Abstract
Objectives
This study aims to describe the types of anterior skull base defects following expanded endoscopic approaches (EEA) and to outline the techniques involved in the repair of these defects.
Design
We retrospectively analyzed 63 cases of endoscopic skull base reconstruction (ESBR) following tumor excision, done from September 2011 to January 2015. These tumors consisted of 14 pituitary adenomas, 20 craniopharyngiomas, and 29 other miscellaneous tumors. The classification of skull base defects by Tabaee et al and the classification of cerebrospinal fluid (CSF) leaks by Esposito et al were considered during the ESBR. Recurrence of CSF leak was considered as failure of reconstruction.
Results
The 63 skull base defects included in this study occurred following EEA for tumor excision. Failure of reconstruction occurred in 6 six patients. All were successfully repaired, however, three patients in this series died due to tumor-related complications.
Conclusion
The adherence to the general principles of reconstruction, appreciating the subtle differences in the nature of the various defects and the ability to adopt different strategies are the prerequisites for the successful closure of skull base defects.
Keywords: CSF leak, skull base, fovea ethmoidalis, cribriform plate, sphenopalatine artery
Introduction
The effectiveness of endoscopic anterior skull base surgery received a major enhancement with the introduction of vascular flaps that aided in the repair of large dural defects resulting after expanded endoscopic approaches (EEA). In this retrospective study, we highlight the general principles of reconstruction, the characteristics of various skull base defects and the application of various techniques for a successful repair.
Material and Methods
We retrospectively analyzed data from a prospectively collected database that included 63 patients undergoing an EEA for various anterior skull base pathologies. These included 14 pituitary adenomas, 20 craniopharyngiomas, and 29 other miscellaneous tumors as shown in Table 1. There were 40 males and 23 females, and 10 were children.
Table 1. Tumors of anterior skull base.
| Craniopharyngioma | 20 |
| Pituitary adenoma | 14 |
| Other miscellaneous tumors | 29 |
| Tuberculum sellae meningioma | 4 |
| Juvenile angiofibroma | 4 |
| Clival chordoma | 4 |
| Trigeminal schwannoma | 3 |
| Olfactory neuroblastoma | 3 |
| Planum sphenoidale meningioma | 2 |
| Neuroendocrine carcinoma | 2 |
| Inverted papilloma | 2 |
| Opticochiasmatic hypothalamic glioma | 1 |
| Adenoid cystic carcinoma | 1 |
| Teratocarcinoma | 1 |
| Astrocytoma | 1 |
| Nasal schwannoma | 1 |
Skull base defects were classified as (1) type I (small skull base defect), (2) type II A (sella defect without cerebrospinal fluid [CSF] leak), (3) type II B (sella defect with CSF leak), (4) type III A (EEA defect without ventricular communication), and (5) type III B (EEA defect with ventricular communication).1 The grade of CSF leak was classified as (1) grade 0 (no CSF leak), (2) grade I (low-pressure leak), (3) grade II (moderate-pressure leak), and (4) grade III (high-pressure leak).2 Anterior skull base defects involving the cribriform plate and fovea ethmoidalis and posterior wall of the frontal sinus were grouped as grade I leaks; defects involving the suprasellar region and the clivus as grade II leaks in view of their communication with suprasellar and prepontine cisterns, respectively, and defects communicating with the third ventricle following craniopharyngioma excision were considered as grade III leaks.
Watertight closure of the dural defect with underlay and overlay grafts followed by an overlay vascular flap placement was the cornerstone of our repair strategy. All EEA defects had placement of vascular flaps as an overlay layer except in three of our initial cases. The nasoseptal flap3 was the most common flap that was used in 54 cases. In a few cases, in addition to the nasoseptal flap, we placed the inferior turbinate flap4 for three revision cases (Fig. 1) and the middle turbinate flap5 in three pediatric cases. In one patient for whom a nasoseptal flap was not available after excision of an olfactory neuroblastoma, a lateral nasal wall flap from the floor of the nose, the entire lateral wall, and inferior turbinate, pedicled on the branches of the sphenopalatine artery, was successfully used. Five cases were addressed with a combined approach—open transcranial and EEA—due to the extent of tumor spread. We used the pericranial flap for skull base reconstruction following frontal craniotomy in two cases of anterior skull base malignancies with intracranial extension. Three cases of juvenile nasopharyngeal angiofibroma with intracranial extension underwent temporal craniotomy, and the temporoparietal flap was used for the repair of these skull base defects.
Fig. 1.
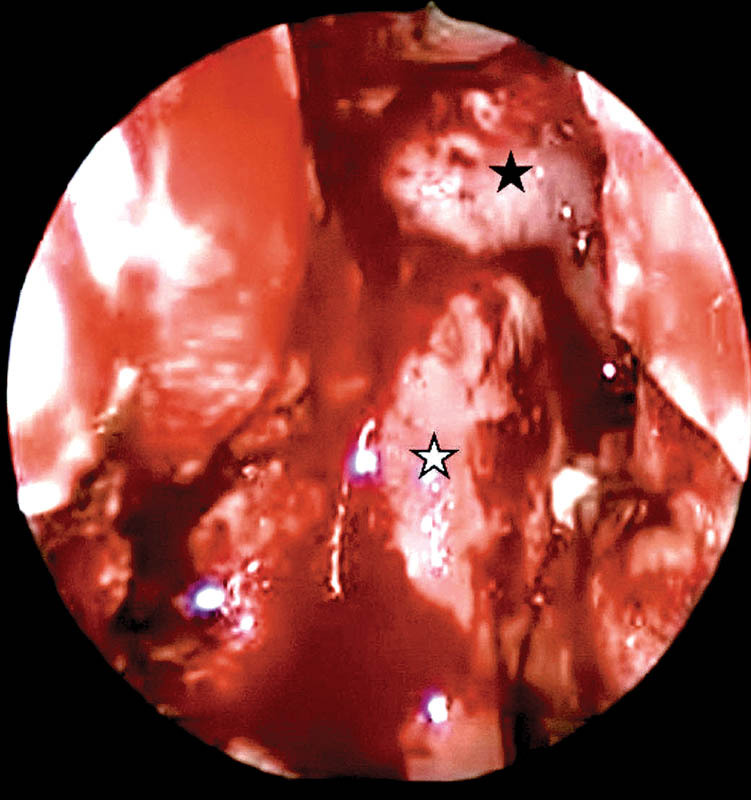
Intraoperative picture of the vascular flaps in skull base reconstruction. White star, inferior turbinate flap; black star, nasoseptal flap.
Results
There were 56 type III A skull base defects and 7 type III B skull base defects in this study. With regard to CSF leak, there were 17 grade 1 leaks, 39 grade II leaks, and 7 grade III leaks among the 63 patients.
Type III A skull base defects in this study had either a grade I or grade II leak depending upon the location. Grade I leaks were located in the anterior cranial fossa floor and grade II leaks in the suprasellar area. Type III B skull base defects, in view of their ventricular communication, were considered to have a grade III leak.
Grade I leaks or anterior cranial fossa floor defects were often the largest defects that occurred, extending from the posterior wall of the frontal sinus up to, but not involving the planum sphenoidale and from one lamina paparacea to the other lamina paparacea (Fig. 2). The primary pathologies in this group were the sinonasal malignancies involving the anterior skull base (Table 1). A multilayered grafting with placement of fascia lata as underlay and overlay grafts across the bony margins of the defect was used for closure of these defects (Fig. 3) followed by the vascular flap placement.
Fig. 2.
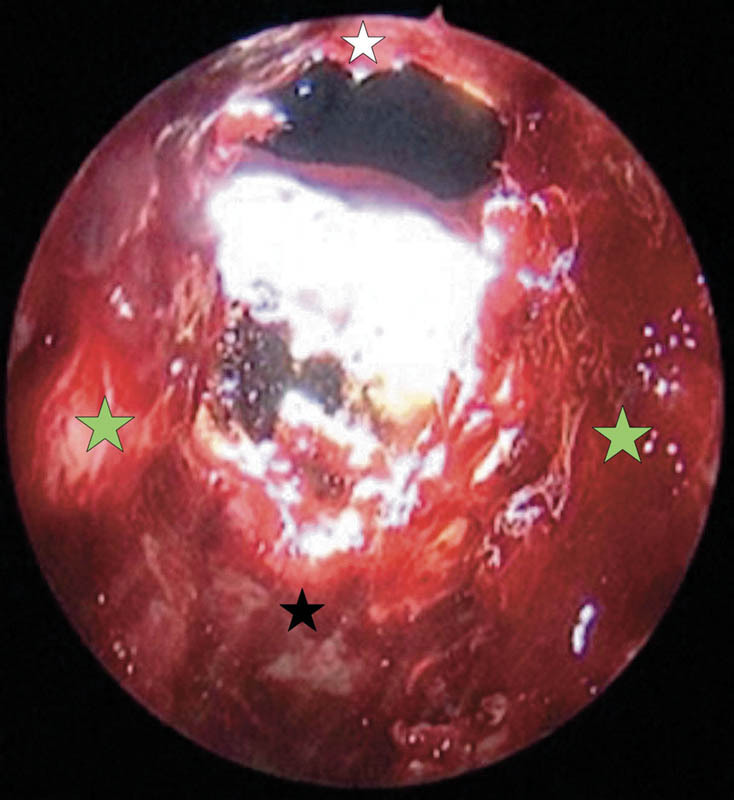
Postoperative anterior cranial fossa floor defect following a combined approach for anterior skull base malignancy excision. White star, posterior wall of frontal sinus; black star, planum sphenoidale; green star, lamina paparacea.
Fig. 3.
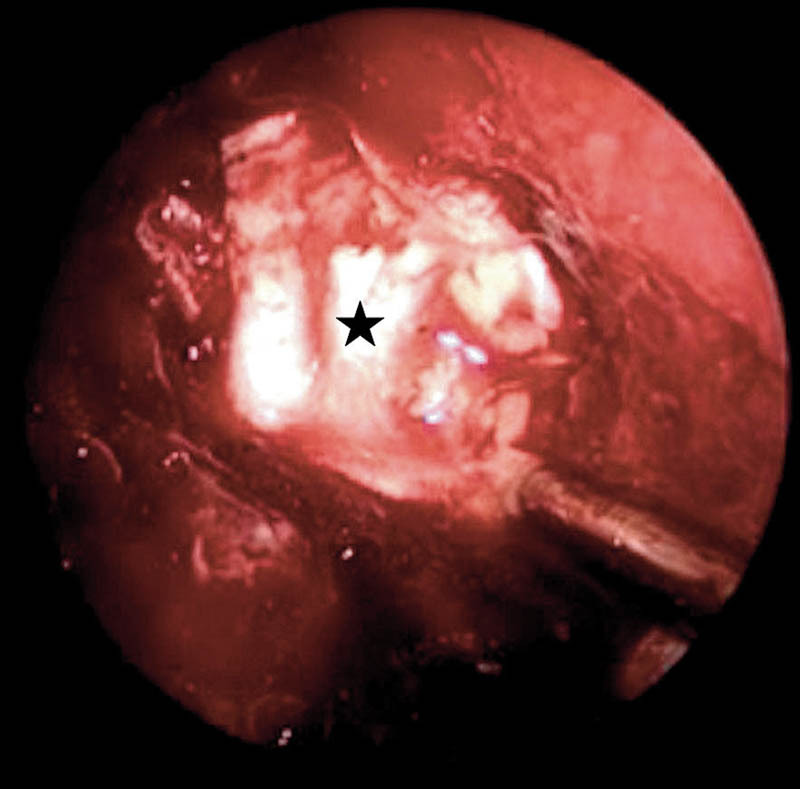
Postoperative anterior cranial fossa floor defect repair with multilayered grafting technique. Black star, fascia lata placed as overlay graft over the underlay grafts.
Grade II leaks or suprasellar defects were defects in the planum sphenoidale and tuberculum sellae (Fig. 4), and these were noted following excision of suprasellar lesions, namely, pituitary adenomas, craniopharyngiomas, and suprasellar meningiomas. These defects were repaired with the bath-plug technique6 (Fig. 5), where a fat graft is placed intradurally and is pulled out with a suture so that the fat graft tightly obliterates the defect. This technique, although originally described for smaller defects, was found to be reliable in larger defects and high-pressure grade II leaks of this series. Fascia lata was placed as an onlay graft over the fat and dural edges. The nasoseptal flap was then placed over the entire repair site onto the bone edges.
Fig. 4.
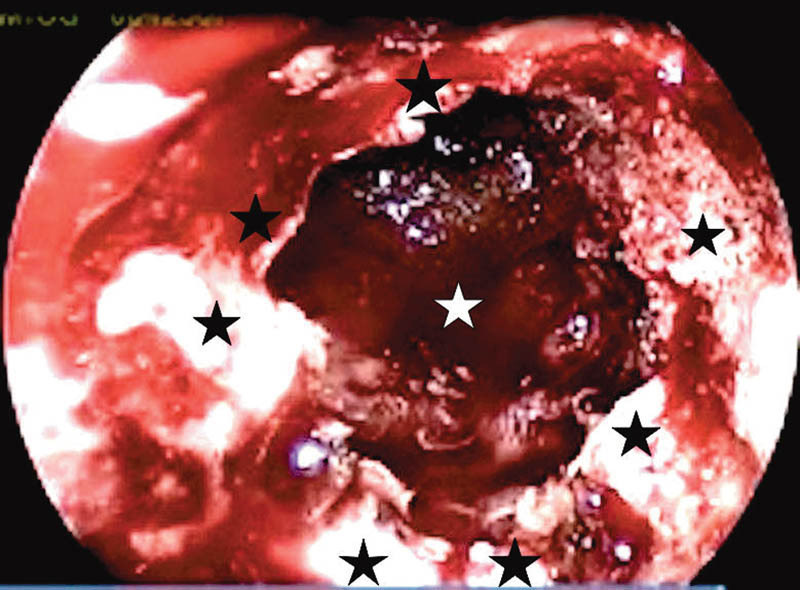
Postoperative suprasellar defect following tuberculum sellae meningioma excision. White star, suprasellar cisternal leak; black star, bony margins of the defect.
Fig. 5.
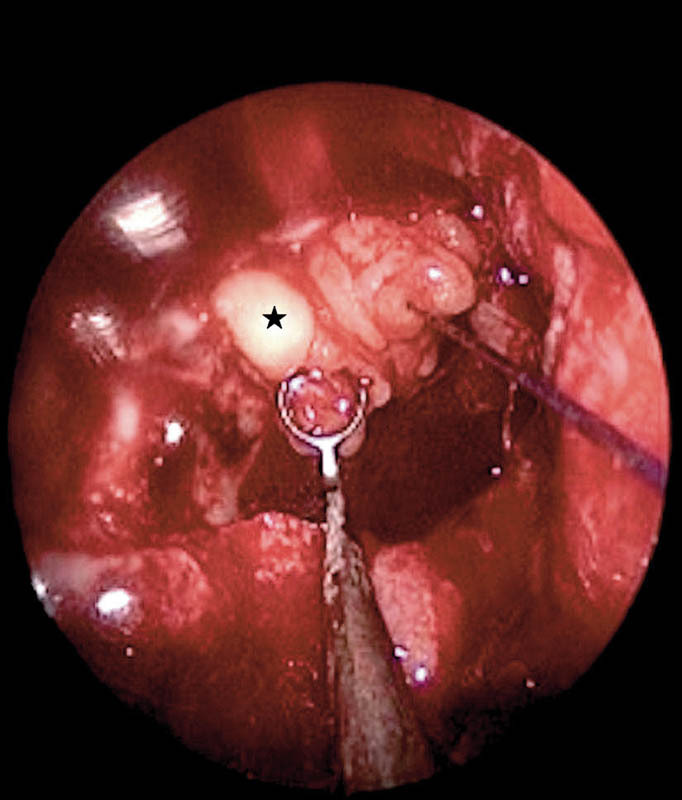
Suprasellar defect repair with bath-plug technique. Black star, fat bath plug tightly sealing the dural defect.
Grade III leaks or defects with third ventricular communication typically seen after craniopharyngioma excisions were the highest pressure leaks encountered (Fig. 6). We used the Gasket seal technique 7 for repair (Fig. 7) in two cases, where a flat piece of cartilage pushes the fascia lata into the defect and holds it in place ensuring a tight seal around the dural edges. However, in the other five cases, where the optic chiasm was closer to the defect, we used a small piece of fat within the defect, covered by fascia lata as an underlay graft and thereafter an overlay fascia lata graft. The pedicled nasoseptal flap was then placed over this area, directly in contact with bony margins of the defect.
Fig. 6.
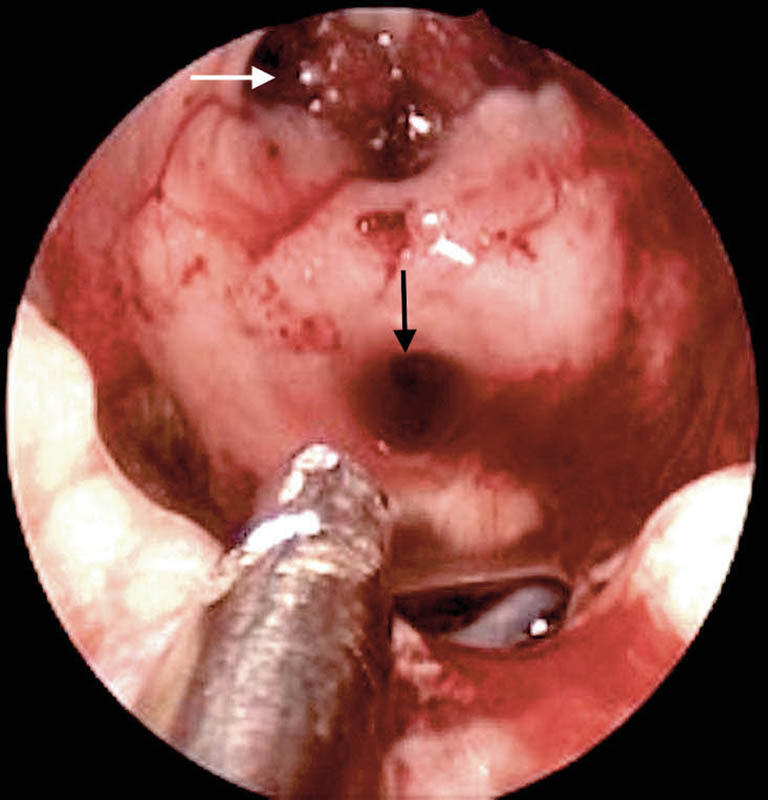
Postoperative defect with ventricular communication following a craniopharyngioma excision. White arrow, foramen of Monro; black arrow, cerebral aqueduct of Sylvius.
Fig. 7.
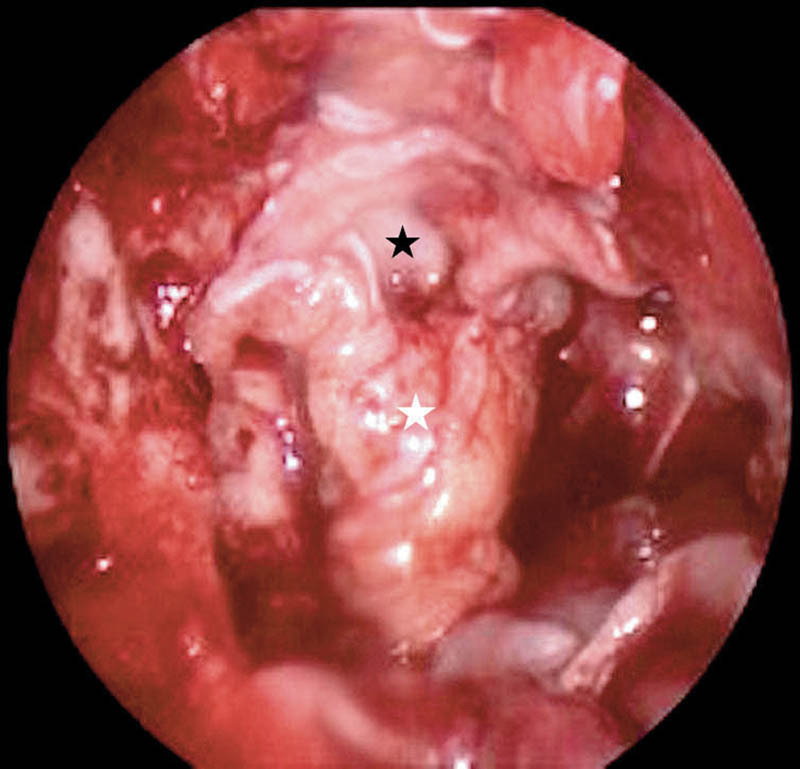
Gasket seal repair of the skull base defect with ventricular communication. White star, fascia lata; black star, septal cartilage pushing the fascia lata.
The vascular flap and repair site were then layered with Surgicel (Ethicon, Johnson & Johnson, United States), tissue glue, and Gelfoam (Pfizer, New York, United States), following which bilateral nasal packing was done with Merocel (Medtronic, United States) packs. A postoperative lumbar drain was placed in all cases and removed along with the nasal packs on the fifth postoperative day. Intravenous ceftriaxone was administered for 5 days. The patients underwent endoscopic nasal cleaning on the 10th and the 14th postoperative days and were kept on saline sprays, antihistamines, and laxatives for 3 months.
Failure of Reconstruction
Recurrence of CSF leak occurred in six patients due to failure of reconstruction. The commonest reason for failure, which occurred in four cases, was an inadequately sized or inappropriately positioned nasoseptal flap. These were rectified by placing additional vascular flaps—inferior turbinate flap in three cases and a transcranial pericranial flap placement in one case. The other two cases were repaired by using fat packing at the exact site of CSF leak. All patients with failure of reconstruction were successfully repaired (Table 2). There were three patients who died in this series. One patient with a craniopharyngioma had a delayed presentation of pneumocephalus at 3 months with a CSF leak due to necrosis of an edge of the nasoseptal flap. We repaired this with placement of fat, but the patient died of septicemia during the recovery period. Another patient with a craniopharyngioma had bleeding from a perforator to the hypothalamus during surgery and subsequently developed a stroke. He died a few months later due to complications of stroke but not due to the repair. The third patient with a chiasmatic hypothalamic glioma had postoperative hypothalamic shock, following which he had a cardiac arrest due to ventricular tachycardia on the third postoperative day.
Table 2. Failure of reconstruction.
| No. | Type of defect | Pathology | Presentation | Reason for failure | Remedial steps |
|---|---|---|---|---|---|
| 1 | Anterior cranial fossa floor defect | Olfactory neuroblastoma | CSF leak on fifth postoperative day following pack removal | Inadequate nasoseptal flap | Transcranial pericranial flap placement |
| 2 | Suprasellar defect | Craniopharyngioma | Meningitis on 14th postoperative day | Inadequate nasoseptal flap | Inferior turbinate flap augmentation |
| 3 | Suprasellar defect | Planum sphenoidale meningioma | CSF leak on fifth postoperative day following pack removal | Migration of bone chip placed to stabilize the fat used in dural defect repair | Fat bath-plug technique used for dural defect repair |
| 4 | Suprasellar defect | Craniopharyngioma | CSF leak on 14th postoperative day | Migration of nasoseptal flap edge | Flap replacement and inferior turbinate flap augmentation |
| 5 | Defect with ventricular communication | Craniopharyngioma | CSF leak on 14th postoperative day | Inadequate nasoseptal flap | Inferior turbinate flap augmentation |
| 6 | Defect with ventricular communication | Craniopharyngioma | Pneumocephalus after 3 months | Necrosis of the edge of nasoseptal flap | Fat plug insertion at the leak site |
Abbreviation: CSF, cerebrospinal fluid.
Discussion
The domain of skull base reconstruction has evolved considerably since the days of the pioneers Walter Dandy, Dohlman, and Oskar Hirsch, who performed the first skull base reconstructions via craniotomy (1926), extracranial approaches (1948), and endoscopic approaches (1952), respectively.8 The transition from the open craniotomy approaches to primarily an endoscopic approach occurred about two decades ago. With the introduction of modified endoscopic Lothrop and transpterygoid approaches, almost every region except the lateral most corners of the frontal sinus could be reached with an endoscopic approach. With the EEA, comes the responsibility of performing extensive repairs to prevent postoperative CSF leaks and meningitis. The technique used for repairing smaller defects with free grafts cannot be applied for larger defects following EEA, since the failure rate is high.8 The introduction of the nasoseptal flap has dramatically reduced the incidence of CSF leaks down to approximately 5 to 10%.9 In our series, the primary reconstruction failure rate was 9.5%, which is within the rate noted above; however, there is scope for improvement with certain centers reporting leak rates of less than 5%.8 10 11 12
In the planning for the method of reconstruction, we considered variables such as age of the patient, size, and location of the defect,8 a grade of CSF leak,8 risk factors such as Cushing disease, postradiotherapy status,8 internal carotid artery exposure,8 raised intracranial pressure, the approach (EEA vs. combined transcranial and EEA), and raised body mass index.13 We found the classifications of skull base defects1 and the grade of CSF leaks2 mentioned earlier useful for the planning of the ESBR. This concept helped us to evaluate the size of the defect and the gradient of the CSF leak. The reconstruction techniques mentioned by Lund et al8 were the basis for using vascular flaps in the ESBR.
When EEA is contemplated in children, the reconstruction has additional challenges as the pedicled flaps tend to be small, often necessitating multiple flaps to close the defect. A similar situation might arise in adults with very large skull base defects as the intranasal flaps can cover only two or three adjacent zones of the defect. The zones in which defect can occur during an EEA are the clivus, sella, tuberculum sellae, planum sphenoidale, cribriform plate, and posterior table of the frontal sinus. Hence, in children and in large skull base defects, we occasionally harvest a triple flap—nasoseptal, ipsilateral middle turbinate, and contralateral limited posterior nasoseptal flaps. This contralateral flap is essentially the nasal septal mucosal flap of the opposite side, harvested posterior to the septotomy site and instead of using it as a “reverse” flap,14 it is made into a smaller nasoseptal flap with the vascular pedicle based on the contralateral sphenopalatine artery. With this strategy, we have had no failure of reconstruction so far in children.
Anterior cranial fossa defects in our series were primarily defects following resection of anterior skull base malignancies. These were low-pressure defects and often presented as rectangular-shaped defects with no significant free space above the defect. Hence, a multilayered grafting technique with the placement of multiple layers of fascia lata gave good results, and the contact of the frontal lobe with the flat surface of the fascia acted as a reinforcement for the defect repair. We avoided the use of intradural fat packing in these defects to avoid the potential displacement of the fascia lata grafts, which could occur when the brain comes in contact with the fat placed above the grafts.
Suprasellar defects in our study were typically circumferential defects in the planum sphenoidale and the tuberculum sellae with a free space above the defect within the suprasellar cistern, making this a moderately high-pressure leak. These defects were repaired with an intradural fat graft after ensuring that we did not recreate the pressure effect of the tumor. The size of the fat plug was tailored to fill the empty space above the defect and was fashioned to be slightly broader than the width of the dural defect; it was then pulled out with a suture to bridge across the dural defect to prevent excess pressure on intracranial structures. The advantage of this bath-plug technique is that the CSF pulsations from the cisternal space cause the fat plug to close the defect more tightly around the onlay fascia lata. Placement of fascia lata alone across these defects may not be effective as they can get displaced by the CSF pulsations resulting in a failure.
High-pressure CSF leaks—type III B defect with grade III leak—typically have a higher tendency for failure because of their ventricular communication. We did not use the bath-plug technique as we felt the CSF pulsations at the repair site were significantly stronger. Hence, we resorted to placing a barrier (fat or cartilage) intradurally to absorb the main force of the CSF pulsations, thus protecting the dural edges of the defect. This allows the placement of multiple layers of fascia lata over the barrier as underlay and overlay grafts, which results in a tight seal at the edges of the defect. An adequate vascular flap cover is imperative in the closure of these defects and if needed, multiple flaps should be used without hesitation. We have experience in repairing seven such high-pressure leaks, of which two failed. We have noticed similar results of failure in reconstruction in other reports,1 9 though there are better rates of successful reconstruction mentioned in the literature.15
ESBR has become the mainstay in the repair of skull base defects, and its domain has enlarged considerably over the years to include larger and high-pressure defects. The role of the different variables involved in the repair of these defects, which we have noted above and their combined presence can make ESBR very challenging. The surgeons need to have significant knowledge regarding the nature of these defects and should be well versed with the various techniques of repair. The ability to harvest different types of vascular flaps—intranasal flaps and extranasal flaps—is essential to achieve good closure rates. These factors are of critical importance, perhaps even the determining factor between success and failure in the repair of these defects.
Conclusion
ESBR involves the repair of various types of skull base defects which differ significantly in their nature. While the principles of repair of smaller defects have been well established, the translation of these principles to defects resulting after EEA need not result in the same success rates. The larger and higher pressure leaks need much more planning, versatile techniques, and ability to innovate to account for the different variables involved in these defects.
Acknowledgment
The authors acknowledge Dr. Anand Job, Professor of ENT for his constant support and guidance for the EEA program, which the authors have been involved with since September 2011.
Note
The authors have no financial disclosures or conflict of interest to declare in submitting this article. This study has been cleared by the institutional review board.
The skull base reconstruction of the initial group of patients in this study was presented in the World Congress of Skull Base Surgery at Brighton, United Kingdom, May 2012. The abstract of the presentation was published in this journal—Successful dural repair following the endoscopic extended transsphenoidal approach for suprasellar meningiomas, Journal of Neurological Surgery—Skull Base, Supplement Number S 2, Volume 73, June 2012, page 120–121.
References
- 1.Tabaee A, Anand V K, Brown S M, Lin J W, Schwartz T H. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117(7):1133–1137. doi: 10.1097/MLG.0b013e31805c08c5. [DOI] [PubMed] [Google Scholar]
- 2.Esposito F Dusick J R Fatemi N Kelly D F Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery Neurosurgery 200760402295–303., discussion 303–304 [DOI] [PubMed] [Google Scholar]
- 3.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 4.Choby G W, Pinheiro-Neto C D, de Almeida J R. et al. Extended inferior turbinate flap for endoscopic reconstruction of skull base defects. J Neurol Surg B Skull Base. 2014;75(4):225–230. doi: 10.1055/s-0033-1358791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M R, Taylor R J, Hackman T G. et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(4):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 6.Wormald P J. New York: Thieme; 2005. Cerebrospinal fluid leak closure; pp. 109–118. [Google Scholar]
- 7.Garcia-Navarro V, Anand V K, Schwartz T H. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80(5):563–568. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Lund V, Stammberger H, Nicolai P. et al. European position paper on endoscopic management of tumours of the nose, paranasal sinus and skull base. Rhinology. Suppl. 2010 22:1–143. [PubMed] [Google Scholar]
- 9.Patel M R, Stadler M E, Snyderman C H. et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel K S, Komotar R J, Szentirmai O. et al. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg. 2013;119(3):661–668. doi: 10.3171/2013.4.JNS13124. [DOI] [PubMed] [Google Scholar]
- 11.Mascarenhas L, Moshel Y A, Bayad F. et al. The transplanum transtuberculum approaches for suprasellar and sellar-suprasellar lesions: avoidance of cerebrospinal fluid leak and lessons learned. World Neurosurg. 2014;82(1–2):186–195. doi: 10.1016/j.wneu.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Ottenhausen M, Banu M A, Placantonakis D G. et al. Endoscopic endonasal resection of suprasellar meningiomas: the importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg. 2014;82(3–4):442–449. doi: 10.1016/j.wneu.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Ivan M E, Iorgulescu J B, El-Sayed I. et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015;22(1):48–54. doi: 10.1016/j.jocn.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Kasemsiri P, Carrau R L, Otto B A. et al. Reconstruction of the pedicled nasoseptal flap donor site with a contralateral reverse rotation flap: technical modifications and outcomes. Laryngoscope. 2013;123(11):2601–2604. doi: 10.1002/lary.24088. [DOI] [PubMed] [Google Scholar]
- 15.Chin D, Harvey R J. Endoscopic reconstruction of frontal, cribriform and ethmoid skull base defects. Adv Otorhinolaryngol. 2013;74:104–118. doi: 10.1159/000342285. [DOI] [PubMed] [Google Scholar]


