Abstract
Objective
The objective of this study was to evaluate hearing outcomes following middle fossa (MF) or retrosigmoid (RS) craniotomy for vestibular schwannoma (VS) removal with the goal of hearing preservation.
Design
This is a retrospective series.
Setting
This study was set at a skull base referral center.
Participants
In this study, 377 sporadic VS patients underwent primary microsurgery for VS from 2002 to 2012 using the MF (n = 305) or RS (n = 72) approaches.
Main Outcome Measures
The main outcome measures were change in pure-tone average (PTA) and word recognition score from pre- to postoperative and surgical complications.
Results
Preoperative hearing did not differ between approaches. Tumors were larger in the RS group (mean = 1.78 cm) than the MF group (mean = 0.97 cm) (p ≤ 0.001). Mean times to last audiometric follow-up were MF 1.0 year and RS 0.7 years. Mean decline in hearing from preoperative to last follow-up was greater in the RS group (55.5 dB in PTA and 45.6% in discrimination) than the MF group (38.9 dB and 31.7%) (p ≤ 0.011 and 0.033, respectively). The effect of surgical approach on hearing outcome remained after controlling for tumor size. Facial nerve outcomes and cerebrospinal fluid leak rates were not significantly different.
Conclusion
Loss of hearing was greater with the RS approach than the MF approach, even when accounting for differences in tumor size. Postoperative facial nerve function and other complications did not differ between approaches.
Keywords: vestibular schwannoma, acoustic neuroma, middle fossa craniotomy, retrosigmoid craniotomy, hearing preservation
Introduction
Surgical strategies for acoustic neuroma include the middle fossa (MF) approach and retrosigmoid (RS) approach when preservation of hearing is considered a worthwhile objective.1 2 The MF approach was developed by Dr. William House in 1961 to approach the internal auditory canal (IAC) and cerebellopontine angle (CPA) for decompression of the IAC in cases of extensive otosclerosis, and he later applied it to resection of vestibular schwannomas (VSs).3 It is typically used for tumors 2 cm and smaller in size when preoperative hearing exists. Reported success of hearing preservation ranges from 55 to 70%.4 5 6
RS craniotomy for hearing preservation is another approach used with good success for small tumors of the IAC and tumors at the CPA.7 Our clinical algorithm for performing a hearing preservation surgery includes both surgical approaches, with selection based on tumor location, patient age, size of tumor, and hearing classification.
Limited data are available comparing these approaches, with few factors predicting hearing preservation.8 9 10 Our goal was to evaluate surgical and hearing outcomes following MF or RS craniotomy for VS removal in non-NF2 patients undergoing primary microsurgery for VS. We report the largest combined series to date from a single institution comparing these surgical modalities.
Methods
A retrospective chart review was performed of patients undergoing MF craniotomy or RS craniotomy for removal of non-NF2 VS with the goal of hearing preservation between 2002 and 2012. Institutional review board approval was obtained (St. Vincent Medical Center, 07–033).
Patient Selection
All patients in the study elected a hearing preservation surgical strategy. The recommendation to use a hearing preservation approach includes consideration of tumor size, pure-tone average (PTA), and word recognition score (WRS). Typically, a hearing preservation strategy is recommended for patients with a PTA of less than 50 dB and WRS of greater than 50%. Tumors greater than 2.5 cm in largest dimension are not considered for hearing preservation as outcomes tend to be poor in patients with larger tumors.11 At our institution, the decision to proceed via either the MF or the RS approach was made based on tumor size and configuration. MF resection was considered for tumors localized primarily within the IAC or with extension into the CPA measuring less than 1 cm. RS resection was considered for tumors localized primarily in the medial IAC and the CPA. Tumors with CPA measurements larger than 1 cm were considered suitable for resection via the RS approach but not the MF approach.
A subset of patients with tumors less than 2.5 cm in greatest dimension, but with CPA components greater than 1 cm along with extension all the way to the fundus of the IAC, were considered poor candidates for hearing preservation surgery. Some of these patients elected to proceed with a hearing preservation surgery, using either the MF or the RS approach, despite understanding the caveats regarding the likelihood of hearing preservation and/or increased risks of cranial neuropathies or other potential complications.
For the group of patients with tumors thought to be good candidates for hearing preservation, treatment was individualized based on either the specific appearance of the tumor on MR imaging or patient preference. There is no way to reliably systematize the method of this individualization of care.
Subjects
From 2002 to 2012, 305 non-NF2 patients with unilateral VS underwent tumor removal by MF, while 72 underwent a RS approach. Eight neurotologists and three neurosurgeons performed the procedures. Patient characteristics were similar between groups (Table 1). However, mean tumor size was smaller in patients undergoing MF (1.0 cm) compared with RS (1. 8 cm) (p ≤ 0.001). Presenting symptoms are also shown in Table 1. Dizziness was more commonly reported as a presenting symptom in the MF group than the RS group, while headache was more common preoperatively in patients undergoing RS. Duration of any preoperative symptoms did not differ between groups, with means for both groups of 18 months. Figs. 1 and 2 display the baseline PTA and WRS values for the MF and RS cohorts, respectively, using the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) scattergram format. Mean preoperative PTA and WRS did not differ between groups.
Table 1. Patient characteristics for the MF and RS groups.
| MF (n = 305) | RS (n = 72) | Statistical significance | |
|---|---|---|---|
| Age (y, mean [SD]) | 48.9 (10.4) | 47.4 (10.7) | NS |
| Sex (% male) | 51.8% | 40.3% | NS (p ≤ 0.089) |
| Ear (% right) | 45.2% | 48.6% | NS |
| Tumor size (cm, mean [SD]) | 1.0 (0.36) | 1. 8 (0.38) | p ≤ 0.001 |
| Duration Sx (mo, mean [SD]) | 18.1 (30.0) | 18.0 (28.46) | NS |
| Presenting symptoms | |||
| Hearing loss | 83.9% | 75.0% | NS (p ≤ 0.09) |
| Dizzy | 41.0% | 25.0% | 0.015 |
| Tinnitus | 77.0% | 73.6% | NS |
| Headache | 16.7% | 27.8% | p ≤ 0.043 |
| Paresthesia | 0.7% | 2.8% | NS |
Abbreviations: MF, middle fossa; NS, not significant; RS, retrosigmoid; SD, standard deviation; Sx, symptoms.
Fig. 1.
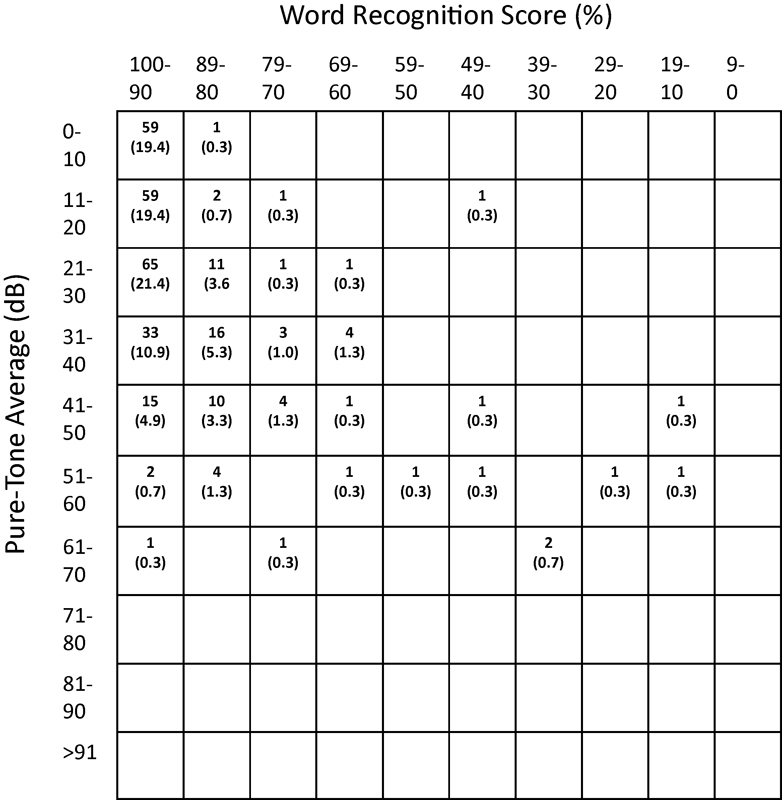
Preoperative hearing for the middle fossa patients. Cells show the number (%) of individual patients with that combination of PTA and WRS. PTA, pure-tone average; WRS, word recognition score.
Fig. 2.
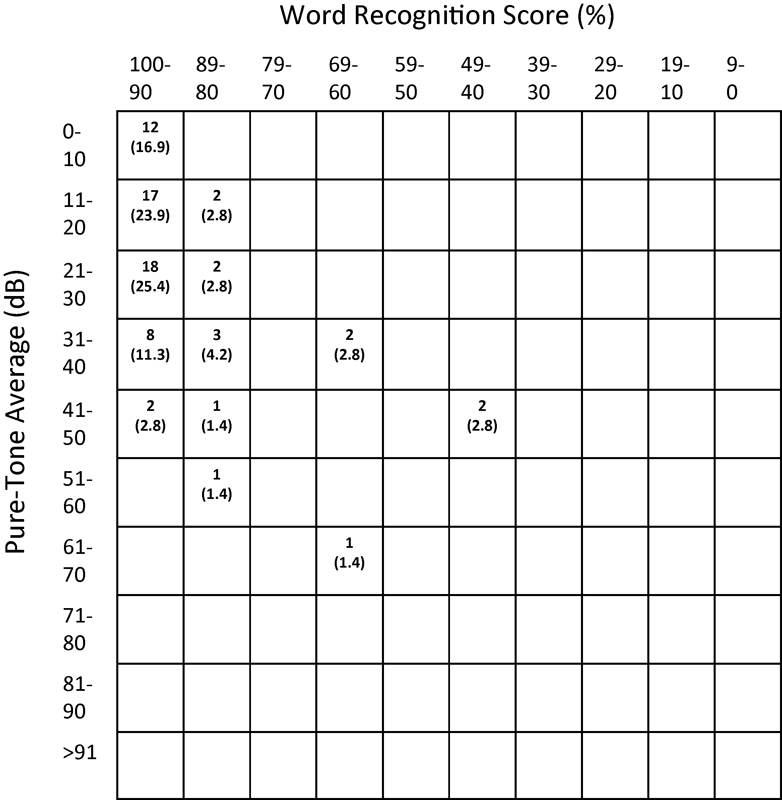
Preoperative hearing for the retrosigmoid patients. Cells show the number (%) of individual patients with that combination of PTA and WRS. PTA, pure-tone average; WRS, word recognition score.
Surgery
Both surgical approaches have been well-described elsewhere.12 13 All patients received intraoperative facial electromyography (EMG) and auditory brain stem response (ABR) monitoring. Intraoperative electrocochleography (ECoG) was used in one RS case. Additional direct eighth nerve monitoring (DENM) was used in 46 MF and 2 RS cases.
At our institution, a standard set of case report forms are completed for all tumor removal surgical cases and entered into an ongoing database. Patients meeting the criteria for this study were identified and preoperative patient demographics and findings at the time of surgery, pre- and postoperative hearing test results, preoperative and 1-year postoperative facial nerve function using the House–Brackmann (H-B) grading system,14 postoperative complications, and extent of resection were obtained from the database. The database includes greatest diameter of tumor, including CPA and IAC portions on magnetic resonance imaging (MRI). Other measurements, including volumetric tumor quantification were not captured.
Audiometric Assessment
Standard PTA and WRS testing (NU-6 prerecorded 25-word lists) are performed for all patients. The preoperative, first postoperative, and last available audiogram were used for this study.
Data Analysis
Data were obtained directly from the tumor database and imported into a statistical program for analysis. Four-frequency PTAs (500, 1,000, 2,000, and 3,000 Hz) were computed for preoperative, postoperative (3–6 weeks), and last available audiometric follow-up. AAO-HNS guidelines for reporting hearing outcomes were followed and the appropriate scattergrams were generated.15 Both parametric and nonparametric statistics were used, as appropriate, to compare the two groups, including chi-square for categorical data and t-tests, correlation, and analysis of variance (ANOVA) for continuous/interval level data. ANOVA with covariate was used to control for tumor size in some analyses. Criterion for statistical significance was set at p ≤ 0.05, two tailed.
Results
Surgical observations are presented in Table 2. Rates of gross total and partial resection did not differ between groups, with the vast majority of patients having total tumor removal. Tumor origin could not be determined (unknown) in 18.4% of cases in the MF cohort and 38.9% of the RS cohort.
Table 2. Operative findings and characteristics.
| MF (n = 305) | RS (n = 72) | Statistical significance | |
|---|---|---|---|
| Removal | NS | ||
| Gross total | 97.0% | 93.1% | |
| Partial/elected | 3.0% | 6.9% | |
| Tumor origin | |||
| Superior vestibular | 36.1% | 26.4% | |
| Inferior vestibular | 44.6% | 33.3% | |
| Cochlear | 0.3% | 0.0% | |
| Unknown | 18.4% | 38.9% | p ≤ 0.005a |
| Other | 0.7% | 1.4% | |
| OR time (h, mean [SD]) | 4.0 (0.78) | 5.2 (1.02) | p ≤ 0.001 |
| Blood loss (mL, mean [SD]) | 291.9 (133) | 251.0 (74.8) | p ≤ 0.013 |
| Hospital stay (d, mean [SD]) | 4.3 (1.3) | 4.1 (0.9) | NS |
| Adherence to facial nerve | p ≤ 0.012 | ||
| None | 10.5% | 4.2% | |
| Minimal | 45.2% | 30.6% | |
| Moderate | 35.7% | 51.4% | |
| Severe | 8.5% | 13.9% | |
| Facial nerve intact | 99.7% | 100% | NS |
Abbreviations: MF, middle fossa; NS, not significant; RS, retrosigmoid; SD, standard deviation.
Unknown versus all others.
Operative time was shorter for the MF group (4.0 hours) than the RS group (5.2 hours) (p ≤ 0.001), but mean blood loss was greater 292 versus, 251 mL, respectively, p ≤ 0.013). Cerebrospinal fluid (CSF) leak rates were 5.9% in the MF group and 1.4% in the RS group, not a significant difference. Only one patient in the MF group required a second surgery to control a CSF leak.
Tumor adherence to the facial nerve was categorized at the time of surgery as none, minimal, moderate, or severe. Adherence categorized as none or minimal was more common in the MF group (p ≤ 0.012). The facial nerve was intact after surgery in nearly all cases. Table 3 shows the immediate postoperative and 1-year facial nerve grade results. A good result (H-B grade I or II) was achieved at 1 year in 97.1 and 100% of patients in the MF and RS groups, respectively, not a significant difference. However, the RS group did have a significantly greater percentage of H-B grade I (p ≤ 0.004).
Table 3. H-B facial nerve grade immediate and 1-year postoperative.
| H-B grade | MF | RS | Statistical significance |
|---|---|---|---|
| Immediate postoperative (MF, n = 305; RS, n = 72) | |||
| I | 88.2 | 97.2 | p ≤ 0.027 |
| II | 6.9 | 2.8 | Good (I/II) NS (p ≤ 0.086) |
| III | 1.6 | 0.0 | |
| IV | 1.0 | 0.0 | |
| V | 1.3 | 0.0 | |
| VI | 1.0 | 0.0 | |
| One year postoperative (MF, n = 241; RS, n = 59) | |||
| I | 88.8% | 100% | p ≤ 0.004 |
| II | 8.3% | 0.0% | Good (I/II) NS |
| III | 1.7% | 0.0% | |
| IV | 1.2% | 0.0% | |
| V | 0.0% | 0.0% | |
| VI | 0.0% | 0.0% | |
Abbreviations: H-B, House–Brackmann; MF, middle fossa; NS, not significant; RS, retrosigmoid.
Standard intraoperative monitoring protocols include both facial nerve EMG and ABR monitoring. Rates and outcomes of monitoring are described in Table 4. ECoG was used for a single RS case. Additional DENM was used in 15.1% of MF cases and 2.8% of RS cases (p ≤ 0.001). Eighth nerve responses during surgery, determined either by ABR, EcoG, or DENM, were characterized as the same, poorer, or disappeared when compared with preoperative monitoring. The RS group more commonly had eighth nerve responses that were poorer or disappeared during surgery (p ≤ 0.001).
Table 4. Reported rates and outcomes of intraoperative monitoring.
| MF | RS | Statistical significance | |
|---|---|---|---|
| Facial Nerve EMG | 100% | 100% | NS |
| Eighth nervea | |||
| ABR | 85.9% | 90.3% | NS |
| EcoG | 0.0% | 1.4% | NS |
| DENM | 15.1% | 2.8% | p ≤ 0.001 |
| Eighth nerve responseb | (n = 223) | (n = 61) | p ≤ 0.001 |
| Same | 58.7% | 24.6% | |
| Poorer | 14.3% | 27.9% | |
| Disappeared | 26.9% | 47.5% | |
Abbreviations: ABR, auditory brain stem response; DENM, direct eighth nerve monitoring; ECoG, electrocochleography; EMG, electromyography; MF, middle fossa; NS, not significant; RS, retrosigmoid.
Percentage represents those for whom the answer was known, with subjects for whom the data were not reported included as “No.”
Combined data for ABR, ECoG, and DENM monitoring.
Hearing change outcomes are illustrated in Figs. 3 and 4, with mean changes shown in Table 5. As noted previously, preoperative hearing did not differ between groups. The last audiometric follow-up occurred at a mean of 1 year and 0.7 years for MF and RS, respectively, not a significant difference. There was considerable variability in time to last follow-up, ranging from no available postoperative audiological test (15% for MF and 25% for RS) or immediate postop (∼1–2 weeks, with no later follow-up for dead ears) to more than 8 years. A decline in mean hearing from preoperative levels to last follow-up was significantly greater in the RS group (55.5 dB in PTA and 44.6% in WRS) than the MF group (38.9 dB and 30.4%) (p ≤ 0.011 and 0.033, respectively). Hearing declined more than 40 dB/40% in 27.5% of MF cases and 41.3% of RS cases, yielding “dead ears” in 29.0 and 40.7%, respectively, a difference approaching statistical significance. The effect of surgical approach on hearing outcome remained even when statistically controlling for the influence of tumor size. In a subgroup analysis of the MF cohort, use of DENM was not associated with superior hearing outcomes. In cases in which DENM was used, the nerve response was poorer or disappeared in 57.8% compared with 64.0% of those cases in which it was not used, not a statistically significant difference.
Fig. 3.
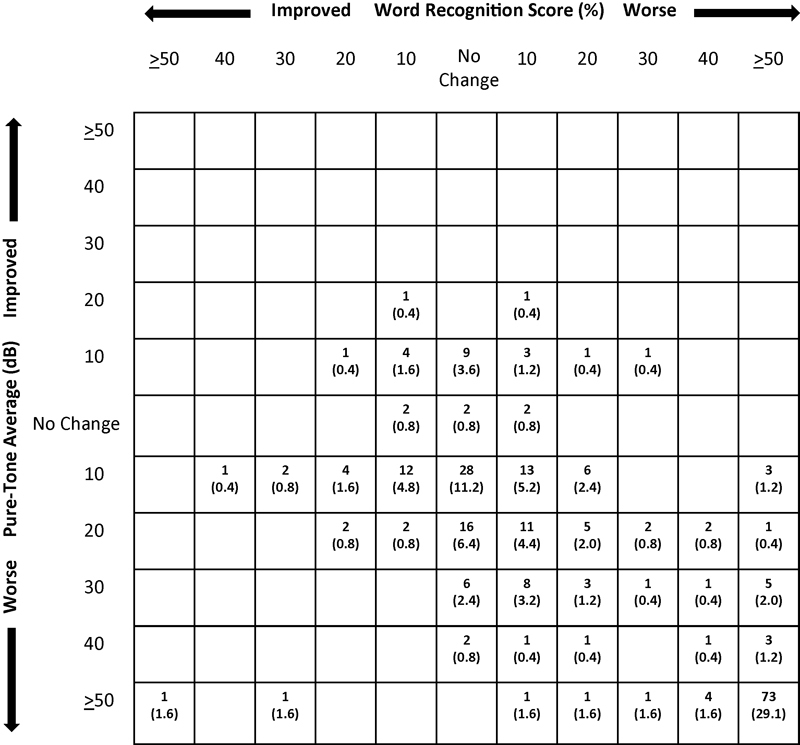
Postoperative scattergram showing the distribution of change in hearing from preoperative to last available follow-up for the middle fossa patients. The number (%) of patients who had a decrease in hearing in WRS, PTA, or both is represented in the boxes of the right lower quadrant. PTA, pure-tone average; WRS, word recognition score.
Fig. 4.
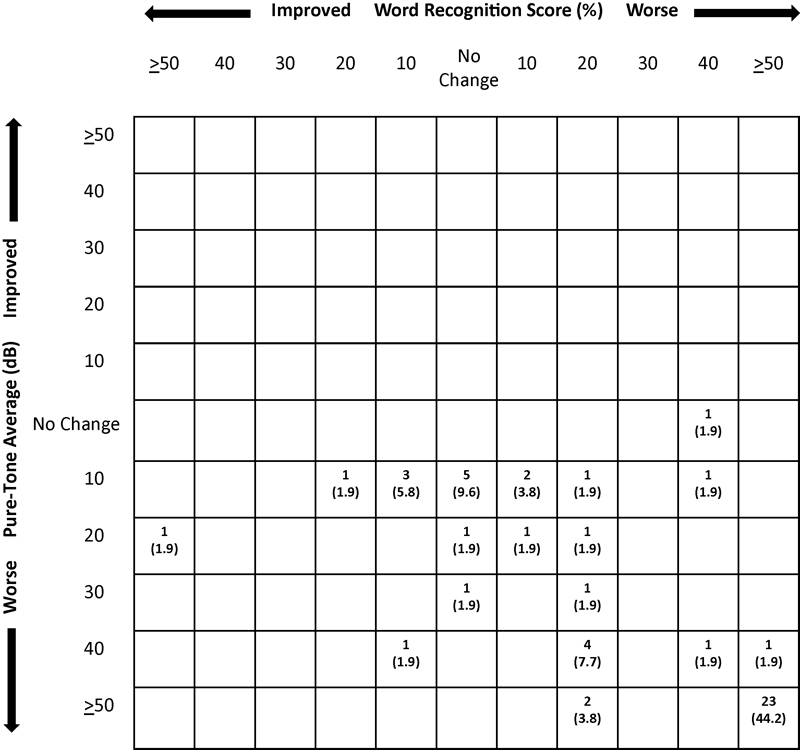
Postoperative scattergram showing the distribution of change in hearing from preoperative to last available follow-up for retrosigmoid patients. The number (%) of patients who had a decrease in hearing in WRS, PTA, or both is represented in the boxes of the right lower quadrant. PTA, pure-tone average; WRS, word recognition score.
Table 5. Hearing outcomes; mean (standard deviation).
| MF | RS | Statistical significance | |
|---|---|---|---|
| Preoperative PTA (dB) | 25.3 (14.6) | 25.5 (15.5) | NS |
| Preoperative WRS (%) | 92.6 (13.4) | 93.1 (11.6) | NS |
| Time to last audio (y) | 1.0 (1.7) | 0.7 (1.2) | NS |
| Last PTA (dB) | 64.4 (44.3) | 81.3 (45.7) | p ≤ 0.012 |
| Last WRS (%) | 60.8 (43.8) | 46.3 (43.7) | p ≤ 0.028 |
| Δ Prelast PTA (dB) | − 38.9 (43.1) | − 55.5 (44.1) | p ≤ 0.011 |
| Δ Prelast WRS (%) | 30.4 (42.1) | 44.6 (43.0) | p ≤ 0.033 |
| Dead ear (PTA > 120 dB) | 29.0% | 40.7% | NS (p ≤ 0.089) |
Abbreviations: MF, middle fossa; NS, not significant; PTA, pure-tone average; RS, retrosigmoid; WRS, word recognition score.
Postoperative rates of CSF leak, meningitis, and tumor recurrence at the last clinical evaluation were recorded. CSF leak occurred in 5.9% of the MF group and 1.4% of the RS group, a difference that was not statistically significant. No patients in either group developed meningitis. Tumor recurrence rates were classified as no residual/recurrence, residual/recurrence, or indeterminate by MRI. Overall, the rate of residual/recurrence was 5.3% and the indeterminate rate was 4.6%. Residual/recurrence rates did not differ significantly between MF and RS groups (MF 4.4% and RS 8.8%).
Discussion
Our data represent a large single institution series comparing the use of MF and RS approaches for resection of VSs with the goal of hearing preservation. Loss of hearing following tumor removal was greater on average with the RS approach than the MF approach, even when controlling for differences in tumor size. Long-term facial nerve outcomes and the incidence of complications such as CSF leak did not differ between approaches.
These surgical modalities also have been compared in several smaller series. Irving et al reported the results of 100 hearing preservation surgeries (50 MF cases and 50 RS cases).16 Consistent with our findings, audiological outcomes were superior in the MF group, with 52% of patients achieving AAO-HNS criteria class B or better hearing compared with 14% of the RS group. Similarly, Staecker et al illustrated superiority of the MF approach with a hearing preservation rate of 57% in the MF group versus 47% in the RS.9 Both of these reports suggest equivalent long-term facial nerve outcomes, consistent with our findings.
Findings in the current study suggest that differences in audiological outcomes are due to events that occur intraoperatively. Patients in the RS group more commonly had eighth nerve responses (ABR, ECoG, and DENM) that were poorer or disappeared during surgery. Several factors relating to surgical approach may account for differences in hearing outcomes. Expertise with a particular surgical modality could be considered but is unlikely. Our group has used both approaches for hearing preservation cases for approximately two decades, although the MF approach is more commonly performed.
Differential rates of intraoperative monitoring are also unlikely to account for superior audiological outcomes among the MF group. Rates of DENM were higher in the MF group. Although the use of DENM has been shown to be associated with higher rates of hearing preservation during VS resection when compared with ABR,17 other reports fail to support these findings.18 In subgroup analyses of our MF cohort, use of DENM was not significantly associated with amount of change in hearing, suggesting that different rates of monitoring did not produce differential outcomes.
Differences in surgical dissection and tumor visualization may be important factors. Data suggest that while tumor origin was more commonly the inferior vestibular nerve in both groups, the nerve of origin could not be determined in a greater number of RS cases—suggesting improved tumor visualization through the MF approach. Clinical and anatomical studies support improved visualization of the fundus through the MF compared with the RS approach, with lateral dissection limited by the posterior semicircular canal is the RS approach.16 19 Tumors that involve the fundus are associated with poor hearing outcomes through either the MF or RS approach.20 21 22 23 Improved tumor visualization may be an important factor in the superior audiological outcomes in the MF group.
Facial nerve outcomes after 1 year were H-B grade I or II in 97.1 and 100% of patients in the MF and RS groups, respectively. Although these rates of good results were not statistically different, the rate of just grade I was higher in the RS group, findings that may have some clinical significance for patients with poorer outcomes in the MF group. In addition, immediate postoperative H-B grade was superior in the RS group, consistent with previous reports.10 16 The MF approach provides early and often direct visualization of the facial nerve during tumor resection. However, a greater degree of manipulation of the facial nerve may be required for tumor resection because the facial nerve often lies in a plane superior to the cochlear nerve and tumor. These principles may account for initial superior facial nerve outcomes in the RS group. Visualization of the facial nerve in the MF approach may also account for the operative finding that tumor adherence to the facial nerve is more frequently characterized as none or minimal in the MF group.
Other factors that may influence the use of a particular surgical approach include complication rates such as CSF leak and surgical considerations such as blood loss, surgical time, and tumor recurrence rates. Although blood loss was greater in the MF group and operative time was greater in the RS group, these factors are not sufficient to counsel for a particular surgical modality. In addition, other centers report longer operative times with MF, suggesting variability among groups.10 Rates of important adverse events such as CSF leak and tumor recurrence were minimal and did not differ between approaches.
As with all retrospective studies, there are several caveats with this study. Previous data show that hearing preservation rates with the MF approach are associated with better preoperative hearing, shorter intra-aural wave V latency, shorter absolute wave V latency, superior vestibular nerve origin, and the presence of fundal fluid.5 22 Our study did not evaluate preoperative ABR findings or the presence of fundal fluid, which could, therefore, be differently represented between MF and RS groups. Our database captured the greatest diameter of tumor, including CPA and IAC portions; however, volumetric analysis was not performed, which could be an important factor for tumor size comparisons.
In addition, extent of intracanalicular or CPA extension was not compared directly between the two groups, and these are clearly factors that may influence hearing outcomes. Individualization of care, without strict criteria assigning patients to one surgical approach or the other, limits our ability to directly compare the effects of treatment with like groups. Given the nature of the disease, the numerous treatment options available, and the patient's inherent role in decision making, it is unlikely that wholly direct comparisons, with possible biases controlled (i.e., random assignment to treatment), is feasible. However, by using data previously entered into an ongoing database, we limited possible reporting or evaluation bias. We also did not use quality of life surveys in the assessment of outcomes, vestibular outcomes, or rates of postoperative headaches. A prospective study is planned at our institution that will incorporate such instruments.
Conclusion
MF craniotomy as a hearing preservation strategy for VS showed superior hearing outcomes when compared with a RS approach. This difference was independent of the influence of tumor size. However, the RS approach remains the approach of choice for tumors that are too large for MF without extension to the fundus. There is a higher risk of transient facial paralysis and minimally poorer 1-year facial nerve grade after MF surgery. Risk of CSF leak did not differ significantly between approaches. This study supports the continued use of both approaches in the management of selected patients with VS where hearing preservation is a goal and may help in the decision-making process for individual patients.
Acknowledgment
The authors are grateful to Karen I. Berliner, PhD, for statistical and editorial assistance.
Note
Eric P. Wilkinson and Daniel S. Roberts contributed equally.
References
- 1.Slattery W H III, Brackmann D E, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol. 1997;18(5):596–601. [PubMed] [Google Scholar]
- 2.Rowed D W, Nedzelski J M. Hearing preservation in the removal of intracanalicular acoustic neuromas via the retrosigmoid approach. J Neurosurg. 1997;86(3):456–461. doi: 10.3171/jns.1997.86.3.0456. [DOI] [PubMed] [Google Scholar]
- 3.House W F. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. Laryngoscope. 1961;71:1363–1385. doi: 10.1288/00005537-196111000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Shelton C Hitselberger W E House W F Brackmann D E Hearing preservation after acoustic tumor removal: long-term results Laryngoscope 1990100(2 Pt 1):115–119. [DOI] [PubMed] [Google Scholar]
- 5.Brackmann D E, Owens R M, Friedman R A. et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21(3):417–424. doi: 10.1016/s0196-0709(00)80054-x. [DOI] [PubMed] [Google Scholar]
- 6.Quist T S, Givens D J, Gurgel R K, Chamoun R, Shelton C. Hearing preservation after middle fossa vestibular schwannoma removal: are the results durable? Otolaryngol Head Neck Surg. 2015;152(4):706–711. doi: 10.1177/0194599814567874. [DOI] [PubMed] [Google Scholar]
- 7.Yamakami I, Ito S, Higuchi Y. Retrosigmoid removal of small acoustic neuroma: curative tumor removal with preservation of function. J Neurosurg. 2014;121(3):554–563. doi: 10.3171/2014.6.JNS132471. [DOI] [PubMed] [Google Scholar]
- 8.Phillips D J, Kobylarz E J, De Peralta E T, Stieg P E, Selesnick S H. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010;31(9):1463–1468. [PubMed] [Google Scholar]
- 9.Staecker H, Nadol J B Jr, Ojeman R, Ronner S, McKenna M J. Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am J Otol. 2000;21(3):399–404. doi: 10.1016/s0196-0709(00)80051-4. [DOI] [PubMed] [Google Scholar]
- 10.Sameshima T Fukushima T McElveen J T Jr Friedman A H Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach Neurosurgery 2010673640–644., discussion 644–645 [DOI] [PubMed] [Google Scholar]
- 11.Yates P D, Jackler R K, Satar B, Pitts L H, Oghalai J S. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol. 2003;24(3):460–464. doi: 10.1097/00129492-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Friedman R A Fayad J N The middle cranial fossa approach to vestibular schwannomas In: Lateral Skull Base Surgery: House Clinic Atlas. New York, NY: Theime; 2012 [Google Scholar]
- 13.Fayad J N Friedman R A Schwartz M S The retrosigmoid approach In: Lateral Skull Base Surgery: House Clinic Atlas. New York, NY: Theime; 2012 [Google Scholar]
- 14.House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 15.Gurgel R K, Jackler R K, Dobie R A, Popelka G R. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg. 2012;147(5):803–807. doi: 10.1177/0194599812458401. [DOI] [PubMed] [Google Scholar]
- 16.Irving R M, Jackler R K, Pitts L H. Hearing preservation in patients undergoing vestibular schwannoma surgery: comparison of middle fossa and retrosigmoid approaches. J Neurosurg. 1998;88(5):840–845. doi: 10.3171/jns.1998.88.5.0840. [DOI] [PubMed] [Google Scholar]
- 17.Danner C, Mastrodimos B, Cueva R A. A comparison of direct eighth nerve monitoring and auditory brainstem response in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2004;25(5):826–832. doi: 10.1097/00129492-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo E, Hiraumi H, Hamada M, Russo A, De Stefano A, Sanna M. Intraoperative cochlear nerve monitoring in vestibular schwannoma surgery—does it really affect hearing outcome? Audiol Neurootol. 2008;13(1):58–64. doi: 10.1159/000108623. [DOI] [PubMed] [Google Scholar]
- 19.Blevins N H, Jackler R K. Exposure of the lateral extremity of the internal auditory canal through the retrosigmoid approach: a radioanatomic study. Otolaryngol Head Neck Surg. 1994;111(1):81–90. doi: 10.1177/019459989411100116. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald C B, Hirsch B E, Kamerer D B, Sekhar L. Acoustic neuroma surgery: predictive criteria for hearing preservation. Otolaryngol Head Neck Surg. 1991;104(1):128. doi: 10.1177/019459989110400123. [DOI] [PubMed] [Google Scholar]
- 21.Nadol J B Jr, Chiong C M, Ojemann R G. et al. Preservation of hearing and facial nerve function in resection of acoustic neuroma. Laryngoscope. 1992;102(10):1153–1158. doi: 10.1288/00005537-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Goddard J C, Schwartz M S, Friedman R A. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31(7):1128–1134. doi: 10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 23.Tringali S, Ferber-Viart C, Fuchsmann C, Buiret G, Zaouche S, Dubreuil C. Hearing preservation in retrosigmoid approach of small vestibular schwannomas: prognostic value of the degree of internal auditory canal filling. Otol Neurotol. 2010;31(9):1469–1472. [PubMed] [Google Scholar]


