Abstract
Background
Dengue virus (DENV) is a mosquito-borne flavivirus that causes significant human disease and mortality in the tropics and subtropics. By examining the effects of virus infection on gene expression, and interactions between virus and vector, new targets for prevention of infection and novel treatments may be identified in mosquitoes. We previously performed a microarray analysis of the Aedes aegypti transcriptome during infection with DENV and found that mosquito ubiquitin protein Ub3881 (AAEL003881) was specifically and highly down-regulated. Ubiquitin proteins have multiple functions in insects, including marking proteins for proteasomal degradation, regulating apoptosis and mediating innate immune signaling.
Methods
We used qRT-PCR to quantify gene expression and infection, and RNAi to reduce Ub3881 expression. Mosquitoes were infected with DENV through blood feeding. We transfected DENV protein expression constructs to examine the effect of Ub3881 on protein degradation. We used site-directed mutagenesis and transfection to determine what amino acids are involved in Ub3881-mediated protein degradation. Immunofluorescence, Co-immunoprecipitation and Western blotting were used to examine protein interactions and co-localization.
Results
The overexpression of Ub3881, but not related ubiquitin proteins, decreased DENV infection in mosquito cells and live Ae. aegypti. The Ub3881 protein was demonstrated to be involved in DENV envelope protein degradation and reduce the number of infectious virions released.
Conclusions
We conclude that Ub3881 has several antiviral functions in the mosquito, including specific viral protein degradation.
General significance
Our data highlights Ub3881 as a target for future DENV prevention strategies in the mosquito transmission vector.
Keywords: Dengue virus, Mosquito transmission, Ubiquitin, Protein degradation, Arbovirus
Graphical abstract

Highlights
-
•
A novel mosquito ubiquitin, Ub3881, is identified in Aedes aegypti.
-
•
Ub3881 is shown to have antiviral functions during dengue virus infection of the mosquito.
-
•
Ub3881 targets a dengue viral protein for degradation during infection.
-
•
Future dengue virus prevention strategies could incorporate Ub3881 as a target to prevent mosquito infection.
1. Introduction
Dengue virus (DENV) is a globally significant mosquito-borne flavivirus responsible for serious disease and mortality. There are over 2.5 billion people at risk of DENV infection worldwide, with 50–100 million new cases each year and with more than 100 endemic countries [1]. There is no specific treatment for DENV infection and efforts to create a vaccine have been hindered by safety issues and antibody-dependent enhancement (ADE) [2], [3]. There are four serotypes of DENV, and infection with one does not provide protection against secondary infection with another serotype. This can potentially lead to immunopathology due to ADE, often resulting in severe forms of dengue diseases, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [2], [3], [4], [5], [6], [7].
Aedes aegypti is the main mosquito vector responsible for transmitting DENV to humans. This mosquito is well-adapted to live in areas of human habitation as it regularly enters residences to feed and prefers humans to other vertebrates. Once a mosquito acquires virus from an infected person, DENV needs 7–10 days to replicate in the vector before the mosquito can transmit virus to a new host [8], [9]. In many dengue-endemic regions, there is intra-household transmission due to the nature of Ae. aegypti and the tendency for many family members to share one house. The standard control measures to prevent the spread of DENV in endemic areas rely on use of insecticides, larval habitat reduction [10], and surveillance, but the incidence of dengue fever (DF) and DHF continues to rise each year. Other, innovative means for vector management and disease prevention are necessary for complete control of this disease [6], [11], [12], [13].
As traditional avenues for prevention and treatment may not prove completely effective against DENV, there exists an opportunity for exploring novel methods of stopping DENV spread by careful evaluation of viral pathogenesis and interaction with the mosquito vector. The targeting, modification or elimination of specific genes in Ae. aegypti could reduce vector competence for virus acquisition [8], [14], [15]. A study by our laboratory identified a number of mosquito genes that had significantly altered expression during flavivirus infection [16]. One of these genes that encodes a ubiquitin (Ub) protein (SeqID AAEL003881) was significantly down-regulated at all time-points examined in Ae. aegypti during infection with DENV. Investigation of the impact of Ub3881 on DENV infection in the mosquito as well as the mechanisms responsible for the down-regulation of Ub3881 during infection will help determine whether the protein can be used to inhibit DENV in the transmission vector.
Ubiquitin is a globular protein that is extremely conserved in all eukaryote cells with a highly homologous protein sequence in all animals from insects to humans. It is comprised of 76 amino acids and usually contains seven conserved lysine (K) residues: K6, K11, K27, K29, K33, K48, and K63 [17]. Ubiquitin has many varying functions, including marking regulatory proteins for degradation, regulating apoptosis, activating the immune system, cell proliferation, cell differentiation, and cell survival [18], [19], [20]. Each ubiquitin lysine attachment creates varied structures and determines the outcome of the target protein [17], [21]. The process of ubiquitination is quite complex with many pathways and functions in virtually every cell type.
Though ubiquitination is an important protein modification for normal cell cycle functions, it is also required for and manipulated by many viruses, including flaviviruses [22], [23], [24]. For example, several coronaviruses cannot exit the endosome with an impaired ubiquitin-proteasome system [25] and proteasomal inhibitors are known to block the endocytosis of influenza virus [26]. The ubiquitin ligase CBLL1 has been shown to be crucial for the internalization of West Nile virus (WNV) in mammalian cells [27]. Both herpes simplex virus type 1 (HSV-1) and human papillomavirus (HPV) are able to direct the tumor suppressor protein p53 for proteasomal degradation through manipulation of ubiquitin ligases [28], and human immunodeficiency virus (HIV) proteins recruit the formation of the host E3 ubiquitin ligase complex to induce degradation of both APOBEC-3G and CD4 [29], [30]. Inhibition of the ubiquitin-proteasome system has also been shown to block various steps in the replication of several viruses, including poxviruses, coxsackie virus 3B, respiratory syncytial virus and WNV [31], [32], [33], [34], [35]. Research in primary human endothelial cells revealed a requirement for a ubiquitin-activating enzyme E1 and a proteasome subunit during DENV infection, demonstrating the important role that ubiquitination plays during the DENV life cycle [36]. There was also a recent report that DENV infection in mosquitoes may be dependent on a functioning ubiquitin proteasome system [37]. However, a comprehensive picture of the role of ubiquitin and individual ubiquitin pathway proteins during DENV infection in the mosquito transmission vector is still lacking.
Here, we examine the impact of varying levels of Ub3881 on DENV infection in mosquito cells and live Ae. aegypti, and examine the requirements for the ubiquitin-protease system during DENV infection of insect cells in vitro. We also explore various mechanisms likely involved in the antiviral action of Ub3881 during DENV infection. Our data sheds light on the role that Ub3881 plays during DENV infection in the mosquito vector and highlights a possible target protein for controlling DENV transmission.
2. Methods
2.1. Cell culture and virus growth
The Aag2 Ae. aegypti cell line was used for transfection and infection studies. The cells were grown at 30 °C and 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Gemini, CA), 1% penicillin-streptomycin and 1% tryptose phosphate broth (Sigma, MO). Dengue virus stock was grown in C6/36 Aedes albopictus cell line using the same media. The dengue strain used was DENV-2 New Guinea C. Cells were infected at an m.o.i. of 1.0, virus was allowed to propagate for 6–8 days, supernatant was removed, spun down and virus stock was stored at − 80 °C until use.
2.2. Mosquito infections
The Rockefeller strain of Ae. aegypti were infected by blood-feeding, using 400 μL of DENV-infected C6/36 cell supernatant (10^4 pfu) added to 1 mL serum-inactivated human blood (purchased from The Blood Center, New Orleans, LA). Blood was tested in our laboratory and was negative for the presence of flavivirus or flaviviral antibodies. Mosquitoes were fed for 20 min at room temperature using a hemotek feeder and maintained in groups of 10 at 30 °C, 80% humidity. Mosquitoes were supplied sucrose water as a source of dietary sugar. At the conclusion of experiments, mosquitoes were briefly washed in 70% ethanol and then rinsed in sterile PBS. Organs were dissected in sterile PBS and transferred to Eppendorf tubes separately. Mosquito organs were stored in PBS with protease inhibitors for protein assays and homogenized in RLT buffer (Qiagen, CA) for gene expression assays.
2.3. qRT-PCR analysis
RNA was isolated from infected Ae. aegypti mosquitoes on Days 1, 2, 7 and purified using RNeasy kit (Qiagen, CA) according to manufacturer's instructions. The quantitative RT-PCR analysis was done using the QuantiFast kit according to manufacturer's instructions (Qiagen, CA). Oligos for the qRT-PCR reactions were:
DENV envelope: F: 5′-CATTCCAAGTGAGAATCTCTTTGTCA-3′, R: 5′-CAGATCTCTGATGAATAACCAACG-3′; Ae. aegypti Actin: F: 5′-GAACACCCAGTCCTGCTGACA-3′, R: 5′-TGCGTCATCTTCTCACGGTTAG-3′; Ae. aegypti Ub3881: F: 5′-CCGGTCTGGCCGAGGACGAAACC-3′, R: 5′-CAGGCCTGGCCACCTCTC-3′.
2.4. Mosquito injections
DNA plasmids were injected according to our published whole-body transfection method [38]. Briefly, Cellfectin II (Invitrogen, CA) was mixed with S2 Schneider's medium at a 1:1 ratio and then keep at RT for 10 min. Plasmid DNA was combined with this mixture and incubated at RT for 30 min before thoracic microinjection into Aedes aegypti. Mosquitoes were injected with 500 ng plasmid/300 nL solution. The siRNA was dissolved in sterile PBS and injected into the thorax in a similar manner at varying concentrations for optimization.
siRNA molecules used were: Ub3881: 5′-aagaagauggucgaagcccgg-3′; GAPDH: proprietary sequence, Ambion catalog # AM4605.
2.5. Immunofluorescence analysis
Aag2 Ae. aegypti cells were infected with DENV at an MOI of 0.1. At 24 h post-infection, infected cells and control cells were fixed in 4% paraformaldehyde for 20 min at RT, washed with PBS(−) and then stained for infection using antibodies against Ub3881, Imd or Relish proteins (made at Yale Keck Center), DENV envelope gene (Millipore, MA) and/or 19S proteasome (Abcam, MA). The antibodies were diluted in 1% BSA at 1/250 and cells were incubated for 20 min at RT. All secondary antibodies used were standard (anti-mouse or anti-rabbit TRITC and FITC), and were diluted according to manufacturer's instructions. Infection was visualized using fluorescent microscopy, equipment and specifics can be found in figure legends.
2.6. Western blots
Solutions were run on a 4–12% SDS-PAGE gel for 1.5 h at 15 mA per gel (unless figure legend indicates otherwise). The proteins were then transferred to PVDF membrane. The membrane was blocked with 5% milk in 1% TBST for 1 h at RT and then incubated with the appropriate primary antibody overnight at 4 °C. The membrane was washed and then incubated with the appropriate horseradish peroxidase secondary antibody for 1 h at RT. The protein blots were incubated with ECL substrates (Amersham, NJ) for 5 min at RT and then detected on Kodak film.
2.7. Transfection of plasmids
The expression plasmids were made from pAc5.1/V5-His A vector (Invitrogen, CA) and cloning was done using PCR along with gene-specific primers as previously described [39]. We used the Qiagen mini-prep kit to isolate DNA from bacterial cultures after transforming DH5-alpha cells. Plasmids were transfected into cells using Effectene (Qiagen, CA) according to manufacturer's instructions. Briefly, for a 10 cm2 plate, 10 μg of DNA was mixed with 500 μL buffer EC and 32 μL enhancer was added. This was allowed to incubate for 5 min on the benchtop. Then, 30 μL Effectene reagent was added and the solution vortexed briefly. After 10 min incubation, the solution was added to the cells. Expression was observed 24 h post-transfection and peaked at 48 h.
2.8. Protein interactions
Antibodies against Ub3881 (Supplementary Table 3) were bound to protein A/G beads and used to probe Ae. aegypti lysate. The bound Ub3881 was eluted and interacting partners in solution were analyzed by LC/MS-MS. All steps were done at 4 °C to maintain the protein interactions. The eluted proteins were analyzed at the Yale University W.M. Keck Foundation core facility. The eluate was subjected to trypsin digestion followed by LC/MS-MS (liquid chromatography and mass spectometry) for peptide sequencing and identification using the recently completed Aedes aegypti mosquito genome [40]. Putative mosquito proteins were identified via amino acid sequence identity to both known mosquito proteins and their mammalian counterparts using the BLAST software on the NCBI website. A portion of the solution was also run on an SDS-PAGE gel and Western blot analysis was done with the Ub3881 antibody. In addition, antibodies against Ub3881 and mosquito Imd proteins were used to precipitate proteins out of DENV-infected mosquito lysate. The solutions were run on an SDS-PAGE gel and Western blot analysis was done using the antibody against Ub3881.
3. Results
3.1. Ub3881 is specifically down-regulated by DENV infection
We previously identified mosquito genes that had differential expression during infection with West Nile virus (WNV), yellow fever virus (YFV) and dengue virus (DENV). Many genes were highly down-regulated during infection with all three viruses, including an ubiquitin protein that we have designated Ub3881 (AAEL003881) (Table S1) [16]. The Ub3881 gene was the only mosquito ubiquitin in our screen to have significantly altered expression during DENV infection (Fig. 1A and Table S2). An alignment of mosquito ubiquitin genes analyzed is shown in Fig. 1B (with the exception of AAEL008920, as the active length is not complete in the published Ae. aegypti genome). Interestingly, the Ub3881 protein has several variations in amino acid sequence when compared to the canonical universal ubiquitin protein sequence (Fig. 1B), including an asparagine (N) in place of the lysine at position 63 (K63) and an alanine inserted at position 19. To confirm that Ub3881 expression is lower during DENV infection of the mosquito, we repeated the infection and analysis. Total RNA was isolated from mosquitoes on 1, 2 and 7 days post-infection (dpi) and qRT-PCR analysis done to quantify levels of mosquito ubiquitin gene expression. We confirmed that Ub3881 is significantly down-regulated during DENV infection of Ae. aegypti at all time-points analyzed and that other ubiquitin genes did not have significantly altered gene expression (Fig. 1C). We also used lysate from homogenized mosquitoes to confirm Ub3881 protein reduction during DENV infection at the same timepoints (Fig. S1).
Fig. 1.
Ubiquitin protein Ub3881 expression is decreased during DENV infection in Ae. aegypti. A. Ae. .aegypti were infected with DENV and microarray analysis for gene expression was done at 1, 2 and 7 days post-infection on isolated RNA, known ubiquitin family genes are shown. P < 0.05. Pooled mosquitoes. Experimental and technical replicates were done in triplicate. B. Alignment using ClustalOmega of the first active length of the ubiquitin genes from 1A. C/D. Mosquitoes were infected with DENV via blood feeding and at 1, 2 and 7 days post-infection, C. RNA was isolated from whole mosquitoes or D. RNA was isolated from dissected midguts and salivary glands. qRT-PCR analysis was done to measure expression of ubiquitin genes. Experimental and technical replicates were done in triplicate. P < 0.05. Statistical analysis done using ANOVA and Students t-tests. Fold-change in expression is shown as compared to uninfected, bloodfed mosquitoes.
Supplementary Fig. 1.

Ub3881 protein changes during DENV infection. Mosquitoes were infected with DENV via blood feeding and at 1, 2 and 7 days post-infection, mosquitoes were homogenized and cell lysates run on SDS-PAGE gel. Western blot analysis was done using the antibody against Ub3881. Mock is uninfected, bloodfed mosquito lysate.
3.2. Ub3881 gene expression and regulation during infection is tissue-specific
During infection of the mosquito, DENV must first infect the midgut (MG), and then disseminate through the body to the salivary glands (SG), which is critical for transmission to a new host. To determine the tissue-specific expression of Ub3881 during infection, we orally infected Ae. aegypti with DENV and dissected MG and SG on 1, 2 and 7 dpi. We performed qRT-PCR analysis on RNA isolated at each time point to determine levels of gene expression (Fig. 1D). On day 1 of DENV infection, Ub3881 was over 8-fold down-regulated in the SG of the mosquitoes. The decrease in Ub3881 expression in the MG was around 2-fold on day 1 (Fig. 1D). On day 2 of infection, the expression of Ub3881 was over 4-fold down-regulated in both the SG and MG (Fig. 1D). On day 7, the expression in the SG was over 6-fold lower in the DENV- infected mosquitoes and expression in MG had around a 5-fold decrease (Fig. 1D). These results suggest a complex balance of Ub3881 gene regulation throughout the mosquito and in the individual organs during DENV infection. This also indicates that, as in humans, the infectious process can lead to systemic pathological changes very early in DENV infection, perhaps through signaling pathways and/or molecules traveling in mosquito hemolymph.
3.3. Overexpression of Ub3881 inhibits DENV infection in mosquito cells and live mosquitoes
We hypothesize that DENV infection may specifically down-regulate Ub3881 expression in the mosquito to combat antiviral effects on the DENV life cycle. To investigate whether Ub3881 has anti-DENV properties, we chose to overexpress the Ub3881 gene and analyze the resulting DENV infection. The coding regions for Ub3881, and the representative ubiquitin gene Ub6511 whose expression was not altered by DENV infection in the mosquito (Fig. 1C), were cloned into the insect expression vector pAc5.1/V5-His (Life Technologies, CA). The expression vectors were transfected into an Ae. aegypti mosquito cell line, Aag2, and the cells were infected with DENV. The overexpression of either protein had no significant cytotoxic effect on the cells. We found that the overexpression of Ub3881 caused a 6-fold reduction in DENV infection as compared to control infection with GFP transfection (Fig. 2A), and overexpression of Ub6551 did not significantly alter infection levels. Next, we analyzed the effects of Ub3881 overexpression on DENV infection in mosquitoes. We injected Ae. aegypti with the expression vectors using a whole-body transfection method that we used with success previously [38]. We first analyzed gene expression in the mosquitoes at 3 and 14 days post-microinjection (dpmi) to confirm that expression of Ub3881 was increased after plasmid transfection. We found that expression of Ub3881 was increased by an average of 1000-fold at 3 and 14 dpmi in the injected mosquitoes (Fig. 2B). We then injected mosquitoes with the Ub3881 expression vector and infected them with DENV via blood feeding at 5 dpmi. Mosquito MG and SG were dissected on 8 dpi, total RNA was isolated and qRT-PCR analysis was done to quantify DENV infection levels. As shown in Fig. 2C, DENV infection was down 10- to 25-fold in the mosquito MG and reduced up to 10-fold in the mosquito SG during Ub3881 overexpression. These data demonstrate that increased Ub3881 reduces DENV infection in Ae. aegypti and that the reduction occurs in both MG and SG, the organs involved in acquisition and transmission of virus.
Fig. 2.
The overexpression of Ub3881 inhibits DENV infection in Ae. aegypti. A. Aag2 mosquito cells were transfected with expression plasmids encoding either Ub3881 or a representative ubiquitin Ub6551 and cells were infected with DENV. qRT-PCR analysis was done to measure DENV infection. P < 0.001. B. Ae. aegypti mosquitoes were transfected with Ub3881 expression plasmid and qRT-PCR analysis was done to measure Ub3881 expression at D3 and D14. C. Mosquitoes overexpressing Ub3881 were infected with DENV via blood feeding at D5 post-transfection. At D8 post-infection, midgut and salivary glands were dissected and analyzed by qRT-PCR to measure DENV infection. P < 0.001. D. Aag2 cells were transfected with either mosquito Ub3881 siRNA or human GAPDH siRNA (control) and infected with DENV 72 h post-transfection. Cells were analyzed by qRT-PCR at various timepoints to measure DENV infection. E. Mosquitoes were injected with siRNA targeting either Ub3881 or human GAPDH and infected with DENV by blood feeding 7 days post-injection. At day 5 post-infection, qRT-PCR analysis was done to measure DENV infection.
3.4. DENV infection efficiently regulates expression of Ub3881
Since DENV infection reduced Ub3881 expression in the mosquito, and increasing Ub3881 reduced DENV infection, we hypothesized that further reduction of Ub3881 may increase levels of infection. To investigate this, we used RNA interference (RNAi) to block the expression of Ub3881 with siRNA molecules designed against the Ub3881 gene. The standard insect RNAi method of injecting dsRNA transcribed from the target gene could not be performed due to the potential of non-specific knock-down. Therefore, an siRNA molecule against a gene-specific coding region of Ub3881 was used so that the inhibition of expression would be specific. We transfected the Ub3881 siRNA into Aag2 cells and infected the cells with DENV 72 h post-transfection (hpt). This timepoint was chosen after carefully examining Ub3881 knockdown at 24–168 hpt. The Ub3881 was over 80% reduced at 72 hpt and expression remained down until the 168 timepoint. At 24, 36, 48, 72 and 96 h post-infection (hpi), cellular RNA was analyzed by qRT-PCR for infection levels. Reduction in Ub3881 resulted in a slight increase in DENV infection in the cells (Fig. 2D) but it was not significant. Next, we used the siRNA in live Ae. aegypti to reduce the Ub3881 levels prior to DENV infection. The siRNA was intrathoracically injected according to our previous methods [16], [41] and mosquitoes were infected with DENV by blood feeding at 7 dpmi. At 5 dpi, RNA was isolated from mosquitoes for qRT-PCR analysis. We found a slight increase in DENV infection in the mosquitoes with reduced Ub3881 (Fig. 2E) but the difference was not statistically significant. This indicated both that Ub3881 may not be inhibitory to initial virus entry and that the Ub3881 down-regulation caused by DENV infection is sufficient for successful infection.
3.5. Ub3881 reduces the number or infectivity of dengue virions produced
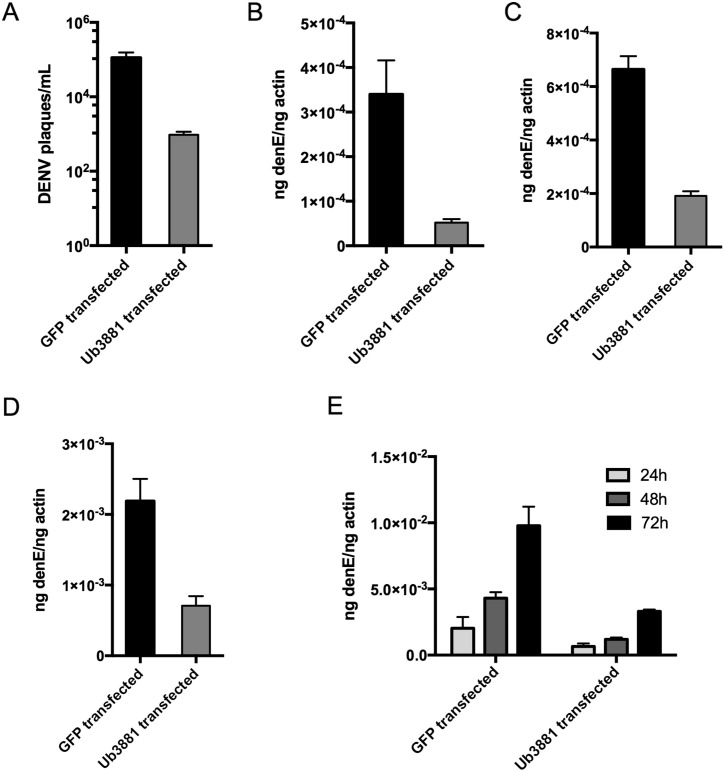
Since we found that Ub3881 may have antiviral activity, we hypothesized that the overexpression of Ub3881 would negatively impact infectious DENV production. To examine this, we transfected the Ub3881 expression plasmid into Aag2 cells and then infected with DENV 24 h post-transfection. A different set of cells was transfected with a GFP plasmid as a control. Since we wanted to investigate the number and quality of virions produced from the cells with and without Ub3881 overexpression, we assayed the infectivity cell supernatants. First, we used the supernatant in a plaque assay on uninfected Vero cells. We found that the supernatant from Aag2 cells that were overexpressing Ub3881 resulted in a lower number of plaques than the supernatant from the control cells (Fig. 3A). This indicated that there is less infectious virus in cells that are overexpressing Ub3881. We next used the supernatant to infect a new set of cells, both Aag2 mosquito and Huh7 human liver cells. We found that, again, the supernatant from cells that were overexpressing Ub3881 resulted in lower infection, as measured by qRT-PCR (Fig. 3B/C). We also measured the DENV in the supernatant from the original transfected/infected Aag2 cells to quantify DENV. We determined that there was less DENV in the supernatant from cells that had Ub3881 transfected than the supernatant from control cells (Fig. 3D). Finally, we performed qRT-PCR analysis to quantify DENV infection over time in the original Aag2 cells, with and without Ub3881 overexpression. We found that DENV infection rapidly increases over time in the control cells and the cells that had Ub3881 transfected showed significantly lower DENV infection, especially at later timepoints (Fig. 3E). Together, this data suggest that Ub3881 expression results in less infectious dengue virions produced in the cells.
Fig. 3.
Ub3881 reduces production of infectious DENV. Aag2 cells were transfected with expression plasmids encoding either Ub3881 or GFP, and 24 h post-transfection cells were infected with DENV. A–D. Cell culture supernatants were collected 48 h post-infection. A. Supernatants were used in a plaque assay on Vero cells to quantify infectious DENV. Plaque forming units per mL (PFU/mL) are shown on log scale. B/C. Supernatants were used to infect Aag2 (B) and Huh7 (C) cells and cell lysates were used in qRT-PCR analysis to quantify DENV infection. D. Supernatants were used in qRT-PCR analysis to quantify virions produced in original infection. E. Original Aag2 cell lysates were used in qRT-PCR to quantify DENV virus growth over time. Lysates were collected at 48 h post-infection. All graphs shown in this figure have P < 0.01 and statistical P-values were calculated using students t tests.
3.6. Ub3881 expression impacts immune signaling in DENV-infected mosquito cells
One function of ubiquitin in insects is to regulate immune signaling [42], [43]. Thus, the suppression of DENV infection by Ub3881 might be due to the activated state of antiviral immune pathways. Ubiquitin can regulate Imd immune signaling via attachment to the Imd protein, which may result in the transcription of innate immune genes, such as antimicrobial peptides (AMPs) [44]. In addition, the Imd pathway was recently shown to be involved in antiviral immunity in insects [45], [46]. Thus, quantification of AMP expression levels was analyzed during Ub3881 overexpression and DENV infection. Aag2 cells were transfected with Ub3881 or Ub6551 expression vectors and infected with DENV 24 hpt. Levels of AMP genes were analyzed by qRT-PCR at 48 hpi. We found that several AMP genes, including cecropin, attacin, defensin and diptericin, had significantly increased levels during DENV infection with the addition of Ub3881 overexpression, as compared to cells overexpressing Ub6551 or DENV infection alone (Fig. 4A, Table S4). We hypothesized that the Ub3881-induced expression of immune genes may be signaled through the Imd pathway. Therefore, we next sought to determine if the Ub3881 directly interacted with the Aedes Imd protein. To investigate this, we used antibody against the Ae. aegypti Imd coding sequence that we had produced (Table S3, Fig. S2) in an immunoprecipitation assay (IP) to pull down Ub3881 binding partners from whole Ae. aegypti lysates. The precipitated solution was sent for LC/MS-MS analysis to determine which proteins interacted with mosquito Ub3881. A Western blot of the eluate is shown in Fig. 4B and a list of the proteins identified in the elution solution is shown in Table 1 . We identified both Aedes Imd protein and several 26S proteasome proteins as putative Ub3881 interactors. Several vitellogenin proteins were also identified as Ub3881 binding partners. To confirm that there was an interaction between Ub3881 and mosquito Imd protein, we used the antibodies we had made against each protein to precipitate them from DENV-infected Ae. aegypti lysates. We ran the precipitated solutions on SDS-PAGE gels and used Western blot analysis with the antibody against Ub3881. The results shown in Fig. 4C demonstrate that Ub3881 protein was pulled out with both the Ub3881 antibody and the antibody against mosquito Imd protein. Next, we used the antibodies to localize both Ub3881 and Imd in Aag2 cell lines during DENV infection (Fig. 4D). These results indicate that there is an interaction between Ub3881 and Imd in the mosquito during DENV infection, and this interaction could be driving increased production of AMPs.
Fig. 4.
Ub3881 is involved in mosquito antiviral pathways. A. Aag2 cells were transfected with expression plasmids encoding either Ub6511 or Ub3881 and infected with DENV 24 h post-transfection. At 48 h post-infection, cells were analyzed by qRT-PCR for expression of select antimicrobial peptides (AMPs). B. Antibodies against Ub3881 were bound to protein A/G beads and used to probe Ae.aegypti lysate. The bound Ub3881 (and partners) was eluted and interacting partners in solution were analyzed by LC/MS-MS (see Table 1). A portion of the solution was also run on an SDS-PAGE gel and Western blot analysis was done with the Ub3881 antibody. C. Antibodies produced against Ub3881 and mosquito Imd proteins were used to precipitate proteins out of DENV-infected mosquito lysate. The solutions were run on an SDS-PAGE gel and Western blot analysis was done using the antibody against Ub3881. D. Aag2 cells were infected with DENV and immunofluorescence analysis was done using antibodies against Ub3881 and mosquito Imd protein to localize the proteins in the cells. A representative image is shown; images were taken at 20 ×. E. Aag2 cells were transfected with Ub3881 and immunofluorescence analysis was done using antibodies against Ub3881 and cleaved caspase-3 to demonstrate increased apoptosis with increased Ub3881 expression. Green cells have overexpression of Ub3881 and red cells have labeled Representative cells are shown; images were taken at 20 ×. F. Aag2 cells were transfected with Ub3881 or a mock expression plasmid and infected with DENV 24 h post-transfection. A group of control cells were not infected. Cell lysates were run on SDS-PAGE gels and Western blot analysis was done with antibodies against cleaved caspase-3 as a marker for apoptosis. Mock = uninfected, mock transfected; Ub3881 = uninfected, Ub3881-transfected; DENV = DENV-infected, mock transfected; and DENV-Ub3881 = DENV-infected, Ub3881-transfected. All Western blots were run with corresponding amounts of protein per lane as loading controls.
Table 1.
Ub3881 binding partners in Ae. aegypti.
| Protein ID | Name | Score | Expectation |
|---|---|---|---|
| gi | 108873755 | Imd | 679 | 2.3E-61 |
| gi | 472308 | Vitellogenin-A | 586 | 4.1E-52 |
| gi | 37528873 | Vitellogenin-B | 1005 | 4.9E-94 |
| gi | 37528871 | Vitellogenin-C | 606 | 4.1E-54 |
| gi | 157110191 | Ub-enzyme E1 | 1097 | 3.3E-103 |
| gi | 157127055 | 26S proteasome subunit 7 | 172 | 1E-10 |
| gi | 157124938 | 26S proteasome non-ATPase subunit | 151 | 1.3E-8 |
| gi | 1709799 | 26S proteasome subunit 8 | 514 | 7.4E-45 |
| gi | 157132413 | 26S proteasome subunit S9 | 489 | 2E-42 |
| gi | 157106603 | 26S proteasome subunit S10b | 482 | 1.5E-41 |
| gi | 157125334 | Importin beta-3 | 1009 | 2E-94 |
| gi | 157126954 | Importin beta-4 | 572 | 1E-50 |
| gi | 157108446 | Coatamer (COPA) | 462 | 1.1E-39 |
Another function of ubiquitin in insects is the initiation of cellular apoptosis, for example, through the ubiquitination of the inhibitor of apoptosis (IAP) protein [47], [48]. To investigate whether Ub3881 may initiate apoptosis in mosquito cells during DENV infection, we transfected Aag2 cells with Ub3881 and/or infected cells with DENV. We then performed immunofluorescence analysis on cells that had overexpression of Ub3881 and measured apoptosis using an antibody recognizing cleaved caspase-3 protein. The cleavage of caspase-3 is commonly used as an indicator of cellular apoptosis and the antibody against human caspase-3 recognizes the cleaved (active) protein in insect cells [49], [50]. We found that the cells with increased Ub3881 expression had increased levels of cleaved caspase-3 as compared to the cells with no Ub3881 overexpression (Fig. 4E), indicating increased apoptosis. Fig. 4E presents 3 mosquito cells, as indicated by DAPI blue fluorescence. Only the single green cell, indicating overexpression of Ub3881, also had red fluorescence, which indicated increased cleaved caspase-3. We then did Western blot analysis on the cell lysates using the same antibody against cleaved caspase-3. We again found that the addition of Ub3881 alone was sufficient to induce apoptosis, as indicated by the increase in cleaved caspase-3 (Fig. 4F). Infection with DENV without the overexpression of Ub3881 resulted in a slight induction in apoptosis but not nearly as substantial as with the increased Ub3881 protein (Fig. 4F). The transfection of Ub3881 along with the infection with DENV caused the greatest increase in cleaved caspase-3 protein (Fig. 4F, far right panel). We then looked at how a loss of Ub3881 function might affect apoptosis during DENV infection by using RNAi to block Ub3881 expression in Aag2 cells. Cells were infected with DENV and Western blot analysis done to check for cleaved caspase-3. Infection with DENV again increased apoptosis as compared to uninfected cells. Interestingly, a reduction in Ub3881 expression lowered the DENV-stimulated increase in apoptosis, providing further evidence that Ub3881 might be involved in apoptosis during DENV infection in mosquito cells (Fig. S3).
Supplementary Fig. 3.

Reduction of Ub3881 decreases apoptosis during DENV infection. Aag2 cells were transfected with siRNA targeting Ub3881 (Lane 3) or a control siRNA (Lanes 1 & 2), and infected with DENV 72 h post-transfection (Lanes 3 & 4). A group of control cells were not infected (Lane 1). Western blot analysis was done on cell lysates with antibodies against cleaved caspase-3 as a marker for apoptosis. Lane 1 = control cells, Lane 2 = DENV-infected cells with control siRNA, Lane 3 = DENV-infected cells with Ub3881 siRNA.
3.7. The antiviral effects of Ub3881 are not blocked by proteasomal inhibition
A known function of ubiquitin is to bind proteins and target them for proteasomal degradation [51]. To this end, we sought to reduce the anti-DENV effects of Ub3881 in mosquito cells by chemically inhibiting the proteasome system. The Ub3881 gene was overexpressed in Aag2 cells and the cells were treated with the proteasome inhibitor carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (MG132) prior to DENV infection. Control cells were transfected with GFP plasmid. Infection levels were analyzed using isolated RNA and qRT-PCR analysis. MG132 has successfully been used to inhibit the proteasome in insect cells previously [52] and we found no cellular toxicity in our assays at the levels used. Surprisingly, blocking proteasomal activity did not reduce the antiviral effects of Ub3881 (Fig. 5A), though blocking activity also reduced DENV infection in cells without Ub3881 overexpression (Fig. 5A). We next looked at the effects of Ub3881 overexpression with proteasomal inhibition over time and found no significant changes within the first 96 h of infection (Fig. 5B). It is important to note that previous research has shown that the proteasome pathway may be required for flavivirus replication [35], thus the reduction of proteasomal activity may have directly caused the inhibition of DENV infection seen here. To confirm that virus release also remained reduced with overexpression of Ub3881 during proteasomal inhibition, we also measured DENV in the supernatant by qRT-PCR. We found that MG132 did not stop Ub3881 from inhibiting DENV virus production in the cells (Fig. 5C). We next examined whether Ub3881 interacts with the 26S proteasome to determine if this ubiquitin is involved in the mosquito proteasome pathway. We looked at the association between the proteasome and Ub3881 in Aag2 cells with and without DENV infection using polyclonal antibodies that we had made against Ub3881 and antibodies recognizing the 19S regulatory complex of the proteasome. Cells were then examined by fluorescence microscopy and co-localization of Ub3881 and the proteasome was seen both with and without DENV infection (Fig. 5D). This suggested that Ub3881 may be involved in the mosquito ubiquitin-proteasome pathway and could be marking proteins for degradation.
Fig. 5.
Proteasomal inhibition does not block antiviral effects of Ub3881. A. Aag2 cells were transfected with Ub3881 expression vector or control GFP vector and then treated with MG132 proteasome inhibitor prior to and throughout DENV infection. Infection was measured by qRT-PCR analysis. B. Aag2 cells were treated with MG132 proteasome inhibitor and then transfected with the Ub3881 expression vector or control GFP vector prior to DENV infection. MG132 treatment was continued throughout infection. RNA was isolated from the cells over time and DENV infection was measured by qRT-PCR analysis. C. Aag2 cells were transfected with the Ub3881 expression vector or a control GFP vector and then treated with MG132 proteasome inhibitor prior to and throughout DENV infection. Cell supernatants were used in qRT-PCR analysis to quantify DENV release from cells. D. Aag2 cells were infected with DENV and immunofluorescence analysis was done using antibodies against the 19S proteasome and Ub3881. Mock (uninfected) cells were used as controls. Representative images are shown; images were taken at 63 ×.
3.8. Ub3881 targets the DENV envelope protein for degradation
We next hypothesized that Ub3881 may be attaching to DENV proteins and labeling them for destruction, thereby inhibiting the viral life cycle. To investigate this, we overexpressed Ub3881 in Aag2 cells, infected the cells with DENV and performed Western blot analysis on cell lysates to detect levels of individual DENV proteins. The cells expressing Ub3881 had much lower levels of all viral proteins than the control cells (Fig. 6A), which suggested that the Ub3881 protein was indeed degrading viral proteins. However, since the overexpression of Ub3881 impairs viral infection, it is possible that the protein levels were lower due to a reduction in viral RNA rather than via protein degradation. To address this, we expressed Ub3881 in Aag2 cells and then separately transfected plasmids expressing each of the individual DENV proteins. We performed Western blot analysis on the cell lysates to measure viral protein levels. This time, the levels of most DENV proteins did not change with increased Ub3881 expression, with the exception of the envelope (E) protein (Fig. 6B). This indicated that the Ub3881 may be specifically targeting E for degradation, which could be one of the main antiviral mechanisms. This also supports that levels of other viral proteins were low due to the reduced viral RNA levels rather than proteasomal degradation. Looking at the sequence of DENV E, we found that there are 3 putative ubiquitination sites within the sequence at lysines K88, K157 and K163 (Fig. S4), which indicates that the ubiquitination of DENV E by Ub3881 is likely. To determine whether these putative ubiquitination sites are involved, we made DENV E mutant genes individually replacing the lysines with alanines, resulting in DENV E K88A, K157A and K163A. We cloned these gene mutants into the insect expression vector and transfected these, with and without the Ub3881 expression vector, into Aag2 cells. We performed Western blot analysis on the cell lysates to determine the levels of E protein. Each of the lysate samples from cells overexpressing mutant E protein, lacking one of the three putative ubiquitination sites, had greater amounts of E protein than the cells expressing wild-type DENV E along with Ub3881 (Fig. 6C), with the K157A mutant retaining the most DENV E relative to the cells expressing wild-type E. This indicated that all three of the putative ubiquitination sites are likely involved in the Ub3881-DENV E interaction and resulting degradation of DENV E, and that the lysine at position 157 is the most likely to be involved. To confirm that Ub3881 interacts with DENV E, we ran eluate pulled from infected Ae. aegypti lysate using the Ub3881 antibody on an SDS-PAGE gel and did Western blotting analysis using an antibody against DENV E protein as the probe. We found that DENV E protein was pulled out of DENV-infected mosquito lysate by Ub3881 protein, confirming an interaction between the two proteins (Fig. 6D). We also used DENV E protein to pull out E from the infected eluate as a control (Fig. 6D, far right panel). Finally, we performed immunofluorescence analysis on Ub3881-transfected and DENV-infected Aag2 cells using antibodies against mosquito Ub3881 and DENV E protein. We found that Ub3881 colocalized with the envelope protein in the infected cells (Fig. 6E). Together, these results indicate that Ub3881 binds DENV E and marks it for destruction and/or degradation.
Fig. 6.
DENV envelope protein is specifically degraded by Ub3881. A. Aag2 cells were transfected with the Ub3881 expression vector or a mock expression vector and infected with DENV. The cell lysate was run on an SDS-PAGE gel and Western blot analysis was done with antibodies against each individual DENV protein. B. Aag2 cells were individually transfected with expression plasmids encoding for each DENV protein, along with the Ub3881 expression plasmid or a mock expression plasmid. Equal amounts of protein from the cell lysates were run on SDS-PAGE gels and Western blot analysis was done with antibodies against each individual DENV protein. C. Aag2 cells were individually transfected with expression plasmids expressing DENV envelope protein, along with the Ub3881 expression plasmid or a mock expression plasmid. The envelope protein coding regions were WT, K88A, K157A or K163A. Cell lysates were run on SDS-PAGE gels and Western blot analysis was done with antibodies against DENV envelope protein. D. Antibodies against Ub3881 and DENV E proteins were used to precipitate proteins out of DENV-infected mosquito lysate. The solutions were run on an SDS-PAGE gel and Western blot analysis was done using the antibody against DENV E protein. E. Aag2 cells were transfected with the Ub3881 expression plasmid and infected with DENV 24 h post-transfection. Cells were analyzed by immunofluorescence microscopy using antibodies against DENV E and Ub3881. A representative image is shown; images were taken at 40 ×. Western blots were run with corresponding amounts of protein per lane as loading control.
4. Discussion
The identification and investigation of host factors regulated during DENV infection of the mosquito may identify new targets and pathways important for insect immunity as well as the viral life cycle within the transmission vector. Here, we investigated the role of the mosquito ubiquitin protein Ub3881 that was found to be highly down-regulated during DENV infection of Ae. aegypti [16]. Ubiquitin proteins and the proteasome system have been known to be required for many stages in a number of viral lifecycles, and have been shown to play both pro- and antiviral roles. Viruses employ several strategies that require host ubiquitin to evade immune system surveillance, undergo replication, for budding, and viral maturation [29], [31], [32], [34]. For example, human cytomegalovirus inhibits MHC antigen presentation through the dislocation of MHC class I from the endoplasmic reticulum. The now cytoplasmic MHC-1 becomes polyubiquitinated and is promptly removed for proteasome degradation [36]. This process known as ubiquitin-dependent ER-associated degradation (ERAD) is typically used to clean up and remove misfolded proteins from the ER [53], and has also been shown to play a role during WNV infection [27]. During Hepatitis C virus (HCV) infection, a ubiquitin ligase may mediate the ubiquitination and degradation of the core protein [54], which is notable in light of our findings that the mosquito Ub3881 can bind and mark DENV envelope protein for degradation. In addition, several enveloped RNA viruses cannot complete budding without exploiting host cellular ubiquitination mechanisms for the virion membrane [55].
Previous research has shown that significant alterations in host protein expression and levels of components of the ubiquitin-proteasome system have been associated with DENV infection in vivo and in vitro [23], [24], [36], [56], [57]. Data demonstrated that pharmacological inhibition of the ubiquitin-proteasome system at different stages of the process (i.e. blocking the proteasome or ubiquitin E1 activity) can greatly reduce DENV and WNV titers [35], [55], [56]. Although the mechanisms are unknown, the ubiquitin-proteasome also appears to be required for flavivirus RNA translation and/or replication during infection [55] and it was recently demonstrated that there is an important role for the ubiquitin proteasome pathway in the regulation of DENV production in the mosquito vector [37]. Consistent with this, we observed that blocking proteasome activity with the proteasome inhibitor MG132 reduced DENV infection in Aag2 mosquito cells without overexpression of Ub3881. Interestingly, this blockage failed to reduce the antiviral effect of Ub3881 in cells overexpressing the ubiquitin protein. This was perhaps due to the need for the proteasome during DENV infection and not because Ub3881 acts independently of the proteasome system. Previous work has demonstrated that inhibiting the proteasome system can increase autophagy in mammalian cells [58]. Ubiquitin is also known to play a role in selective cellular autophagy pathways [59]. For instance, the ubiquitin protein ligase Parkin has been reported to target substrates for both autophagic and proteosomal degradation, and this degradation is dependent on linkage through K48 [60]. If the mosquito Ub3881 does have antiviral roles that do not involve the proteasome system, a role in autophagy would be an interesting avenue to investigate.
A recent study looking at host gene expression during DENV infection in patients and in cell lines found that several genes involved in the ubiquitin-proteasome pathway were upregulated, both in vivo and in vitro, and that inhibition of the ubiquitin-proteasome pathway critically hinders dengue viral replication in mammalian cells [56]. The proteasome inhibitors MG-132 and ALLN significantly reduced DENV replication in the human liver cell lines tested. However, it is unclear if at this time whether the ubiquitin-proteasome system is antiviral or if dengue virus uses components of the system for its advantage, perhaps for replication [56]. Even though the proteasome might be necessary for DENV infection, the use of ubiquitylation to degrade viral proteins through the proteasome would not be beneficial to the life cycle. This is just one illustration of how complex the ubiquitin-proteasome system is.
There are seven lysine residues considered integral to the prototypical ubiquitin molecule (K6, K11, K27, K29, K33, K48 and K63), any of which the carboxy-terminal glycine of ubiquitin can link to form polyubiquitin chains [61]. Generally speaking, K63-linked ubiquitination is involved in signaling and the regulation of kinase activation while ubiquitin chains linked through lysine 6, 11, 27, 29, 33 or 48 usually target a substrate protein for proteasomal degradation [62], [63]. Linear ubiquitin chains linked via the N-terminal amino group can also play important nonproteolytic functions. We found that the mosquito ubiquitin Ub3881 has lysines at position 6, 27, 29 and 48 but not K11, K33 or K63. In addition, an inserted alanine at position 19 shifts the position of the downstream lysines by one position. As Ub3881 does not have a lysine at position 63 but does have K48, we hypothesized that the protein may likely be acting to target proteins for degradation through the proteasome rather than participating in cell signaling or protein delivery to endosomal-lysosomal pathways [64]. We found that the Ub3881 was in fact acting to label the dengue virus envelope protein for degradation. Whether any monoubiquitination of dengue viral envelope protein can lead to relocation or protein interactions within an infected cell remains to be investigated.
The Imd pathway is an innate immune response in insects that can stimulate the production of antimicrobial peptides (AMP) and is rapidly triggered during bacterial infection in Drosophila by a peptidoglycan in the cell wall of Gram-negative and some Gram-positive bacteria [65], [66]. Interestingly, the Imd pathway is also protective against some RNA viruses, though it is not entirely clear how viruses prompt the Imd pathway and in what way Imd establishes resistance in return [66]. In Drosophila, a polyubiquitin chain linked via K63 has been found to recruit and activate the TAK1-TAB2 complex which then further activates sections of the immune deficiency (Imd) pathway [65], [66]. The mosquito Ub3881 does not have a lysine at positon 63 that would be suggestive of an involvement in cell signaling or immune response. In spite of this, we found that Ub3881 associated with Ae. aegypti Imd protein, and increased amounts of Ub3881 resulted in increased AMP signaling. Perhaps ubiquitination via a lysine other than K63 may be the mechanism behind the Ub3881 and Imd interaction and resulting AMP gene expression during DENV infection. Further investigation into which lysine residue may be involved in the various functions of Ub3881, especially with regard to dengue virus infection, is an anticipated future direction. Another component of the insect Imd pathway is the inhibitor of apoptosis protein (IAP), which blocks caspases from the initiation of cell apoptosis and can activate the innate immune system through polyubiquitination [65]. We found that that increased amounts of mosquito Ub3881 caused increased apoptosis, especially in cells infected by DENV. We also saw that the reduction of Ub3881 during DENV infection resulted in a reduction in apoptosis. This may suggest an intriguing role for Ub3881 in cell death during DENV infection. It is interesting to speculate that the increased apoptosis could result from ubiquitination of IAP by Ub3881, though the mechanisms behind this phenomenon are not entirely clear as of yet. If the Ub3881 blocks mosquito Relish activity, this may also result in apoptosis, as Relish is the insect form of NFKB and an increase in NFKB activity in mammalian cells may result in decreased apoptosis [67]. We plan to investigate the relationship between Ub3881 and mosquito Relish protein in the near future.
It's very interesting that loss of function experiments did not impact DENV infection in our assays. This suggests that Ub3881 may not be inhibitory to DENV early in infection or that the repressive effects DENV has on Ub3881 expression could be sufficient to negate the antiviral effect. As lowering Ub3881 levels before infection didn't have any benefit for DENV infection levels, one reason could be that there is no involvement of the protein during early steps such as attachment and entry, but rather that Ub3881 plays a role during replication, assembly or budding. Once DENV proteins are made, they likely act quickly and efficiently to reduce expression of Ub3881 to levels that are optimal for the later steps in infection. The reduction of almost 100-food at one timeout during virus infection as well as the devastating effects on infection due to Ub3881 overexpression both indicate that this mosquito protein is extremely harmful to the DENV life cycle. The lack of benefit from additional knock down could also indicate that the virus may have evolved its mechanisms of host manipulation rather perfectly. The action of DENV on Ub3881 expression might be enough to prevent any antiviral effects. The loss of Ub3881 function did reduce the level of apoptosis during DENV infection, which again points to a role for Ub3881 in apoptosis of the mosquito cells during infection.
This investigation revealed that Ub3881 plays an important role during DENV infection in the mosquito, targeting DENV E for destruction and reducing the production of quality infectious virions. The reduction in quality infectious virions is likely directly linked to the low availability of envelope protein during Ub3881 overexpression, as newly produced virus requires a specific amount of DENV E. The Ub3881 protein may also function as part of the innate immune response against flaviviruses. The targeting of DENV E for degradation and increased AMP expression are both ways in which the viral life cycle would benefit from the down-regulation of Ub3881 during infection. The data presented here adds to our knowledge of dengue virus pathogenesis in the mosquito transmission vector. Although a traditional vaccine for DENV may soon be licensed for use by many nations, and the Sanofi Pasteur vaccine is currently licensed for use in Mexico, Brazil and the Philippines [68], [69], additional strategies involving the elimination of infection in the transmission vector could serve as strong complement vaccine approaches. Understanding the effects of dengue infection on the mosquito, responses both common and unique to individual flaviviruses, will aid in developing broadly applicable methods to treat and prevent infection of the mosquito vector in hopes of stopping virus spread before human transmission. In future studies, we aim to further examine the ubiquitination of the dengue viral envelope protein as well as the putative binding of Ub3881 to mosquito IAP during cellular apoptosis. We also hope to test Ub3881 peptides as inhibitors of DENV infection in mosquitoes.
The following are the supplementary data related to this article.
Genes that were highly downregulated during flavivirus infection in Ae. Aegypti.
Expression of ubiquitin genes in Ae. aegypti during DENV infection.
Ae. aegypti peptide sequences used for antibody production.
Antimicrobial peptide expression during DENV infection.
Antibody binding in immunflourescence (IF) assays. Aag2 cells were infected with DENV and then fixed in 4% paraformaldehyde 24 h after infection. Cells were stained with A. Ub3881 antibody, B. Relish antibody and C. Imd antibody to test function of each in mosquito cells for subsequent assays.
Putative ubiquitination sites in DENV envelope protein. Using Ubpred.org, we were able to identify three putative ubiquitination sites in DENV envelope protein at lysines K88, K157 and K163. The sites are highlighted in red. UbPred is a random forest-based predictor of potential ubiquitination sites in proteins.
Transparency Document
Transparency document.
Acknowledgements
This work was supported by NIH grants K22 AI103067-01 and UO1 AI070343. E.F. is an Investigator of the Howard Hughes Medical Institute. We thank Dr. John F. Anderson and the CAES (New Haven, CT) as well as Drs. Patricia Scaraffia and Dawn Wesson at Tulane University School of Public Health for mosquito rearing assistance and experimental insights. Author Contributions: T.M.C., A.T. and B.L.R. designed the experiments; T.M.C., M.J.C., E.C. and B.L.R. infected the mosquitoes with virus; T.M.C., A.T. and M.J.C. did mosquito injections; T.M.C., B.L.R. and M.J.C. did mosquito tissue dissections; T.M.C. and A.T. performed all other experiments; S.H., D.vL. and E.F. assisted with discussion and editing; A.T. and T.M.C. wrote the paper.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Organization WH . WHO Fact Sheet; 2014. Dengue and Dengue Hemorrhagic Fever. [Google Scholar]
- 2.Flipse J., Wilschut J., Smit J.M. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic. 2013;14:25–35. doi: 10.1111/tra.12012. [DOI] [PubMed] [Google Scholar]
- 3.Kyle J.L., Harris E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rey F.A. Dengue virus: two hosts, two structures. Nature. 2013;497:443–444. doi: 10.1038/497443a. [DOI] [PubMed] [Google Scholar]
- 6.Maciel-de-Freitas R., Valle D. Challenges encountered using standard vector control measures for dengue in Boa Vista, Brazil. Bull. World Health Organ. 2014;92:685–689. doi: 10.2471/BLT.13.119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejnirattisai W., Jumnainsong A., Onsirisakul N., Fitton P., Vasanawathana S. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang S., Shields A.R., Jupatanakul N., Dimopoulos G. Suppressing dengue-2 infection by chemical inhibition of Aedes aegypti host factors. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gubler D.J., Rosen L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am.J.Trop. Med. Hyg. 1976;25:146–150. doi: 10.4269/ajtmh.1976.25.146. [DOI] [PubMed] [Google Scholar]
- 10.Kay B., Vu S.N. New strategy against Aedes aegypti in Vietnam. Lancet. 2005;365:613–617. doi: 10.1016/S0140-6736(05)17913-6. [DOI] [PubMed] [Google Scholar]
- 11.Baron O.L., Ursic-Bedoya R.J., Lowenberger C.A., Ocampo C.B. Differential gene expression from midguts of refractory and susceptible lines of the mosquito, Aedes aegypti, infected with dengue-2 virus. J. Insect Sci. 2010;10:41. doi: 10.1673/031.010.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter C.J. Stop the biting: targeting a mosquito's sense of smell. Cell. 2014;156:878–881. doi: 10.1016/j.cell.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Moreira L.A., Iturbe-Ormaetxe I., Jeffery J.A., Lu G., Pyke A.T. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Olson K.E., Higgs S., Gaines P.J., Powers A.M., Davis B.S. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 15.Sessions O.M., Barrows N.J., Souza-Neto J.A., Robinson T.J., Hershey C.L. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colpitts T.M., Cox J., Vanlandingham D.L., Feitosa F.M., Cheng G. Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann A. The role of ubiquitylation for the control of cell death in Drosophila. Cell Death Differ. 2010;17:61–67. doi: 10.1038/cdd.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson M.A., Gross T.L., Myles K.M., Adelman Z.N. Validation of novel promoter sequences derived from two endogenous ubiquitin genes in transgenic Aedes aegypti. Insect Mol. Biol. 2010;19:441–449. doi: 10.1111/j.1365-2583.2010.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosas B., Hanna J., Kirkpatrick D.S., Zhang D.P., Tone Y. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. doi: 10.1016/j.cell.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Meyer H.J., Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157:910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Mosqueda J., Dikic I. Deciphering functions of branched ubiquitin chains. Cell. 2014;157:767–769. doi: 10.1016/j.cell.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Ribet D., Cossart P. Pathogen-mediated posttranslational modifications: a re-emerging field. Cell. 2010;143:694–702. doi: 10.1016/j.cell.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choy M.M., Zhang S.L., Costa V.V., Tan H.C., Horrevorts S. Proteasome inhibition suppresses dengue virus egress in antibody dependent infection. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nag D.K., Finley D. A small-molecule inhibitor of deubiquitinating enzyme USP14 inhibits dengue virus replication. Virus Res. 2012;165:103–106. doi: 10.1016/j.virusres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Yu G.Y., Lai M.M. The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J. Virol. 2005;79:644–648. doi: 10.1128/JVI.79.1.644-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Zhuang X. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11790–11795. doi: 10.1073/pnas.0803711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan M.N., Ng A., Sukumaran B., Gilfoy F.D., Uchil P.D. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ching W., Koyuncu E., Singh S., Arbelo-Roman C., Hartl B. A ubiquitin-specific protease possesses a decisive role for adenovirus replication and oncogene-mediated transformation. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huthoff H., Towers G.J. Restriction of retroviral replication by APOBEC3G/F and TRIM5alpha. Trends Microbiol. 2008;16:612–619. doi: 10.1016/j.tim.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomaguchi M., Fujita M., Adachi A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes Infect. 2008;10:960–967. doi: 10.1016/j.micinf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Satheshkumar P.S., Anton L.C., Sanz P., Moss B. Inhibition of the ubiquitin-proteasome system prevents vaccinia virus DNA replication and expression of intermediate and late genes. J. Virol. 2009;83:2469–2479. doi: 10.1128/JVI.01986-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teale A., Campbell S., Van Buuren N., Magee W.C., Watmough K. Orthopoxviruses require a functional ubiquitin-proteasome system for productive replication. J. Virol. 2009;83:2099–2108. doi: 10.1128/JVI.01753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Si X., Gao G., Wong J., Wang Y., Zhang J. Ubiquitination is required for effective replication of coxsackievirus B3. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupfer C., Pastey M.K. Decreased replication of human respiratory syncytial virus treated with the proteasome inhibitor MG-132. Virus Res. 2010;149:36–41. doi: 10.1016/j.virusres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Garcia M.D., Meertens L., Bonazzi M., Cossart P., Arenzana-Seisdedos F. Appraising the roles of CBLL1 and the ubiquitin/proteasome system for flavivirus entry and replication. J. Virol. 2011;85:2980–2989. doi: 10.1128/JVI.02483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanlaya R., Pattanakitsakul S.N., Sinchaikul S., Chen S.T., Thongboonkerd V. The ubiquitin-proteasome pathway is important for dengue virus infection in primary human endothelial cells. J. Proteome Res. 2010;9:4960–4971. doi: 10.1021/pr100219y. [DOI] [PubMed] [Google Scholar]
- 37.Choy M.M., Sessions O.M., Gubler D.J., Ooi E.E. Production of infectious dengue virus in Aedes aegypti is dependent on the ubiquitin proteasome pathway. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng G., Liu L., Wang P., Zhang Y., Zhao Y.O. An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colpitts T.M., Cox J., Nguyen A., Feitosa F., Krishnan M.N. Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector. Virology. 2011;417:179–187. doi: 10.1016/j.virol.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nene V., Wortman J.R., Lawson D., Haas B., Kodira C. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng G., Cox J., Wang P., Krishnan M.N., Dai J. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meinander A., Runchel C., Tenev T., Chen L., Kim C.H. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J. 2012;31:2770–2783. doi: 10.1038/emboj.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thevenon D., Engel E., Avet-Rochex A., Gottar M., Bergeret E. The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe. 2009;6:309–320. doi: 10.1016/j.chom.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Myllymaki H., Valanne S., Ramet M. The Drosophila imd signaling pathway. J. Immunol. 2014;192:3455–3462. doi: 10.4049/jimmunol.1303309. [DOI] [PubMed] [Google Scholar]
- 45.Carissimo G., Pondeville E., McFarlane M., Dietrich I., Mitri C. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E176–E185. doi: 10.1073/pnas.1412984112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avadhanula V., Weasner B.P., Hardy G.G., Kumar J.P., Hardy R.W. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paquette N., Broemer M., Aggarwal K., Chen L., Husson M. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol. Cell. 2010;37:172–182. doi: 10.1016/j.molcel.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tenev T., Ditzel M., Zachariou A., Meier P. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 2007;14:1191–1201. doi: 10.1038/sj.cdd.4402118. [DOI] [PubMed] [Google Scholar]
- 49.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 50.Collins H., Moon N.S. The components of Drosophila histone chaperone dCAF-1 are required for the cell death phenotype associated with rbf1 mutation. G3 (Bethesda) 2013;3:1639–1647. doi: 10.1534/g3.113.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voges D., Zwickl P., Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 52.Schauer S., Burster T., Spindler-Barth M. N- and C-terminal degradation of ecdysteroid receptor isoforms, when transiently expressed in mammalian CHO cells, is regulated by the proteasome and cysteine and threonine proteases. Insect Mol. Biol. 2012;21:383–394. doi: 10.1111/j.1365-2583.2012.01144.x. [DOI] [PubMed] [Google Scholar]
- 53.Doroudgar S., Volkers M., Thuerauf D.J., Khan M., Mohsin S. Hrd1 and ER-associated protein degradation, ERAD, are critical elements of the adaptive ER stress response in cardiac myocytes. Circ. Res. 2015;117:536–546. doi: 10.1161/CIRCRESAHA.115.306993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao L., Tu H., Shi S.T., Lee K.J., Asanaka M. Interaction with a ubiquitin-like protein enhances the ubiquitination and degradation of hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 2003;77:4149–4159. doi: 10.1128/JVI.77.7.4149-4159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilfoy F., Fayzulin R., Mason P.W. West Nile virus genome amplification requires the functional activities of the proteasome. Virology. 2009;385:74–84. doi: 10.1016/j.virol.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fink J., Gu F., Ling L., Tolfvenstam T., Olfat F. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl. Trop. Dis. 2007;1 doi: 10.1371/journal.pntd.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu M.W., Shih H.M., Yang T.H., Yang Y.L. The type 2 dengue virus envelope protein interacts with small ubiquitin-like modifier-1 (SUMO-1) conjugating enzyme 9 (Ubc9) J. Biomed. Sci. 2007;14:429–444. doi: 10.1007/s11373-007-9151-9. [DOI] [PubMed] [Google Scholar]
- 58.Pandey U.B., Nie Z., Batlevi Y., McCray B.A., Ritson G.P. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 59.Kraft C., Peter M., Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat. Cell Biol. 2010;12:836–841. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- 60.Moore D.J. Parkin: a multifaceted ubiquitin ligase. Biochem. Soc. Trans. 2006;34:749–753. doi: 10.1042/BST0340749. [DOI] [PubMed] [Google Scholar]
- 61.Pickart C.M., Eddins M.J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Xu G., Jaffrey S.R. Proteomic identification of protein ubiquitination events. Biotechnol. Genet. Eng. Rev. 2013;29:73–109. doi: 10.1080/02648725.2013.801232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulman B.A., Harper J.W. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nathan J.A., Kim H.T., Ting L., Gygi S.P., Goldberg A.L. Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? EMBO J. 2013;32:552–565. doi: 10.1038/emboj.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falschlehner C., Boutros M. Innate immunity: regulation of caspases by IAP-dependent ubiquitylation. EMBO J. 2012;31:2750–2752. doi: 10.1038/emboj.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleino A., Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014;42:25–35. doi: 10.1016/j.dci.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 68.Fink K., Shi P.Y. Live attenuated vaccine: the first clinically approved dengue vaccine? Expert Rev. Vaccines. 2014;13:185–188. doi: 10.1586/14760584.2014.870888. [DOI] [PubMed] [Google Scholar]
- 69.Villar L., Dayan G.H., Arredondo-Garcia J.L., Rivera D.M., Cunha R. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes that were highly downregulated during flavivirus infection in Ae. Aegypti.
Expression of ubiquitin genes in Ae. aegypti during DENV infection.
Ae. aegypti peptide sequences used for antibody production.
Antimicrobial peptide expression during DENV infection.
Antibody binding in immunflourescence (IF) assays. Aag2 cells were infected with DENV and then fixed in 4% paraformaldehyde 24 h after infection. Cells were stained with A. Ub3881 antibody, B. Relish antibody and C. Imd antibody to test function of each in mosquito cells for subsequent assays.
Putative ubiquitination sites in DENV envelope protein. Using Ubpred.org, we were able to identify three putative ubiquitination sites in DENV envelope protein at lysines K88, K157 and K163. The sites are highlighted in red. UbPred is a random forest-based predictor of potential ubiquitination sites in proteins.
Transparency document.