Abstract
Objective
Diabetic cardiomyopathy is a major cause of morbidity, but limited data are available on early cardiac abnormalities in type 1 diabetes (T1D). We investigated differences in myocardial strain in adolescents with and without T1D. We hypothesized that adolescents with T1D would have worse strain than their normoglycemic peers, which boys would have worse strain than girls, and that strain would correlate with glycemic control and adipokines.
Methods
We performed fasting laboratory measures and echocardiograms with speckle tracking to evaluate traditional echocardiographic measures in addition to longitudinal (LS) and circumferential (CS) strain, and in adolescents (15±2 years) with (19 boys; 22 girls) and without (16 boys; 32 girls) type 1 diabetes.
Results
Compared to controls, adolescents with type 1 diabetes had significantly lower CS (−20.9 vs. −22.7%, p=0.02), but not LS (p=0.83). Boys with T1D had significantly lower LS than girls with T1D (−17.5 vs. −19.7%, p=0.047), adjusted for Tanner stage. The significant sex differences observed in indexed left ventricular mass, left end-diastolic volume, diastolic septal and posterior wall thickness in our controls were lacking in adolescents with T1D.
Conclusions
Our observations suggest that youth with T1D have worse myocardial strain than normoglycemic peers. In addition, the relatively favorable cardiac profile observed in girls vs. boys in the control group, was attenuated in T1D. These early cardiovascular changes in youth with T1D are concerning and warrant longitudinal and mechanistic studies.
Keywords: Left ventricular Strain, Myocardial Mechanics, Type 1 Diabetes, Diabetic Cardiomyopathy
Introduction
Type 1 diabetes is a major cause of morbidity and mortality, most often due to cardiovascular disease (CVD). While type 1 diabetes (T1D) is known to increase risk of premature death from CVD (1), little is known about the early stages of diabetic cardiomyopathy in T1D. Diabetic cardiomyopathy is considered to be the development of clinical left ventricular dysfunction independent of complications of diabetes or coronary artery disease. While the exact pathophysiology of diabetic cardiomyopathy remains unclear, data suggest that the diabetic myocardium is more susceptible to injury, and that the final common pathway leading to heart failure is likely mitochondrial dysfunction (2). Diabetic cardiomyopathy is difficult to predict and measure. Traditionally, left ventricular function has been evaluated with echocardiography by measuring ejection fraction. This method of determining myocardial dysfunction identifies those with systolic dysfunction relatively late in the disease process. Speckle tracking is an echocardiography technique that measures myocardial strain and noninvasively allows identification of ventricular dysfunction preceding a decline in ejection fraction (3, 4).
We and others have shown that adolescents and adults with T1D have decreased maximal exercise capacity and increased prevalence of left ventricular hypertrophy compared to normoglycemic peers (5–8). In addition, we have previously reported evidence of diastolic dysfunction in a smaller cohort of adolescents with T1D (6). We have also reported worse circumferential strain (CS) in adolescents with type 2 diabetes (T2D), including relationships between strain, leptin and adiponectin(9). There are also a few reports on myocardial strain in adolescents with T1D (10–12), but to our knowledge there are currently no data published on sex differences in early echocardiographic markers of diabetic cardiomyopathy, including myocardial strain in adolescents with T1D.
Women with T2D are twice as likely as men to have coronary heart disease (13). The Coronary Artery Risk Development in Young Adults (CARDIA) study demonstrated better strain in women compared to men, suggesting less cardiomyopathy in females (14), however it is unclear whether similar sex-related differences in strain begin earlier in life, or hold true in people with T1D. Accordingly, we hypothesized that adolescents with T1D would have worse strain than their normoglycemic peers, that adolescents boys would have worse strain than girls, and that among adolescents with T1D, strain would correlate with glycemic control and adipokines.
Materials and Methods
i. Participants
A total of 89 pubertal adolescents between the ages of 12 and 19 years with and without T1D from the RESistance to InSulin in Type 1 And Type 2 diabetes (RESISTANT) study who had echocardiograms with speckle tracking were included in this analysis. Participants with T1D were recruited from the University of Colorado Anschutz pediatric diabetes clinics, the Barbara Davis Center for Diabetes and by advertisement. Type 1 diabetes was defined by American Diabetes Association criteria, insulin requirement, and the presence of glutamic acid decarboxylase (GAD), islet cell (ICA-2) and/or insulin (IAA) autoantibodies. Controls were healthy nondiabetic adolescents without a family history of diabetes. They were selected as a group to be similar in age, body mass index (BMI), Tanner stage, level of habitual physical activity, and sex as the group with diabetes. Absence of diabetes was confirmed in controls by a 2-hour, 75-gram oral glucose tolerance test. Inclusion criteria included Tanner stage > 1 and sedentary status (<3 h regular exercise/wk) to minimize pubertal and training effects. Exclusions included resting blood pressure higher than 140/90 mm Hg or higher than 190/100 mm Hg during exercise, hemoglobin lower than 9 mg/dl, serum creatinine higher than 1.5 mg/dl, glycosylated hemoglobin (HbA1c) higher than 11%, smoking, medication-dependent asthma, other conditions precluding exercise testing, medications affecting insulin resistance (oral steroids, metformin, thiazolidinediones, or atypical antipsychotics), anti-hypertensive medications, oral contraceptives, pregnancy, breastfeeding, plans to alter exercise or diet during the study, and family history of T2D. The study was approved by the University of Colorado Denver Institutional Review Board and the Children’s Hospital of Colorado Scientific Advisory Review Committee. Parental informed consent and participant assent was obtained from all participants less than 18 years old and participant consent from those aged 18 years and above.
ii. Laboratory measures and imaging
Hemoglobin A1c (HbA1c) were measured by (DCCT-calibrated) ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, Calif). Leptin and adiponectin were measured on a morning fasting sample with a radioimmunoassay kit from Milipore.
iii. Activity questionnaires and body composition
A 3 day pediatric physical activity recall questionnaire was used to estimate habitual physical activity (15, 16), reported as a 3-d average of daily metabolic equivalents (METS). Height and weight were measured for determination of BMI. BMI z-score was calculated using BMI, sex and age (17). Pubertal development was assessed by a pediatric endocrinologist using the criteria established by Tanner and Marshall for pubic hair and breast development. Testicular volume was measured using an orchidometer. Body composition was assessed by DEXA scan as previously reported (15, 18).
iv. Echocardiography
Resting supine two-dimensional and tissue Doppler echocardiography was performed by a cardiologist expert in these methods (UT), using a Vivid 7 (General Electric, Waukesha, WI) ultrasound system, to exclude left ventricular systolic dysfunction (ejection fraction <50%), regional wall motion abnormalities, pericardial disease, or significant valvular pathology. Studies were performed fasting and following 3 days of controlled diet and restricted physical activity to standardize output. Image analysis was completed with EchoPAC software (General Electric, Waukesha, WI). Left ventricular dimensions and ejection fraction were obtained by standard m-mode and two-dimensional volumetric method-of-discs analysis (19). Left ventricular mass was calculated as left ventricular mass (LVM) = 0.8 * 1.05 * [(IVSd + PWd + LVIDd)3 −LVIDd3)] (19). Indexed LVM (LVMI) was calculated as LVM / height2.7 (20). Left ventricular hypertrophy (LVH) was defined as LVMI values greater than 90th percentile for age and gender-specific reference data, or IVSd and/or LVPWd greater than 1 cm (21). Using traditional pulse wave blood and tissue Doppler, the mitral inflow peak E and A wave velocities, deceleration time, and myocardial systolic (S’) and early diastolic (E’) velocities at the lateral and septal mitral valve annuli were measured using standard protocols (22).
Speckle tracking was performed with EchoPAC software, which uses the LaGrangian strain approach (23). Global longitudinal strain curves were obtained from each of the 2 standard apical views and 1 parasternal long axis view. The parasternal short axis view at the papillary muscles was used to obtain circumferential strain. In each view the endocardium was traced in end-diastole, defined by the closure of the mitral valve. The epicardium was traced by defining the myocardial thickness such that the entire myocardium was included while excluding the pericardium. The software then generated strain curves by tracking and averaging the relative speed and location of defined patterns or “speckles” within each segment. Only those segments that had an adequate number of traceable speckles were included. In order to obtain valid results, adequate tracking in at least 4 of the 6 segments had to be verified; otherwise, values from that view were discarded. Per standard techniques (24), peak strain was measured on strain curves at the time of aortic valve closure. Global circumferential strain was obtained from the curves in the short axis view that measured global strain as if the entire left ventricle was one segment (rather than an average of the individual segments). Longitudinal strain was calculated as an average of the maximum global strain from the 3 views. There was no particular segment that poorly tracked in either the T1D or control group. Strain is interpreted as a percentage, with a strain of −15% being worse than a strain of −16%, for example.
v. Statistical analysis
Analyses were performed in SAS (version 9.4 for Windows; SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality using normal plots. Two-sample t-tests were employed to compare means of continuous parametric variables between lean adolescents and adolescents with T1D. The Wilcoxon rank-sum test was used for unadjusted continuous non-normally distributed variables (e.g. diabetes duration and CS). Skewed independent variables were natural log transformed in adjusted generalized linear regression models (e.g. CS). In order to examine the differences in echocardiographic variables between boys and girls with T1D, we employed linear regression models and adjusted for Tanner stage due to the difference in pubertal status between boys and girls. We also evaluated for effect modification by diabetes status and sex with a 2-way ANOVA procedure, and considered an interaction significant if p<0.10. Interaction terms that did not meet significance were dropped from the models. Analyses were considered exploratory and hypothesis generating and adjustments for multiple comparisons were not employed. Significance was based on an alpha-level of ≤0.05, unless otherwise specified.
Results
i. Differences between adolescents with T1D and controls
Participant characteristics stratified by T1D status are shown in Table 1. By design, adolescents with T1D and control adolescents were similar in age, sex, BMI, Tanner stage, and baseline level of physical activity (Table 1). While leptin and fat mass were not significantly different between the two groups, adolescents with T1D had greater lean mass than their normoglycemic peers. As expected, HbA1c and adiponectin were significantly higher in adolescents with T1D compared to controls (Table 1).
Table 1.
Differences in clinical parameters between controls and adolescents with T1D.
| Type 1 Diabetes (N=41) | Controls (N=48) | P-value | |
|---|---|---|---|
| Age (years) | 15.0 ± 1.9 | 15.1 ± 2.1 | 0.80 |
| Sex (% female) | 54% | 67% | 0.21 |
| BMI percentile | 58±27 | 57±22 | 0.78 |
| BMI z-score* | 0.5 (−0.2–0.9) | 0.4 (−0.2–0.8) | 0.79 |
| Lean mass (kg) | 44.1 ± 7.7 | 39.5 ± 8.4 | 0.01 |
| Fat mass (kg) | 15.7 ± 7.8 | 18.0 ± 14.7 | 0.38 |
| Tanner stage* | 4.5 (4–5) | 4.0 (4–5) | 0.48 |
| Diabetes Duration (years)* | 4.6 (1.7–7.8) | – | – |
| HbA1c (%) | 8.3 ± 1.4 | 5.2 ± 0.3 | <0.0001 |
| Leptin (ng/mL)* | 13.1 (5.8–16.4) | 10.1 (3.4–13.4) | 0.09 |
| Adiponectin (μg/mL) | 11.5 ± 6.0 | 9.0 ± 3.4 | 0.02 |
| Physical Activity (METs) | 63.8 ± 11.8 | 59.5 ± 13.6 | 0.15 |
| Heart Rate (min−1) | 67 ± 13 | 67 ± 24 | 0.94 |
| Resting systolic blood pressure (mmHg) | 116 ± 12 | 112 ± 8 | 0.08 |
| Resting diastolic blood pressure (mmHg) | 67 ± 8 | 66 ± 7 | 0.45 |
Data presented as mean and SD unless otherwise specified.
Median, P25–P75.
Differences in echocardiographic parameters between adolescents with T1D and controls are shown in Table 2. The left ventricular end-systolic linear dimension (LVIDs) was significantly larger in adolescents with T1D (Table 2). Although all parameters were numerically higher in adolescents with T1D, there were no statistically significant differences in left ventricular end-diastolic dimension (LVIDd) or in volumetric analysis of left ventricular end-diastolic volume, or end-systolic volume, nor were there differences in ejection fraction (Table 2). LVM (irrespective of whether it was expressed in absolute terms, or indexed to body surface area or to m2.7), as well as diastolic septal and posterior wall thickness (IVSd and LVPWd) were significantly greater in adolescents with T1D compared to controls (Table 2).
Table 2.
Differences in echocardiogram parameters between controls and adolescents with T1D.
| Measures of left ventricular size and function | |||
|---|---|---|---|
| Type 1 Diabetes (n=41) | Controls (n=48) | P-value | |
| LVIDd (cm) | 4.4 ± 0.4 | 4.3 ± 0.3 | 0.09 |
| LVIDs (cm) | 2.9 ± 0.4 | 2.8 ± 0.4 | 0.03 |
| FS (%) | 33.6 ± 6.0 | 35.8 ± 7.1 | 0.12 |
| IVSd (cm) | 0.84 ± 0.14 | 0.77 ± 0.14 | 0.03 |
| LVPWd (cm) | 0.83 ± 0.12 | 0.78 ± 0.12 | 0.04 |
| End-diastolic volume (cm3) | 79.2 ± 20.2 | 73.4 ± 18.5 | 0.17 |
| End-systolic volume (cm3) | 27.1 ± 8.0 | 25.2 ± 8.0 | 0.28 |
| Ejection fraction (%) | 65.7 ± 6.3 | 66.0 ± 5.0 | 0.86 |
| LVM (g) | 119.9 ± 29.9 | 103.3 ± 27.7 | 0.008 |
| Indexed LVM (g/m2.7) | 29.9 ± 7.1 | 27.0 ± 6.1 | 0.04 |
| Indexed LVM (g/BSA) | 71.6 ± 18.8 | 63.9 ± 14.4 | 0.03 |
| LVH (%) | 10% | 8% | 0.82 |
| IVSd >1 cm (%) | 10% | 0% | 0.03 |
| LVPWd >1 cm (%) | 7% | 2% | 0.23 |
| Speckle Tracking Parameters | |||
| Longitudinal strain (%) | −18.5 ± 2.5 | −18.4 ± 2.3 | 0.82 |
| Circumferential strain (%)* | −20.9 (−22.3, −18.1) | −22.7 (−25.5, −19.7) | 0.02 |
| Apical rotation (%) | 7.8 ± 3.7 | 6.7 ± 4.3 | 0.33 |
| Traditional Echocardiogram and Tissue Doppler Measurements | |||
| Mitral Peak E velocity (m/s) | 0.92 ± 0.18 | 0.91 ± 0.15 | 0.86 |
| Mitral Peak A velocity (m/s) | 0.38 ± 0.10 | 0.41 ± 0.08 | 0.12 |
| Mitral inflow E/A | 2.6 ± 0.7 | 2.3 ± 0.7 | 0.15 |
| Deceleration Time (ms) | 217 ± 51 | 197 ± 40 | 0.04 |
| Lateral peak E’ (cm/s) | 18.8 ± 3.3 | 18.0 ± 2.6 | 0.19 |
| Lateral peak A’ (cm/s) | 5.9 ± 1.7 | 5.8 ± 1.5 | 0.94 |
| Lateral E/E’ | 5.1 ± 1.3 | 5.2 ± 1.0 | 0.66 |
| Septal E/E’ | 7.0 ± 1.5 | 6.6 ± 1.3 | 0.20 |
| Septal E/A | 2.4 ± 0.7 | 2.7 ± 0.8 | 0.12 |
Data presented as mean and SD unless otherwise specified.
Median, P25–P75.
Using traditional pulse wave blood and tissue Doppler, a significantly increased deceleration time was observed in adolescents with T1D, consistent with diastolic dysfunction, but there were no significant differences in other traditional echo parameters (Table 2). Speckle tracking analysis demonstrated significantly lower CS in adolescents with T1D compared to controls (CS: −20.9 [−22.3, −18.1] vs. −22.7 [−25.5, −19.7], p=0.02) [Figure 1]. Conversely, no differences in LS were observed between the two groups (Figure 2).
Figure 1. CS in adolescents with and without type 1 diabetes.
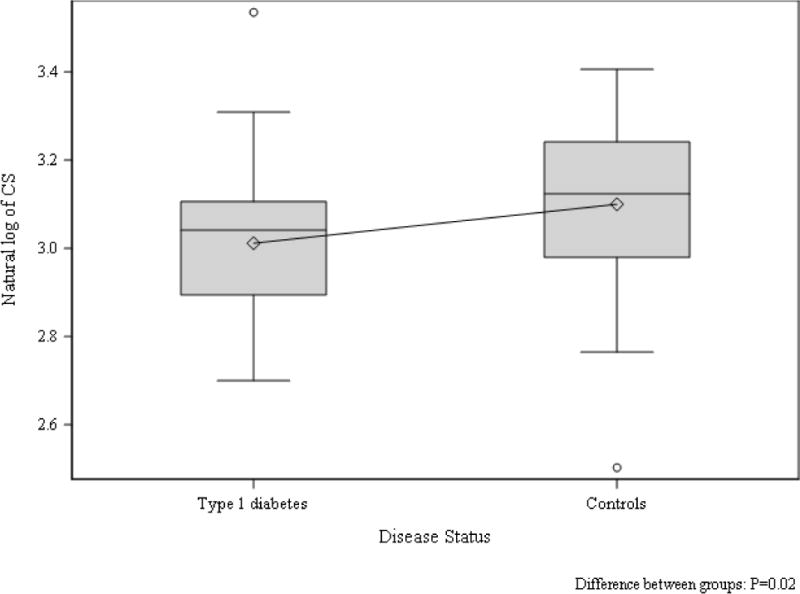
Group difference p=0.02
Figure 2. LS in adolescents with and without type 1 diabetes.
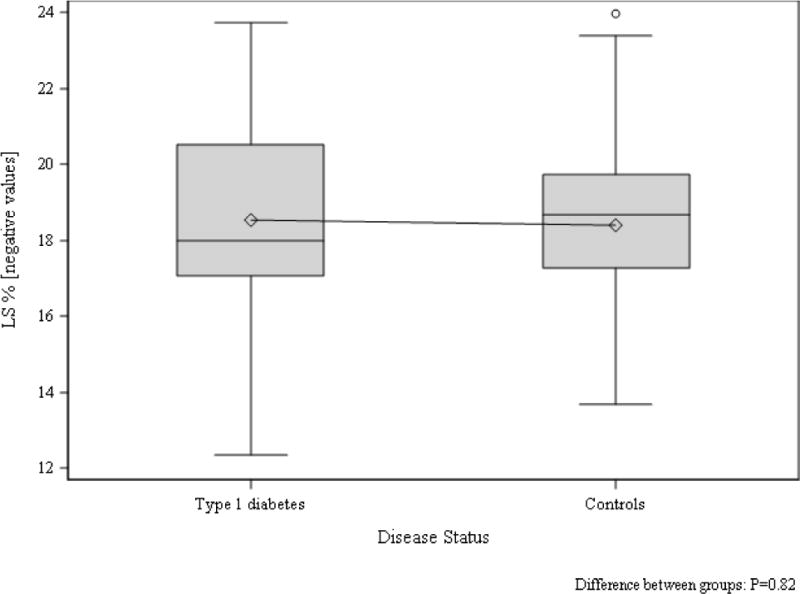
Group difference p=0.82
ii. Sex differences in adolescents without T1D
Characteristics stratified by sex for control adolescents are shown in Table 3. While the girls were of similar age to the boys in the control group, as expected, the girls had more advanced pubertal status (Table 3). Moreover, girls also had higher BMI percentile, BMI z-score, fat mass and leptin concentration than the boys (Table 3). Boys in the control group had greater lean mass than the girls (Table 3).
Table 3.
Differences in clinical parameters between adolescent boys and girls with and without T1D.
| Type 1 Diabetes (n=41) | Control (n=48) | |||||
|---|---|---|---|---|---|---|
| Females (n=22) | Males (n=19) | P-value | Females (n=32) | Males (n=16) | P-value | |
| Age (years) | 15.0 ± 2.1 | 14.9 ± 1.8 | 0.93 | 15.2 ± 2.1 | 14.8 ± 2.0 | 0.44 |
| BMI percentile | 58±26 | 58±30 | 0.95 | 63±17 | 46±26 | 0.01 |
| BMI z-score* | 0.29 (−0.10–0.83) | 0.45 (−0.26–1.05) | 0.98 | 0.42 (0.11–0.89) | −0.17 (−0.75–0.70) | 0.03 |
| Lean mass (kg) | 39.7 ± 4.8 | 49.0 ± 7.5 | <0.0001 | 36.6 ± 4.0 | 45.5 ± 11.8 | 0.0004 |
| Fat mass (kg) | 16.7 ± 6.9 | 14.6 ± 8.7 | 0.38 | 21.9 ± 16.1 | 9.5 ± 4.8 | 0.0003 |
| Tanner stage* | 5 (4–5) | 4 (3–5) | 0.001 | 5 (4–5) | 4 (3–4) | 0.001 |
| Diabetes Duration (years) | 3.8 (1.1–10.6) | 5.7 (1.9–7.5) | 0.36 | – | – | – |
| HbA1c (%) | 8.2 ± 1.3 | 8.4 ± 1.6 | 0.57 | 5.2 ± 0.2 | 5.0 ± 0.4 | 0.04 |
| Leptin (ng/mL)* | 14.5 (12.2–18.8) | 3.3 (2.9–13.3) | 0.003 | 11.2 (9.4–16.2) | 2.5 (1.6–6.7) | <0.0001 |
| Adiponectin (μg/mL) | 12.2 ± 7.0 | 10.6 ± 4.4 | 0.39 | 8.3 ± 2.9 | 10.5 ± 4.0 | 0.048 |
| Physical Activity (METs) | 65.2 ± 13.8 | 62.0 ± 8.9 | 0.41 | 59.2 ± 15.1 | 60.3 ± 109 | 0.82 |
| Heart Rate (min−1) | 71 ± 14 | 62 ± 10 | 0.07 | 70 ± 27 | 58 ± 9 | 0.24 |
| Resting systolic blood pressure (mmHg) | 114 ± 13 | 119 ± 11 | 0.26 | 112 ± 8 | 114 ± 8 | 0.52 |
| Resting diastolic blood pressure (mmHg) | 68 ± 9 | 67 ± 8 | 0.69 | 66 ± 7 | 67 ± 7 | 0.66 |
Median, Q25–Q75.
Corrected for serum glucose.
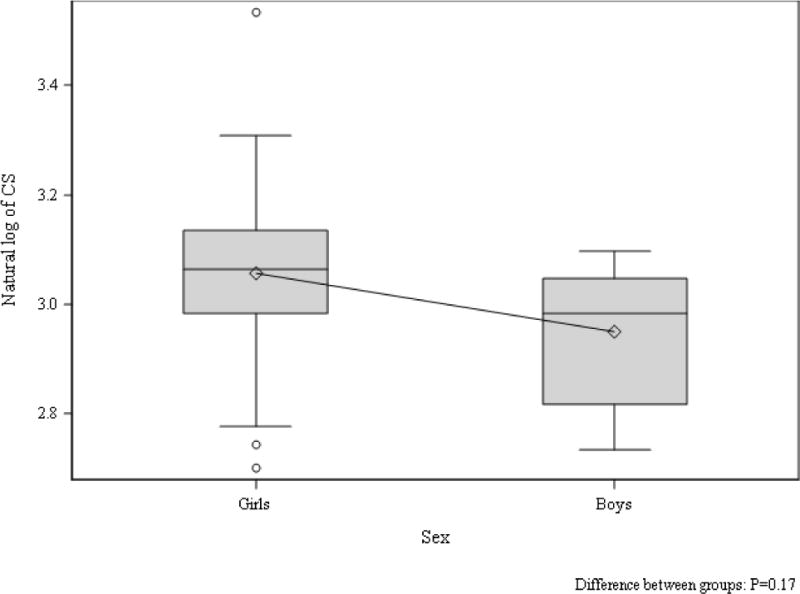
Tanner stage-adjusted differences in echocardiographic parameters between girls and boys in the control group are shown in Table 4. Despite the girls being heavier than the boys in the control group, the boys demonstrated significantly greater LVIDd, LVIDs, left ventricular end-diastolic and end-systolic volume (Table 4). Furthermore, LVM (absolute and indexed to body surface area) as well IVSd and LVPWd were significantly greater in boys than girls in the control group (Table 4). Using traditional pulse wave blood and tissue Doppler, a significantly increased septal peak A’ was also appreciated in control boys compared to girls (Table 4). The only difference in speckle tracking measurements between control boys and girls was reduced apical rotation in the boys (Table 4, Figure 3 and 4).
Table 4.
Differences in echo parameters between adolescent boys and girls with and without T1D adjusted by Tanner stage.
| Measures of left ventricular size and function | ||||||
|---|---|---|---|---|---|---|
| Type 1 diabetes | Control | |||||
| Females (n=22) | Males (n=19) | P-value | Females (n=32) | Males (n=16) | P-value | |
| LVIDd (cm) | 4.3 ± 0.1 | 4.5 ± 0.1 | 0.15 | 4.1 ± 0.1 | 4.4 ± 0.1 | 0.006 |
| LVIDs (cm) | 2.8 ± 0.1 | 3.0 ± 0.1 | 0.09 | 2.5 ± 0.1 | 2.8 ± 0.1 | 0.04 |
| FS (%) | 34.7 ± 2.2 | 33.5 ± 1.8 | 0.59 | 38.0 ± 2.1 | 36.2 ± 1.9 | 0.53 |
| IVSd (cm) | 0.8 ± 0.1 | 0.9 ± 0.0 | 0.04 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.009 |
| LVPWd (cm) | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.06 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.003 |
| End-diastolic volume (cm3) | 66.3 ± 6.3 | 83.2 ± 5.3 | 0.01 | 58.1 ± 4.4 | 85.3 ± 4.0 | <0.0001 |
| End-systolic volume (cm3) | 23.4 ± 2.7 | 28.0 ± 2.2 | 0.10 | 18.8 ± 1.8 | 31.0 ± 1.6 | <0.0001 |
| Ejection fraction (%) | 64.5 ± 2.3 | 65.6 ± 1.9 | 0.63 | 67.3 ± 1.5 | 64.2 ± 1.4 | 0.15 |
| LVM (g) | 108.8 ± 8.8 | 134.7 ± 7.3 | 0.006 | 85.4 ± 7.0 | 124.3 ± 6.3 | <0.0001 |
| Indexed LVM (g/m2.7) | 31.9 ± 2.2 | 32.6 ± 1.8 | 0.74 | 26.2 ± 1.8 | 30.2 ± 1.6 | 0.11 |
| Indexed LVM (g/BSA) | 75.1 ± 5.5 | 80.0 ± 4.5 | 0.38 | 59.5 ± 3.8 | 75.6 ± 3.5 | 0.004 |
| Speckle tracking parameters | ||||||
| Longitudinal strain (%) | −19.7 ± 0.9 | −17.5 ± 1.0 | 0.047 | −19.1 ± 0.8 | −17.3 ± 0.9 | 0.17 |
| Circumferential strain (%)* | −21.4 (−24.6, −18.7) | − 19.0 (−21.8, −16.4) | 0.10 | −22.2 (−26.8, −19.9) | −23.1 (−25.3, −19.4) | 0.71 |
| Apical rotation (%)** | 10.4 ± 1.4 | 9.3 ± 1.1 | 0.52 | 9.5 ± 1.2 | 4.3 ± 1.4 | 0.005 |
| Traditional echo and tissue Doppler measurements | ||||||
| Mitral Peak E velocity (m/s) | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.14 | 0.9 ± 0.0 | 1.0 ± 0.0 | 0.19 |
| Mitral Peak A velocity (m/s) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.77 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.27 |
| Mitral inflow E/A | 3.0 ± 0.2 | 2.9 ± 0.2 | 0.47 | 2.3 ± 0.2 | 2.6 ± 0.2 | 0.17 |
| Deceleration Time (ms) | 207 ± 18 | 221 ± 15 | 0.46 | 200 ± 12 | 184 ± 11 | 0.32 |
| Lateral peak E’ (cm/s) | 18.8 ± 1.1 | 18.8 ± 0.9 | 0.97 | 18.6 ± 0.8 | 18.6 ± 0.7 | 0.99 |
| Lateral peak A’ (cm/s) | 5.9 ± 0.6 | 5.8 ± 0.5 | 0.78 | 6.0 ± 0.5 | 5.7 ± 0.4 | 0.98 |
| Lateral E/E’ | 5.6 ± 0.4 | 5.2 ± 0.4 | 0.41 | 5.0 ± 0.3 | 5.3 ± 0.3 | 0.48 |
| Septal A/E | 2.5 ± 0.2 | 2.8 ± 0.2 | 0.25 | 3.2 ± 0.2 | 2.7 ± 0.2 | 0.08 |
| Septal E/E’ | 7.8 ± 0.5 | 6.9 ± 0.4 | 0.06 | 6.6 ± 0.4 | 6.6 ± 0.4 | 0.96 |
Data presented as least squares means (LSM) and SE unless otherwise specified.
Geometric least squares means and 95% CI.
Model include interaction between sex and diabetes status.
Figure 3. CS in boys and girls without type 1 diabetes.
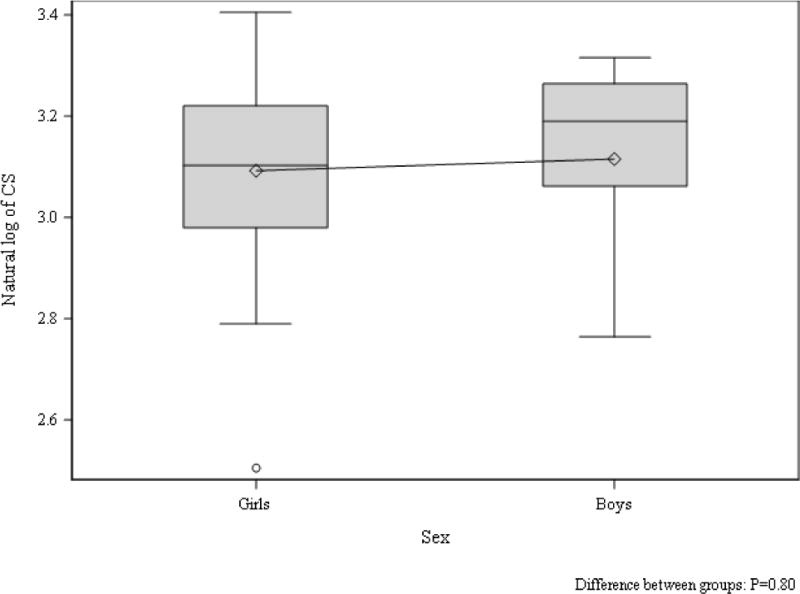
Tanner stage adjusted group difference p=0.71
Figure 4. LS in boys and girls without type 1 diabetes.
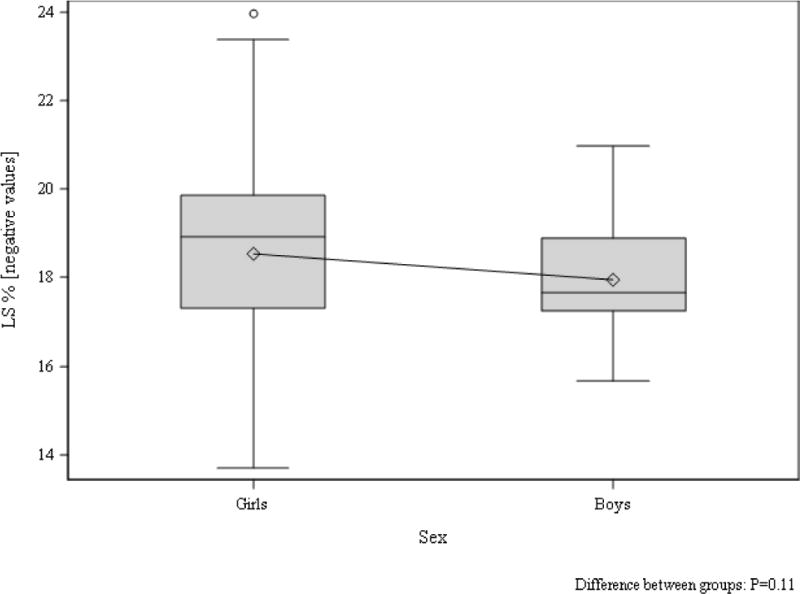
Tanner stage adjusted group difference p=0.17
iii. Sex differences in adolescents with T1D
Characteristics stratified by sex for adolescents with T1D are shown in Table 3. Boys and girls with T1D were similar in age, BMI and diabetes duration, but as expected, girls were more advanced in pubertal development (Table 3). HbA1c, fat mass and baseline level of physical activity were also similar between boys and girls with T1D (Table 3). Boys with T1D had greater lean mass and lower leptin concentrations compared to girls with T1D (Table 3).
Tanner stage-adjusted differences in echocardiographic parameters between boys and girls with T1D are shown in Table 4. Several of the significant sex differences observed in the control group were attenuated in adolescents with T1D. LVIDd and LVIDs and left ventricular end-systolic volume were similar in the girls and boys with T1D, but boys demonstrated a larger left ventricular end-diastolic volume than girls (Table 4). In fact, the relatively favorable cardiac measures in girls compared to boys observed in the participants without T1D were less prominent in adolescents with T1D. While IVSd was significantly greater in boys compared to girls with T1D, the sex difference was smaller in adolescents with T1D compared to those without (Table 4). Furthermore, the sex difference in LVPWd did not reach statistical significance in adolescents with T1D (Table 4). There was a difference in absolute LVM between girls and boys, but the difference disappeared when indexed to body surface area or m2.7. With traditional pulse wave blood and tissue Doppler, there were no significant differences between girls and boys with T1D (Table 4).
Speckle tracking analysis demonstrated significantly lower LS in boys compared to girls with T1D (−17.5±1.0 vs. −19.7±0.9, p=0.047, Figure 5) after adjusting for Tanner stage, but there were no differences observed in CS (Figure 6) or apical rotation by sex in adolescents with T1D (Table 4).
Figure 5. LS in boys and girls with type 1 diabetes.
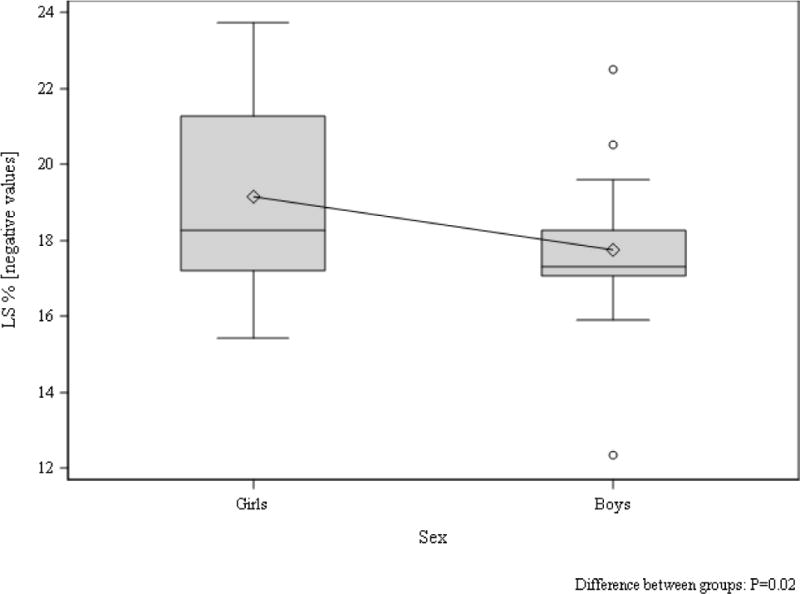
Tanner stage adjusted group difference p=0.047
Figure 6. CS in boys and girls with type 1 diabetes.
Tanner stage adjusted group difference p=0.10
iv. Associations between metabolic risk factors and cardiac function in adolescents with T1D
In a univariable analysis, HbA1c tended to be positively associated with CS, but the association did not reach statistical significance (β±SE: 1.0±0.5, p=0.06, R2 = 0.12), and no significant associations were observed with LS or indexed LVM. When stratifying participants into tertiles of HbA1c (low: <7.6%, mid: 7.6–8.6% and high >8.6%), participants in the high tertile had worse CS than those in the low tertile (−18.3±1.2 vs. 21.7±1.2%, p=0.04), however the difference lost statistical significance with adjustments for sex and Tanner stage (p=0.06). No significant differences in LS or indexed LVM observed across tertiles HbA1c (data not shown). In contrast to our observations with adolescents with T2D (9), adiponectin was not significantly associated with CS, LS or LVM in T1D (data not shown). Natural log of leptin was negatively associated with indexed LVM (β±SE: −9.9±3.2, p=0.003, R2 = 0.21), but not CS or LS (data not shown). Neither lean mass nor fat mass was significantly associated with indexed LVM, CS or LS (data not shown).
Discussion
In our cohort, adolescents with T1D of short duration and no evidence of clinical heart disease had worse CS and greater LVM compared to lean normoglycemic controls. These abnormalities occurred despite relatively normal tissue Doppler velocities, suggesting that strain imaging may be an early, sensitive marker of cardiac dysfunction in T1D. Boys with T1D had worse LS than girls with T1D, despite similar glycemic control, diabetes duration, and BMI, which may represent early cardiovascular sex-differences in T1D. Furthermore, significant sex differences observed in adolescents without T1D for indexed LVM, left end-diastolic volume, LVIDd, LVIDs, LVPWd and IVSd were either attenuated or not significant in adolescents with T1D, arguing that T1D may preferentially worsen some aspects of cardiac physiology in females.
These observations suggest that adolescents with T1D have myocardial mid-wall fiber changes which mirror the defects of adults with more complicated and longer duration diabetes. Furthermore, we confirmed, in a larger sample size, our previous findings of greater LVM in adolescents with T1D with an even shorter disease duration (6). Increased LVM and left ventricular hypertrophy are strong independent predictors of future CVD (25, 26). The relatively favorable cardiac values observed in girls compared to boys in the control group were tempered in T1D.
The derangements in myocardial mechanics observed in adolescents with T1D are concerning as the participants are young with a relatively short duration of diabetes. In our previous report in a smaller cohort, we found more differences in traditional tissue Doppler markers, indicating early diastolic dysfunction (6). However, the participants in the former study had a significantly longer duration of diabetes. The complexities of myocardial mechanical abnormalities in diabetic cardiomyopathy are not well understood. Since ejection fraction, the traditional measurement of systolic function, is typically a late marker of disease, more recent studies have focused on diastolic parameters in evaluating people with diabetes at risk for overt cardiomyopathy (5, 27–29). Mitral inflow pattern and diastolic tissue velocities correlate with disease severity better than ejection fraction (29, 30), but even these parameters are imprecise (31, 32). In this study, we employed speckle tracking, which permits measurement of myocardial systolic deformation, or strain, in multiple directions. Previous studies in adults with heart failure have linked abnormal strain, identified by speckle tracking, with increased morbidity; for example, abnormal strain predicted outcomes in patients with both systolic cardiomyopathy and with heart failure with normal ejection fraction, a quarter of which had diabetes (33, 34). In studies of diabetic cardiomyopathy, strain is a more sensitive marker of disease severity than either ejection fraction or tissue Doppler (4) and may be the earliest marker of cardiac dysfunction. In our group of adolescents with relatively short duration of disease and no clinically evident cardiovascular problems, there were impairments of strain, but in contrast to other populations (18, 29), there were not yet differences in tissue Doppler velocities or ejection fraction. Strain imaging has been used in a few studies of adults with T2D, as well is our recent publication in adolescents with T2D (35), and a few of reports in adolescents with T1D (10–12), but this is the first report to our knowledge to examine sex differences in early echocardiographic markers of diabetic cardiomyopathy. We have previously reported a similar pattern of changes in CS with preservation of LS in adolescents with T2D (9). This pattern is also seen in patients with hypertension and left ventricular hypertrophy (36). In contrast to T1D, in adolescents with T2D, low adiponectin, rather than conventional risk factors, was associated with CS, a relationship we did not observe in our cohort of adolescents with T1D, who are known to have higher adiponectin than controls (35). The mechanisms and implications of a higher than expected adiponectin level for the degree of insulin resistance in T1D are not yet understood.
In adults with T2D, strain imaging has also demonstrated that early in the disease process, among those with well-controlled diabetes without significant complications, longitudinal left ventricular function is decreased, while there is a paradoxical increase in circumferential contraction, preserving overall left ventricular ejection fraction (3). The subendocardial fibers, which mediate longitudinal motion, appear to be affected first by diabetes (37). Others have shown that in those with a longer duration of diabetes and more co-morbidity, circumferential motion is most affected, and may not include abnormalities in longitudinal parameters (38, 39). Abnormalities in circumferential motion are consistent with dysfunction of mid-wall fibers and may signify increased damage to deeper layers in people with more severe or longstanding diabetes (37–39). Furthermore, girls with T1D demonstrated greater LS compared to boys with T1D. The mechanism(s) contributing to the sex difference in LS are unlikely to be explained by conventional risk factors, as boys and girls with T1D in our cohort had similar glycemic control, diabetes duration, blood pressure and baseline physical activity. While we did not observe a significant relationship between HbA1c, LS and CS in adolescents with T1D in our study, others have reported an association between acute glycemia and myocardial strain (11, 12). It is known that the diabetic myocardium is more susceptible to injury, and perhaps there also is loss of the preferential female myocardial adaption to stress in T1D (40–42).
In contrast to our findings, a previous report demonstrated more pronounced early echocardiographic signs of diabetic cardiomyopathy in girls compared to boys with T1D, however they did not have data on myocardial strain (43).
Our study does have important limitations. To minimize the effect of moderate sample size, we used careful and detailed physiological measurements, including speckle echocardiography and controlled pre-study diet and physical activity. Another limitation to the present study is the cross-sectional design which prevents determination of causality and whether the association holds true longitudinally; for that reason the data should be viewed as hypothesis generating. While not statistically significant there were a greater proportion of girls in the control group compared to the T1D group which may have confounded our findings. Furthermore, speckle tracking to determine strain may be confounded by angle, position and respiratory artifacts, for which reason the images were obtained by the same research technician with all participants positioned supine. Results from this study may also not be generalizable to adults with T1D. It is also possible that the difference in myocardial function is due to a difference in baseline fitness between groups, but with similar resting heart rates, blood pressures and habitual level of physical activity in adolescents with and without T1D, this appears unlikely to have a large impact. Future directions for this study include examining changes in myocardial strain in adolescents with T1D over time, and how the sex differences change over time.
In conclusion, we found that strain imaging with speckle tracking demonstrated worse CS and greater LVM in adolescents with T1D compared to controls, unrelated to glycemic control. While adolescent boys with T1D had worse LS compared to girls with T1D, the relatively favorable values for indexed LVM, left end-diastolic volume, LVIDd, LVIDs, LVPWd and IVSd observed in girls compared to boys in the control group was lost in T1D. Further research should continue to use speckle echocardiography to examine longitudinal sex-specific relationships between strain and important risk-factors in T1D to determine the causes as well as possible therapeutic approaches.
Acknowledgments
Support for this study was provided by NCRR K23 RR020038, NIH BIRCWH K12 5K12HD057022, ADA 7-11-CD-08, JDRF Award #11-2010-343, R56 DK088971, ADA 1-11-JF-23 and NIH/NCATS Colorado CTSI UL1 TR000154, Center for Women’s Health Research, VA Merit. Drs. Nadeau and Bjornstad are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Bjornstad, Dorosz, Cree-Green, Pyle, Regensteiner, Reusch and Nadeau, Ms. Baumgartner and Mr. Coe have no conflict of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose.
Author Contributions
PB wrote, formulated analytic plan, contributed to discussion and analytic plan, and reviewed/edited the manuscript; JLD contributed to discussion and analytic plan, and reviewed/edited the manuscript; MCG contributed to discussion and analytic plan, and reviewed/edited the manuscript; AB researched and reviewed/edited the manuscript; GC researched and reviewed/edited the manuscript; LP reviewed/edited the analysis plan and manuscript; JGR contributed to the discussion, and reviewed/edited the manuscript; JER contributed to the discussion, and reviewed/edited the manuscript; KN researched, wrote, formulated analytic plan, contributed to the discussion and analytic plan, and reviewed/edited the manuscript.
References
- 1.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111(25):3489–93. doi: 10.1161/CIRCULATIONAHA.104.529651. Epub 2005/06/29. [DOI] [PubMed] [Google Scholar]
- 2.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart failure clinics. 2012;8(4):619–31. doi: 10.1016/j.hfc.2012.06.007. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104(10):1398–401. doi: 10.1016/j.amjcard.2009.06.063. Epub 2009/11/07. [DOI] [PubMed] [Google Scholar]
- 4.Andersen NH, Poulsen SH, Eiskjaer H, Poulsen PL, Mogensen CE. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with Type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci (Lond) 2003;105(1):59–66. doi: 10.1042/CS20020303. Epub 2003/03/18. [DOI] [PubMed] [Google Scholar]
- 5.Rowland TW, Martha PM, Jr, Reiter EO, Cunningham LN. The influence of diabetes mellitus on cardiovascular function in children and adolescents. Int J Sports Med. 1992;13(5):431–5. doi: 10.1055/s-2007-1021293. Epub 1992/07/11. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. The Journal of clinical endocrinology and metabolism. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. Epub 2009/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia. 2008;51(7):1317–20. doi: 10.1007/s00125-008-1012-1. Epub 2008/05/01. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau KJ, Reusch JE. Cardiovascular function/dysfunction in adolescents with type 1 diabetes. Curr Diab Rep. 2011;11(3):185–92. doi: 10.1007/s11892-011-0180-4. Epub 2011/02/22. [DOI] [PubMed] [Google Scholar]
- 9.Bjornstad P, Truong U, Dorosz JL, Cree Green M, Baumgartner A, Coe G, et al. Cardiopulmonary dysfunction in obese adolescents and adolescents with type 2 diabetes. Journal of American Heart Association. 2016 doi: 10.1161/JAHA.115.002804. Accepted – in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jedrzejewska I, Krol W, Swiatowiec A, Wilczewska A, Grzywanowska-Laniewska I, Dluzniewski M, et al. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. European heart journal cardiovascular Imaging. 2015 doi: 10.1093/ehjci/jev164. Epub 2015/07/15. [DOI] [PubMed] [Google Scholar]
- 11.Hensel KO, Grimmer F, Jenke AC, Wirth S, Heusch A. The influence of real-time blood glucose levels on left ventricular myocardial strain and strain rate in pediatric patients with type 1 diabetes mellitus – a speckle tracking echocardiography study. BMC cardiovascular disorders. 2015;15(1):175. doi: 10.1186/s12872-015-0171-5. Epub 2015/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labombarda F, Leport M, Morello R, Ribault V, Kauffman D, Brouard J, et al. Longitudinal left ventricular strain impairment in type 1 diabetes children and adolescents: a 2D speckle strain imaging study. Diabetes & metabolism. 2014;40(4):292–8. doi: 10.1016/j.diabet.2014.03.007. Epub 2014/05/13. [DOI] [PubMed] [Google Scholar]
- 13.Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, et al. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000343. Epub 2015/12/09. [DOI] [PubMed] [Google Scholar]
- 14.Nwabuo C, Choi E-Y, Venkatesh BA, Kishi S, Almeida AL, Moreira HT, et al. Gender and Race Differences in Aging-Related Alteration in Myocardial Systolic Function Assessed by 2D Sp eckle Tracking Echocardiograp hy Over a 20 Year Follow-up: The CARDI A Study. American College of Cardiology. 2015 Abstract. [Google Scholar]
- 15.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin Resistance in Adolescents with Type 1 Diabetes and Its Relationship to Cardiovascular Function. Journal of Clinical Endocrinology & Metabolism. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin Resistance in Adolescents with Type 2 Diabetes Is Associated with Impaired Exercise Capacity. Journal of Clinical Endocrinology & Metabolism. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. Epub 2000/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. The Journal of clinical endocrinology and metabolism. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. Epub 2009/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. doi: 10.1016/j.echo.2014.10.003. Epub 2015/01/07. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–60. doi: 10.1016/0735-1097(92)90385-z. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 21.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22(6):709–14. doi: 10.1016/j.echo.2009.03.003. Epub 2009/05/09. [DOI] [PubMed] [Google Scholar]
- 22.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–84. doi: 10.1067/mje.2002.120202. Epub 2002/02/12. [DOI] [PubMed] [Google Scholar]
- 23.Tee N, Gu Y, Murni Shim W. Comparative myocardial deformation in 3 myocardial layers in mice by speckle tracking echocardiography. BioMed research international. 2015;2015:148501. doi: 10.1155/2015/148501. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun JP. Myocardial Imaging: Future applications of speckle tracking echocardiography. 1st. Malden, MA: Blackwell Publishing; 2007. [Google Scholar]
- 25.Verdecchia P, Carini G, Circo A, Dovellini E, Giovannini E, Lombardo M, et al. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38(7):1829–35. doi: 10.1016/s0735-1097(01)01663-1. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 26.Meijs MF, Vergouwe Y, Cramer MJ, Vonken EJ, Velthuis BK, Verton DJ, et al. A prediction model for left ventricular mass in patients at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17(6):621–7. doi: 10.1097/HJR.0b013e328332d4bc. Epub 2010/09/04. [DOI] [PubMed] [Google Scholar]
- 27.Poirier P, Bogaty P, Philippon F, Garneau C, Fortin C, Dumesnil JG. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism. 2003;52(8):1056–61. doi: 10.1016/s0026-0495(03)00091-x. Epub 2003/08/05. [DOI] [PubMed] [Google Scholar]
- 28.Poirier P, Garneau C, Bogaty P, Nadeau A, Marois L, Brochu C, et al. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2000;85(4):473–7. doi: 10.1016/s0002-9149(99)00774-2. Epub 2000/03/23. [DOI] [PubMed] [Google Scholar]
- 29.Raev DC. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17(7):633–9. doi: 10.2337/diacare.17.7.633. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 30.Vazeou A, Papadopoulou A, Miha M, Drakatos A, Georgacopoulos D. Cardiovascular impairment in children, adolescents, and young adults with type 1 diabetes mellitus (T1DM) Eur J Pediatr. 2008;167(8):877–84. doi: 10.1007/s00431-007-0603-z. Epub 2007/10/30. [DOI] [PubMed] [Google Scholar]
- 31.Muranaka A, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography. 2009;26(3):262–71. doi: 10.1111/j.1540-8175.2008.00805.x. Epub 2008/11/20. [DOI] [PubMed] [Google Scholar]
- 32.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105(10):1195–201. doi: 10.1161/hc1002.105185. Epub 2002/03/13. [DOI] [PubMed] [Google Scholar]
- 33.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54(7):618–24. doi: 10.1016/j.jacc.2009.04.061. Epub 2009/08/08. [DOI] [PubMed] [Google Scholar]
- 34.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54(1):36–46. doi: 10.1016/j.jacc.2009.03.037. Epub 2009/06/27. [DOI] [PubMed] [Google Scholar]
- 35.Bjornstad P, Truong U, Dorosz JL, Cree Green M, Baumgartner A, Coe G, et al. Cardiopulmonary dysfunction in obese adolescents and adolescents with type 2 diabetes. Circulation. 2015 doi: 10.1161/JAHA.115.002804. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93(2):259–65. doi: 10.1161/01.cir.93.2.259. Epub 1996/01/15. [DOI] [PubMed] [Google Scholar]
- 37.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45(3):248–63. doi: 10.1136/hrt.45.3.248. Epub 1981/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351–69. doi: 10.1016/j.echo.2010.02.015. quiz 453–5. Epub 2010/04/07. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca CG, Dissanayake AM, Doughty RN, Whalley GA, Gamble GD, Cowan BR, et al. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol. 2004;94(11):1391–5. doi: 10.1016/j.amjcard.2004.07.143. Epub 2004/11/30. [DOI] [PubMed] [Google Scholar]
- 40.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. Journal of nuclear cardiology: official publication of the American Society of Nuclear Cardiology. 2007;14(4):573–81. doi: 10.1016/j.nuclcard.2007.03.001. Epub 2007/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, et al. Sex differences in endothelial STAT3 mediate sex differences in myocardial inflammation. American journal of physiology Endocrinology and metabolism. 2007;293(3):E872–7. doi: 10.1152/ajpendo.00251.2007. Epub 2007/06/28. [DOI] [PubMed] [Google Scholar]
- 42.Wallen WJ, Cserti C, Belanger MP, Wittnich C. Gender-differences in myocardial adaptation to afterload in normotensive and hypertensive rats. Hypertension. 2000;36(5):774–9. doi: 10.1161/01.hyp.36.5.774. Epub 2000/11/18. [DOI] [PubMed] [Google Scholar]
- 43.Suys BE, Katier N, Rooman RP, Matthys D, Op De Beeck L, Du Caju MV, et al. Female children and adolescents with type 1 diabetes have more pronounced early echocardiographic signs of diabetic cardiomyopathy. Diabetes Care. 2004;27(8):1947–53. doi: 10.2337/diacare.27.8.1947. Epub 2004/07/28. [DOI] [PubMed] [Google Scholar]


