Summary
Chlamydiae comprise important pathogenic and symbiotic bacteria that alternate between morphologically and physiologically different life stages during their developmental cycle. Using electron cryotomography, we characterize the ultrastructure of the developmental stages of three environmental chlamydiae: Parachlamydia acanthamoebae, Protochlamydia amoebophila and Simkania negevensis. We show that chemical fixation and dehydration alter the cell shape of Parachlamydia and that the crescent body is not a developmental stage, but an artefact of conventional electron microscopy. We further reveal type III secretion systems of environmental chlamydiae at macromolecular resolution and find support for a chlamydial needle-tip protein. Imaging bacteria inside their host cells by cryotomography for the first time, we observe marked differences in inclusion morphology and development as well as host organelle recruitment between the three chlamydial organisms, with Simkania inclusions being tightly enveloped by the host endoplasmic reticulum. The study demonstrates the power of electron cryotomography to reveal structural details of bacteria–host interactions that are not accessible using traditional methods.
Introduction
All chlamydiae share an obligate intracellular life style and depend on a eukaryotic host for replication (Horn, 2008). Chlamydial ancestors adapted to this life inside a host more than 700 million years ago probably thriving in ancient protists (Horn and Wagner, 2004; Horn et al., 2004; Kamneva et al., 2012; Subtil et al., 2013). For a long time Chlamydiae were thought to consist of only human and certain animal pathogens (the Chlamydiaceae). In the past two decades, a novel class of ‘environmental’ chlamydiae have been identified in contaminated cell cultures, in an aborted bovine fetus, in fish gills, and as symbionts of arthropods and amoebae (Rourke et al., 1984; Michel et al., 1994; Kahane and Friedman, 1995; Amann et al., 1997; Rurangirwa et al., 1999; Fritsche et al., 2000; Horn et al., 2000; Draghi et al., 2004; Kostanjsek et al., 2004; Thomas et al., 2006; Karlsen et al., 2008). While pathogenic chlamydiae are a homogeneous phylogenetic group, the genomes of environmental chlamydiae are more diverse (Bertelli et al., 2010; Collingro et al., 2011). It is still unclear to what extent this genomic variation manifests as variations in cell structure.
The biphasic chlamydial life cycle starts with the infection of a host cell by the elementary body (EB) (Horn, 2008). After uptake by the host, the EB resides inside a host-derived vacuole (termed ‘inclusion’) (Hackstadt et al., 1997) and differentiates into a reticulate body (RB), the replicative developmental stage. The RB then divides several times by binary fission before redifferentiating into EBs, which leave the host cell by lysis or exocytosis to start a new round of infection (Abdelrahman and Belland, 2005; Hybiske and Stephens, 2007; Horn, 2008). An additional infectious developmental stage, the sickle-shaped crescent body, was reported for a number of environmental chlamydiae (Greub and Raoult, 2002; Lamoth and Greub, 2010; Nakamura et al., 2010).
Once inside their host, chlamydiae perturb the organelle organization of the host cell in various ways. Chlamydia trachomatis inclusions, for instance, cause fragmentation of the Golgi (Heuer et al., 2009), which facilitates the acquisition of cholesterol and sphingomyelin (Hackstadt et al., 1995; Carabeo et al., 2003). They also recruit the host’s rough endoplasmic reticulum (rER), eventually resulting in a translocation of rER proteins into the inclusion (Dumoux et al., 2012). Mitochondria are recruited to the inclusions of C. psittaci and Waddlia chondrophila (Friis, 1972; Peterson and de la Maza, 1988; Matsumoto et al., 1991; Croxatto and Greub, 2010).
Internalization, inclusion development and host-organelle recruitment are all mediated by the secretion of effector proteins into the inclusion membrane and/or host cytoplasm by the type III secretion (T3S) system (Peters et al., 2007). While the genes that encode this needle-like secretion system are present in all chlamydial genomes (Collingro et al., 2011), T3S structures have not been seen in environmental chlamydiae, and few structural details are known about the T3S systems of pathogenic chlamydiae (Matsumoto, 1979; Nichols et al., 1985; Dumoux et al., 2012).
Studying chlamydial cell biology is challenging because of their obligate intracellular lifestyle and the lack of routine genetic tools (Binet and Maurelli, 2009; Kari et al., 2011; Wang et al., 2011; Nguyen and Valdivia, 2012). While many insights have come from conventional electron microscopy (EM) studies, the chemical fixation, dehydration, plastic embedding, thin sectioning and heavy-metal staining involved can lead to membrane artefacts, misleading representations of the nucleoid structure or loss of entire cellular components (Pilhofer et al., 2010). Here, we investigated three environmental chlamydiae, Protochlamydia amoebophila, Parachlamydia acanthamoebae and Simkania negevensis, by electron cryotomography (ECT), which allows cells to be imaged in a near-native, ‘frozen-hydrated’ state. This approach revealed not only new structural details of these obligate intracellular bacteria at macromolecular resolution and in three dimensions but also provided new perspectives on the bacteria-host interface.
Results
Developmental stages of environmental chlamydiae
To investigate the ultrastructure of isolated cells, chlamydiae were purified from amoeba cultures, plunge-frozen on EM grids and imaged intact. Twenty-five, twenty and twenty tomograms were collected on purified Simkania, Parachlamydia and Protochlamydia cells respectively. EBs and RBs could be distinguished by their size, morphology and the granularity of their cytoplasm. EBs were coccoid and had diameters of 450 nm (Simkania, ±22, n = 9), 678 nm (Parachlamydia, ±32, n = 5) and 625 nm (Protochlamydia, ±170, n = 5) (Fig. 1A, D and G). RBs were more pleomorphic and larger (667 nm, Simkania, ±46, n = 5; 838 nm, Parachlamydia, ±135, n = 4; 884 nm, Protochlamydia, ±88, n = 6) (Fig. 1C, F and I). EBs exhibited regions of concentrated filamentous material (presumably condensed DNA), with different texture from the rest of the EB cytoplasm. Smaller but otherwise similar regions were also occasionally seen in RBs. Because of an irregularly shaped outer membrane, the thickness of the RB periplasm was more variable than that seen in EBs. Large numbers of ribosomes were found throughout the cytoplasm of both EBs and RBs except in the region of the putatively condensed DNA within EBs. We also observed cells with features characteristic of both developmental stages (Fig. 1B, E and H), including multiple small patches of condensed DNA, probably representing intermediate stages in the process of differentiation or redifferentiation.
Fig. 1.
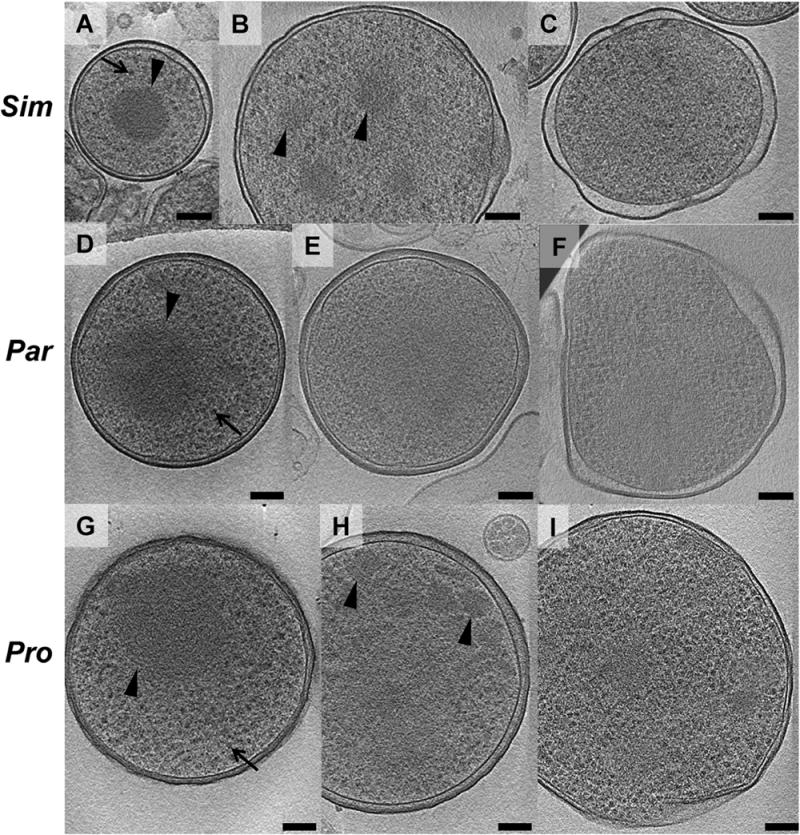
Developmental stages of environmental chlamydiae. Simkania (Sim, A–C), Parachlamydia (Par, D–F) and Protochlamydia (Pro, G–I) cells were purified from asynchronously infected amoeba cultures, plunge-frozen and imaged by ECT. EBs (A, D, G) and RBs (C, F, I) were identified by differences in cell size, cell shape, thickness of periplasm and cytoplasmic granularity. EBs had a smaller diameter, a spherical shape, a uniformly thin periplasm, a condensed nucleoid (arrowheads) and a large number of ribosomes (arrows). RBs had a larger cell size, a polymorphic shape, a periplasm with varying thickness, a wavier outer membrane and a large number of ribosomes. Intermediate stages are shown in B, E, H. Shown are slices through cryotomograms. Bar, 100 nm.
Previous studies using conventional EM have reported that some chlamydiae exhibit crescent shapes, and these ‘crescent bodies’ were suggested to represent an infectious life stage of Simkania, Parachlamydia and Protochlamydia (Greub and Raoult, 2002; Lamoth and Greub, 2010; Nakamura et al., 2010). Surprisingly, while our tomograms of intact as well as cryosectioned cells (see later) allowed for the identification of EBs, RBs and intermediate stages (Fig. 1), we never saw any crescent bodies (Fig. 1, Fig. 2A and B, Figs 3–5). We therefore explored if crescent bodies could be an artefact of chemical fixation and dehydration/embedding.
Fig. 2.
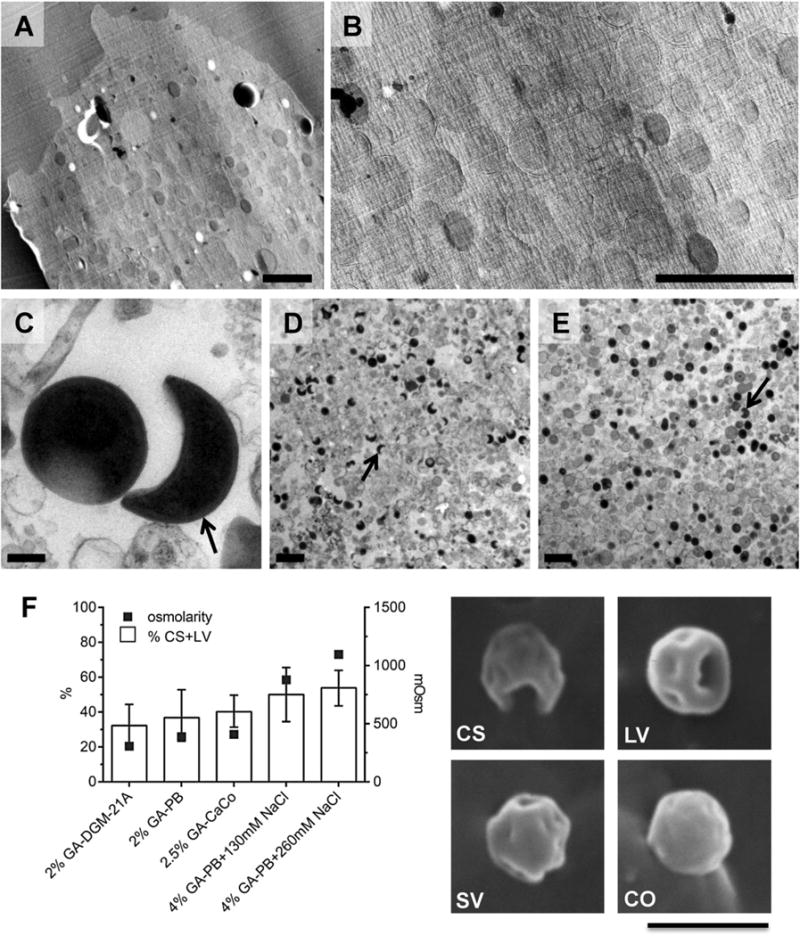
Crescent bodies are not a developmental stage but rather artefacts of conventional EM. Crescent bodies were not observed in cryotomograms of either plunge-frozen (Fig. 1, Movie S1) or cryosectioned cells (Figs 3 and 4). A two-dimensional overview image of an infected amoeba cell only showed roundish structures, representing chlamydial cells or mitochondria (A, B enlarged). In contrast, when purified Parachlamydia cells were fixed, dehydrated, plastic-embedded and imaged as in Greub and Raoult (2002), crescent bodies (C, D arrows) were observed frequently (47% ± 10). The use of a low-osmolarity, low-fixative concentration buffer resulted in far fewer (2% ± 2) crescent bodies (E). Higher osmolarity and higher fixative concentration also resulted in higher percentages of crescent bodies or cells with large invaginations as observed by scanning electron microscopy (F); the graph displays the percentage of purified Parachlamydia cells forming crescent shapes or large invaginations after fixation with different buffers (error bars indicate the 95% confidence intervals of percentages; buffer osmolarity is indicated on the right vertical axis). Representative scanning electron microscopy images of purified Parachlamydia cells with different degrees of invagination are shown. CS, crescent shape; LV, large invaginations; SV, small invaginations; CO, coccoid; GA, glutaraldehyde; PB, phosphate buffer; CaCo, cacodylate buffer. Bars 2 μm (A, B, D, E, F) or 200 nm (C).
Fig. 3.
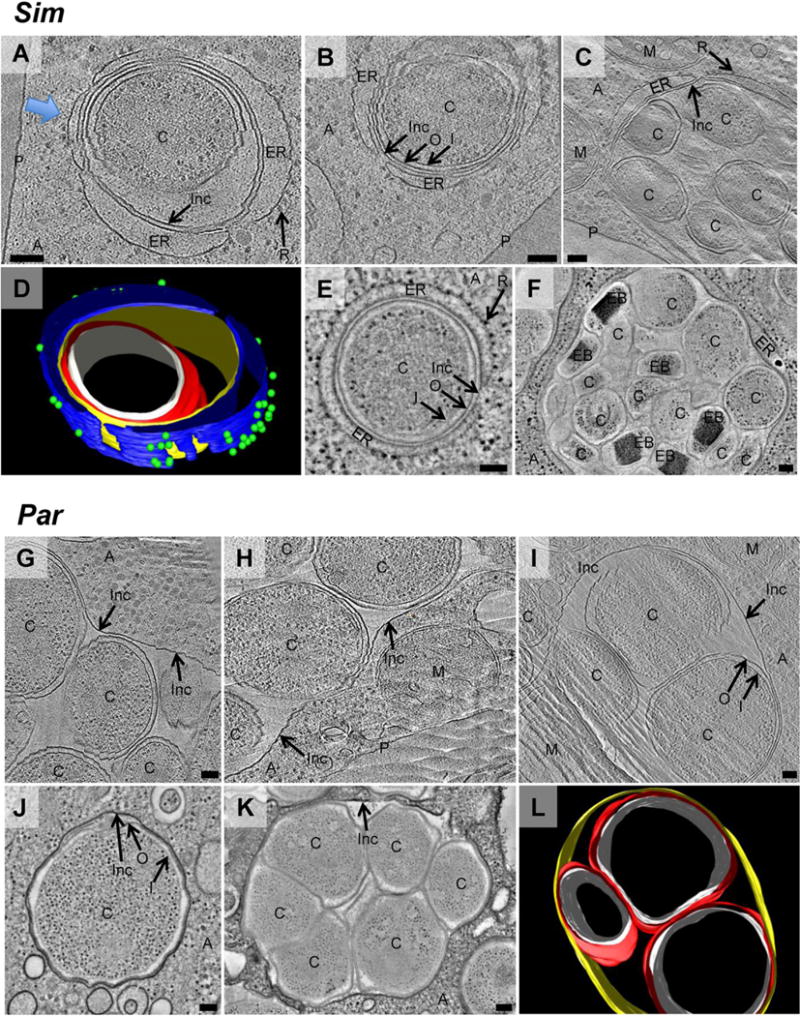
Simkania (Sim) and Parachlamydia (Par) form multicellular inclusions, but only Simkania inclusions recruit the host endoplasmic reticulum. To investigate Simkania (A–F) and Parachlamydia (G–L) inside their host, infected amoeba cultures were high-pressure-frozen, cryosectioned and imaged by ECT (cryotomographic slices are shown in A–C, G–I). For comparison, another sample was high-pressure-frozen, freeze-substituted, plastic-embedded, stained and imaged at room temperature (tomographic slices are shown in E, F, J, K). Simkania and Parachlamydia cells ‘C’ were found inside unicellular (A, B, E, J) and multicellular (C, F, G, H, K, L) inclusions. Inc, inclusion membrane; I, chlamydial inner membrane; O, chlamydial outer membrane; P, amoeba plasma membrane; A, amoeba cytoplasm; M, mitochondrion. Every Simkania inclusion (A–F) was observed in close association with cisternae-like ribosome (‘R’)-studded structures, likely host rER (‘ER’). The host ER almost entirely enveloped the inclusion [three-dimensional (3D) model of A in D, blue arrow indicating viewing direction; see also Movie S2]. Note that membranes in close proximity (e.g. Inc and rER membrane in E) cannot be identified as two separate membranes in conventional EM images (E, F). L is a 3D model of I (Movie S3). Colours in models: white ‘I’, red ‘O’, yellow ‘Inc’, blue ‘ER’, green ‘R’. Bars, 100 nm.
Fig. 5.
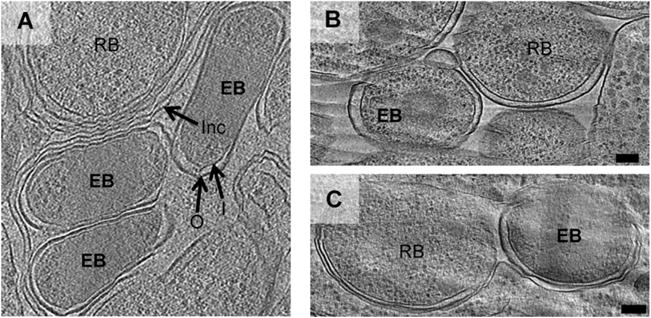
Simkania EBs show an elongated cell shape in densely packed inclusions inside the host cell. A–C show cryotomographic slices of cryosectioned amoebae infected with Simkania, Parachlamydia or Protochlamydia in which EBs could be identified by the granularity of the cytoplasm. In contrast with the coccoid shape of purified EBs (Fig. 1), Simkania EBs inside host cells (A and Fig. 3F) were sometimes elongated. Protochlamydia and Parachlamydia EBs were always coccoid, both purified (Fig. 1) and inside amoebae (B and C respectively). Bars, 100 nm.
Parachlamydia cells were purified from asynchronous amoeba cultures and split into two aliquots. One sample was processed with procedures similar to the original study describing crescent bodies (Greub and Raoult, 2002): cells were fixed with glutaraldehyde and osmium tetroxide, dehydrated, plastic-embedded, thin-sectioned, stained and imaged at room temperature. Crescent bodies made up 47 ± 10% of all putative chlamydial cells (n = 813) and had the typical shape and dimensions reported previously (Greub and Raoult, 2002) (arrows in Fig. 2C and D). The second sample was treated in the same way, except that the osmolarity and fixative concentration in the fixation buffer was reduced. Crescent bodies were still present but only at a frequency of 2 ± 2% (n = 1355) (Fig. 2E). Osmolarity and fixative concentration therefore influence the abundance of crescent bodies. This was further supported by scanning EM (SEM) of purified chlamydial cells, where buffers with higher osmolarity and higher fixative concentration also resulted in an increased percentage of crescent bodies (Fig. 2F).
To distinguish between the effects of fixation and dehydration/plastic embedding, two further aliquots of purified cells were cryopreserved (i.e. plunge-frozen) and imaged in a frozen-hydrated state in an electron cryomicroscope. No crescent bodies were found in projection images of 584 cells, which were directly plunge-frozen after purification (Movie S1). When cells were fixed with glutaraldehyde and osmium tetroxide before plunge-freezing, crescent bodies were still absent (data not shown). Lysed cells were observed in some cases, but none of those had a typical crescent shape or continuous intact membranes, which are characteristic for crescent bodies. We conclude that crescent bodies are an artefact of the combined effect of chemical fixation and dehydration/embedding.
Architecture of inclusions
Next chlamydiae were imaged inside their host. Because ECT is limited to thin (less than ~500 nm) samples, asynchronously infected amoeba cultures were pelleted, mixed with cryoprotectant, high-pressure frozen, sectioned at cryotemperatures (150 nm section thickness) and then imaged. Twenty-one, four and twenty-seven tomograms were collected of vitreous cryosections of Simkania-, Parachlamydia- and Protochlamydia-infected amoebae respectively. For comparison, we also collected tomograms of parallel samples prepared by high-pressure freezing, freeze-substitution, plastic-embedding, thin-sectioning and staining.
First, the localization of chlamydiae inside their host was investigated. Intracellular bacterial cells were always seen surrounded by an inclusion membrane and never directly in the cytoplasm (Figs 3 and 4, and Movies S2, S3 and S4). Single-celled inclusions and inclusions packed with up to 17 chlamydial cells were observed in amoebae infected with Simkania (Fig. 3A–F) or Parachlamydia (Fig. 3G–L). RBs were the predominant stage within the inclusions, some of them dividing by binary fission. In contrast, 92% (n = 52) of the inclusions in amoebae infected with Protochlamydia contained a single bacterial cell (Fig. 4A, D and E). The 8% of inclusions harbouring more than one bacterium were not roundish and tightly packed with bacteria, as seen for Parachlamydia and Simkania (Fig. 3); they rather appeared like inclusions in the process of starting separation, with membranes contracting in between the bacteria (Fig. 4B and C), or in a stage where two single-cell inclusions were connected by a narrow membrane tube (the percentage of single-cell inclusions in all cases might actually be slightly lower than noted, as extensions of the inclusions above and below the section cannot be visualized).
Fig. 4.
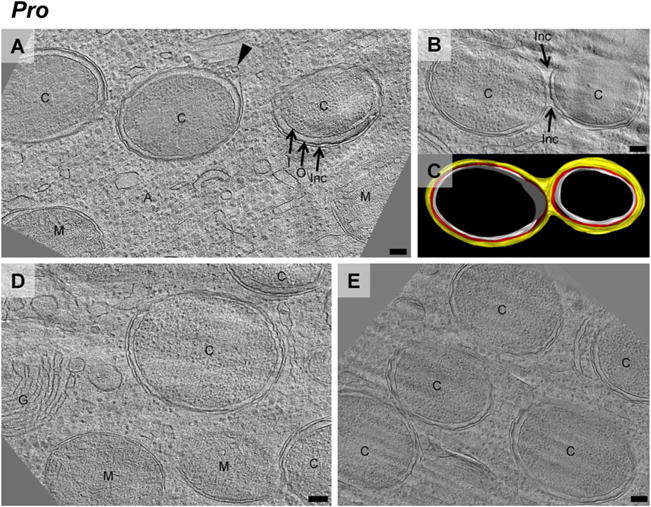
Protochlamydia (Pro) inclusions divide with chlamydial cells. (A, B, D, E) are cryotomographic slices of cryosectioned amoeba cells infected with Protochlamydia. In contrast with Simkania and Parachlamydia, Protochlamydia cells ‘C’ were found only inside unicellular inclusions, or in bicellular inclusions that were either in the process of division or fusion. Inc, inclusion membrane; I, chlamydial inner membrane; O, chlamydial outer membrane; P, amoeba plasma membrane; A, amoeba cytoplasm; G, golgi; M, mitochondria. In some cases, the inclusion membrane showed budding vesicles (arrowhead), suggesting more active inclusion membrane dynamics than in Simkania or Parachlamydia. C is a three-dimensional-model of B (white ‘I’, red ‘O’, yellow ‘Inc’; Movie S3).
Interestingly, we observed a difference in cell shape between Simkania EBs imaged inside densely packed inclusions within their host versus after purification. While EBs imaged inside these inclusions were frequently rod-shaped or elongated (cells labelled ‘EB’ in Figs 3F and 5A), purified EBs were always spherical (Fig. 1A). Parachlamydia and Protochlamydia EBs, in contrast, were always coccoid (Fig. 1D and G, and cells labelled ‘EB’ in Fig. 5B and C). RBs of all species had a somewhat polymorphic, spherical shape inside the host cell.
Recruitment of ER by Simkania
Chlamydiae associate not only with the inclusion membrane, but some also recruit and reshape entire host organelles. We found that inclusions of Simkania were always enveloped by an additional membranous structure (Fig. 3A–F). The granularity inside the cisternae-like membrane sacs was different from the rest of the eukaryotic cytoplasm, suggesting that they were part of a separate compartment. The endoplasmic reticulum (ER)-like membrane architecture and the presence of many ribosomes on the cytoplasmic side of the distal membrane identified the compartment as rER. Segmentations showed that the inclusions were not entirely surrounded by the rER, however, leaving small patches of direct connections between inclusion and amoeba cytoplasm (Fig. 3D). rER was found associated with single-cellular (Fig. 3A, B and E) and multicellular inclusions filled with EBs and RBs (Fig. 3C and F), indicating that ER recruitment occurs early after internalization and remains throughout the intracellular stage.
In contrast with Simkania, no direct association of Parachlamydia or Protochlamydia inclusions and any host organelle was detected. Mitochondria were occasionally observed in their vicinity (Figs 3G–L and 4A–E), but a specific co-localization was not suggested by fluorescent labelling of mitochondria (Fig. S1).
Secretion systems
Translocation of chlamydial effector proteins into the inclusion membrane and into the host cytoplasm is crucial for chlamydiae to shape their intracellular environment. In a cryotomogram of a Simkania EB, we observed a structure with characteristics typical of a T3S apparatus (Fig. 6A and B) (Marlovits et al., 2004). A density in the periplasm was found to be similar to T3S basal bodies and connected to an extracellular needle-like structure. The needle (length 63 nm, diameter 9 nm) seemed to be engaged with a membranous structure, possibly a remnant of the host cell. The dimensions of the apparatus were similar to projections seen on the surface of infectious C. psittaci cells (Matsumoto, 1979). Interestingly, the otherwise relatively narrow distance between the inner and outer membrane (13 nm) in Simkania EBs required a bulging (41 nm) of the cytoplasmic membrane to accommodate the basal body. Such widening of the periplasm was observed frequently in the same and other EBs (Figs 6A and S2). Basal body-like densities inside these bulges indicate that they likely represented T3S structures as well, but the corresponding needles were probably sheared off during purification. A pronounced widening of the periplasmic space was also reported for T3S structures of C. trachomatis (Dumoux et al., 2012).
Fig. 6.
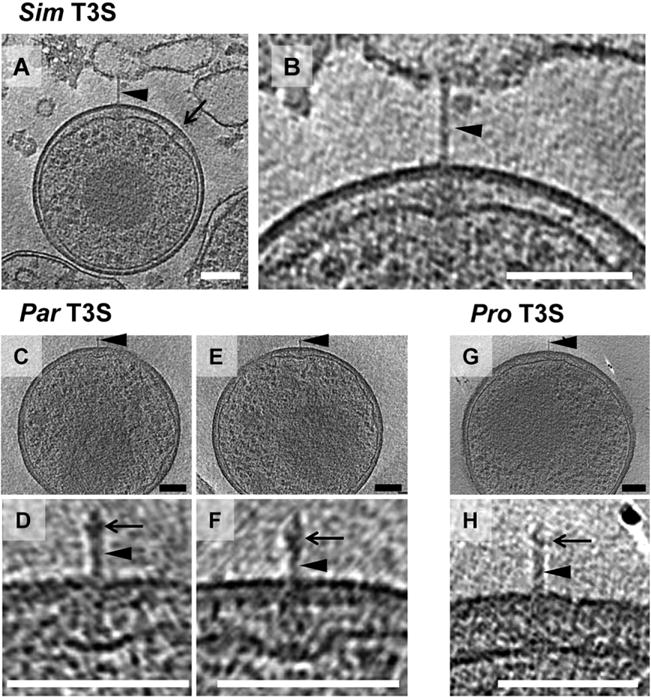
Type III secretion systems. Shown are slices through cryotomograms of purified Simkania (Sim) (A and B enlarged), Parachlamydia (Par) (C, E and D, F enlarged) and Protochlamydia (Pro) (G and H enlarged) EBs. All species show T3S structures (arrowheads in A–H). A pronounced widening of the periplasm was observed in Simkania (B) and Parachlamydia (D, F) to accommodate the T3S basal body. Often, such widening with periplasmic densities was observed in the absence of a needle in Simkania EBs (arrow in A and Fig. S2), suggesting that some needles are sheared off during purification. Parachlamydia and Protochlamydia T3S needles exhibited a bulge (arrows in D, F, H) where needle-tip proteins have been found in other bacteria.
Putative T3S systems in Parachlamydia showed a similar bulging of the periplasm in the region of the basal body (36–44 nm rather than 17 nm) (Fig. 6C–F). The needle structure was substantially different compared with Simkania, with a length of 38–42 nm, a diameter of 6–7 nm and a widening (12 nm) of the needle 7 nm from its tip (Fig. 6D and F). A T3S-like structure found on a Protochlamydia EB comprised a 52-nm-long, 7-nm-diameter needle, and may have also had a widening close to the needle tip (Fig. 6G and H). Periplasmic bulging was not observed, however, as the width of the periplasm in Protochlamydia was already ~40 nm.
Discussion
EBs and RBs in a life-like state
Early conventional EM studies suggested that the DNA in EBs is condensed (Moulder, 1966), which was later found to be mediated by histone-like proteins (Barry et al., 1992). However, nucleoid structure in particular is prone to artefacts introduced by fixation, dehydration and staining in conventional EM (Pilhofer et al., 2010). While a more recent ECT study imaged C. trachomatis cells preserved in a near-native, frozen-hydrated state, such ultrastructural details were unfortunately not resolved probably due to instrumental limitations (Huang et al., 2010). Again plunge-freezing cells, but using higher electron energies and energy filtration, here we have confirmed that EB genomes are indeed densely packed. Our finding of ribosomes in EBs is consistent with the notion that they are metabolically active to some degree (Haider et al., 2010; Sixt et al., 2013) rather than completely dormant (Hatch et al., 1985). Besides size differences of EBs and RBs not observed before for environmental chlamydiae, another distinguishing feature between the developmental stages we noted was the variable periplasmic width in RBs. This is consistent with the notion that lower abundances of stabilizing cysteine-rich proteins in RBs result in more flexible outer membranes (Hatch et al., 1986). Similarly, the more flexible shape and deformation of Simkania EBs inside host cells compared with Protochlamydia and Parachlamydia might be a consequence of differences in cell envelope architecture, such as the absence of the cysteine-rich proteins that Simkania lacks in contrast with all other chlamydiae (Collingro et al., 2011).
Crescent bodies are an artefact
While crescent-shaped cells had been seen previously and thought to represent a distinct developmental stage (Greub and Raoult, 2002; Lamoth and Greub, 2010; Nakamura et al., 2010), here we showed that they are artefacts of conventional EM methods. While no crescent bodies have been reported for the pathogenic Chlamydiaceae, EBs with peculiar stellate outlines were found occasionally. The previous hypothesis that this morphology could be attributed to EM preparation methods as well (Matsumoto, 1988) is supported by our results. The reason for crescent bodies not being observed in Chlamydiaceae could be differences in their outer membrane protein composition compared with environmental chlamydiae (Heinz et al., 2009; Collingro et al., 2011), leading to different effects during chemical fixation and dehydration/embedding.
Trapezoidal, dumbbell-shaped and elongated intracellular Simkania EBs have also been described in the past (Kahane et al., 2001; Michel et al., 2005; Henning et al., 2007). While we found elongated morphologies especially in cells tightly packed in inclusions, trapezoidal and dumbbell-shaped forms were never seen, suggesting that those are also artefacts. Conventional EM studies of other environmental chlamydiae EBs have reported head-and-tail, star and rod shapes (Kostanjsek et al., 2004; Karlsen et al., 2008; Lienard et al., 2011). It remains unclear whether these morphologies are natural.
Shapes of bacteria from other phyla have also been reported to be affected by chemical fixation and dehydration. For instance, crescent-shaped cells were observed for Gemmata obscuriglobus (Lindsay et al., 1995), a member of the chlamydial sister-phylum Planctomycetes, and the mollicute Acholeplasma ladlawii (Lemcke, 1972), showing that fixation conditions must be chosen carefully to preserve the cell shape and that the description of new shapes based on fixed cells should be handled with caution.
Diversity of the intracellular niche of environmental chlamydiae
Most chlamydiae are known to reside inside the host-derived membranous inclusion after host cell invasion (Hackstadt et al., 1997), but Parachlamydia and Simkania have also been reported to be localized directly in the cytoplasm (Michel et al., 1994; Greub and Raoult, 2002). Here, intracellular chlamydiae were always seen surrounded by an inclusion membrane, supporting the importance of this host-bacterium interface for intracellular survival and replication. Inclusions in Protochlamydia infections were exclusively unicellular, but Simkania and Parachlamydia cells were more commonly found in multicellular inclusions.
Some chlamydial species are known to recruit and reshape entire host organelles including mitochondria, Golgi stacks or the ER (Peterson and de la Maza, 1988; Matsumoto et al., 1991; Heuer et al., 2009; Croxatto and Greub, 2010; Dumoux et al., 2012). We found that Simkania inclusions are almost entirely enveloped by the rER (Fig. 3), adding additional layers to the host-bacterial interface. In this way, Simkania might use a similar strategy as the facultative intracellular pathogens Legionella pneumophila and Brucella abortus (Swanson and Isberg, 1995; Abu Kwaik et al., 1998; Roy, 2002). However, in contrast with L. pneumophila and B. abortus phagosomes, the Simkania inclusion does not fuse with ER-derived vesicles, and Simkania thus remains inside the inclusion. The tight association of the Simkania inclusion with the ER could nevertheless provide similar benefits such as prevention from fusing with lysosomes. Interestingly, the abilities to recruit ER and to replicate in human and insect cells coincide in Simkania (Kahane et al., 2007; Sixt et al., 2012) and members of the pathogenic chlamydiae (Dumoux et al., 2012), but are absent in Parachlamydia and Protochlamydia.
Species-specific differences in inclusion morphology and recruitment of host organelles are likely due to the presence of different effector proteins in the inclusion membrane (Rockey et al., 1997; Betts et al., 2009; Heinz et al., 2010). Adaptation to different hosts likely drove the diversification of environmental chlamydiae (Bertelli et al., 2010; Collingro et al., 2011).
T3S systems
Translocation of chlamydial effector proteins through the elaborate cell envelope and the inclusion membrane requires a secretion system and is thought to be accomplished by the T3S system. T3S systems are encoded in all known chlamydial genomes (Peters et al., 2007; Collingro et al., 2011), and T3S proteins were detected during all stages of infection in members of the pathogenic chlamydiae (Fields et al., 2003). For the first time, we detected T3S-like structures in environmental chlamydiae, providing evidence for its conservation and crucial role in the infectious life cycle of modern and likely ancient chlamydiae. Fewer T3S-like structures were observed by ECT in environmental chlamydiae than by conventional transmission electron microscopy in pathogenic chlamydiae (Matsumoto, 1982; Wilson et al., 2006).
T3S needle tip proteins in other bacteria are known to be highly adapted to the host (Abby and Rocha, 2012). It has been unclear whether the chlamydial T3S needle harbours a tip protein at all. To date, only one candidate for a chlamydial needle tip protein has been identified, but it remains unclear whether it rather functions as an effector (Markham et al., 2009; Stone et al., 2012). The subterminal widening of the needle in Parachlamydia and Protochlamydia seen here indicates that the chlamydial T3S apparatus likely does include a needle tip protein. Interestingly, T3S structures were not seen on purified or cryosectioned RBs perhaps because the juxtaposition of the RB outer membrane and inclusion membrane effects the length of the needle.
Imaging bacteria–host interactions in a near-native state
Finally, this is the first study to image bacteria inside their host in a near-native, frozen-hydrated state. In addition to avoiding and uncovering artefacts, this approach provided novel insights into the nature of the host-bacterial interface. Because amoebae can also serve as hosts for important pathogens such as Legionella pneumophila, Vibrio cholerae, mycobacteria, Francisella tularensis, Pseudomonas aeruginosa and Helicobacter pylori as well as bacterial symbionts like Amoebophilus asiaticus, Paracaedibacter symbiosus or Procabacter acanthamoebae (Horn and Wagner, 2004; Schmitz-Esser et al., 2008), our approach should prove helpful in the study of many other important bacteria–host interactions in the future.
Experimental procedures
Cultivation of organisms and staining of mitochondria
Acanthamoeba castellanii UWC1 infected with Parachlamydia acanthamoebae UV7 or Simkania negevensis and A. castellanii Neff infected with Protochlamydia amoebophila UWE25 were cultivated in TSY (trypticase soy broth with yeast extract) medium (30 g l−1 trypticase soy broth, 10 g l−1 yeast extract, pH 7.3) at 20°C. Amoebal growth was monitored by light microscopy and medium was exchanged every 3–6 days. The presence and identity of the chlamydial symbionts was checked regularly by fluorescence in situ hybridization (FISH) combined with 4′,6-diamidino-2-phenylindole staining of infected cultures using specific probes for the respective symbiont as described previously (Schmitz-Esser et al., 2008). In addition, the identity of the symbionts was verified by isolation of DNA from cultures followed by amplification and sequencing of the 16S rRNA genes. For staining of mitochondria, A. castellanii infected with chlamydial symbionts were incubated with 2 μM MitoTracker Orange CMTMRos (Molecular Probes) in TSY for 45 min. Cells were fixed with 4% paraformaldehyde, followed by FISH with specific probes.
Purification of chlamydiae
Infected A. castellanii cultures were harvested by centrifugation (7197 × g, 10 min), washed in Page’s amoebic saline (PAS) (Page, 1976), centrifuged and resuspended in PAS. Amoeba cells were ruptured by vortexing with an equal volume of glass beads for 3 min. Glass beads and cell debris were removed by centrifugation (5 min, 300 × g). The supernatant was filtered through a 1.2 μm filter and centrifuged at maximum speed for 10 min. The obtained pellet was resuspended in PAS.
Conventional transmission EM
To analyse the impact of fixation and to compare the effect of different fixation buffers on the morphology of Parachlamydia, chlamydiae were purified from their amoeba hosts, and the sample was divided into three parts. One part was immediately plunge-frozen (see later). The second part was fixed in 4% glutaraldehyde in phosphate buffered saline (PBS, 130 mM NaCl, 10 mM NaxPO4; pH 7.2–7.4) for 1 h, washed in PBS and further fixed in 1% osmium tetroxide in PBS for 1 h followed by two washing steps. The third part was fixed in the same way as the second sample, except that 2% glutaraldehyde in phosphate buffer (10 mM NaxPO4; pH 7.2–7.4) was used as first fixative and that the 10 mM phosphate buffer replaced PBS in the following washing and fixation steps. Samples were dehydrated in ethanol and acetone through a graded series, embedded in Epon-Araldite (Electron Microscopy Sciences, Port Washington, PA), thin-sectioned with a UC6 ultramicrotome (Leica, Vienna, Austria), and stained with uranyl acetate and lead citrate. Two-dimensional images were recorded on a Tecnai T12 TEM (FEI, Eindhoven, the Netherlands).
For room temperature EM of high-pressure frozen/freeze substituted samples, infected amoeba cells were high-pressure frozen (see later). The frozen domes were transferred under liquid nitrogen to cryotubes containing 2% or 0.04% glutaraldehyde in acetone. The tubes were placed in a model AFS freeze-substitution machine (Leica) and freeze-substituted at −90°C for 60 h, then warmed to −20°C over 10 h. Cells were rinsed 3× with cold acetone, then post-fixed with 2.5% osmium tetroxide in acetone at −20°C for 24 h. The samples were then warmed to 4°C over 2 h, rinsed 3× with cold acetone, and embedded in Epon-Araldite resin (Electron Microscopy Sciences). Following polymerization, semi-thin (200 nm) sections were cut with a UC6 ultramicrotome (Leica) and placed on Formvar-coated, copper/rhodium 1 mm slot grids (Electron Microscopy Sciences). Sections were stained with uranyl acetate and lead citrate, and imaged in a Tecnai T12 TEM (FEI). Dual-axis tilt-series were acquired using SerialEM (Mastronarde, 2005), then subsequently calculated and analysed using IMOD (Kremer et al., 1996) on an Apple MacPro computer.
Plunge-freezing
For plunge-freezing, copper/rhodium EM grids (R2/2 or R2/1, Quantifoil, Jena, Germany) were glow-discharged for 1 min. A 20×-concentrated bovine serum albumin-treated solution of 10 nm colloidal gold (Sigma, St Louis, MO) was added to purified chlamydiae (1:4 v/v) immediately before plunge freezing. A 4 μl droplet of the mixture was applied to the EM grid, then automatically blotted and plunge-frozen into a liquid ethane-propane mixture (Tivol et al., 2008) using a Vitrobot (FEI) (Iancu et al., 2006).
Cryosectioning
Acanthamoeba castellanii cells continuously infected with either Simkania, Parachlamydia or Protochlamydia were mixed with uninfected amoeba cells at a ratio of 1:1 and incubated for 24 h. For Parachlamydia, the ratio of infected to uninfected cells was 5:1. Amoebae were harvested (7197 × g, 10 min), and the pellet was mixed with 40% dextran (w/v) in PAS. The samples were transferred to brass planchettes and rapidly frozen in a HPM010 high-pressure freezing machine (Bal-Tec, Leica). Cryosectioning of the vitrified samples was done as previously described (Ladinsky et al., 2006; Ladinsky, 2010). Semi-thin (90–200 nm) cryosections were cut at −145°C or −160°C with a 25°Cryo diamond knife (Diatome, Biel, Switzerland), transferred to grids (continuous carbon-coated 200-mesh copper grids or 700-mesh uncoated copper grids) and stored in liquid nitrogen.
ECT
Images were collected using a Polara 300 kV FEG transmission electron microscope (FEI) equipped with an energy filter (slit width 20 eV; Gatan, Pleasanton, CA) on a lens-coupled 4 k × 4 k UltraCam charge-coupled device (CCD) (Gatan) or K2 Summit direct electron detector (Gatan). Pixels on the CCD represented 0.95 nm (22 500×) or 0.63 nm (34 000×) at the specimen level. Typically, tilt series were recorded from −60° to +60° with an increment of 1° at 10 μm under-focus. The cumulative dose of a tilt-series was 180–220 e−/Å2 (for whole cells) or 100–150 e−/Å2 (for cryosections). UCSFTOMO (Zheng et al., 2007) was used for automatic acquisition of tilt-series and two-dimensional projection images. Three-dimensional reconstructions were calculated using the IMOD software package (Kremer et al., 1996) or Raptor (Amat et al., 2008). Tomograms of cryosections were reconstructed using IMOD’s patch tracking to generate the aligned stack (Kremer et al., 1996). Tomograms were visualized and segmented using 3dMOD (Kremer et al., 1996).
SEM
Glass coverslips (12-mm diameter) were cleaned in acidic ethanol, dried for 1 h at 60°C and coated with 0.01% poly-L-Lysine solution for 10 min. Two hundred microlitres of purified Parachlamydia in the respective buffer were spotted onto the dry coverslip. After 10 min non-attached cells were removed, and remaining cells were fixed for 1 h at room temperature using the following fixatives: 2% glutaraldehyde in 10 mM phosphate buffer (pH 7.2), 2.5% glutaraldehyde in 3 mM cacodylate buffer (pH 7.2), 2% glutaraldehyde in DGM-21A-defined medium (Haider et al., 2010), 4% glutaraldehyde in 10 mM phosphate buffer with 130 mM NaCl, and 4% glutaraldehyde in 10 mM phosphate buffer with 260 mM NaCl. After three washing steps (5 min each) in the respective buffer, cells were further fixed in 1% osmium tetroxide in the respective buffer for 1 h at room temperature and washed again three times. Samples were dehydrated in acetone and chemically dried in hexamethyldisilazane. Glass slides were gold coated for 160 s using default settings (Agar sputter coater B7340) and analysed using a Philips XL-30 ESEM. For analysis, 10 or more random SEM images with 36 or more individual putative bacterial cells in total were taken. Roundish or crescent-shaped objects with a diameter of 0.5–1 μm were counted as bacterial cells. Each cell was then classified into one out of four morphological types (crescent shape, large invaginations, small invaginations, coccoid), and the percentage of each type was determined. Osmolarity measurements of buffers and fixatives were performed using an Advanced Micro 3MO plus osmometer (Block Scientific, New York, NY). Samples and standards were measured three times each.
Supplementary Material
Fig. S1. Simkania, Parachlamydia and Protochlamydia do not recruit mitochondria to their inclusions. MitoTracker staining of mitochondria (red) was combined with detection of chlamydiae (green) and the amoeba host (blue) by fluorescence in situ hybridization. No significant clustering of mitochondria around inclusions of Simkania (A), Parachlamydia (B) or Protochlamydia (C) was detected in A. castellanii. Bars 10 μm.
Fig. S2. More examples of putative Simkania T3S structures with sheared-off needles. Shown are slices through cryotomograms of purified Simkania. Often, a widening of the periplasm and basal body-like densities were observed in the absence of a T3S needle, suggesting some needles are sheared off during purification. Bar 100 nm.
Movie S1. Crescent bodies are not seen in projection images of plunge-frozen Parachlamydia cells.
Movie S2. Cryotomogram and three-dimensional model of the Simkania inclusion from Fig. 3D.
Movie S3. Cryotomogram and three-dimensional model of the Parachlamydia inclusion from Fig. 3L.
Movie S4. Cryotomogram and three-dimensional model of the Protochlamydia inclusion from Fig. 4C.
Acknowledgments
This work was funded by the Austrian Science Fund FWF (Y277-B03 to MH), the European Research Council (ERC StG ‘EvoChlamy’ to MH), the Caltech Center for Environmental Microbiology Interactions (to GJJ, MP), and a gift from the Gordon and Betty Moore Foundation to Caltech.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Abby SS, Rocha EPC. The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet. 2012;8:e1002983. doi: 10.1371/journal.pgen.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–959. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Abu Kwaik Y, Gao LY, Stone BJ, Venkataraman C, Harb OS. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol. 1998;64:3127–3133. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R, Springer N, Schonhuber W, Ludwig W, Schmid EN, Muller KD, Michel R. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol. 1997;63:115–121. doi: 10.1128/aem.63.1.115-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat F, Moussavi F, Comolli LR, Elidan G, Downing KH, Horowitz M. Markov random field based automatic image alignment for electron tomography. J Struct Biol. 2008;161:260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barry CE, Hayes SF, Hackstadt T. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science. 1992;256:377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- Bertelli C, Collyn F, Croxatto A, Ruckert C, Polkinghorne A, Kebbi-Beghdadi C, et al. The Waddlia genome: a window into chlamydial biology. PLoS ONE. 2010;5:e10890. doi: 10.1371/journal.pone.0010890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts HJ, Wolf K, Fields KA. Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr Opin Microbiol. 2009;12:81–87. doi: 10.1016/j.mib.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of Chlamydia psittaci using recombinant DNA introduced by electroporation. Proc Natl Acad Sci USA. 2009;106:292–297. doi: 10.1073/pnas.0806768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci USA. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingro A, Tischler P, Weinmaier T, Penz T, Heinz E, Brunham RC, et al. Unity in variety – the pan-genome of the Chlamydiae. Mol Biol Evol. 2011;28:3253–3270. doi: 10.1093/molbev/msr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto A, Greub G. Early intracellular trafficking of Waddlia chondrophila in human macrophages. Microbiology. 2010;156:340–355. doi: 10.1099/mic.0.034546-0. [DOI] [PubMed] [Google Scholar]
- Draghi A, 2nd, Popov VL, Kahl MM, Stanton JB, Brown CC, Tsongalis GJ, et al. Characterization of ‘Candidatus Piscichlamydia salmonis’ (order Chlamydiales), a chlamydia-like bacterium associated with epitheliocystis in farmed Atlantic salmon (Salmo salar) J Clin Microbiol. 2004;42:5286–5297. doi: 10.1128/JCM.42.11.5286-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoux M, Clare DK, Saibil HR, Hayward RD. Chlamydiae assemble a pathogen synapse to hijack the host endoplasmic reticulum. Traffic. 2012;13:1612–1627. doi: 10.1111/tra.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields KA, Mead DJ, Dooley CA, Hackstadt T. Chlamydia trachomatis type III secretion: evidence for a functional apparatus during early-cycle development. Mol Microbiol. 2003;48:671–683. doi: 10.1046/j.1365-2958.2003.03462.x. [DOI] [PubMed] [Google Scholar]
- Friis RR. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972;110:706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche TR, Horn M, Wagner M, Herwig RP, Schleifer KH, Gautom RK. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl Environ Microbiol. 2000;66:2613–2619. doi: 10.1128/aem.66.6.2613-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Raoult D. Crescent bodies of Parachlamydia acanthamoebae and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl Environ Microbiol. 2002;68:3076–3084. doi: 10.1128/AEM.68.6.3076-3084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Scidmore MA, Rockey DD. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- Haider S, Wagner M, Schmid MC, Sixt BS, Christian JG, Häcker G, et al. Raman microspectroscopy reveals long-term extracellular activity of chlamydiae. Mol Microbiol. 2010;77:687–700. doi: 10.1111/j.1365-2958.2010.07241.x. [DOI] [PubMed] [Google Scholar]
- Hatch TP, Miceli M, Silverman JA. Synthesis of protein in host-free reticulate bodies of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1985;162:938–942. doi: 10.1128/jb.162.3.938-942.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch TP, Miceli M, Sublett JE. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1986;165:379–385. doi: 10.1128/jb.165.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E, Tischler P, Rattei T, Myers G, Wagner M, Horn M. Comprehensive in silico prediction and analysis of chlamydial outer membrane proteins reflects evolution and life style of the Chlamydiae. BMC Genomics. 2009;10:634. doi: 10.1186/1471-2164-10-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E, Rockey DD, Montanaro J, Aistleitner K, Wagner M, Horn M. Inclusion membrane proteins of Protochlamydia amoebophila UWE25 reveal a conserved mechanism for host cell interaction among the Chlamydiae. J Bacteriol. 2010;192:5093–5102. doi: 10.1128/JB.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning K, Zöller L, Hauröder B, Hotzel H, Michel R. Hartmanella vermiformis (Hartmannellidae) harboured a hidden chlamydia-like endosymbiont. Endocytobiosis Cell Res. 2007;18:1–10. [Google Scholar]
- Heuer D, Lipinski AR, Machuy N, Karlas A, Wehrens A, Siedler F, et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Horn M. Chlamydiae as symbionts in eukaryotes. Annu Rev Microbiol. 2008;62:113–131. doi: 10.1146/annurev.micro.62.081307.162818. [DOI] [PubMed] [Google Scholar]
- Horn M, Wagner M. Bacterial endosymbionts of free-living amoebae. J Eukaryot Microbiol. 2004;51:509–514. doi: 10.1111/j.1550-7408.2004.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Horn M, Wagner M, Muller KD, Schmid EN, Fritsche TR, Schleifer KH, Michel R. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology. 2000;146(Part 5):1231–1239. doi: 10.1099/00221287-146-5-1231. [DOI] [PubMed] [Google Scholar]
- Horn M, Collingro A, Schmitz-Esser S, Beier CL, Purkhold U, Fartmann B, et al. Illuminating the evolutionary history of chlamydiae. Science. 2004;304:728–730. doi: 10.1126/science.1096330. [DOI] [PubMed] [Google Scholar]
- Huang Z, Chen M, Li K, Dong X, Han J, Zhang Q. Cryo-electron tomography of Chlamydia trachomatis gives a clue to the mechanism of outer membrane changes. J Electron Microsc (Tokyo) 2010;59:237–241. doi: 10.1093/jmicro/dfp057. [DOI] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci USA. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu CV, Tivol WF, Schooler JB, Dias DP, Henderson GP, Murphy GE, et al. Electron cryotomography sample preparation using the Vitrobot. Nat Protocols. 2006;1:2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- Kahane S, Dvoskin B, Mathias M, Friedman MG. Infection of Acanthamoeba polyphaga with Simkania negevensis and S. negevensis survival within amoebal cysts. Appl Environ Microbiol. 2001;67:4789–4795. doi: 10.1128/AEM.67.10.4789-4795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane S, Fruchter D, Dvoskin B, Friedman MG. Versatility of Simkania negevensis infection in vitro and induction of host cell inflammatory cytokine response. J Infect. 2007;55:e13–e21. doi: 10.1016/j.jinf.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Kahane SEM, Friedman MG. Evidence that the novel microorganism ‘Z’ may belong to a new genus in the family Chlamydiaceae. FEMS Microbiol Lett. 1995;126:203–208. doi: 10.1111/j.1574-6968.1995.tb07417.x. [DOI] [PubMed] [Google Scholar]
- Kamneva OK, Knight SJ, Liberles DA, Ward NL. Analysis of genome content evolution in pvc bacterial super-phylum: assessment of candidate genes associated with cellular organization and lifestyle. Genome Biol Evol. 2012;4:1375–1390. doi: 10.1093/gbe/evs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari L, Goheen MM, Randall LB, Taylor LD, Carlson JH, Whitmire WM, et al. Generation of targeted Chlamydia trachomatis null mutants. Proc Natl Acad Sci USA. 2011;108:7189–7193. doi: 10.1073/pnas.1102229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen M, Nylund A, Watanabe K, Helvik JV, Nylund S, Plarre H. Characterization of ‘Candidatus Clavochlamydia salmonicola’: an intracellular bacterium infecting salmonid fish. Environ Microbiol. 2008;10:208–218. doi: 10.1111/j.1462-2920.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- Kostanjsek R, Strus J, Drobne D, Avgustin G. ‘Candidatus Rhabdochlamydia porcellionis’, an intracellular bacterium from the hepatopancreas of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda) Int J Syst Evol Microbiol. 2004;54:543–549. doi: 10.1099/ijs.0.02802-0. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Ladinsky MS. Chapter eight – micromanipulator-assisted vitreous cryosectioning and sample preparation by high-pressure freezing. In: Jensen GJ, editor. Methods in Enzymology. Amsterdam, the Netherlands: Elsevier; 2010. pp. 165–194. [DOI] [PubMed] [Google Scholar]
- Ladinsky MS, Pierson JM, McIntosh JR. Vitreous cryo-sectioning of cells facilitated by a micromanipulator. J Microsc. 2006;224:129–134. doi: 10.1111/j.1365-2818.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- Lamoth F, Greub G. Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol Rev. 2010;34:260–280. doi: 10.1111/j.1574-6976.2009.00207.x. [DOI] [PubMed] [Google Scholar]
- Lemcke RM. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972;110:1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard J, Croxatto A, Prod’hom G, Greub G. Estrella lausannensis, a new star in the Chlamydiales order. Microbes Infect. 2011;13:1232–1241. doi: 10.1016/j.micinf.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Lindsay MR, Webb RI, Hosmer HM, Fuerst JA. Effects of fixative and buffer on morphology and ultrastructure of a freshwater planctomycete, Gemmata obscuriglobus. J Microbiol Methods. 1995;21:45–54. [Google Scholar]
- Markham AP, Jaafar ZA, Kemege KE, Middaugh CR, Hefty PS. Biophysical characterization of Chlamydia trachomatis CT584 supports its potential role as a type III secretion needle tip protein. Biochemistry. 2009;48:10353–10361. doi: 10.1021/bi901200y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlovits TC, Kubori T, Sukhan A, Thomas DR, Galán JE, Unger VM. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Recent progress of electron microscopy in microbiology and its development in future: from a study of the obligate intracellular parasites, chlamydia organisms. J Electron Microsc (Tokyo) 1979;28:57–64. [Google Scholar]
- Matsumoto A. Electron microscopic observations of surface projections on Chlamydia psittaci reticulate bodies. J Bacteriol. 1982;150:358–364. doi: 10.1128/jb.150.1.358-364.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. Structural Characteristics of Chlamydial Bodies. Boca Raton, FL, USA: CRC Press; 1988. [Google Scholar]
- Matsumoto A, Bessho H, Uehira K, Suda T. Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J Electron Microsc (Tokyo) 1991;40:356–363. [PubMed] [Google Scholar]
- Michel R, Hauröder-Philippczyk B, Müller K-D, Weishaar I. Acanthamoeba from human nasal mucosa infected with an obligate intracellular parasite. Eur J Protistol. 1994;30:104–110. [Google Scholar]
- Michel R, Müller KD, Zöller L, Walochnik J, Hartmann M, Schmid EN. Free-living amoebae serve as a host for the Chlamydia-like bacterium Simkania negevensis. Acta Protozool. 2005;44:113–121. [Google Scholar]
- Moulder JW. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Matsuo J, Hayashi Y, Kawaguchi K, Yoshida M, Takahashi K, et al. Endosymbiotic bacterium Protochlamydia can survive in acanthamoebae following encystation. Environ Microbiol Rep. 2010;2:611–618. doi: 10.1111/j.1758-2229.2010.00182.x. [DOI] [PubMed] [Google Scholar]
- Nguyen BD, Valdivia RH. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc Natl Acad Sci USA. 2012;109:1263–1268. doi: 10.1073/pnas.1117884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BA, Setzer PY, Pang F, Dawson CR. New view of the surface projections of Chlamydia trachomatis. J Bacteriol. 1985;164:344–349. doi: 10.1128/jb.164.1.344-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page FC. An Illustrated Key to Freshwater and Soil Amoebae. Ambleside, UK: Freshwater Biological Association; 1976. [Google Scholar]
- Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Peterson EM, de la Maza LM. Chlamydia parasitism: ultrastructural characterization of the interaction between the chlamydial cell envelope and the host cell. J Bacteriol. 1988;170:1389–1392. doi: 10.1128/jb.170.3.1389-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilhofer M, Ladinsky MS, McDowall AW, Jensen GJ. Chapter 2 – bacterial TEM: new insights from cryo-microscopy. In: Müller-Reichert T, editor. Methods in Cell Biology. Amsterdam, the Netherlands: Elsevier; 2010. pp. 21–45. [DOI] [PubMed] [Google Scholar]
- Rockey DD, Grosenbach D, Hruby DE, Peacock MG, Heinzen RA, Hackstadt T. Chlamydia psittaci IncA is phosphorylated by the host cell and is exposed on the cytoplasmic face of the developing inclusion. Mol Microbiol. 1997;24:217–228. doi: 10.1046/j.1365-2958.1997.3371700.x. [DOI] [PubMed] [Google Scholar]
- Rourke AW, Davis RW, Bradley TM. A light and electron microscope study of epitheliocystis in juvenile steelhead trout, Salmo gairdneri Richardson. J Fish Dis. 1984;7:301–309. [Google Scholar]
- Roy CR. Exploitation of the endoplasmic reticulum by bacterial pathogens. Trends Microbiol. 2002;10:418–424. doi: 10.1016/s0966-842x(02)02421-6. [DOI] [PubMed] [Google Scholar]
- Rurangirwa FR, Dilbeck PM, Crawford TB, McGuire TC, McElwain TF. Analysis of the 16S rRNA gene of microorganism WSU 86–1044 from an aborted bovine foetus reveals that it is a member of the order Chlamydiales: proposal of Waddliaceae fam. nov., Waddlia chondrophila gen. nov., sp. nov. Int J Syst Bacteriol. 1999;49:577–581. doi: 10.1099/00207713-49-2-577. [DOI] [PubMed] [Google Scholar]
- Schmitz-Esser S, Toenshoff ER, Haider S, Heinz E, Hoenninger VM, Wagner M, Horn M. Diversity of bacterial endosymbionts of environmental Acanthamoeba isolates. Appl Environ Microbiol. 2008;74:5822–5831. doi: 10.1128/AEM.01093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt BS, Hiess B, König L, Horn M. Lack of effective anti-apoptotic activities restricts growth of Parachlamydiaceae in insect cells. PLoS ONE. 2012;7:e29565. doi: 10.1371/journal.pone.0029565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt BS, Siegl A, Müller C, Watzka M, Wultsch A, Tziotis D, et al. Metabolic features of Protochlamydia amoebophila elementary bodies – a link between activity and infectivity in Chlamydiae. PLoS Pathog. 2013;9:e1003553. doi: 10.1371/journal.ppat.1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CB, Sugiman-Marangos S, Bulir DC, Clayden RC, Leighton TL, Slootstra JW, et al. Structural characterization of a novel Chlamydia Pneumoniaetype III secretion-associated protein, Cpn0803. PLoS ONE. 2012;7:e30220. doi: 10.1371/journal.pone.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Collingro A, Horn M. Tracing back to primordial chlamydiae, extinct parasites of plants? Trends Plant Sci. 2013 doi: 10.1016/j.tplants.2013.10.005. (in press) [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Casson N, Greub G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ Microbiol. 2006;8:2125–2135. doi: 10.1111/j.1462-2920.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- Tivol WF, Briegel A, Jensen GJ. An improved cryogen for plunge freezing. Microsc Microanal. 2008;14:375–379. doi: 10.1017/S1431927608080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DP, Timms P, McElwain DL, Bavoil PM. Type III secretion, contact-dependent model for the intracellular development of chlamydia. Bull Math Biol. 2006;68:161–178. doi: 10.1007/s11538-005-9024-1. [DOI] [PubMed] [Google Scholar]
- Zheng SQ, Keszthelyi B, Branlund E, Lyle JM, Braunfeld MB, Sedat JW, Agard DA. UCSF tomography: an integrated software suite for real-time electron microscopic tomographic data collection, alignment, and reconstruction. J Struct Biol. 2007;157:138–147. doi: 10.1016/j.jsb.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Simkania, Parachlamydia and Protochlamydia do not recruit mitochondria to their inclusions. MitoTracker staining of mitochondria (red) was combined with detection of chlamydiae (green) and the amoeba host (blue) by fluorescence in situ hybridization. No significant clustering of mitochondria around inclusions of Simkania (A), Parachlamydia (B) or Protochlamydia (C) was detected in A. castellanii. Bars 10 μm.
Fig. S2. More examples of putative Simkania T3S structures with sheared-off needles. Shown are slices through cryotomograms of purified Simkania. Often, a widening of the periplasm and basal body-like densities were observed in the absence of a T3S needle, suggesting some needles are sheared off during purification. Bar 100 nm.
Movie S1. Crescent bodies are not seen in projection images of plunge-frozen Parachlamydia cells.
Movie S2. Cryotomogram and three-dimensional model of the Simkania inclusion from Fig. 3D.
Movie S3. Cryotomogram and three-dimensional model of the Parachlamydia inclusion from Fig. 3L.
Movie S4. Cryotomogram and three-dimensional model of the Protochlamydia inclusion from Fig. 4C.


