Abstract
It is unknown whether inflammatory/hemostatic biomarkers are associated with coronary artery calcium (CAC) progression. Our purpose was to evaluate the associations of baseline levels of C-reactive protein, fibrinogen, plasminogen-activator inhibitor 1 (PAI-1), tissue plasminogen activator antigen, and circulating factor VII with CAC progression in healthy midlife women. Inflammatory/hemostatic biomarkers were measured at baseline. CAC was quantified by CT scans at baseline and after 2.3 ± 0.5 years of follow-up. Significant CAC progression was defined as present if 1) follow-up CAC Agatston score was >0 if baseline CAC score = 0, 2) annualized change in CAC score was ≥10 if baseline CAC score >0 to <100, and 3) annualized percent change in CAC score was ≥10% if baseline CAC score ≥100. Extent of CAC progression was defined as [log(CAC(follow-up)+25) – log(CAC(baseline)+25)] / year. Logistic and linear regression models were used as appropriate and the final models were adjusted for baseline CAC score, age, study site, race/ethnicity, menopausal status, sociodemographics, traditional cardiovascular disease (CVD) risk factors, family history of CVD, and CVD medication use. The study included 252 women (baseline age 51.2 ± 2.6 years; 67.5% white; 56.4% pre- or early perimenopausal). In final models, only log(PAI-1) was associated with presence of CAC progression (OR: 1.91; 95% CI: 1.24–2.93; per 1 log unit increase in PAI-1; p=0.003). Additionally, higher log(PAI-1) was marginally associated with greater extent of CAC progression (p=0.06). In conclusion, PAI-1 is associated with the presence of CAC progression in middle-aged women. Targeting PAI-1 may decrease atherogenesis beyond conventional CVD risk factors.
Keywords: epidemiology, inflammation, hemostasis, prevention, risk factor
INTRODUCTION
Nearly half of middle-aged women afflicted with coronary heart disease (CHD) have zero or only one traditional risk factor.1 Coronary artery calcium (CAC) progression, or serial measurement of CAC scores, assesses temporal changes in subclinical atherosclerosis and has been demonstrated to correlate with CHD events.2,3 Biomarkers of inflammation and hemostasis have been linked to atherosclerosis, but have not been widely integrated into current CHD prevention algorithms due to lack of substantial evidence that they improve risk assessment.4–6 In women, additional studies considering menopausal status and race/ethnicity are needed.7 The goal of this study was to assess associations between baseline levels of five biomarkers of inflammation and hemostasis with CAC progression in apparently healthy women of black and white race/ethnicity transitioning through menopause who were free of known cardiovascular disease (CVD). Biomarkers included C-reactive protein (CRP), fibrinogen, plasminogen-activator inhibitor 1 (PAI-1), tissue plasminogen activator antigen (tPA-ag), and circulating factor VII (factor VIIc).
METHODS
The Study of Women’s Health Across the Nation (SWAN) is a multi-center, multi-racial/ethnic prospective cohort study in the United States that was designed to examine the menopausal transition.8 Seven clinical sites recruited women who were white and one additional pre-determined race/ethnicity (blacks in Boston, Chicago, Detroit, Pittsburgh; Chinese in Oakland; Hispanic in New Jersey; Japanese in Los Angeles). Eligibility criteria for the SWAN parent study included age 42–52 years, an intact uterus, menstruating within the prior 3 months, not using reproductive hormones, and not pregnant or lactating. Those with prior hysterectomy or bilateral oophorectomy were excluded. Study protocols were approved by the institutional review board at each site and participants provided written informed consent.
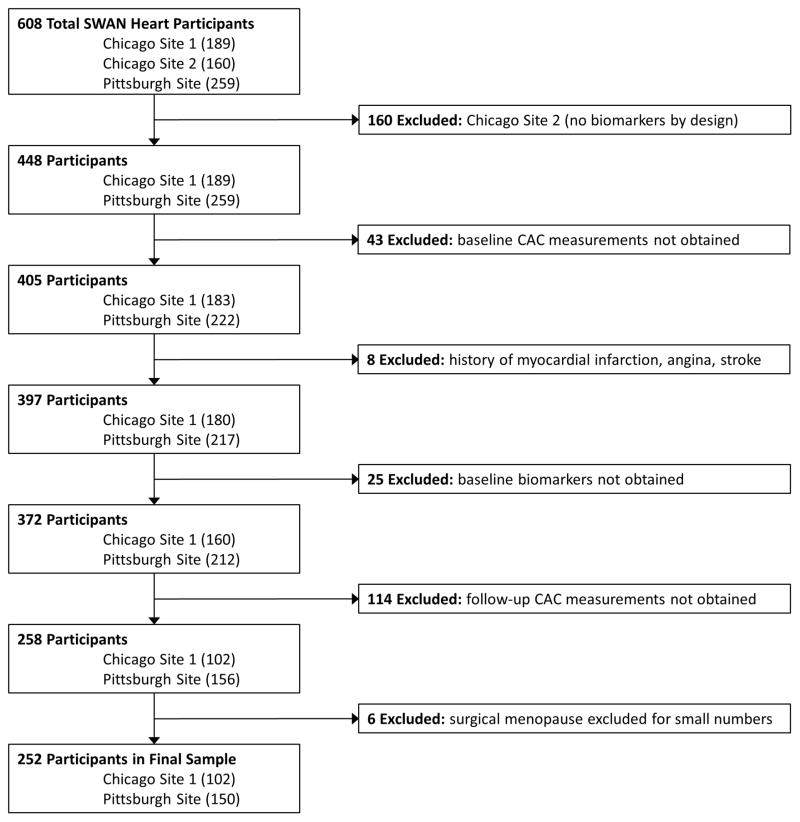
The SWAN Heart ancillary study was designed to evaluate subclinical atherosclerosis during the menopausal transition. It was performed at the Pittsburgh and Chicago sites and enrolled 608 self-identified white and black women. This present study utilized data from both baseline and follow-up visits of the SWAN Heart study. The baseline SWAN Heart visit occurred during SWAN parent study annual visits 4 through 7, while the follow-up SWAN Heart visits occurred during SWAN parent study annual visits 6 through 9.9 The Pittsburgh site had one recruiting location and the Chicago site had two recruiting locations. Only the Pittsburgh and one of the Chicago locations, by design, obtained inflammatory/hemostatic biomarkers and consisted of 448 women who were considered to be eligible for the current study. Women were excluded from the current analyses if they did not have baseline CAC measurements (n=43); reported myocardial infarction, angina or stroke at either the baseline or follow-up SWAN Heart visits (n=8); did not have baseline levels of CRP, fibrinogen, PAI-1, tPA-ag, and factor VIIc (n=25); or did not have follow-up CAC measurements (n=114). Women with surgical menopause were excluded due to small numbers (n=6). Thus, the final sample size for those with both baseline and follow-up CAC measurements and baseline inflammatory/hemostatic biomarkers was 252 participants (Figure 1).
Figure 1. Participant Flow Chart.
CAC = coronary artery calcium; SWAN = Study of Women’s Health Across the Nation.
Women in the final sample, when compared to the 196 excluded women, were older (51.7 ± 2.9 years vs. 50.6 ± 2.5 years; p=0.0002), had higher systolic blood pressure (121.4 ± 17.5 mmHg vs. 115.1 ± 16.0 mmHg; p=0.0002), had lower LDL-C (117.2 ± 32.5 mg/dL vs. 125.1 ± 33.3 mg/dL; p=0.01), and were less likely to have a family history of CVD (58.7% vs. 75.0%; p=0.0003). They were similar with respect to race/ethnicity, BMI, HDL-C, triglycerides, diabetes, and smoking.
A CT scanner (C-150 Ultrafast CT Scanner, GE Imatron, San Francisco, California) was used for CAC quantification. Thirty to forty contiguous 3-mm thick transverse images were obtained from the level of the aortic root to the apex of the heart. Images were obtained during a maximal breath hold, using electrocardiographic triggering so each 100-millisecond exposure was obtained during the same phase of the cardiac cycle (60%) of the R-R interval. All scan data were saved to an optical disk for central scoring, using a DICOM workstation and software by AcuImage, Inc (South San Francisco, California). This software program implements the Agatston scoring method.10 CAC was defined as a hyper-attenuating lesion >130 Hounsfield units with an area of ≥3 pixels. The Agatston unit (U) score was calculated by multiplying the lesion area (mm2) by a density factor (between 1 and 4). The total calcium score was the sum of the individual scores for the four major epicardial coronary arteries. Under the supervision of a cardiologist, a technologist scored the scans. This technique was demonstrated at the Pittsburgh site to have an intraclass correlation of 0.99.11
Phlebotomy was performed in the morning after an overnight fast within 2–5 days of a spontaneous menstrual cycle. If a timed sample could not be obtained because of irregular or cessation of menstrual cycles, a random fasting sample was taken within 90 days of the annual visit. CRP, fibrinogen, PAI-1, t-PA-ag, and factor VIIc were all measured in plasma. CRP was measured using ultrasensitive rate immunonephelometry (Dade-Behring, Marburg, Germany). The sensitivity of the assay was 0.03 mg/dL, and the interassay coefficient of variations (CVs) at CRP concentrations of 0.05 and 2.2 mg/dL were 10% to 12% and 5% to 7%, respectively. Fibrinogen and factor VIIc were measured in frozen citrated plasma using a clot-based turbidometric detection system (MLA ELECTRA 1400C; Medical Laboratory Automation Inc., Mt. Vernon, NY). Fibrinogen monthly interassay CVs were 2.3 to 3.5% and 2.6 to 3.6% at mean concentrations of 250 and 140 mg/dL, respectively, and factor VIIc monthly interassay CVs were 7.8%, 5%, and 4% for mean activities of 8%, 45%, and 99%, respectively. PAI-1 was measured using a solid phased monoclonal antibody and a second enzyme-labeled goat antiserum for detection (IMUBIND plasma PAI-1 enzyme-linked immunosorbent assay; American Diagnostica, Greenwich, Connecticut). PAI-1 monthly interassay CVs were 5% to 9% and 4% to 9% at mean concentrations of 7 and 22.5 ng/dL, respectively. A double antibody in an enzyme-linked immunosorbent assay (American Diagnostica) measured t-PA-ag, with a human single chain t-PA-ag as a standard calibrated against an international standard (National Institute for Biological Standards and Control, Hertfordshire, United Kingdom). Monthly interassay CVs were 4.7% to 8.7% and 3.8% to 7.8% at mean concentrations of 5.6 and 11 ng/dL, respectively. Total serum cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C,) triglycerides, insulin, and glucose were determined from a fasting blood sample with standard methods described previously.12 The Homeostasis Model Assessment (HOMA) insulin resistance index was calculated using the following equation: [insulin x glucose] / 22.5. Glucose in mg/dL was converted to millimoles per liter by multiplying by 0.0555. HOMA represents a computer model of the glucose-insulin feedback system during a fasting state, specifically regarding the functions of the tissues and organs related to glucose regulation.
Blood pressure was measured twice and the average was used. Smoking was coded into current versus past or never smoker. BMI was derived from in-clinic measures of weight and height, and was calculated by [mass (kg)] / [height (m)]2. Income was recorded into three categories: <$50,000; $50,000 to <$100,000, and ≥$100,000. Education was recorded as high school graduate or less, some college or college graduate, and graduate degree. CVD medication use (blood pressure medication use and cholesterol medication use), and family history of CVD were self-reported. Diabetes was defined as the use of any medication for diabetes or a fasting blood glucose of >125 mg/dl.
Menopausal status, obtained annually from reported bleeding patterns in the year preceding the visit, was categorized as 1) pre-menopausal (bleeding in previous 3 months with no past year change in cycle predictability), 2) early perimenopausal (bleeding the previous 3 months with decrease in cycle predictability in the past year), 3) late perimenopausal (<12 to >3 months of amenorrhea), 4) natural post-menopausal (≥12 months of amenorrhea), 5) surgical post-menopausal (for those who had undergone hysterectomy with/without oophorectomy), and 6) indeterminate status (for women pre-/peri-menopausal who reported taking hormones in the past year because of the impact of hormone use, even if discontinued, on bleeding patterns). For the current analyses, pre-menopausal and early perimenopausal women were combined into one group, as were late perimenopausal and post-menopausal women.13 Additionally, those who were indeterminate status and post-menopausal women who were on hormone therapy were collapsed into a single category as hormone therapy users.
Data was summarized by CAC progression presence using frequencies, mean with standard deviation (SD), and median with first quartile (Q1) and third quartile (Q3) as appropriate. Differences between groups were assessed by the Wilcoxon-Mann-Whitney test for continuous variables and the chi-square or Fisher’s exact tests for categorical variables. All inflammatory/hemostatic markers had skewed distributions and were therefore log transformed.
CAC progression was evaluated as 1) a categorical outcome for presence of CAC progression, and 2) a continuous outcome for extent of CAC progression. For the presence of CAC progression analyses, a dichotomous variable of significant progression was created. Significant CAC progression was defined as present when 1) CAC score was >0 at follow-up if baseline CAC = 0, 2) annualized change in CAC score was ≥10 if baseline CAC score >0 to <100, and 3) annualized percent change in CAC score was ≥10% if baseline CAC score ≥100. This definition has been previously used in a study of low-risk subjects.14
The distribution of the changes in CAC score per year using the original scale was significantly skewed. Therefore, extent of CAC progression per year was calculated as follows15:
This method has been shown to predict all-cause mortality.16 Logistic regression and linear regression models were utilized as appropriate to assess associations between inflammatory/hemostatic biomarkers (separate model for each biomarker) as related to CAC progression and extent of CAC progression per year. For the multivariable analyses, covariates known to be associated with CHD and those that were found to be univariably significantly associated with the outcomes at a p<0.25 were considered. Study site and menopausal status were forced as it was part of the SWAN design.8 BMI and baseline CAC were introduced separately given known association with biomarkers of inflammation and hemostasis, and known association with CAC progression, respectively. Due to small numbers of diabetic cases in our sample, this variable was not considered in multivariable analyses. Interactions were assessed for race/ethnicity and menopausal status on the relationship between inflammatory/hemostatic biomarkers and CAC progression; neither was significant. A 2-tailed value of p<0.05 was considered significant for all analyses. Goodness-of-fit was assessed with the p-value from the Hosmer-Lemeshow Test for logistic regression and R2 for linear regression. To account for missing data on outcome at the follow-up visit (n=114 women with no CAC score at the follow-up visit) we conducted an inverse probability weighting (IPW) sensitivity analyses. In this method complete cases are weighted by the inverse of their probability of being a complete case (Supplemental table). Results were very consistent with the main analyses excluding missing data. These sensitivity analyses support that our findings were not biased by those who missed the follow-up CAC score. SAS version 9.4 (Cary, North Carolina) was used for statistical analyses.
RESULTS
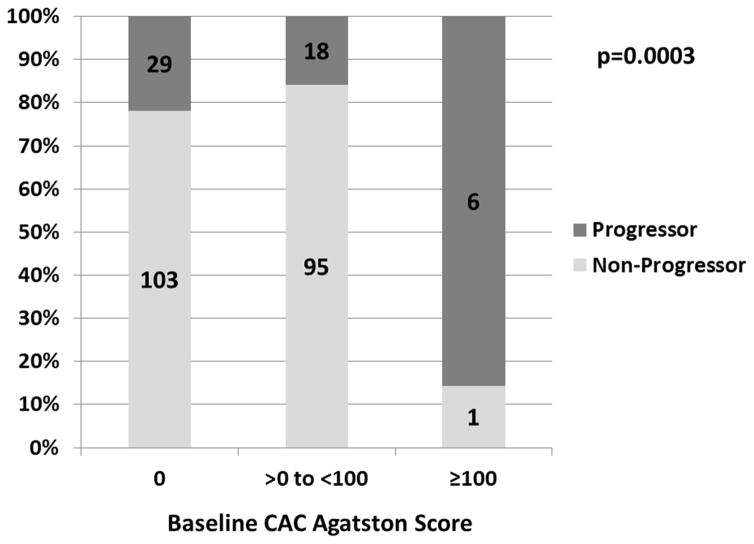
Baseline characteristics are presented in Table 1. The mean average follow-up time between scans was 2.3 ± 0.5 years. Of the 132 women with CAC=0 at baseline, 29 (22.0%) had any CAC on the follow-up measurement. Of the 113 women with 0<baseline CAC<100, there were 18 women (15.9%) who demonstrated an annualized increase of ≥10 U. Of the 7 women who had baseline CAC ≥100, there were 6 (85.7%) who demonstrated progression (annualized increase of ≥10% compared to baseline). Presence of CAC progression by baseline CAC is presented in the Figure 2. Women who had CAC progression had a worse baseline CV profile.
Table 1.
Baseline characteristics by presence or absence of CAC progression.
| Characteristics | Total (n = 252) | CAC Stable (n = 199) | CAC Progressed† (n = 53) | p-value* |
|---|---|---|---|---|
| Age at baseline (years) | 51.2 (2.6) | 51.1 (2.6) | 51.7 (2.9) | 0.2 |
| Site | 0.6 | |||
| Chicago | 102 (40.5%) | 79 (39.7%) | 23 (43.4%) | |
| Pittsburgh | 150 (59.5%) | 120 (60.3%) | 30 (56.6%) | |
| Race/ethnicity | 0.6 | |||
| Black | 82 (32.5%) | 63 (31.7%) | 19 (35.9%) | |
| White | 170 (67.5%) | 136 (68.3%) | 34 (64.2%) | |
| Menopausal status | 0.1 | |||
| Pre- and early perimenopausal | 142 (56.4%) | 113 (56.8%) | 29 (54.7%) | |
| Late peri- and post-menopausal | 77 (30.6%) | 56 (28.1%) | 21 (39.6%) | |
| Hormone therapy user | 33 (13.1%) | 30 (15.1%) | 3 (5.7%) | |
| Income | 0.7 | |||
| <$50,000 | 82 (32.7%) | 65 (32.7%) | 17 (32.7%) | |
| $50,000-<$100,000 | 96 (38.3%) | 74 (37.2%) | 22 (42.3%) | |
| ≥$100,000 | 73 (29.1%) | 60 (30.2%) | 13 (25.0%) | |
| Education | 0.5 | |||
| High school or less | 41 (16.3%) | 32 (16.1%) | 9 (17.0%) | |
| Some college or college | 133 (52.8%) | 102 (51.3%) | 31 (58.5%) | |
| Graduate | 78 (31.0%) | 65 (32.7%) | 13 (24.5%) | |
| Systolic blood pressure (mmHg) | 117.1 (15.8) | 116.0 (15.8) | 121.4 (14.8) | 0.009 |
| Body mass index (kg/m2) | 29.4 (6.5) | 28.8 (6.4) | 31.4 (6.7) | 0.008 |
| High density lipoprotein cholesterol (mg/dL) | 57.2 (13.4) | 58.3 (13.6) | 53.4 (11.7) | 0.02 |
| Low density lipoprotein cholesterol (mg/dL) | 119.3 (34.4) | 116.8 (33.2) | 128.5 (37.5) | 0.08 |
| Triglycerides (mg/dL) | 98.0 (74.0, 136.5) | 97.0 (73.0, 133.0) | 105.0 (76.0,170.0) | 0.1 |
| Cardiovascular medication use | 56 (22.2%) | 40 (20.1%) | 16 (30.2%) | 0.1 |
| Diabetes mellitus | 9 (3.6%) | 4 (2.0%) | 5 (9.4%) | 0.01 |
| Family history of cardiovascular disease | 166 (65.9%) | 132 (66.3%) | 34 (64.2%) | 0.8 |
| Current smoker | 30 (11.9%) | 22 (11.1%) | 8 (15.1%) | 0.4 |
| Homeostasis Model Assessment | 1.9 (1.5, 3.1) | 1.8 (1.4, 2.9) | 2.4 (1.5, 3.9) | 0.05 |
| Baseline coronary artery calcium (U) | 0 (0, 7.2) | 0 (0, 5.8) | 0 (0, 31.2) | 0.2 |
| C-reactive protein (mg/L) | 2.1 (1.0, 5.9) | 2.1 (1.0, 5.9) | 2.5 (1.3, 5.6) | 0.5 |
| Fibrinogen (mg/dL) | 273 (242, 312) | 273 (238, 316) | 279 (247, 300) | 0.7 |
| Plasminogen-activator inhibitor 1 (ng/mL) | 13.0 (7.2, 23.5) | 12.0 (6.6, 21.8) | 20.2 (10.0, 52.0) | 0.0004 |
| Tissue plasminogen activator antigen (ng/mL) | 6.6 (5.0, 9.4) | 6.6 (4.7, 9.2) | 6.8 (5.8, 10.1) | 0.06 |
| Factor VIIc (%) | 120 (102, 137) | 115 (98, 134) | 123 (110, 140) | 0.03 |
Continuous variables presented as mean with standard deviation in parenthesis or median with first and third quartiles in parenthesis. Categorical variables presented as number with percent in parenthesis.
p-value for CAC groups - Wilcoxon rank-sum test for continuous variables; χ 2 test or Fisher's exact test for categorical variables
CAC progression: any CAC for baseline CAC=0, ≥10 for 0<baseline CAC<100, ≥10% for baseline CAC≥100
CAC = coronary artery calcium
Figure 2. Percentage Of Women With CAC Progression By Baseline CAC Agatston Score.
The numbers in columns are women. The p-value was calculated by Fisher’s Exact Test. CAC = coronary artery calcium.
Univariable logistic regression demonstrated significant positive associations between the presence of CAC progression and log(PAI-1) and log(tPA-ag), but not log(CRP) or log(fibrinogen) (Table 2). Of the inflammatory/hemostatic biomarkers, only log(PAI-1) was significantly associated with extent of CAC progression. In the multivariable model, log(tPA-ag) was no longer significantly associated with CAC progression after adjusting for baseline CAC, age, site, race/ethnicity, menopausal status, income, education, and SBP (Table 3). Of these covariates, log(CAC+1) at baseline attenuated the relationship between log(tPA-ag) and CAC progression. In contrast, log(PAI-1) maintained a significantly positive relationship independent of all covariates included in the model.
Table 2.
Univariable logistic regression analyses for presence of CAC progression and linear regression analyses for extent of CAC progression/year.
| Presence of CAC Progression†
|
Extent of CAC Progression
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | β (SE) | p-value | |
| Age at baseline (years) | 1.09 (0.97–1.22) | 0.1 | 0.005 (0.003) | 0.04 |
| Pittsburgh sitea | 0.86 (0.47–1.59) | 0.6 | 0.003 (0.014) | 0.8 |
| Whiteb | 0.83 (0.44–1.57) | 0.6 | 0.011 (0.015) | 0.4 |
| Menopausal statusc | 0.1 | 0.2 | ||
| Late peri- and post-menopausal | 1.46 (0.77–2.79) | 0.3 | 0.028 (0.015) | 0.07 |
| Hormone therapy user | 0.39 (0.11–1.37) | 0.1 | 0.0004 (0.021) | 1.0 |
| Incomed | 0.7 | 1.0 | ||
| $50,000-<$100,000 | 1.14 (0.56–2.32) | 0.7 | −0.003 (0.016) | 0.9 |
| ≥$100,000 | 0.83 (0.37–1.85) | 0.6 | 0.002 (0.017) | 0.9 |
| Educatione | 0.5 | 0.3 | ||
| Some college or college | 1.08 (0.47–2.51) | 0.9 | −0.012 (0.019) | 0.5 |
| Graduate | 0.71 (0.28–1.84) | 0.5 | −0.030 (0.021) | 0.2 |
| Systolic blood pressure (mmHg) | 1.02 (1.002–1.04) | 0.03 | 0.0005 (0.0004) | 0.3 |
| Body mass index (kg/m2) | 1.06 (1.01–1.11) | 0.01 | 0.003 (0.001) | 0.01 |
| High density lipoprotein cholesterol (mg/dL) | 0.97 (0.94–1.00) | 0.02 | −0.0004 (0.0005) | 0.4 |
| Low density lipoprotein choleterol (mg/dL) | 1.01 (1.001–1.02) | 0.03 | 0.0002 (0.0002) | 0.3 |
| Triglyceridesf (mg/dL) | 2.08 (1.13–3.82) | 0.02 | 0.037 (0.014) | 0.009 |
| Cardiovascular medication use | 1.72 (0.87–2.40) | 0.1 | 0.015 (0.016) | 0.4 |
| Diabetes mellitus | 5.08 (1.31–19.63) | 0.02 | 0.056 (0.037) | 0.1 |
| Family history of cardiovascular disease | 0.91 (0.48–1.71) | 0.8 | 0.0004 (0.014) | 1.0 |
| Current smoker | 1.43 (0.60–3.42) | 0.4 | 0.003 (0.021) | 0.9 |
| Homeostasis Model Assessmentf | 1.49 (0.91–2.43) | 0.1 | 0.009 (0.011) | 0.5 |
| Baseline coronary artery calcium+1f (U) | 1.29 (1.06–1.56) | 0.01 | 0.011 (0.005) | 0.01 |
| C-reactive proteinf (mg/L) | 1.06 (0.83–1.36) | 0.6 | 0.003 (0.006) | 0.6 |
| Fibrinogenf (mg/dL) | 1.29 (0.28–5.95) | 0.7 | −0.010 (0.036) | 0.8 |
| Plasminogen-activator inhibitor-1f (ng/mL) | 1.94 (1.39–2.72) | 0.0001 | 0.020 (0.007) | 0.006 |
| Tissue plasminogen activator antitgenf (ng/mL) | 2.21 (1.06–4.61) | 0.03 | 0.012 (0.016) | 0.4 |
| Factor VIIcf (%) | 2.50 (0.78–8.05) | 0.1 | 0.055 (0.028) | 0.051 |
Pittsburgh versus Chicago;
White versus Black;
Referenced to pre- and early perimenopausal;
Referenced to <$50,000;
Referenced to high school or less;
Log transformed
CAC Progression: any CAC for baseline CAC=0, ≥10 for 0<baseline CAC<100; ≥10% for baseline CAC ≥100
CAC = coronary artery calcium; CI = confidence interval; OR = odds ratio; SE = standard error
Table 3.
Multivariable logistic regression analyses for presence of CAC progression and linear regression analyses for extent of CAC progression/year.
| Presence of CAC Progression†
|
Extent of CAC Progression
|
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | β (SE) | p-value | |
| Model 1: Log(baseline coronary artery calcium+1), Age, Site, Race/ethnicity, Menopausal status, Income, Education, Systolic blood pressure | ||||
| C-reactive protein (mg/L) | 0.96 (0.72–1.27) | 0.8 | 0.0001 (0.006) | 1.0 |
| Fibrinogen (mg/dL) | 0.82 (0.15–4.56) | 0.8 | −0.017 (0.037) | 0.6 |
| Plasminogen-activator inhibitor-1 (ng/mL) | 1.93 (1.32–2.83) | 0.0007 | 0.018 (0.008) | 0.02 |
| Tissue plasminogen activator antigen (ng/mL) | 1.90 (0.83–4.31) | 0.1 | 0.005 (0.017) | 0.8 |
| Factor VIIc (%) | 2.43 (0.56–10.57) | 0.2 | 0.034 (0.031) | 0.3 |
| Model 2: Model 1 + Body mass index | ||||
| C-reactive protein (mg/L) | 0.89 (0.65–1.21) | 0.5 | −0.006 (0.007) | 0.4 |
| Fibrinogen (mg/dL) | 0.81 (0.14–4.65) | 0.8 | −0.035 (0.038) | 0.4 |
| Plasminogen-activator inhibitor-1 (ng/mL) | 1.93 (1.31–2.85) | 0.0009 | 0.016 (0.008) | 0.06 |
| Tissue plasminogen activator antigen (ng/mL) | 1.81 (0.76–4.30) | 0.2 | −0.005 (0.018) | 0.8 |
| Factor VIIc (%) | 2.43 (0.56–10.55) | 0.2 | 0.034 (0.031) | 0.3 |
| Model 3: Model 2 + Log(Homeostasis Model Assessment), Family history of cardiovascular disease, High density lipoprotein cholesterol, Low density lipoprotein cholesterol, Log(triglycerides), Cardiovascular medication use, Current smoker | ||||
| C-reactive protein (mg/L) | 0.86 (0.61–1.22) | 0.4 | −0.0004 (0.007) | 0.6 |
| Fibrinogen (mg/dL) | 0.77 (0.11–5.20) | 0.8 | −0.040 (0.040) | 0.3 |
| Plasminogen-activator inhibitor-1 (ng/mL) | 1.91 (1.24–2.93) | 0.003 | 0.017 (0.009) | 0.06 |
| Tissue plasminogen activator antigen (ng/mL) | 1.42 (0.53–3.84) | 0.5 | −0.007 (0.020) | 0.7 |
| Factor VIIc (%) | 1.58 (0.32–7.82) | 0.6 | 0.030 (0.034) | 0.4 |
All inflammatory/hemostatic biomarkers were log transformed.
CAC Progression: any CAC for baseline CAC=0, ≥10 for 0<baseline
CAC<100; ≥10% for baseline CAC ≥100
CAC = coronary artery calcium; CI = confidence interval; OR = odds ratio; SE = standard error
Similarly, in multivariable linear regression analysis, only log(PAI-1) was positively and significantly associated with yearly extent of CAC progression after adjusting for baseline CAC, age, study site, race/ethnicity, menopausal status, income, education, and SBP (Table 3). When BMI was added to the model, the association was attenuated.
DISCUSSION
In the current study, baseline levels of PAI-1 were significantly associated with the presence of CAC progression and marginally associated with the yearly extent of CAC progression, independent of traditional CV risk factors and socio demographics. To the best of our knowledge, no studies to date have assessed the relationships of PAI-1, tPA-ag and factor VIIc with CAC progression. We have previously shown that PAI-1 was not associated with presence or extent of baseline CAC in women at midlife.17 Others demonstrated a positive relationship between PAI-1 level and presence of CAC on a single measurement in young diabetics, but not non-diabetics.18 Our findings of a relationship between levels of PAI-1 and CAC progression in the current analyses, but not CAC prevalence in a previous work suggests that PAI-1 may be an important temporal marker for healthy midlife women who are at elevated risk for aggressive atherosclerotic development.17 Some have postulated that a single measure of CAC is a representation of long-term, or lifetime, atherosclerotic plaque burden whereas CAC progression is more indicative of short-term, or current, disease activity.19
Relationships between inflammatory biomarkers and incident CAC is mostly mediated through BMI.20 In women, CRP appears to be associated with incident CAC in those of normal BMI, but not overweight or obese.21 The influence of BMI on the relationships between inflammatory biomarkers and CAC progression is less studied.2 In our study, adjusting for BMI attenuated the association between PAI-1 and extent of CAC progression, but not presence of CAC progression.
CHD events and CAC progression continue despite optimization of traditional risk factors, including statin use. Statins have failed to demonstrate significant decreases in CAC progression.22 Lipid regulation may not adequately affect calcification in coronary atheromas, which occurs late in the atherosclerotic process. Mechanisms that prevent worsening CAC burden may ultimately improve upon current state-of-the-art CHD prevention recommendations. Whether targeting hemostatic abnormalities, as evidenced by elevated PAI-1 levels, may slow CAC progression and consequently decrease CHD events will require further investigation.
The potential mechanisms for the detected association between levels of PAI-1 and CAC progression are not completely clear. Inhibition of fibrinolysis through PAI-1 has been postulated as a pathway to increase arterial fibrin deposition and thrombosis.23 PAI-1 may thus be an important factor in CAC progression in late stage atherosclerotic lesions as altered healing of ruptured atheromas may contribute to increasing CAC. CAC progression has been correlated with mortality and morbidity, and CAC magnitude has been correlated with atherosclerotic plaque burden.3,16,24
Increased activation of the renin-angiotensin-aldosterone system RAAS has been known to be associated with abnormalities in the fibrinolytic system, particularly endothelial expression of PAI-1 by angiotensin.25 Prior studies suggest that angiotensin-converting enzyme inhibition alone or a combination of angiotensin II type I (AT1) receptor and aldosterone receptor inhibition have the potential to chronically reduce elevated levels of PAI-1.25–27 This may theoretically relieve PAI-1 mediated suppression of fibrinolysis.
Decreasing PAI-1 activity has also improved vascular structure in mouse models. AT1 receptor blockade with azilsartan decreased PAI-1 expression in the aortic wall of mice fed a high fat diet.28 In addition, mice fed azilsartan had more stable plaques as evidenced by greater cellularity and collagen than mice that were fed placebo. Administration of apelin to mice has been shown to attenuate angiotensin II-induced perivascular fibrosis in coronary artery sections.29 The observed effect was postulated to be at least in part due to decreased PAI-1 levels by blocking angiotensin II-mediated PAI-1 gene expression. These findings add evidence to the potential histologic stability, and prevention of calcification, that may be conferred to atherosclerotic plaques by controlling PAI-1 expression. Future studies should assess this question.
Our study only included women who were white and black. The results, therefore, may not be generalizable to individuals of other racial/ethnic groups. The participants were recruited from a narrow age range to study the menopausal transition. The findings may not be applicable to women who are younger or older. There were very few diabetics in our study. Diabetics are of particular interest for primary prevention measures given the high prevalence of CHD in this population and the increasing incidence of this condition in developed countries. Excluded women were more likely to be younger, have higher systolic blood pressure, and smokers. This may have introduced selection bias. Finally, our small sample size and short follow-up time may have resulted in inadequate statistical power due to few women developing the outcome. Some small but meaningful associations may not have achieved statistical significance, thereby resulting in negative findings for some independent variables.
In conclusion, the association of PAI-1 with CAC progression in our study suggests that abnormalities in the fibrinolytic system may play an important role in CAC progression in middle-aged women undergoing the menopausal transition. Future studies should evaluate the association of PAI-1 levels with subclinical atherosclerosis in a broader population. If similar relationships are present, additional studies with the goal of decreasing CAC progression in those with elevated PAI-1 levels may be warranted.
Supplementary Material
Acknowledgments
FUNDING SOURCES: SWAN has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heart was supported by grants from the NHLBI (HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIA, NINR, ORWH, or the NHLBI.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair Chris
Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary artery disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 2.McEvoy JW, Blaha MJ, DeFilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement?: a review of published reports. J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borissoff JI, Spronk HMH, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Mosca L, Benjamin EJ, Berra K, Bazason JL, Dolo J, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang L, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DF, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo FA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 9.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 11.Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, Givens L, Matthews KA. Usefulness of electron bean tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol. 2001;87:560–564. doi: 10.1016/s0002-9149(00)01431-4. [DOI] [PubMed] [Google Scholar]
- 12.Matthews KA, Santoro N, Lasley B, Chang Y, Crawford S, Pasternak RC, Sutton-Tyrrell K, Sowers M. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789–1796. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 13.El Khoudary SR, Shields KJ, Chen H, Matthews KA. Menopause, complement, and hemostatic markers in women at midlife: The Study of Women’s Health Across the Nation. Atherosclerosis. 2013;231:54–58. doi: 10.1016/j.atherosclerosis.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, Lloyd-Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with short-term but high lifetime estimated risk for cardiovascular disease: the Coronary Artery Risk Development in Young Adults Study and Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronmal KA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 16.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, DeMoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. J Am Coll Cardiol Img. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Wang NC, Matthews KA, Barinas-Mitchell EJM, Chang CH, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African-American and white race/ethnicity: the Study of Women’s Health Across the Nation (SWAN) heart study. Menopause. 2016 doi: 10.1097/GME.0000000000000605. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratte KA, Barón AE, Ogden LG, Hassell KL, Rewers M, Hokanson JE. Plasminogen activator inhibitor-1 is associated with coronary artery calcium in type 1 diabetes. J Diabetes Complications. 2009;23:387–393. doi: 10.1016/j.jdiacomp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Anand DV, Lim E, Darko D, Bassett P, Hopkins D, Lipkin D, Corder R, Lahiri A. Determinants of progression of coronary artery calcification in type 2 diabetes: role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218–2225. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumental RS, Nasir K. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Gupta NK, de Lemos JA, Ayers CR, Abdullah SM, McGuire DK, Khera A. The relationship between C-reactive protein and atherosclerosis differs on the basis of body mass index: the Dallas Heart Study. J Am Coll Cardiol. 2012;60:1148–1155. doi: 10.1016/j.jacc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Henein MY, Owen A. Statins moderate coronary stenosis but not coronary calcification: results from meta-analyses. Int J Cardiol. 2011;153:31–35. doi: 10.1016/j.ijcard.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 24.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Greegoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 25.Kerins DM, Hao Q, Vaughan DE. Angiotensin induction of PAI-1expression in endothelial cells is mediated by the hexapeptide angiotensin IV. J Clin Invest. 1995;96:2515–2520. doi: 10.1172/JCI118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown NJ, Kumar S, Painter CA, Vaughan DE. ACE inhibition versus angiotensin type I receptor antagonism: differential effects on PAI-1 over time. Hypertension. 2002;40:859–865. doi: 10.1161/01.hyp.0000040264.15961.48. [DOI] [PubMed] [Google Scholar]
- 27.Sawathiparnich P, Murphey LJ, Kumar S, Vaughan DE. Effect of combined AT1 receptor and aldosterone receptor antagonism on plasminogen activator inhibitor-1. J Clin Endocrinol Metab. 2003;88:3867–3873. doi: 10.1210/jc.2003-030374. [DOI] [PubMed] [Google Scholar]
- 28.French CJ, Zaman T, Sobel BE. The angiotensin receptor blocker, azilsartan medoxomil (TAK-491), suppresses vascular wall expression of plasminogen activator inhibitor type-1 protein potentially facilitating the stabilization of atherosclerotic plaques. J Cardiovasc Pharmacol. 2011;58:143–148. doi: 10.1097/FJC.0b013e31821dcbea. [DOI] [PubMed] [Google Scholar]
- 29.Siddiquee K, Hampton J, Khan S, Zadory D, Gleaves L, Vaughan DE, Smith LH. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J Hypertens. 2011;29:724–731. doi: 10.1097/HJH.0b013e32834347de. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.