Abstract
Objectives
Operative time, previously identified as a risk factor for postoperative morbidity, is examined in patients undergoing benign cranial nerve tumor resection.
Design/Setting/Participants
This retrospective cohort analysis included patients enrolled in the ACS-NSQIP registry from 2007 through 2013 with a diagnosis of a benign cranial nerve neoplasm.
Main Outcome Measures
Primary outcomes included postoperative morbidity and mortality. Readmission and reoperation served as secondary outcomes.
Results
A total of 565 patients were identified. Mean (median) operative time was 398 (370) minutes. The 30-day complication, readmission, and return to the operating room rates were 9.9%, 9.9%, and 7.3%, respectively, on unadjusted analyses. CSF leak requiring reoperation or readmission occurred at a rate of 3.1%. On multivariable regression analysis, operations greater than 413 minutes were associated with an increased odds of overall complication (OR 4.26, 95% CI 2.08–8.72), return to the operating room (OR 2.65, 95% CI 1.23–5.67), and increased length of stay(1.6 days, 95% CI 0.94–2.23 days). Each additional minute of operative time was associated with an increased odds of overall complication (OR 1.004, 95% CI 1.002–1.006) and increased length of stay (0.006 days, 95% CI 0.004–0.008).
Conclusion
Increased operative time in patients undergoing surgical resection of a benign cranial nerve neoplasm was associated with an increased rate of complications.
Keywords: acoustic neuroma, vestibular schwannoma, skull base, operative time, complications, national surgical quality improvement program
Introduction
Identifying modifiable risk factors is the pinnacle of surgical quality improvement. Certain factors have previously been established as increasing risk for postoperative complications. These include age, anesthesia physical status classification, and comorbidities.1 Operative time has been identified in orthopedic, vascular, and spine surgery as a risk factor for postoperative complications, but it has not been reviewed in the benign cranial nerve tumor population.2 3 4
Benign cranial nerve tumors comprise a minority of intracranial neoplasms, with the most prevalent among these being vestibular schwannomas (VSs), accounting for 6 to 8% of all intracranial neoplasms and approximately 90% of all cerebellopontine angle tumors.5 6 7 8 VSs are benign nerve sheath tumors that arise from the eighth cranial nerve and proposed treatment algorithms are linked to size. Tumors smaller than 1.5 cm may undergo observation or patients may elect to proceed with treatment options of radiation or surgery.9 Tumors from 1.5 to 2.5 cm are typically treated with radiosurgery or microsurgery. Tumors greater than 2.5 cm are most commonly treated with microsurgery and often are associated with higher treatment morbidity; however, this increase in morbidity is likely secondary to the complexity of the surgery as the tumor grows more intimate with the surrounding brainstem and cranial nerves. This complexity, given the intimate relationships of critical structures and tumor tissue, may require longer operative times relative to other elective intracranial operations.
In other surgical specialties, operative time has been linked to increased rates of infection, length of stay, and morbidity.2 3 4 Longer operative times in patients undergoing VS surgery have been associated with increased incidence of cerebrospinal fluid (CSF) leaks postoperatively.10 We hypothesize that increased operative time may be associated with higher rates of other postoperative complications as well. The current study is the first to investigate the impact of operative time in benign cranial nerve tumor surgery outcomes in a large, national, multi-institutional cohort.
Methods
Inclusion and Exclusion Criteria
The American College of Surgeons' National Surgical Quality Improvement Program (ACS-NSQIP) dataset was used for this retrospective cohort analysis. Since its initiation by the Department of Veterans Affairs in 1994, NSQIP has evolved through the efforts of the ACS into the leading program for surgical quality measurements and improvement in the private sector.11 12 13 Through collection of preoperative characteristics, operative variables, and outcome measures from more than 250 participating institutions, NSQIP is the first “national, validated, outcome-based, risk-adjusted, peer-controlled program” for the improvement of surgical care.12 14 15 16 More than 130 variables are collected on surgical patients from participating institutions. Preoperative risk factors are included in the data, in addition to intraoperative variables and 30-day postoperative morbidity and mortality outcomes.17 Data collectors receive specialized training and are employed at each site to abstract and submit the various data points. Quality-control processes, primarily through audits by Surgical Clinical Reviewers, are practiced to ensure inter-rater reliability.11 18 19 Further information regarding the dataset, collected variables, internal clinical reviewer standards, and participating sites is available on the ACS-NSQIP Web site at https://www.facs.org/quality-programs/acs-nsqip?.
Patients enrolled in the NSQIP data registry between 2007 and 2013 with an International Classification of Disease, 9th Revision (ICD-9) code indicating a primary postoperative diagnosis code of 225.1 (benign neoplasm of a cranial nerve) were included for analysis.
Patients with an American Society of Anesthesiologists (ASA) physical status classification of four or greater (n = 10), denoting patients with severe systemic disease that is a constant threat to life or moribund patients, were excluded from the analysis.20
Outcomes
The development of at least one of the following complications within the 30-day postoperative period defined our primary outcome: mortality, surgical site infection, wound dehiscence, septic shock, venous thromboembolism, stroke, cardiac arrest, coma, requirement for perioperative blood transfusion, urinary tract infection (UTI), failure to wean ventilatory support within 48 hours, unplanned reintubation, and pneumonia.
Instances of reoperation or readmission were the secondary outcomes of interest. CSF leak, hydrocephalus, and facial nerve injury requiring reoperation or readmission were featured outcomes, as was meningitis requiring readmission. The overall rate of return to the operating room and unplanned readmission within 30 days were also studied. Data were not available prior to the year 2012 for these secondary outcome measures.
Covariates
Preoperative patient factors available for analysis included the following: age, race, body mass index (BMI) as defined by the World Health Organization (WHO) classification, sex, history of diabetes, hypertension requiring medication, history of severe chronic obstructive pulmonary disease (COPD), smoking status, history of chronic corticosteroid use within the 30 days of the operation, dyspnea at baseline, and history of a bleeding disorder. Operative data available for analysis included ASA physical status classification and total operative time in minutes.
Operative time was evaluated using two definitions. First, operative time was investigated as a continuous variable in minutes. Second, it was considered as a dichotomized variable with the dividing time as 413 minutes. The time threshold for dichotomization was determined by identifying the time that simultaneously optimized sensitivity and specificity of time with respect to any morbidity. This can be identified as the point that minimizes the distance between the receiver operator characteristic (ROC) curve and the upper left-hand corner in Fig. 1.
Fig. 1.
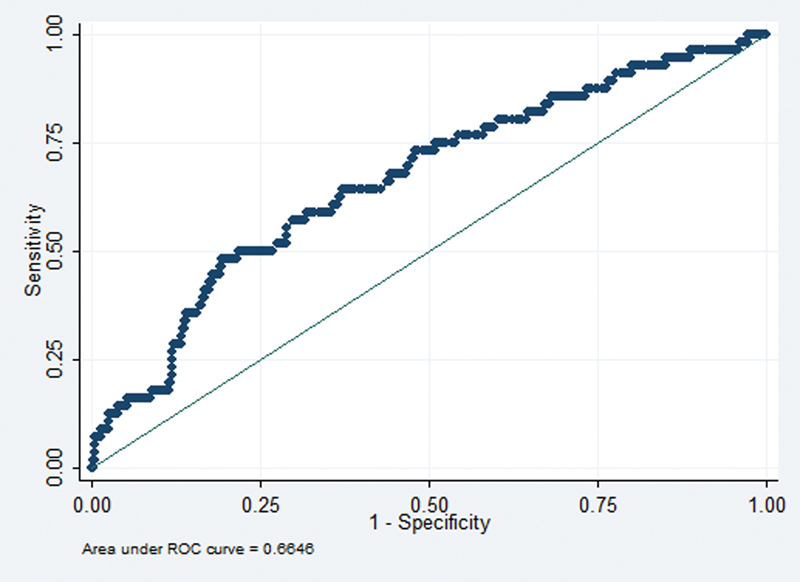
Receiver operator characteristic (ROC) curve utilized to derive the time threshold for dichotomization.
Statistical Analysis
Unadjusted bivariate analyses of both primary and secondary outcomes were conducted with respect to operative time using the two definitions established previously. For outcomes that occurred at an incidence of 5% or greater (any morbidity, return to the operating room, and 30-day readmission), multivariable logistic regression analysis was performed to investigate the association between operative time and outcomes in a risk-adjusted manner. For each outcome, separate multivariable models were performed for each of the respective operative time definitions. Multivariable models controlled for available patient and operative variables.
As the national NSQIP data are de-identified, this analysis was exempt from review by our Institutional Review Board (IRB). Commercially available software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, Texas: StataCorp LP) was used for statistical analysis with significance established at 0.05.17 Student t-tests were used for continuous variables, and chi-square tests were used for categorical variables in the unadjusted bivariate analysis.
Results
Between 2007 and 2013, 565 patients were identified as having received surgical resection of a benign cranial nerve neoplasm in the NSQIP dataset. The mean (median) operative time was 398 (370) minutes. Additional patient demographic and operative characteristics are presented in Table 1. Distribution of operative time is shown in Fig. 2.
Table 1. Patient demographic and operative characteristics.
| Available data (n) | Percent available (%) | Value | |
|---|---|---|---|
| Age, mean (median) | 565 | 100.00 | 50.98 (53) |
| Total operative time, mean (median) | 565 | 100.00 | 398.29 (370) |
| Gender, no. (col %) | |||
| Male | 565 | 100.00 | 245 (43.36) |
| Female | 320 (56.64) | ||
| BMI category (WHO classification), no. (col %) | |||
| < 18.5 | 563 | 99.64 | 4 (0.71) |
| 18.5–24.9 | 152 (27) | ||
| 25.0–29.9 | 188 (33.39) | ||
| 30.0–34.9 | 125 (22.2) | ||
| 35.0–39.9 | 59 (10.48) | ||
| 40+ | 35 (6.22) | ||
| Race (all years), no. (col %) | |||
| White | 500 | 88.50 | 446 (89.2) |
| Hispanic | 8 (1.6) | ||
| Black or African American | 20 (4) | ||
| Asian or Pacific Islander | 25 (5) | ||
| Native American | 1 (0.2) | ||
| ASA class, no. (col %) | |||
| 0 | 565 | 100.00 | 27 (4.78) |
| 1 | 303 (53.63) | ||
| 2 | 235 (41.59) | ||
| Comorbidities, no. (col %) | |||
| Smoker | 565 | 100.00 | 59 (10.44) |
| Diabetes | 31 (5.49) | ||
| Hypertension | 178 (31.5) | ||
| History of COPD | 6 (1.06) | ||
| Chronic corticosteroid use | 36 (6.37) | ||
| Dyspnea | 15 (2.65) | ||
| Bleeding disorder | 4 (0.71) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; WHO, World Health Organization.
Fig. 2.
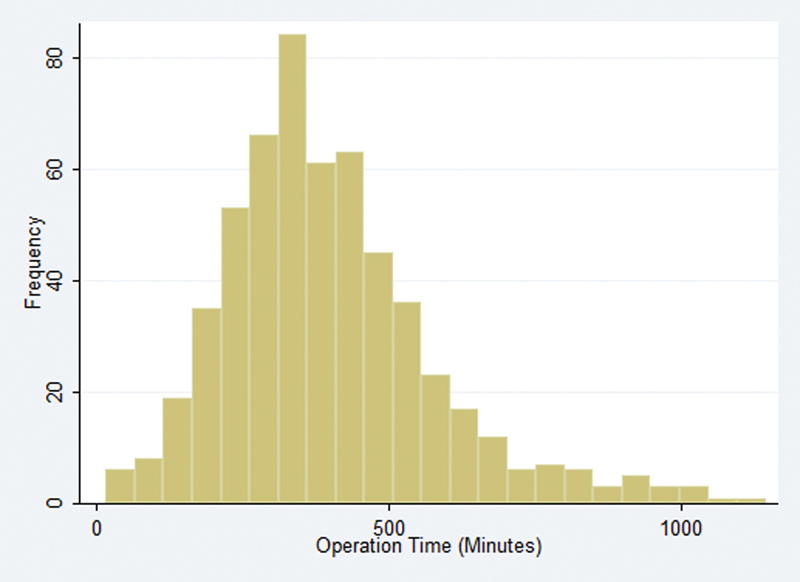
Distribution of operative times.
Within this cohort, 56 (9.91%) patients experienced at least one of the following 30-day complications: mortality, surgical site infection, wound dehiscence, septic shock, venous thromboembolism, stroke, cardiac arrest, coma, requirement for perioperative blood transfusion, UTI, failure to wean ventilatory support at 48 hours, unplanned reintubation, and pneumonia (Table 2). Separately, the mortality rate was 0.88%. Unplanned readmission within 30 days occurred at a rate of 9.89%. CSF leak, meningitis, hydrocephalus, and facial nerve injury resulting in reoperation or readmission occurred at a rate of 3.11%, 0.85%, 0.56%, and 1.41%, respectively. Fig. 3 details the complication rates across the spectrum of operative times in our cohort. The longest 20% of operations had a higher mean composite morbidity. The top quintile of operative times, approximately 12 hours and longer, had a mean rate of morbidity approaching 30%.
Table 2. Postoperative mortality and morbidity.
| Available data (n) | Percent available (%) | Value | |
|---|---|---|---|
| Complication, no. (col %) | |||
| Any complication | 565 | 100.00 | 56 (9.91) |
| Mortality | 5 (0.88) | ||
| Surgical site infection | 9 (1.59) | ||
| Wound dehiscence | 6 (1.06) | ||
| Septic shock | 11 (1.95) | ||
| Venous thromboembolism | 11 (1.95) | ||
| Stroke | 5 (0.88) | ||
| Cardiac arrest | 2 (0.35) | ||
| Coma | 2 (0.35) | ||
| Requirement for perioperative blood transfusion | 14 (2.48) | ||
| UTI | 8 (1.42) | ||
| Failure to wean ventilator within 48 h | 11 (1.95) | ||
| Unplanned reintubation | 6 (1.06) | ||
| Pneumonia | 9 (1.59) | ||
| Return to the operating room | 41 (7.26) | ||
| DVT | 10 (1.77) | ||
| 30-d readmission | 354 | 62.65 | 35 (9.89) |
| CSF leak resulting in reoperation or readmission | 11 (3.11) | ||
| Meningitis resulting in reoperation or readmission | 3 (0.85) | ||
| Hydrocephalus resulting in reoperation or readmission | 2 (0.56) | ||
| Facial nerve injury resulting in reoperation or readmission | 5 (1.41) |
Abbreviations: CSF, cerebrospinal fluid leak; DVT, Deep vein thrombosis; UTI, urinary tract infection.
Fig. 3.
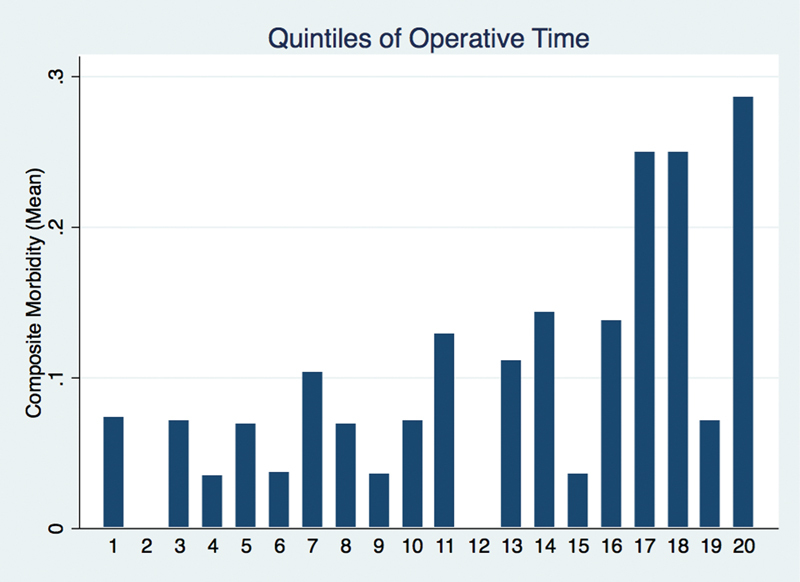
Mean composite morbidity across the spectrum of operative times.
When the individual components of the composite morbidity variable were considered separately, several complications were associated with greater operative times (Table 3). Mortality (414 vs. 398 minutes, p < 0.001), wound dehiscence (514 vs. 397 minutes, p < 0.001), septic shock (532 vs. 396 minutes, p = 0.001), cardiac arrest (482 vs. 398 minutes, p < 0.001), failure to wean ventilatory support within 48 hours of the operation (506 vs. 396 minutes, p < 0.001), transfusion (627 vs. 393, p = 0.023), and unplanned reintubation (459 vs. 398 minutes, p < 0.001) were all associated with prolonged mean operative times. The mean operative time for patients with and without surgical site infection, however, was not statistically significant (395 vs. 398 minutes, p = 0.065). The morbidity of coma postoperatively was associated with shorter operations (162 vs. 399 minutes, p < 0.001). CSF leak requiring reoperation or readmission and overall 30-day readmission, with data only available for 2012, were also associated with longer operative times (518 vs. 387 minutes, p < 0.001 and 435 vs. 386 minutes, p = 0.029, respectively).
Table 3. Mean operative times for postoperative mortality and morbidity.
| Mean total operative time without complication | Standard deviation | Mean total operative time with complication | Standard deviation | p value | |
|---|---|---|---|---|---|
| Any complication | 386.9 | 171.9 | 501.9 | 220.5 | 0.175 |
| Mortality | 398.2 | 179.5 | 414.0 | 289.6 | < 0.001 |
| Surgical site infection | 398.3 | 181.2 | 395.4 | 128.3 | 0.065 |
| Wound dehiscence | 397.1 | 178.5 | 513.7 | 316.4 | < 0.001 |
| Septic shock | 395.6 | 179.0 | 532.3 | 209.0 | 0.001 |
| Venous thromboembolism | 397.5 | 181.2 | 440.1 | 133.9 | 0.668 |
| Stroke | 398.7 | 180.7 | 348.4 | 154.9 | 0.073 |
| Nerve injury | 398.4 | 180.6 | 341.0 | 0 | 1 |
| Cardiac arrest | 398.0 | 179.5 | 482.0 | 476.6 | < 0.001 |
| Coma | 399.1 | 180.2 | 162.0 | 62.2 | < 0.001 |
| Requirement for perioperative blood transfusion | 392.5 | 175.2 | 627.1 | 234.4 | 0.023 |
| UTI | 396.4 | 179.3 | 530.4 | 221.5 | 1 |
| Failure to wean ventilator within 48 h | 396.2 | 177.0 | 505.5 | 300.3 | < 0.001 |
| Unplanned reintubation | 397.6 | 178.5 | 459.3 | 331.2 | < 0.001 |
| Pneumonia | 396.2 | 177.3 | 526.4 | 306.5 | 0.065 |
| Return to the operating room | 393.3 | 179.9 | 462.4 | 176.8 | 0.504 |
| 30-d readmission | 385.9 | 162.5 | 435.1 | 161.9 | 0.029 |
| CSF leak resulting in reoperation or readmission | 386.7 | 160.5 | 518.1 | 191.8 | < 0.001 |
| Meningitis resulting in reoperation or readmission | 391 | 163.0 | 369.7 | 179.2 | 0.997 |
| Hydrocephalus resulting in reoperation or readmission | 391.2 | 163.2 | 321.0 | 100.4 | 0.996 |
| 7th cranial nerve injury resulting in reoperation or readmission | 389.4 | 163 | 489.8 | 133.0 | 0.426 |
| DVT | 397.8 | 181.2 | 425.0 | 131.0 | 0.949 |
Abbreviations: CSF, cerebrospinal fluid leak; DVT, Deep vein thrombosis; UTI, urinary tract infection.
Evaluated as a continuous variable and in “long operations” (> 413 minutes), associations between operative time and postoperative outcomes were investigated in a risk-adjusted manner using multivariable logistic regression analysis controlling for available patient characteristics and demographics (Table 4). When assessed as a continuous variable, each additional minute of operative time was associated with an increased odds of morbidity (odds ratio [OR] 1.004, 95% confidence interval [CI] 1.002–1.006). Increased length of total hospital stay was found on linear regression to be associated with longer operative times (0.006 days, 95% CI 0.004–0.008). Multivariable analysis with operative time as a dichotomized variable revealed associations between operations greater than 413 minutes and increased odds of morbidity (OR 4.258, 95% CI 2.079–8.724) and increased rates of return to the operating room (OR 2.645, 95% 1.233–5.674) relative to patients with an operative time less than 413 minutes. Increased length of total hospital stay was also noted in patients with operative times exceeding 413 minutes (1.581 days, 95% CI 0.935–2.226).
Table 4. Multivariable logistic regression analysis interrogating associations between operative time and morbidity.
| Variable | Pr > chi-square | Odds ratio estimate | Lower 95% confidence limit for odds ratio | Upper 95% confidence limit for odds ratio |
|---|---|---|---|---|
| Total operative time | – | – | – | – |
| Any complication | < 0.001 | 1.004 | 1.002 | 1.006 |
| Return to operating room | 0.06 | 1.002 | 1.000 | 1.004 |
| 30-d readmission | 0.382 | 1.001 | 0.999 | 1.004 |
| Operative time > 413 min | – | – | – | – |
| Any complication | < 0.001 | 4.258 | 2.079 | 8.724 |
| Return to operating room | 0.012 | 2.645 | 1.233 | 5.674 |
| 30-d readmission | 0.179 | 1.75 | 0.774 | 3.955 |
Discussion
Identifying significant factors that play a role in postoperative morbidity and mortality is a crucial aspect of any successful surgical practice. Some risk factors that correlate with complications are rigid and fixed (e.g., age), whereas others are modifiable. A patient may be able to mitigate risk by losing weight or controlling their blood glucose, whereas a surgeon may optimize outcomes by increasing efficiency in the operating room and eliminating unnecessary delays. Increasing efficiency is the optimal method of expediting operative time, as speed that sacrifices technique and safety can only be detrimental for outcomes. In the current study we demonstrated that prolonged operative times are associated with increased rates of morbidity. We hypothesize that longer operative times may relate to certain nonmodifiable tumor-specific characteristics (e.g., size, consistency, and relationship to cranial nerves and the brainstem) as well as modifiable surgeon characteristics (e.g., operative technique, efficiency, and experience), all of which influence patient outcomes.
Our findings are consistent with prior reports that have identified increases in operative time as a predictor of adverse events perioperatively.2 3 4 10 One previous study reported a significant relationship between operative time and the rate of reintubation in neurosurgical patients. More than 17,000 neurosurgical patients were identified in NSQIP, and preoperative and operative variables were analyzed to detect risk factors for postoperative reintubation. Operative time exceeding 3 hours was among the significant risk factors identified. Prolonged cases had nearly three times higher likelihood of reintubation.21 While the aforementioned study demonstrated the association between operative time and complications across a broad mix of neurosurgical procedures, our current study examines the association between operative time and outcomes in a specific patient population.
Examination of benign cranial nerve tumor resections allows for dedicated analysis of a specific population of interest. Operative time itself is multifactorial with variation secondary to surgeon technical skill and expertise, tumor size, tumor consistency, nerve involvement, and patient specific operative goals. Recognizing and acknowledging the impact of operative time as an independent variable on postoperative morbidity empowers surgeons to improve their patients' outcomes. In considering operative risk, surgeons can factor operative time as a contributor to overall outcome among the multitude of other patient-specific and surgical factors. While the relative attribution of the independent effect of modifiable components of increased operative time (e.g., operating team skill and experience) compared with unmodifiable patient specific factors (e.g., tumor size, consistency, and involvement of critical structures) cannot be fully addressed in this study, we do note that the association with many non-neurologic complications suggests an independent effect of increased operative time.
Well established as a factor for postoperative complications, increasing tumor size has been associated with CSF leaks, meningitis, hematomas, and cerebellar edema following VS surgery.22 23 24 25 A previous NSQIP study examining benign cranial nerve tumor resection found that prolonged operative times were associated with increased length of stay. While acknowledging the inability to control for tumor size, the authors proposed that prolonged operative times may be a surrogate marker for larger, unfavorable tumors.19 A single institution series, with analyses controlling for several covariates including tumor size, identified prolonged operative time as an independent risk factor for CSF leaks postoperatively. Surgeon fatigue versus excess blood and proteinaceous debris interfering with proper CSF flow were proposed as possible mechanisms.10 In our analysis of mean composite morbidity across the spectrum of operative times, those in the 80th percentile and above had an increased mean composite morbidity; the longest 5% of operative times in our cohort exhibited a mean composite morbidity of nearly 30%, almost threefold the overall mean composite morbidity of 9.9%. This acceleration of complication profile in operative times exceeding 12 hours may provide reason for a staged approach when expecting lengthy operative times. We acknowledge that operative time may be both a surrogate marker for more challenging resections (increased size, intimate involvement of critical structures, tumor consistency) as well as an independent risk factor for postoperative morbidity.
Composite morbidity detailed in our analysis includes respiratory, cardiac, wound-related, and infectious adverse events. In cases with prolonged surgical duration, there was an increased odds of morbidity for every-minute increase in operative time. These complications result in multiple downstream effects, proving detrimental to patient outcomes as well as negatively impacting the patient's economic burden by increasing the length of hospital stay. Increased rates of reintubation, difficulty with ventilator weaning, cardiac arrest, and septic shock all require either an intensive care unit (ICU) admission or an extended ICU stay. Prolonged operative times significantly increased lengths of stay and per minute of additional operative time, and length of stay was noted to increase. In this cohort, outside of the composite morbidity, these respiratory and cardiac complications independently correlated with increased operative time. Higher rates of wound dehiscence as well as CSF leak requiring readmission and reoperation are also observed in cases with prolonged operative times. Reoperation overall was noted to be higher in operative cases exceeding 413 minutes. However, readmission was not significantly associated with operative duration. This may be secondary to the fact that many reoperations take place within the same initial admission; for example, CSF leaks and postoperative hematomas may be addressed with revision surgery during the primary admission. Though overall morbidity may be increased with prolonged operative times, many complications that occur are successfully managed during the primary admission.
While increased mean operative times were found to be statistically significant with respect to mortality (414 vs. 398 minutes, p < 0.001), the 16-minute difference was not clinically significant from a surgeon's perspective. An additional period of 16 minutes may be secondary to unavoidable delays such as equipment adjustments and supply retrieval, and over the course of a 6- to 7-hour operation (mean 398 minutes), it is likely not influential in the patient outcome. Given the analysis was performed in minutes for operative time, a large number of data points were included. This likely explains why the difference of 16 minutes, though not clinically significant, yielded a p value denoting statistical significance. In regards to the morbidity of coma being associated with shorter operations, this may be secondary to aborted procedures as a result of intraoperative vascular or anesthetic complications, though this is purely speculative given the limitations of the data.
Working with a national, multi-institutional database has its advantages and disadvantages. Innately the data are retrospective, thus lending to the biases that accompany this type of study. Moreover, we are limited by coding for diagnoses as a designated code for “Acoustic Neuroma” or “Vestibular Schwannoma” is not included in the International Classification of Diseases codes (ICD 9). This pathology falls under code 225.1, “benign neoplasm of a cranial nerve.” The vast majority of benign cranial nerve neoplasms are VSs, with schwannomas of other cranial nerves being exceptionally rare. Trigeminal schwannomas comprise only 0.2% of all intracranial neoplasms, and lower cranial nerve schwannomas account for an even smaller fraction.6 26 The epidemiologic data support our assumption that the majority of the NSQIP cohort of benign cranial nerve neoplasms are in fact VSs, and thus we relate our findings specifically to VS resection and related outcomes.27
Operating within the confines of the database, we are also limited in the covariates we can assess. We are unable to control for extent of resection, tumor size, or prior resection greater than 30 days before the index operation; these factors have been related to VS outcomes postoperatively and may be confounders in analyzing the magnitude of the effect of increased operative time.10 28 29 30 Though unable to control for tumor volume, we recognize that the operative time relationship with morbidity may be a reflection of its surrogacy for tumor size and complexity. We propose that in addition to a surrogate effect, operative duration may also be an independent risk factor.
While the 30-day readmission, CSF leak, hydrocephalus, and facial nerve injury data are limited to the 2012–2013 year, its inclusion delineates this study from other administrative databases where information following the primary admission is not available for analysis. These are also complications specific to VS resection and, thus, important to study. The 30-day interval captured by NSQIP data collection may miss delayed complications as well as readmissions and reoperations required for wound infection, CSF leak, hydrocephalus, and facial reanimation. Facial nerve injury that occurs without requiring readmission or reoperation, which is the usual case, may not be recorded, explaining the lower than expected rate of injury. Patients requiring revision surgeries for VS growth, despite other therapies, may also not be captured by NSQIP.
Despite its limitations, the NSQIP dataset provides a large cohort with a multitude of covariates that allow for robust analysis of targeted populations. Exploring hypotheses within this database provides for a strong analysis and exposes areas of clinical questions that can be refined through further studies. Future expansions on our findings may include exploration of significant contributors to operative time and how these individual components may be mitigated. A large series with patient and tumor characteristics, anatomical variation, and surgical approach data would provide adequate content to analyze the effect of operative time both as a surrogate and as an independent risk factor. This type of detailed study would require prospective data collection with specific attention to tumor characteristics as well as the variety of time intervals that compose overall operative duration. Examining postoperative morbidity in the context of controlling for detailed tumor characteristics would allow for a stronger conclusion in regards to operative time as an independent risk factor.
Conclusion
Patients undergoing surgical resection of a benign cranial nerve neoplasm demonstrated increased rates of complications postoperatively with lengthened procedure times. Specifically, prolonged operations were associated with postoperative wound, cardiac, and respiratory complications, as well as an increased length of total hospital stay and reoperation. While prolonged operative times may be reflective of overall tumor size and complexity, we argue that given our findings, operative duration may also play a role as an independent risk factor for postoperative morbidity in benign cranial nerve neoplasm resections.
Acknowledgments
Internal funding was utilized without commercial sponsorship or support.
References
- 1.Daneman N, Simor A E, Redelmeier D A. Validation of a modified version of the national nosocomial infections surveillance system risk index for health services research. Infect Control Hosp Epidemiol. 2009;30(6):563–569. doi: 10.1086/597523. [DOI] [PubMed] [Google Scholar]
- 2.Shen J, Liang J, Yu H, Qiu G, Xue X, Li Z. Risk factors for delayed infections after spinal fusion and instrumentation in patients with scoliosis. Clinical article. J Neurosurg Spine. 2014;21(4):648–652. doi: 10.3171/2014.6.SPINE13636. [DOI] [PubMed] [Google Scholar]
- 3.Kalish J A, Farber A, Homa K. et al. Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) J Vasc Surg. 2014;60(5):1238–1246. doi: 10.1016/j.jvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Daines B K, Dennis D A, Amann S. Infection prevention in total knee arthroplasty. J Am Acad Orthop Surg. 2015;23(6):356–364. doi: 10.5435/JAAOS-D-12-00170. [DOI] [PubMed] [Google Scholar]
- 5.Carlson M L, Link M J, Wanna G B, Driscoll C LW. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2015;48(3):407–422. doi: 10.1016/j.otc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz O, Iyer A K, Kano H, Talbott E O, Lunsford L D. Epidemiology and environmental risk factors associated with vestibular schwannoma. World Neurosurg. 2015;84(6):1674–1680. doi: 10.1016/j.wneu.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Lin D, Hegarty J L, Fischbein N J, Jackler R K. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131(3):241–244. doi: 10.1001/archotol.131.3.241. [DOI] [PubMed] [Google Scholar]
- 8.Memari F, Hassannia F, Abtahi S HR. Surgical outcomes of cerebellopontine angle tumors in 50 cases. Iran J Otorhinolaryngol. 2015;27(78):29–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Stangerup S-E, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257–268, vii. doi: 10.1016/j.otc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Copeland W R, Mallory G W, Neff B A, Driscoll C LW, Link M J. Are there modifiable risk factors to prevent a cerebrospinal fluid leak following vestibular schwannoma surgery? J Neurosurg. 2015;122(2):312–316. doi: 10.3171/2014.10.JNS14432. [DOI] [PubMed] [Google Scholar]
- 11.Khuri S F. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837–843. doi: 10.1016/j.surg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Khuri S F, Daley J, Henderson W. et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink A S Campbell D A Jr Mentzer R M Jr et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility Ann Surg 20022363344–353., discussion 353–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bydon M, Abt N B, De la Garza-Ramos R. et al. Impact of resident participation on morbidity and mortality in neurosurgical procedures: an analysis of 16,098 patients. J Neurosurg. 2015;122(4):955–961. doi: 10.3171/2014.11.JNS14890. [DOI] [PubMed] [Google Scholar]
- 15.Bydon M, Abt N B, Macki M. et al. Preoperative anemia increases postoperative morbidity in elective cranial neurosurgery. Surg Neurol Int. 2014;5:156. doi: 10.4103/2152-7806.143754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abt N B, Bydon M, De la Garza-Ramos R. et al. Concurrent neoadjuvant chemotherapy is an independent risk factor of stroke, all-cause morbidity, and mortality in patients undergoing brain tumor resection. J Clin Neurosci. 2014;21(11):1895–1900. doi: 10.1016/j.jocn.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 17.McCutcheon B A, Ciacci J D, Marcus L P. et al. Thirty-day perioperative outcomes in spinal fusion by specialty within the NSQIP database. Spine. 2015;40(14):1122–1131. doi: 10.1097/BRS.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 18.Shiloach M, Frencher S K Jr, Steeger J E. et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell B P, Rizk H G, Stevens S M, Nguyen S A, Meyer T A. The relation between obesity and hospital length of stay after elective lateral skull base surgery: an analysis of the American College of Surgeons National Surgical Quality Improvement Program. ORL J Otorhinolaryngol Relat Spec. 2015;77(5):294–301. doi: 10.1159/000435786. [DOI] [PubMed] [Google Scholar]
- 20.Griffith K E. Preoperative assessment and preparation. Int Anesthesiol Clin. 1994;32(3):17–36. doi: 10.1097/00004311-199432030-00004. [DOI] [PubMed] [Google Scholar]
- 21.Shalev D, Kamel H. Risk of reintubation in neurosurgical patients. Neurocrit Care. 2015;22(1):15–19. doi: 10.1007/s12028-014-0053-1. [DOI] [PubMed] [Google Scholar]
- 22.Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004;25(3):379–386. doi: 10.1097/00129492-200405000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Slattery W H III, Francis S, House K C. Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol. 2001;22(6):895–902. doi: 10.1097/00129492-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Brennan J W, Rowed D W, Nedzelski J M, Chen J M. Cerebrospinal fluid leak after acoustic neuroma surgery: influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001;94(2):217–223. doi: 10.3171/jns.2001.94.2.0217. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers G K, Luxford W M. Factors affecting the development of cerebrospinal fluid leak and meningitis after translabyrinthine acoustic tumor surgery. Laryngoscope. 1993;103(9):959–962. doi: 10.1288/00005537-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Fukaya R, Yoshida K, Ohira T, Kawase T. Trigeminal schwannomas: experience with 57 cases and a review of the literature. Neurosurg Rev. 2010;34(2):159–171. doi: 10.1007/s10143-010-0289-y. [DOI] [PubMed] [Google Scholar]
- 27.Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J Neurosurg. 2002;96(3):453–463. doi: 10.3171/jns.2002.96.3.0453. [DOI] [PubMed] [Google Scholar]
- 28.Darrouzet V, Martel J, Enée V, Bébéar J-P, Guérin J. Vestibular schwannoma surgery outcomes: our multidisciplinary experience in 400 cases over 17 years. Laryngoscope. 2004;114(4):681–688. doi: 10.1097/00005537-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Yashar P, Zada G, Harris B, Giannotta S L. Extent of resection and early postoperative outcomes following removal of cystic vestibular schwannomas: surgical experience over a decade and review of the literature. Neurosurg Focus. 2012;33(3):E13. doi: 10.3171/2012.7.FOCUS12206. [DOI] [PubMed] [Google Scholar]
- 30.Ansari S F, Terry C, Cohen-Gadol A A. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus. 2012;33(3):E14. doi: 10.3171/2012.6.FOCUS12163. [DOI] [PubMed] [Google Scholar]


