0.88) | <.001 | 0.86 (0.69, 1.06) | .16 | ||
| Yes | 122/1248 | 0.90 (0.71, 1.14) | .39 | 0.98 (0.71, 1.33) | .88 | .64 | |
|
| |||||||
| Anatomical location | 555/4970 | ||||||
| Proximal colon | 216/1968 | 1.02 (0.42, 1.23) | .83 | 0.93 (0.70, 1.24) | .63 | .45 | |
| Distal colon | 151/1811 | 0.70 (0.56, 0.86) | .001 | 0.75 (0.56, 1.02) | .07 | ||
| Colon unspecified | 3/81 | 0.30 (0.09, 0.95) | .04 | 0.59 (0.13, 2.63) | .49 | ||
| Rectum | 151/1987 | 0.66 (0.53, 0.82) | <.001 | 0.97 (0.72, 1.29) | .84 | ||
|
| |||||||
| Tumor MMR status | 555/4970 | ||||||
| Proficient | 314/3790 | 0.74 (0.63, 0.87) | <.001 | 0.86 (0.68, 1.09) | .20 | .54 | |
| Deficient | 71/631 | 0.99 (0.73, 1.34) | .93 | 0.96 (0.63, 1.49) | .87 | ||
OR, Odds ratio; CI, confidence interval, MMR, mismatch repair; n, number of individuals with cholecystectomy; N, total number
for stratified analysis by sex: it was calculated using likelihood ratio tests comparing unconditional multivariable logistic regression models with and without an interaction term; for stratified analyses by first-degree family history, anatomical location and tumor MMR status, it was calculated using likelihood ratio tests on multinomial logistic regression models.
adjusted for sex (women or men, except for stratified analysis by sex), first-degree family history (no, yes; except for stratified analysis by family history), age at study recruitment (continuous), alcohol consumption (per standard drink per day), BMI (WHO categories), BMI at age 20 years (WHO categories), cigarette smoking (never, former, current), aspirin (non-users, <5 years use, ≥5 years use), ibuprofen (non-users, <5 years use, ≥5 years use), diabetes status (no, yes), recruitment center (Cancer Care Ontario, Fred Hutchinson Cancer Research Center, Mayo Clinic or University of Melbourne), regular physical activity (no, yes), and red meat consumption (serving per day)
For women, further adjusted for hormonal contraceptive use (non-users, <5 years use, ≥5 years use) and number of live birth (never, 1, 2, 3, ≥4)
Moreover, there was no evidence for a difference in the time since cholecystectomy between cases and controls (14.5 (SD 12.1) years vs. 15 (SD 11.6) years; mean difference: −0.0003, 95%CI: −0.004, 0.003 years, P=.86), after adjusting for confounding factors.
Discussion
We found no evidence for an association between cholecystectomy and CRC risk, overall or by sex, first-degree family history of CRC, anatomical location or tumor MMR status. This study is the first to examine the association according to tumor MMR status.
Although a few studies have reported a positive association between cholecystectomy and CRC risk [12] [13], previous meta-analyses have reported that cholecystectomy was not associated with the risk of proximal colon cancer [7], rectal cancer [8] or colorectal adenoma [9]. However, most of the previous studies adjusted only for age and sex [10]. In our current study, we adequately adjusted for additional important confounding factors such as obesity, diabetes, and family history.
The strengths of our study include large sample size and high quality data of the Colon Cancer Family Registry, which employs standardized protocols for data and biospecimens collection [11]. One limitation of our study is the possibility of recall bias because cholecystectomy information was self-reported and not validated by medical records. However, a previous study reported 99.6% agreement between self-report and medical records for cholecystectomy [14]. Further, given approximately 90% of our study participants are Caucasians from developed countries, the study results may not be applicable to other populations.
In conclusion, our findings suggest that cholecystectomy is not a substantial risk factor for CRC, regardless of sex, family history, anatomical location, or tumor MMR status.
ACKNOWLEDGEMENTS
The authors thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
FUNDING
This work was supported by grant UM1 CA167551 from the National Cancer Institute, National Institutes of Health (NIH) and through collaborating centers: Australasian Colorectal Cancer Family Registry (U01/U24 CA097735), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01/U24 CA074800), Ontario Familial Colorectal Cancer Registry (U01/U24 CA074783), and Seattle Colorectal Cancer Family Registry (U01/U24 CA074794).
Seattle Colorectal Cancer Family Registry research was also supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which was funded by Control Nos. N01-CN-67009 (1996-2003) and N01-PC-35142 (2003-2010) and Contract No. HHSN2612013000121 (2010-2017) from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000035C awarded to the University of Southern California. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute or their Contractors and Subcontractors is not intended nor should be inferred.
This work was also supported by Centre for Research Excellence grant APP1042021 and program grant APP1074383 from the National Health and Medical Research Council (NHMRC), Australia. AKW is a NHMRC Early Career Fellow. MAJ is a NHMRC Senior Research Fellow. JLH is a NHMRC Senior Principal Research Fellow. DDB is a University of Melbourne Research at Melbourne Accelerator Program (R@MAP) Senior Research Fellow.
Footnotes
DISCLAIMER
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
DISCLOSURE
The authors have no conflict of interest to declare with respect to this manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. doi:10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lin OS. Acquired risk factors for colorectal cancer. Methods Mol Biol. 2009;472:361–372. doi: 10.1007/978-1-60327-492-0_16. doi:10.1007/978-1-60327-492-0_16. [DOI] [PubMed] [Google Scholar]
- 3.Gurusamy KS, Davidson BR. Surgical treatment of gallstones. Gastroenterology clinics of North America. 2010;39(2):229–244. viii. doi: 10.1016/j.gtc.2010.02.004. doi:10.1016/j.gtc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Barie PS, Eachempati SR. Acute acalculous cholecystitis. Gastroenterology clinics of North America. 2010;39(2):343–357. x. doi: 10.1016/j.gtc.2010.02.012. doi:10.1016/j.gtc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut and liver. 2012;6(2):172–187. doi: 10.5009/gnl.2012.6.2.172. doi:10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511(2):145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 7.Reid FD, Mercer PM, harrison M, Bates T. Cholecystectomy as a risk factor for colorectal cancer: a meta-analysis. Scand J Gastroenterol. 1996;31(2):160–169. doi: 10.3109/00365529609031981. doi:10.3109/00365529609031981. [DOI] [PubMed] [Google Scholar]
- 8.Chiong C, Cox MR, Eslick GD. Gallstone disease is associated with rectal cancer: a meta-analysis. Scand J Gastroenterol. 2012;47(5):553–564. doi: 10.3109/00365521.2012.660538. doi:10.3109/00365521.2012.660538. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Ge Z, Wang Y, Qian J. Meta-analysis of observational studies on cholecystectomy and the risk of colorectal adenoma. Eur J Gastroenterol Hepatol. 2012;24(4):375–381. doi: 10.1097/MEG.0b013e328350f86b. doi:10.1097/MEG.0b013e328350f86b. [DOI] [PubMed] [Google Scholar]
- 10.Coats M, Shimi SM. Cholecystectomy and the risk of alimentary tract cancers: a systematic review. World J Gastroenterol. 2015;21(12):3679–3693. doi: 10.3748/wjg.v21.i12.3679. doi:10.3748/wjg.v21.i12.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D, Colon Cancer Family Registry Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. doi:10.1158/1055-9965.epi-07-0648. [DOI] [PubMed] [Google Scholar]
- 12.Goldacre MJ, Wotton CJ, Abisgold J, Yeates DG, Collins J. Association between cholecystectomy and intestinal cancer: a national record linkage study. Ann Surg. 2012;256(6):1068–1072. doi: 10.1097/SLA.0b013e3182759efb. doi:10.1097/SLA.0b013e3182759efb. [DOI] [PubMed] [Google Scholar]
- 13.Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. 2005;100(8):1813–1820. doi: 10.1111/j.1572-0241.2005.41610.x. doi:10.1111/j.1572-0241.2005.41610.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Sweetland S, Beral V, Green J, Balkwill A, Casabonne D. Self-reported information on joint replacement and cholecystectomy agrees well with that in medical records. J Clin Epidemiol. 2007;60(11):1190–1194. doi: 10.1016/j.jclinepi.2007.02.007. doi:10.1016/j.jclinepi.2007.02.007. [DOI] [PubMed] [Google Scholar]