Abstract
Background
Receptor interacting protein kinase-3 (RIP3) is a key mediator of necroptosis, a form of regulated cell death recently implicated in murine models of renal ischemia-reperfusion injury and transfusion-associated endothelial injury. The importance of necroptosis in human AKI is unknown. We hypothesized that plasma RIP3 concentrations would be associated with acute kidney injury (AKI) after severe trauma.
Methods
We performed a case-control study nested in a prospective cohort of critically ill trauma patients. AKI was defined by AKI Network creatinine criteria within 6 days of presentation. Of 158 cohort subjects, we selected 13 who developed AKI stages 2 or 3, 27 with AKI stage 1, and 40 without AKI. We compared plasma RIP3 concentrations across these groups at presentation and 48 hours. Since red blood cell (RBC) transfusion is an AKI risk factor, we also tested the association of RBCs transfused during resuscitation with RIP3 levels.
Results
Median plasma RIP3 concentration rose more than ten-fold from presentation (15.6 (IQR 15.6-41.3) pg/mL) to 48 hours (164.7 (66.9-300.6) pg/mL; p<0.001). RIP3 concentrations at 48 hours were associated with AKI stage (no AKI: 144.8 (58.6-234.9) pg/mL; AKI stage 1: 165.8 (43.0-310.9) pg/mL; AKI stage 2-3: 365.5 (155.1-727.5) pg/mL; p=0.010) whereas this association was not seen at presentation (p=0.324). RBC transfusions were also associated with 48-hour plasma RIP3 (no RBCs: 99.4 (15.6-166.1) pg/mL; 1-5 units: 182.6 (98.5-274.1) pg/mL; >5 units: 341.8 (150.1-423.8) pg/mL; p<0.001).
Conclusions
In critically ill trauma patients, plasma levels of the necroptosis mediator RIP3 at 48 hours were associated with AKI stage and RBC transfusions.
Keywords: necrosis, apoptosis, cell death, reperfusion injury, humans, caspases
Introduction
Acute kidney injury (AKI), a syndrome of acute renal dysfunction following various insults, is common in critically ill patients and associated with increased morbidity and mortality even in its mildest forms (1-3). Specific therapies for AKI, however, are lacking. While many strides have been made in understanding AKI pathophysiology in pre-clinical models, identification of effective therapeutic targets in humans has remained challenging (4-6).
Necroptosis is a caspase-independent form of regulated necrotic cell death characterized by cellular swelling, rupture, and release of immunogenic damage-associated molecular patterns (DAMPs) which can incite and perpetuate tissue injury (7-9). More than two decades ago, animal studies demonstrated the importance of programmed cell death pathways in acute renal injury (10, 11). These studies focused on apoptotic, caspase-dependent pathways, which classically involve limited release of immunogenic material. Recent work by Linkermann et al has shown that murine models of renal ischemia-reperfusion injury (IRI) are characterized by death receptor-mediated activation of necroptosis and other regulated necrosis pathways, more so than by apoptosis (12-14). Receptor-interacting protein kinase 3 (RIP3) is a molecule essential for necroptosis execution, and RIP3 knockout mice are protected from renal ischemia-reperfusion injury (8, 13). The extent to which necroptosis and its mediator RIP3 play a role in human AKI, however, is unknown.
Critically ill trauma patients are a logical population in which to test the potential contributions of necroptosis to human AKI pathophysiology. Renal IRI from hemorrhage-related hypovolemic shock is common after major trauma (15). Red blood cell (RBC) transfusion is also a significant independent risk factor for AKI in this population, and we recently showed that banked human RBCs induce necroptosis in cultured human endothelial cells (16-18). Since cells undergoing necroptosis release RIP3, we reasoned that plasma concentrations of RIP3 might serve as a useful measure of necroptosis in humans. The primary objective of this study was to determine if plasma concentrations of RIP3, measured on presentation and at 48 hours, would be associated with AKI in a cohort of critically ill trauma patients. We also sought to determine the association of RBC transfusions with plasma RIP3 in this population.
Materials and Methods
Study population
We conducted a case-control study nested in an ongoing prospective cohort of critically ill trauma patients. Details of the cohort have been published previously (17). Briefly, all patients admitted to the Penn Level I Trauma Center ICU were screened for eligibility. Key inclusion criteria were injury severity score (ISS) ≥16, age≥14, and admission within 24 hours of trauma. Patients were excluded for death or discharge within 24 hours of ICU admission or isolated severe head injury. Demographic, medical history, trauma mechanism and severity, laboratory, physiologic, and therapy data were collected on each subject by review of the medical record. The Institutional Review Board of the University of Pennsylvania approved this study with a waiver of informed consent.
AKI was defined and staged by Acute Kidney Injury Network (AKIN) creatinine or renal replacement therapy (RRT) consensus criteria, utilizing data through the fifth day after presentation (5). If AKI criteria were met, we collected data for staging over the 7 following days (5). We did not use urine output criteria for AKI, since they are thought to be the least specific for true renal injury and therefore would have carried significant risk of misclassification bias (5).
Among 158 subjects with plasma available at presentation and 48 hours, we selected all 13 subjects who developed AKI stages 2 or 3, 27 subjects with stage 1 AKI and 40 subjects without AKI (controls). The stage 1 and no AKI groups were frequency-matched (2:1 and 3:1, respectively) with the AKI stage 2-3 group by age range, history of chronic kidney disease, and trauma mechanism, with subjects randomly selected from within each of the matching sub-strata.
Plasma collection
Blood samples drawn for clinical purposes at presentation to the trauma bay and approximately 48 hours later were sent to the lab in citrated vacutainers and centrifuged at 3000g. Residual supernatant was immediately refrigerated at 4C. Within 24 to 48 hours, study personnel froze these samples at -80°C.
RIP3 measurement
We used a commercially available enzyme-linked immunosorbent assay (CUSABIO, Wuhan, China) to measure plasma RIP3 as previously described (18). Performing personnel were blind to each subject's AKI status. Plasma concentrations below the limit of detection (15.6 pg/mL) were set to 15.6 pg/mL for statistical analysis, and are reported with this value in the results.
Statistical analysis
We tested differences in baseline characteristics across AKIN stage using Kruskal-Wallis and Fisher's exact tests, as appropriate. Differences in RIP3 levels by baseline characteristics were tested using Wilcoxon rank-sum and Kruskal-Wallis tests and Spearman's rank correlation, as appropriate. We used the Wilcoxon signed-rank test to determine the differences in RIP3 levels from presentation to 48 hours. Using Cuzick's non-parametric test of trend, we tested for trends across AKIN stages in presentation RIP3, 48-hour RIP3, and the difference in RIP3 between these time points (19). We used the Wilcoxon rank-sum test for pairwise comparisons of RIP3 between any two AKIN stages. Since injury severity and age differences remaining after frequency matching could confound the association of RIP3 with AKI, we used multivariable ordinal logistic regression to test the association of natural log-transformed RIP3 concentrations with AKIN stage adjusted for ISS and age. For all of these analyses, stages 2 and 3 were combined as in the subject sampling strategy.
We tested the association of RBC units during resuscitation (day of and day after presentation) with both presentation and 48-hour RIP3 concentrations using Cuzick's non-parametric test of trend. We categorized RBC units as none, 1-5, and >5 since 5 units was the median number received by transfused subjects.
Since all subjects with AKI stages 2-3 were male, we repeated our analyses restricted to male subjects to determine any impact on the results. Two-tailed p<0.05 was considered significant for all analyses.
Results
Baseline characteristics of AKI cases and controls are shown in Table 1. The median age was 37.5 (IQR 26.5-61.5) years, median ISS was 25 (18-29), 69 subjects (86.3%) were male, and 50 (62.5%) had blunt trauma. Forty-six subjects (57.5%) received intravenous contrast in the ED and 46 (60.5%) received at least one RBC transfusion during resuscitation. AKI developed early, with a median time to meeting AKI criteria of 15.1 (IQR 10.2-24.9) hours. ISS was similar across AKI stages. Eleven subjects (13.8%) died during the hospitalization, nine (81.8%) of whom had developed AKI.
Table 1. Baseline patient characteristics by acute kidney injury status.
| No AKI (n=40) | AKI Stage 1 (n=27) | AKI Stages 2-3 (n=13) | pf | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Age, years | 32 (25-61) | 46 (26-63) | 47 (31-60) | 0.541g |
| Male sex | 31 (78) | 25 (93) | 13 (100) | 0.079 |
| Race | 0.831 | |||
| Caucasian | 14 (35) | 13 (48) | 4 (31) | |
| African American | 22 (55) | 12 (44) | 8 (62) | |
| Othera | 4 (10) | 2 (7) | 1 (8) | |
| Body mass index (kg/m2) | 24.5 (22.0-29.1) | 25.7 (23.7-29.1) | 27.4 (23.9-33.9) | 0.273 |
|
| ||||
| Medical History | ||||
| Hypertension | 10 (25) | 10 (37) | 4 (36)e | 0.526 |
| Diabetes mellitus | 2 (5) | 3 (11) | 2 (18)e | 0.237 |
| Chronic kidney disease | 1 (3) | 2 (7) | 1 (8)e | 0.472g |
| Congestive heart failure | 1 (3) | 2 (8)d | 1 (8) | 0.792 |
|
| ||||
| Trauma and physiology | ||||
| Blunt trauma mechanism | 15 (38) | 10 (37) | 5 (38) | >0.999g |
| Injury Severity Score | 25 (18-29) | 22 (18-26) | 25 (19-33) | 0.475 |
| Low SBP in ED (mmHg) | 104 (89-117) | 86 (77-108) | 85 (77-112) | 0.177 |
| Glasgow Coma Scale | 14.5 (12-15) | 15 (11-15) | 14 (4-15) | 0.809 |
| First serum Cr (mg/dL) | 1.2 (1.0-1.3) | 1.2 (0.9-1.5) | 1.4 (1.2-1.7) | 0.146 |
|
| ||||
| Treatment and outcome | ||||
| OR prior to ICU admission | 13 (33) | 17 (63) | 8 (62) | 0.027 |
| IV contrastb | 27 (68) | 14 (52) | 5 (38) | 0.135 |
| Crystalloid, litersb | 2.3 (1.4-4.7) | 3.8 (2.5-5.9) | 4.6 (2.4-7.3) | 0.138 |
| RBC units transfusedc | 2 (0-5) | 3 (0-12) | 1 (0-4) | 0.397 |
| Hospital mortality | 2 (5) | 5 (19) | 4 (31) | 0.035 |
Data are shown as n (%) for categorical variables, and median (interquartile range) for continuous variables. Definition of abbreviations: AKI=acute kidney injury; SBP=systolic blood pressure; ED=emergency department; Cr=creatinine; OR=operating room; IV=intravenous; RBC= red blood cell.
Asian (n=4) and Unknown (n=3).
Administered prior to ICU arrival.
Administered during the calendar day of and the day after presentation.
Medical history of congestive heart failure was not obtainable for one subject with AKI stage 1.
Medical history of hypertension and diabetes mellitus was not obtainable for two subjects with AKI stages 2-3, and chronic kidney disease and was not obtainable for one subject with AKI stages 2-3.
Fisher's exact test for categorical variables, Kruskal-Wallis test for continuous variables, comparing across three groups (no AKI, stage 1, stage 2-3).
The three groups (no AKI, stage 1, stages 2-3) were frequency-matched during subject selection on age range, CKD history, and trauma mechanism, so the similarities between the groups are not random.
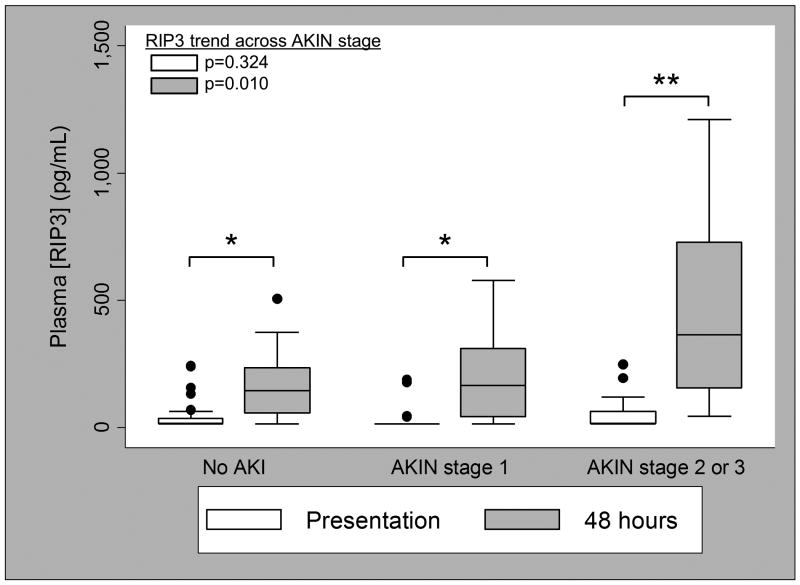
Plasma RIP3 concentrations at trauma bay presentation were not significantly different by AKI stage (no AKI: median 15.6 (IQR 15.6-36.4) pg/mL; AKI stage 1: 15.6 (15.6-15.6) pg/mL; AKI stage 2-3: 15.6 (15.6-64.0) pg/mL; p=0.324; Figure 1). By 48 hours, however, there was a significant difference in RIP3 concentrations across AKI stage (no AKI: 144.8 (58.6-234.9) pg/mL; AKI stage 1: 165.8 (43.0-310.9) pg/mL; AKI stage 2-3: 365.5 (155.1-727.5) pg/mL; p=0.010; Figure 1). Each natural log increase in 48-hour RIP3 was associated with increased odds of higher AKI stage (odds ratio 1.54; 95% CI 1.05-2.26; p=0.026), and this association persisted after adjustment for ISS and age (odds ratio 1.68; 95% CI 1.12-2.51; p=0.012). While 58 subjects (72.5%) had a presentation RIP3 level below the limit of detection (15.6 pg/mL), RIP3 increased at 48 hours in all but two subjects (median increase 129.2; range -89.6 to 1194.6 pg/mL; p<0.001) such that only 12 subjects (15%) had undetectable RIP3 at 48 hours. Like absolute 48-hour levels, the difference in RIP3 from presentation to 48 hours was significantly associated with AKI stage (no AKI: 108.1 (37.7-177.2) pg/mL; AKI stage 1: 129.0 (0-274.7) pg/mL; AKI stage 2-3: 301.5 (139.5-533.9) pg/mL: p=0.008). Pairwise comparisons of RIP3 by AKI stage are noted in the Figure 1 legend.
Figure 1.
Plasma RIP3 concentration at presentation (white boxes) and 48 hours (gray boxes) by Acute Kidney Injury Network (AKIN) stage. *p<0.001 for difference between presentation and 48 hours; **p=0.002 for difference between presentation and 48 hours. Pairwise comparisons for plasma RIP3 concentration at presentation: AKI stage 1 v. no AKI, p=0.365; AKI stage 2-3 v. no AKI, p=0.145; AKI stage 2-3 v. stage 1, p=0.042. Pairwise comparisons for plasma RIP3 concentration at 48 hours: AKI stage 1 v. no AKI, p=0.682; AKI stage 2-3 v. no AKI, p=0.004; AKI stage 2-3 v. stage 1, p=0.016.
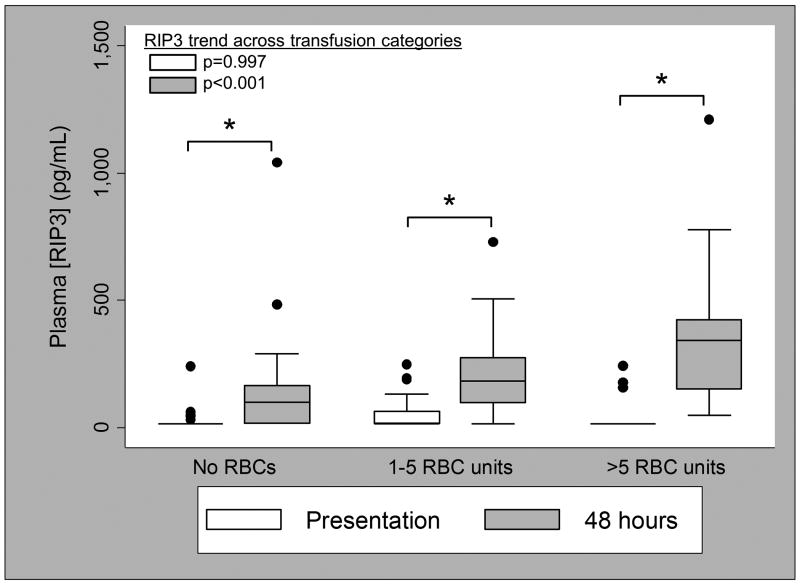
RBC transfusions on the day of and the day after presentation did not correlate with RIP3 measured at presentation, prior to blood product administration (no RBCs: median 15.6 (IQR 15.6-15.6) pg/mL; 1-5 units: 15.6 (15.6-64.0) pg/mL; >5 units: 15.6 (15.6-15.6) pg/mL; p=0.997; Figure 2), but did correlate with higher plasma RIP3 at 48 hours in a dose-dependent fashion (no RBCs: 99.4 (15.6-166.1)pg/mL; 1-5 units: 182.6 (98.5-274.1) pg/mL; >5 units: 341.8 (150.1-423.8) pg/mL; p<0.001; Figure 2). The difference in RIP3 from presentation to 48 hours increased significantly with increasing RBC transfusion (no RBCs: 53.0 (0-139.5) pg/mL; 1-5 units: 144.7 (43.3-248.2) pg/mL; >5 units: 267.8 (118.0-408.2) pg/mL; p<0.001).
Figure 2.
Plasma RIP3 concentration at presentation (white boxes) and 48 hours (gray boxes) by RBC units transfused during resuscitation (day of and day after presentation). Thirty-four subjects received no transfusions, 26 received 1-5 units, 20 received >5 units. *p<0.001 for difference between presentation and 48 hours. Pairwise comparisons for plasma RIP3 concentrations at presentation: 1-5 RBC units v. no RBC units, p=0.074; >5 RBC units v. no RBC units, p=0.629; >5 RBC units v. 1-5 RBC units, p=0.094. Pairwise comparisons for plasma RIP3 concentrations at 48 hours: 1-5 RBC units v. no RBC units, p=0.004; >5 RBC units v. no RBC units, p<0.001; >5 RBC units v. 1-5 RBC units, p=0.051.
Since all subjects with AKI stage 2-3 were male, we repeated analyses limited to the 69 male subjects and found no substantial differences. Specifically, there was no significant association of presentation RIP3 concentration with AKI stage (p=0.852) or RBC transfusions (p=0.513), while the associations of 48-hour RIP3 with AKI stage (p=0.018) and RBC transfusions (p<0.001) remained similar and statistically significant.
Of the baseline characteristics from Table 1, presentation RIP3 levels were associated only with a history of congestive heart failure (median 113.7 (IQR 33.4-209.6) pg/mL, n=4, v. 15.6 (15.6-29.6) pg/mL for no CHF, p=0.014) and Glasgow Coma Scale score (Spearman's rho -0.32, p=0.004). The 11 subjects who died in the hospital had higher RIP3 levels than those who survived, both at presentation (40.9 (15.6-176.3) pg/mL v. 15.6 (15.6-15.6) pg/mL, respectively, p=0.014) and 48 hours (362.1 (143.6-479.7) pg/mL v. 155.1 (53.3-263.8) pg/mL, respectively, p=0.027).
Discussion
We showed that among critically ill trauma patients, plasma RIP3 concentrations at 48 hours were significantly associated with AKI stage. We also demonstrated an association of RBC transfusions administered during the first two study days and subsequent plasma RIP3 concentrations measured at 48 hours. These findings suggest that the emerging in vitro and animal data implicating necroptosis in renal IRI may have relevance in human AKI after severe trauma, and that necroptosis may contribute to the mechanisms underlying prior findings that RBC transfusions are associated with AKI (12, 16, 17).
The source of RIP3 detected in plasma is not known. The elevated RIP3 we found in subjects with AKI may have reflected regulated necrotic cell death in the kidneys with subsequent release into circulating plasma. Linkermann et al have used murine models to show that RIP3 is expressed in proximal tubular and glomerular endothelial cells, and that necroptosis of glomerular endothelial cells can be inhibited by siRNA interference with RIP3, but plasma RIP3 was not reported in these models (12). Alternatively, the association of plasma RIP3 with AKI in our study may have been a reflection of global activation of necroptosis pathways after trauma, more so in patients who developed AKI. In vitro and animal studies have demonstrated RIP3-mediated cell death in multiple tissues, triggered by ligation of tumor necrosis factor receptor 1, toll-like receptors, and through other pathways activated in the setting of major trauma (8, 20). Such an explanation raises the possibility that AKI occurs as a downstream effect of necroptosis and consequent DAMP release from other organs and could implicate necroptosis as a mechanism in multiple organ dysfunction syndrome, evaluation of which is outside the scope of this study. Since RIP3 is normally intracellular, detection of extracellular RIP3 could also be a reflection of necrotic cell death in general, rather than a specific reflection of necroptosis. Recent data show contributions of other forms of regulated necrosis to in vitro and animal models of renal IRI, which could be studied alongside necroptosis in larger human studies of AKI (14).
It is notable that almost three-quarters of subjects had undetectable RIP3 levels on admission, rising to a median level more than ten-fold higher by 48 hours. The levels at presentation may reflect findings in the healthy state, given the typically short interval from injury to presentation, though data on baseline plasma RIP3 in humans are lacking. The dramatic rise by 48 hours still leaves unanswered the question of whether RIP3 is an initiator of AKI, is part of its pathophysiologic consequences, or if both AKI and RIP3 elevations are separate consequences of broader post-injury processes. Serial plasma sampling during the first 48 hours to determine whether the RIP3 rise precedes or follows the development of AKI may help elucidate both mechanistic implications and any utility of RIP3 as a predictor of AKI. RIP3 testing of human urine and renal tissue samples may also help to clarify the presence and timing of necroptotic activity in the kidneys and its contribution to AKI pathogenesis in humans.
The association of RBC transfusion with AKI after trauma, independent of injury severity and other confounders, has been well-described by us and others, and has been demonstrated in non-trauma populations as well (16, 17, 21, 22). In the current study, only plasma RIP3 levels at 48 hours, rather than on presentation, were associated with RBC transfusions, consistent with the hypothesis that transfusion augments necroptotic pathway activation and RIP3 release in humans. This relationship is mechanistically plausible: we have previously shown that RBC transfusion can cause necroptosis in endothelial cells, and that plasma RIP3 increases significantly in mice after RBC transfusion (18). Larger studies may further elucidate the potential contribution of necroptosis to mechanisms underlying the association of RBC transfusion with AKI.
Our study has several limitations, the most important being its limited sample size. This restricted our ability to account for potential confounders of the relationship between RIP3 and AKI stage. Even our limited multivariable analysis may have overfit the data due to the relatively few patients with stage 2-3 AKI. Baseline characteristics affecting RIP3 levels in humans are not well described, since human studies of RIP3 are very limited (18). Studies with greater numbers, particularly of those with moderate to severe AKI, are needed to adequately power adjusted analyses of the RIP3-AKI association and to establish generalizability of our findings. Sample size may also have limited our ability to detect a statistically significant difference in plasma RIP3 levels between stage 1 and no AKI in pairwise comparison. However, stage 1 AKIN criteria were conceived as having a high sensitivity but lower specificity for true renal injury, making those with higher stage AKI a better population in which to evaluate pathogenic mechanisms (5). Given the small number of women in our cohort, larger studies would also be needed to determine if our findings are generalizable to female trauma patients. Finally, as with any plasma biomarker, RIP3 may increase due to decreased renal clearance in the setting of AKI; that is, it may be a marker of functional renal impairment, like serum creatinine, rather than an indicator of AKI pathogenesis. While pre-clinical data implicate necroptosis as an important AKI mechanism in IRI, further study of RIP3 clearance from plasma would be helpful to inform this possibility.
Conclusions
We demonstrated that in critically ill trauma patients, plasma RIP3 levels at 48 hours were associated with AKI stage and RBC transfusions administered during resuscitation. These findings provide evidence that necroptosis may be an important pathogenic contributor to AKI in severely injured populations, and that RBC transfusion-associated necroptotic cell death may contribute to the previously demonstrated association of transfusions with AKI. Given the consistency of our findings with observations in experimental AKI models, along with ongoing development of therapies targeting necroptosis (8), further study is warranted of this mechanistic pathway in humans.
Acknowledgments
Conflicts of Interest and Source of Funding: Drs. Shashaty, Reilly, Sims, Holena, Meyer, Lanken, Feldman, Christie, and Mangalmurti report institutional funding from National Institutes of Health grants. Dr. Sims reports funding from the National Trauma Institute and the Amercian College of Surgeons. Dr. Meyer reports institutional funding from GlaxoSmithKline. Dr. Lanken reports textbook royalties from Elsevier. Dr. Feldman reports payment for lectures at Case Western Reserve University and George Washington University and travel/accommodation/meeting expenses from the American College of Epidemiology. Dr. Christie reports payments from various law firms for asbestos litigation as well as institutional funding from GlaxoSmithKline. Dr. Mangalmurti reports institutional funding from the Department of Defense. This study was funded by NIH grants P50-HL60290, P01-HL079063, K24-HL115354, K12-HL109009, K23-DK097307, K08-HL098362, F32-HL122075, the Department of Defense-PR141324, and by the McCabe Fund Pilot Award.
Footnotes
Reprints will not be ordered
References
- 1.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Critical Care. 2006;10(3) doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009;302(11):1179–85. doi: 10.1001/jama.2009.1322. Epub 2009/09/17. [DOI] [PubMed] [Google Scholar]
- 3.Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med. 2014;189(9):1075–81. doi: 10.1164/rccm.201311-2097OC. Epub 2014/03/08. [DOI] [PubMed] [Google Scholar]
- 4.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nature reviews Nephrology. 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16. Epub 2011/03/03. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care (London, England) 2007;11(2) doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A Unified Theory of Sepsis-Induced Acute Kidney Injury: Inflammation, Microcirculatory Dysfunction, Bioenergetics, and the Tubular Cell Adaptation to Injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature reviews Molecular cell biology. 2010;11(10):700–14. doi: 10.1038/nrm2970. Epub 2010/09/09. [DOI] [PubMed] [Google Scholar]
- 8.Linkermann A, Green DR. Necroptosis. The New England journal of medicine. 2014;370(5):455–65. doi: 10.1056/NEJMra1310050. Epub 2014/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. Journal of the American Society of Nephrology : JASN. 2014;25(12):2689–701. doi: 10.1681/asn.2014030262. Epub 2014/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratych RE, Bulkley GB, Williams GM. Ischemia/reperfusion injury in the kidney. Prog Clin Biol Res. 1986;224:263–89. Epub 1986/01/01. [PubMed] [Google Scholar]
- 11.Schumer M, Colombel MC, Sawczuk IS, Gobe G, Connor J, O'Toole KM, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;140(4):831–8. Epub 1992/04/01. [PMC free article] [PubMed] [Google Scholar]
- 12.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81(8):751–61. doi: 10.1038/ki.2011.450. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 13.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110(29):12024–9. doi: 10.1073/pnas.1305538110. Epub 2013/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–41. doi: 10.1073/pnas.1415518111. Epub 2014/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40(9):912–8. doi: 10.1016/j.injury.2009.05.024. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 16.Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252(1):158–65. doi: 10.1097/SLA.0b013e3181deb6bc. Epub 2010/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shashaty MG, Meyer NJ, Localio AR, Gallop R, Bellamy SL, Holena DN, et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care. 2012;27(5):496–504. doi: 10.1016/j.jcrc.2012.02.002. Epub 2012/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qing DY, Conegliano D, Shashaty MG, Seo J, Reilly JP, Worthen GS, et al. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med. 2014;190(11):1243–54. doi: 10.1164/rccm.201406-1095OC. Epub 2014/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzick J. A wilcoxon-type test for trend. Statistics in Medicine. 1985;4(4):543–7. doi: 10.1002/sim.4780040416. [DOI] [PubMed] [Google Scholar]
- 20.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. Epub 2005/05/25. [DOI] [PubMed] [Google Scholar]
- 21.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33(8):1749–56. doi: 10.1097/01.ccm.0000171531.06133.b0. Epub 2005/08/13. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495–502. doi: 10.1161/circulationaha.108.786913. Epub 2009/01/21. [DOI] [PubMed] [Google Scholar]