Highlights
-
•
First-in-man study of seasonal influenza vaccine containing Advax delta inulin-based adjuvant.
-
•
Advax adjuvant was safe and well tolerated.
-
•
Advax adjuvant enabled up to 10-fold antigen dose-sparing.
-
•
First use of antibody landscapes to compare adjuvanted and unadjuvanted vaccine groups.
Keywords: Vaccine, Influenza, Adjuvant, Delta inulin, Advax, Safety, Immunogenicity
Abstract
Influenza vaccines are usually non-adjuvanted but addition of adjuvant may improve immunogenicity and permit dose-sparing, critical for vaccine supply in the event of an influenza pandemic. The aim of this first-in-man study was to determine the effect of delta inulin adjuvant on the safety and immunogenicity of a reduced dose seasonal influenza vaccine. Healthy male and female adults aged 18–65 years were recruited to participate in a randomized controlled study to compare the safety, tolerability and immunogenicity of a reduced-dose 2007 Southern Hemisphere trivalent inactivated influenza vaccine formulated with Advax™ delta inulin adjuvant (LTIV + Adj) when compared to a full-dose of the standard TIV vaccine which does not contain an adjuvant. LTIV + Adj provided equivalent immunogenicity to standard TIV vaccine as assessed by hemagglutination inhibition (HI) assays against each vaccine strain as well as against a number of heterosubtypic strains. HI responses were sustained at 3 months post-immunisation in both groups. Antibody landscapes against a large panel of H3N2 influenza viruses showed distinct age effects whereby subjects over 40 years old had a bimodal baseline HI distribution pattern, with the highest HI titers against the very oldest H3N2 isolates and with a second HI peak against influenza isolates from the last 5–10 years. By contrast, subjects >40 years had a unimodal baseline HI distribution with peak recognition of H3N2 isolates from approximately 20 years ago. The reduced dose TIV vaccine containing Advax adjuvant was well tolerated and no safety issues were identified. Hence, delta inulin may be a useful adjuvant for use in seasonal or pandemic influenza vaccines.
Australia New Zealand Clinical Trial Registry: ACTRN12607000599471
1. Introduction
Despite widespread national immunization programs, seasonal influenza continues to cause significant morbidity and mortality in susceptible populations including those with chronic diseases and the elderly [1]. A restriction to the routine use of seasonal influenza vaccines in the developing world is the relatively high cost, reflecting the logistics of producing large quantities of influenza virus in eggs or cell-culture. We hypothesized that adjuvant addition permits antigen dose-sparing and thereby cost of influenza vaccines, making them more affordable and providing support for design of low-dose future pandemic influenza vaccines.
Advax is a novel polysaccharide adjuvant based on semi-crystalline particles of inulin [2], [3], [4] and has been shown in preclinical studies to enhance vaccine immunogenicity and protection across a broad range of pathogens including Japanese encephalitis [5], West Nile virus [6], HIV [7], hepatitis B [8], SARS coronavirus [9], listeria [10] and anthrax [11]. Advax adjuvant has also shown benefit in influenza, providing enhanced vaccine protection of ferrets against high pathogenicity avian influenza (H5N1) [12]. When given with influenza vaccine to pregnant dams it provided enhanced passive protection of their pups [13] and it also helped overcome neonatal immune immaturity when used to vaccinate seven-day-old mouse pups [14]. In human studies, when added to a pandemic H1N1/2009 human vaccine trial it enhanced seroconversion and seroprotection rates [15] and similarly had beneficial effects on the immunogenicity of a hepatitis B vaccine [16] and insect sting allergy desensitisation treatment [17].
This first-in-man Phase 1 study was undertaken to assess the safety, tolerability and efficacy of Advax adjuvant when included in a reduced-dose seasonal TIV vaccine.
2. Methods
2.1. Vaccine composition
The study used a 2007 Southern hemisphere trivalent inactivated influenza vaccine containing A/New Caledonia/20/99 (H1N1)-like, A/Wisconsin/67/2005 (H3N2)-like and B/Malaysia/2506/2004-like strains (2007 Fluvax®, CSL Ltd, Australia). Single-dose vials of Advax adjuvant containing delta inulin 20 mg in a bicarbonate buffer were supplied by Vaxine Pty Ltd, Adelaide, Australia. Adjuvant was mixed with the TIV vaccine by drawing both up into a common syringe on the day of immunization.
2.2. Study design, subjects and study procedures
The study was conducted as a randomized, subject- and observer-blinded, parallel-group trial at Flinders Medical Centre, Adelaide, Australia, to assess safety, immunogenicity and antigen-sparing of Advax-adjuvanted TIV vaccine in healthy adult subjects aged 18–65 years. The study was approved by the Flinders Clinical Research Ethics Committee. Exclusions included pregnancy, immuno-suppressive therapy, diabetes mellitus, any immunodeficiency disorder, oral corticosteroids, HIV infection, drug or alcohol abuse, history of vaccine or egg allergy, or prior receipt of a 2007 seasonal influenza vaccine. In the initial stage of the study, subjects were randomized 1:1 to receive either a standard full-dose TIV vaccine (45 μg HA) or a 1/3rd dose of the same TIV antigen (15 μg HA) formulated with 20 mg Advax™ delta inulin adjuvant. After completion of these initial study arms, subjects were also recruited into a 1/10th TIV dose (4.5 μg HA) + Advax adjuvant arm. After signed informed consent, randomisation and venesection for baseline serology, a single vaccine dose was administered by intramuscular injection of 0.5 ml vaccine into the non-dominant deltoid muscle. At 1, 3 and 12 weeks post-immunization blood was obtained to assess the anti-influenza immune response.
2.3. Safety assessments
Solicited local and systemic reactions were collected with a 7-day memory aid. Serious adverse events were collected throughout the study period. Causality of adverse events was assessed by a blinded investigator.
2.4. ELISA assays
Influenza virus specific antibodies were determined by ELISA, as previously described [18]. Briefly, 96-well ELISA plates were coated with each betapropiolactone (BPL) inactivated vaccine component or reference viral antigen (kindly supplied by the WHO Collaborating Influenza for Reference and Research on Influenza, Melbourne, Australia) overnight at 4 °C, and blocked for 1 h using 2% v/v bovine serum albumin in PBS. Serum samples were added in duplicate at 1:1000 v/v dilution in PBS for 2 h, washed and biotinylated mouse anti-human IgG or IgM (Abcam, USA) was added and incubated for 1 h followed by washing and addition of HRP-conjugated streptavidin for 1 h, plates were washed again, and tetramethylbenzidine substrate added for 10 min then stopped with 1 M phosphoric acid. The optical density (OD) of each well was read at 450 nm (OD450nm) with a spectrophotometer plate reader (VersaMax, Molecular Device.) and analyzed using SoftMax Pro Software with the mean OD calculated for each dilution.
2.5. Hemagglutination inhibition assay
Antibody titers were measured by hemagglutination inhibition (HI) assay, as previously described [18]. The titer was expressed as the reciprocal of the highest serum dilution inhibiting hemagglutination. Serum was assayed for responses against each of the three vaccine viruses and also against variant influenza strains (all viruses tested were propagated in embryonated hens eggs and were representative of each vaccine serotype).
2.6. Statistical analysis
The three co-primary efficacy endpoints were seroprotection (HI titer ⩾40), seroconversion (⩾4-fold increase and HI titer ⩾40), and fold increase in geometric mean HI titer (GMT). Data analysis was performed with Prism version 5.0 (Graphpad Software Inc., CA, USA). Baseline characteristics were compared using t-tests, chi-square, or ANOVA. Exact binomial confidence intervals were reported for all proportional end points. Reported p-values are two-sided, with no adjustment for multiple testing; p ⩽ 0.05 was considered significant. Geometric mean (GMT) and 95% confidence intervals were computed by taking the exponent of the mean and of the lower and upper limits of the 95% confidence intervals of the loge-transformed titers.
2.7. Construction of antibody landscapes
Antibody landscapes were constructed from pre and post vaccination samples for 32 subjects in the standard TIV and 38 subjects in the LTIV(1/3rd) + Adj study arms in order to compare the magnitude of vaccine response throughout the broader range A/H3N2 antigenic space. The A/H3N2 antigenic map and methodology were used as previously described [19], with methodological updates as detailed in the Supporting online material.
3. Results
3.1. Study population
A total of 98 subjects were enrolled in the initial two study arms, being standard TIV or LTIV(1/3rd) + Adj. After completion of these arms, an additional 15 subjects were subsequently recruited into the LTIV(1/10th) + Adj arm. Baseline characteristics of the study population are shown in Table 1 . Subjects had a median age of 45 years and were predominantly Caucasian (92.8%) with a relatively even split between males (47.2%) and females (52.8%). There were no significant differences between study groups in baseline characteristics or HI titers against vaccine component or related strains.
Table 1.
Subject baseline characteristics.
| All subjects | Standard TIV | LTIV (1/3rd) + Adj | p Value⁎ | LTIV (1/10th) + Adj | |
|---|---|---|---|---|---|
| Number of subjects | N = 109 | N = 48 | N = 50 | N = 11 | |
| Age, median (IQR) | 45(26, 55) | 44(26, 52) | 44.5(23, 55) | n.s. | 51(42, 55) |
| Gender | |||||
| Males, n (%) | 52(48) | 22(45.8) | 23(46) | n.s. | 7(64) |
| Females, n (%) | 57(52) | 26(54.2) | 27(54) | (36) | |
| Race, n (%) | |||||
| Caucasian | 101(92.8) | 46(95.8) | 45(90) | n.s. | 10(90) |
| Asian | 8(7.2) | 2(4.2) | 5(10) | 1(10) | |
| Mean baseline HI titer | |||||
| A/NC/20/99a | 23.4 | 23.6 | 23.8 | n.s. | 22.1 |
| A/SI/3/2006 | 11.4 | 10.8 | 12.1 | n.s. | 11.1 |
| A/W/67/2005a | 16.8 | 20.7 | 15.7 | n.s. | 11.3 |
| A/U/716/2007 | 8.9 | 10.1 | 9.0 | n.s. | 6.4 |
| B/M/2506/2004a | 20.2 | 23.8 | 16.5 | n.s. | 22.6 |
| B/F/4/2006 | 37.3 | 44.4 | 36.6 | n.s. | 24.5 |
Vaccine strains.
Stated p values are for the comparison of baseline characteristics of standard TIV and LTIV(1/3rd) + Adj groups. IQR = interquartile range, n.s = not significant.
3.2. Serological response to TIV immunization
IgM and IgG responses to immunisation were assessed by ELISA day 7 and 21 post-immunization. There were no significant baseline differences in IgM or IgG levels against each of the three vaccine components in the standard TIV and LTIV(1/3rd) + Adj groups (Fig. 1 ). Both vaccine groups demonstrated a significant rise in IgM and IgG at 7 days post-immunization (7 dpv) when compared to baseline levels. As expected, IgM peaked in both groups at 7 dpv and had declined by 21 dpv. By contrast, IgG reached a peak at 21dpv. Similar patterns of IgM and IgG responses were observed for each of the 3 vaccine components. However, LTIV(1/3rd) + Adj was associated with significantly lower IgM at 7dpv and 21 dpv. Nevertheless, with the exception of influenza B titers at 7 dpv, IgG levels were not significantly different between standard TIV and LTIV(1/3rd) + Adj arms.
Fig. 1.
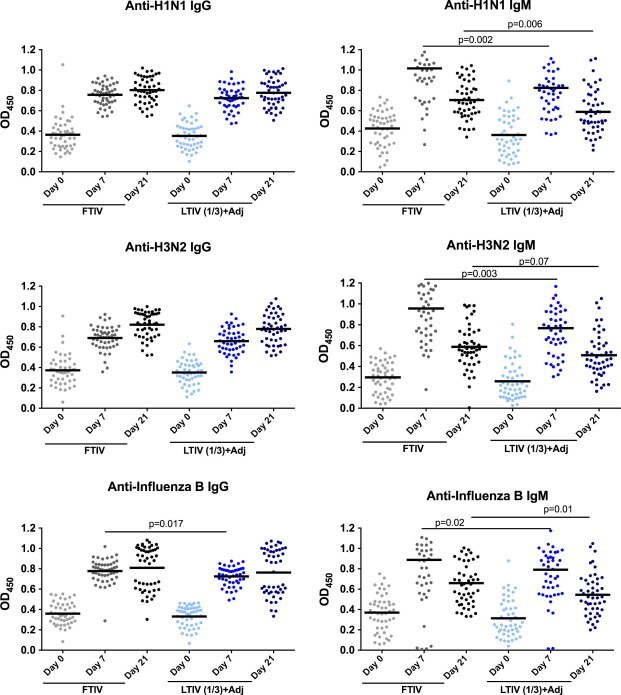
Comparison of pre-, 7-dpv and 21-dpv antibody levels. Anti-influenza IgG and IgM levels by ELISA (mean OD450nm shown as bar) against each of the vaccine strains.
3.3. Haemagglutination inhibition (HI) antibody titers
Whereas ELISA measures total quantity of antibody binding to inactivated influenza antigen, HI assays measure levels of specific functional antibodies that bind to the HA head and prevent its binding to the host sialic acid receptors. There was no difference between groups in baseline mean GMT (Table 1). Both the standard TIV and LTIV(1/3rd) + Adj groups had robust HI responses 3–4 weeks post-immunization and would have passed the European Union Committee for Medicinal Products for Human Use (CHMP) seasonal influenza vaccine criteria for adults 18–60 years old which require seroprotection ⩾70%, seroconversion ⩾40%, and GMT fold rise ⩾2.5. Even the LTIV(1/10th) + Adj group exceeded all the CHMP criteria (Table 2 ). Overall, no significant differences were seen between HI titers in the LTIV(1/3rd) + Adj or LTIV(1/10th) + Adj groups when compared to the standard TIV group.
Table 2.
HI responses 3 weeks post-immunisation to vaccine and variant strains.
| A/H1N1 |
A/H3N2 |
B |
||||
|---|---|---|---|---|---|---|
| A/NC/20/99 | A/SI/3/2006 | A/W/67/2005 | A/U/716/2007 | B/M/2506/2004 | B/Florida 4/2006 | |
| Mean GMT fold increase | ||||||
| Standard TIV | 8.4 | 4.8 | 8.4 | 4.8 | 10.7 | 4.4 |
| LTIV (1/3rd) + Adj | 8.4 | 3.8 | 10.8 | 6.1 | 13.5 | 4.2 |
| LTIV (1/10th) + Adj | 7.7 | 2.9 | 6.7 | 6.1 | 5.6 | 6.1 |
| Seroconversion (%) | ||||||
| Standard TIV | 61% | 50% | 63% | 50% | 63% | 44% |
| LTIV (1/3rd) + Adj | 79% | 50% | 87% | 70% | 85% | 52% |
| LTIV (1/10th) + Adj | 71% | 35% | 65% | 65% | 53% | 65% |
| Seroprotection (%) | ||||||
| Standard TIV | 94% | 72% | 95% | 60% | 96% | 93% |
| LTIV (1/3rd) + Adj | 94% | 62% | 91% | 67% | 100% | 93% |
| LTIV (1/10th) + Adj | 100% | 42% | 76% | 53% | 94% | 94% |
HI responses to vaccine strains are shaded in gray and to variant strains in white. GMT increase shown as fold change relative to baseline.
3.4. Immunity to more recent seasonal virus strains
We next measured HI responses against more recent influenza strains, namely two drifted influenza A strains (A/Solomon Island/3/2006 for A/New Caledonia/20/99, A/Uruguay/716/2007 for A/Wisconsin/77/2005) and a variant B/Yamagata-lineage virus, B/Florida/4/2006, for B/Malaysia/2506/2004 which is a B/Victoria-lineage virus. All vaccine groups showed increases in HI titers against the variant influenza A and B strains although the GMT rise was lower against the more recent influenza A and B strains than the corresponding vaccine strain (Table 2). Notably, both the standard TIV and the LTIV(1/3rd) + Adj but not the LTIV(1/10th) + Adj group, still passed at least 2 of the 3 CHMP criteria for the variant A and B strains.
HI assays were also performed on blood collected 3 months post-immunization to assess antibody persistence in the groups that had received TIV or LTIV(1/3rd) + Adj. HI titers to the three vaccine and three variant strains all remained elevated above baseline at 3 months post-immunization (Fig. 2 ).
Fig. 2.
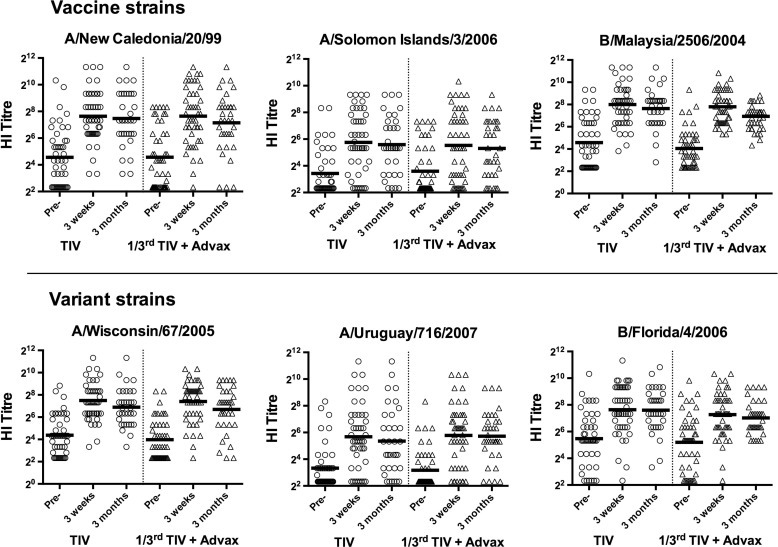
Maintenance of protective HI levels at 3 months post-immunisation. HI titers against vaccine (top figures) and variant (lower figures) strains at baseline, and 3-weeks and 3 months post-immunisation in subjects receiving either standard TIV or LTIV (1/3rd) + Adj.
3.5. Responses across A/H3N2 antigenic space assessed through antibody landscapes
Antibody landscapes have recently been used as a technique to better characterise the full extent of the antibody response induced by influenza infection and/or immunisation and explore such phenomena in detail [19]. Individual antibody landscapes are influenced by the subject’s age and past exposures to influenza infection and/or immunisation, but average group responses can give a robust comparison of the response to different vaccination strains. Although previously used to compare responses between differing vaccine strains, the effect that an adjuvant may have on the global antibody responses to influenza has never been analyzed in this way. We therefore sought to compare in a subset of 70 trial subjects (those aged 60 years or less) the complete HI antigenic map against 81 different H3N2 viruses, with the oldest dating back to the first H3N2 strains to infect humans in 1968. At baseline pre-immunisation HI titers against the 81 H3N2 viruses were similar in the two vaccine groups, with the exception that the standard TIV group had slightly higher levels of reactivity against the very oldest H3N2 strains (Fig. 3 & Supporting online material). When subjects were analyzed by age > or ⩽40 years, marked differences in the pattern of baseline HI titers were seen (Supporting online material). As would be expected due to different exposure histories, in both sets of vaccine recipients the older group showed higher titers against the oldest A/H3N2 strains tested. However, titers against more recent viruses were significantly higher in the ⩽40 year age category – a pattern not so easily attributable to different exposure histories. Notably, subjects >40 years old had a bimodal baseline HI distribution pattern, with the highest baseline HI titers against the very oldest H3N2 isolates and with a second HI peak against influenza isolates from the last 5–10 years. By contrast, subjects <40 years had a unimodal baseline HI distribution with peak recognition of H3N2 isolates from approximately 20 years ago (Supporting online material).
Fig. 3.
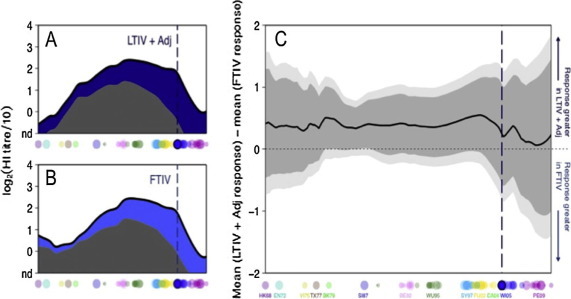
Comparison of antibody landscapes. (A) The mean pre-vaccination and post-vaccination antibody landscapes after vaccination with LTIV (1/3rd) + Adj (32 individuals) or (B) with standard TIV (38 individuals) for each antigenic location on a summary path taken through A/H3N2 antigenic space. In both A and B, the solid black line represents the degree of antibody reactivity measured post-vaccination, gray shows the baseline pre-vaccination average in each group and blue shading shows the additional reactivity at 21 dpv. (C) Comparison of HI titer increase after vaccination with standard TIV or LTIV (1/3rd) + Adj for each position along the antigenic summary path. When above the horizontal midpoint, the black line indicates a higher response in the group vaccinated with LTIV (1/3rd) + Adj; when below the midpoint (not present), it denotes a higher response in the group vaccinated with standard TIV. Data were calculated from the average titer increase between each individual’s paired pre- and post-vaccination titers, with 95% (dark gray) and 99% (light gray) t test-based confidence intervals. The t-test was weighted to consider only those subjects with a pre-titer in the detectable range at any given antigenic point from whom post-pre could therefore be reliably calculated. In each plot, dots along the x axes indicate the antigenic position of the 81 viruses used to generate these landscapes, labelled also by antigenic cluster below panel C. The vertical dotted lines indicate the antigenic position of the vaccine virus, also circled below.
When the change 3 weeks post-immunisation in HI titers was mapped and compared, the differences in response across antigenic space between recipients of either the LTIV(1/3rd) + Adj or standard TIV vaccine did not reach significance. However, there was a trend for recipients of LTIV(1/3rd) + Adj to have higher HI responses across the complete H3N2 antigenic spectrum. When split by age, there was again no significant difference in response between vaccine groups, although the smaller sizes of these group subsets decreased the sensitivity of this analysis.
3.6. Vaccine tolerability and safety
No serious adverse events (SAE) and no unexpected adverse events were observed during the study. Analysis of biochemical and haematological safety tests revealed no clinically relevant changes from baseline in any of the study groups (data not shown). The number of solicited systemic adverse effects was not significantly different in the standard TIV alone group (21 events) compared to the LTIV(1/3rd) + Adj group (15 events) (Table 3 ). There was a non-significant trend to higher rates of fever/pyrexia and arthralgia in the standard TIV group, which could reflect its 3-fold higher RNA content and hence higher TLR7 agonist activity [20]. When frequencies of solicited local reactions were compared between groups, both standard TIV and LTIV(1/3rd) + Adj were extremely well tolerated with a low rate of local reactions. Other local reactions (redness, swelling) had frequencies of less than 6% and were not significantly different between groups. No grade 3 local reactions were reported.
Table 3.
Listing of systemic adverse events.
| Systemic AEs | Standard TIV n = 48 | LTIV (1/3rd) + Adj n = 50 | p Valueb | LTIV (1/10th) + Adj n = 11 |
|---|---|---|---|---|
| Solicited AE – n (%) | ||||
| Headache | 7(14.6) | 7(14.0) | 1.0 | 1(9.0) |
| Fever/chills | 4(8.3) | 1(2.0) | 0.2 | 1(9) |
| Fatigue | 4(8.3) | 3(6.0) | 0.71 | 1(9) |
| Myalgia | 2(4.2) | 3(6.0) | 1.0 | 0(0) |
| Arthralgia | 3(6.3) | 0(0.0) | 0.11 | 0(0) |
| Nausea | 0(0) | 1(2.0) | 1.0 | 1(9) |
| Total n (%) | 20(42%) | 15(30%) | 0.29 | 4(36%) |
| Unsolicited AE – n (%) | ||||
| Diarrhoea | 1(2.1) | 0(0) | 1.0 | 0(0) |
| URTI | 7(14.6) | 6(12.0) | 0.77 | 0(0) |
| Cough | 1(2.1) | 0(0) | 1.0 | 1(9) |
| Othera | 3(6.3) | 2(4.0) | 0.67 | 0(0) |
Other systemic adverse events included conjunctivitis, earache, rash, itch, hayfever, chest pain and an infected tooth.
Standard TIV and LTIV (1/3rd) + Adj groups compared by Fishers exact test.
4. Discussion
The current study was undertaken to assess the safety and dose-sparing capability of Advax, a novel delta inulin-based polysaccharide adjuvant. This was the first time Advax adjuvant was tested in humans with a seasonal TIV vaccine. Vaccine formulated with Advax adjuvant remained immunogenic even when reduced to 1/3rd or even 1/10th the standard TIV dose. Whilst HI titers are regarded as the best predictor of influenza protection, ELISA assays provide an opportunity to explore effects on antibody isotypes. Interestingly, although LTIV(1/3rd) + Adj was associated with significantly lower anti-influenza IgM levels at both 7- and 21-dpv, this did not translate into lower IgG or HI titers. As LTIV without adjuvant was not included as a study arm, it is not possible to say whether the lower IgM response might have been due to the lower vaccine dose and/or the presence of the Advax adjuvant. Previous analysis of vaccination responses across antigenic space through the use of antibody landscapes revealed that a large part of the antibody response to influenza vaccination appears to be a result of surprisingly extensive recall of prior immunity, termed “backboosting” [19]. This study corroborates these findings, with detectable vaccination responses reaching back all the way to the very oldest influenza A/H3N2 strains in both study groups. It was previously unknown what the effect of an adjuvant would be on such phenomena, whether it would for example favor responses against novel or future strains or disproportionately increase the backboosting effect and responses against older strains. The findings from this study indicate that neither is strongly favored and all responses were maintained by the addition of adjuvant despite a reduced antigen dose. The baseline pre-immunisation antibody landscapes, clearly demonstrated the impact of past influenza exposures on each individual’s pattern of HI titers, with older subjects most likely to recognise the oldest H3N2 variants. Why older individuals should have lower baseline titers against more recent strains is less clear however and may represent the decay of antibody titers over time. Indeed, some sera from elderly individuals showed very little detectable antibody reactivity to any of the 81 strains, despite having lived through four decades of A/H3N2 circulation. The fact that no differences were seen between age groups is reassuring support of the efficacy of the LTIV + Adj but may also be influenced by the lack of very elderly individuals in the study. As such it would be informative to use antibody landscapes to examine the effect of adjuvanted vaccines in such recipients, where phenomena such as immune-senescence may play a larger role.
Enhanced vaccine immunogenicity or antigen-sparing should not be at the expense of tolerability or safety. The only currently approved adjuvanted TIV vaccines contain squalene oil emulsion formulations (MF59 adjuvant) approved for use in the elderly [21]. TIV vaccines containing MF59 adjuvant have been extensively studied and shown to enhance vaccines immunogenicity in both the elderly and in young children. Whilst MF59 adjuvant has a well recognised propensity to cause modestly increased injection site pain and muscle aches, meta-analyses have confirmed its positive safety profile [22]. This includes a lack of association of MF59-adjuvanted vaccines with an increased risk of narcolepsy in immunised subjects [23], a finding of high importance given the increased narcolepsy seen in children immunized with the H1N1/2009pdm vaccine (Pandemrix®, GSK, Belgium) adjuvanted with AS03, a squalene-based adjuvant that also contains tocopherol [24]. Despite the well-demonstrated benefits of adjuvants for vaccine immunogenicity, the high level of community apprehension regarding adjuvant safety needs to be acknowledged and considerable consumer education is likely to be required to successfully introduce any new adjuvant into the seasonal influenza market.
Advax is made from the delta isoform of inulin, a sugar that has been used intravenously for over 80 years for testing of kidney function [25]. Reassuringly, the frequency of systemic adverse effects was not significantly different in the LTIV(1/3rd) + Adj when compared to the standard TIV group. The mechanism of action of Advax adjuvant remains under intense study, with current evidence pointing toward a non-inflammatory enhancement of antigen presenting cell function as the means by with it enhances vaccine action [26].
The current study has limitations due to its being a Phase 1 study primarily conducted to establish the safety of Advax adjuvant when combined with seasonal influenza vaccine. The study did not assess long-term vaccine seroprotection, which requires at least 6–12 months of follow-up. The study design did not include control groups at the reduced 1/3rd or 1/10th TIV doses without adjuvant. However, a previous study of an intradermally administered vaccine containing 6 μg HA g (2/5th standard dose) or 3 μg HA (1/5th standard dose) induced inferior HI responses to a standard 15 μg HA vaccine [27], suggesting that the good immunogenicity seen in our study even at the 1/10th TIV + Advax dose, was likely to be the effect of the adjuvant. Larger studies with reduced TIV alone control groups will be required to exactly quantitate Advax’s antigen-sparing effects. Another limitation is that measurement of HI responses is not a perfect predictor of influenza protection. influenza-specific CD4 T cells have recently being shown in human studies to be predictive of influenza protection [28], [29]. It will be important to measure the effect of Advax adjuvant on T-cell responses in future studies.
This first-in-man Phase 1 study confirmed Advax adjuvant combined with a seasonal influenza vaccine was well tolerated and safe in human subjects. Planned future studies will assess the benefits of Advax adjuvant for seasonal influenza vaccines in elderly high-risk subjects as well as for use in pandemic influenza vaccines.
Acknowledgements
Development of Advax adjuvant was supported by Contracts U01-AI061142 and HHSN272200800039C from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, United States. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. We thank the clinical trial coordinators and research staff including Sharen Pringle and Kylie Bragg for their expert assistance. We thank Dr. Sue Heinzel for her contribution to study planning and implementation. We thank all the study subjects for their participation. We thank WHO Collaborating Centre for Reference and Research on Influenza Melbourne for supplying influenza typing kits.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.05.071.
Appendix A. Supplementary material
References
- 1.Fleming D.M., Elliot A.J. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005;23(Suppl 1):S1–S9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 → 1] poly(fructo-furanosyl) alpha-d-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper P.D., Barclay T.G., Ginic-Markovic M., Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1164–1174. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper P.D., Barclay T.G., Ginic-Markovic M., Gerson A.R., Petrovsky N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydr Polym. 2014;103:392–397. doi: 10.1016/j.carbpol.2013.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larena M., Prow N.A., Hall R.A., Petrovsky N., Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8+ T cells and pre-exposure neutralizing antibody. J Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristillo A.D., Ferrari M.G., Hudacik L., Lewis B., Galmin L., Bowen B. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33:1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 11.Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin Vaccine Immunol. 2014;21:580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Layton R.C., Petrovsky N., Gigliotti A.P., Pollock Z., Knight J., Donart N. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda-Okubo Y., Kolpe A., Li L., Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda-Okubo Y., Ong C.H., Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine. 2015;38:4892–4900. doi: 10.1016/j.vaccine.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M., Heinzel S. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012;30:5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. Sep 27. pii: S0264-410X(14)01293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heddle R., Russo P., Petrovsky N., Hanna R., Smith A. Immunotherapy – 2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. World Allergy Organ J. 2013;6:P158. [Google Scholar]
- 18.Honda-Okubo Y., Saade F., Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonville J.M., Wilks S.H., James S.L., Fox A., Ventresca M., Aban M. Antibody landscapes after influenza virus infection or vaccination. Science. 2014;346:996–1000. doi: 10.1126/science.1256427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama S., Ishii K.J., Kumar H., Tanimoto T., Coban C., Uematsu S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 21.De Donato S., Granoff D., Minutello M., Lecchi G., Faccini M., Agnello M. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17:3094–3101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 22.Frey S., Poland G., Percell S., Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21:4234–4237. doi: 10.1016/s0264-410x(03)00456-0. [DOI] [PubMed] [Google Scholar]
- 23.Tsai T.F., Crucitti A., Nacci P., Nicolay U., Della Cioppa G., Ferguson J. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis. 2011;43(9):702–706. doi: 10.3109/00365548.2011.580777. [DOI] [PubMed] [Google Scholar]
- 24.Nohynek H., Jokinen J., Partinen M., Vaarala O. AS03 Adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price M. Inulin clearance as a screening test for kidney function. Arch Phys Med Rehabil. 1974;55:522–523. [PubMed] [Google Scholar]
- 26.Petrovsky N., Cooper P.D. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frenck R.W., Jr., Belshe R., Brady R.C., Winokur P.L., Campbell J.D., Treanor J. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone(R)) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29:5666–5674. doi: 10.1016/j.vaccine.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altenburg A.F., Rimmelzwaan G.F., de Vries R.D. Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine. 2015;33:500–506. doi: 10.1016/j.vaccine.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson T.M., Li C.K., Chui C.S., Huang A.K., Perkins M., Liebner J.C. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


