Abstract
Objective
To determine whether there is a racial difference in the risk of acute kidney injury between hospitalized black and white adults with diabetes mellitus in the United States
Research Design and Methods
We analyzed cross-sectional data from the 2000–2010 National Hospital Discharge Survey (NHDS) to compare the odds of AKI among hospitalized black and white adults with diabetes. After excluding records in which race status was missing, race was other than white or black, discharge status was not provided, or end-stage renal disease was a diagnosis, we identified 276,138 eligible records for analysis. Multivariable logistic regression was used to analyze the association between race, AKI, and in-hospital mortality. Multivariable linear regression was used to analyze the association between length of stay and race among discharge records with a diagnosis of AKI.
Results
In this nationally representative sample of hospitalized U.S. adults with diabetes, blacks had a 50% higher age- and sex-adjusted odds of AKI compared to whites (odds ratio: 1.51; 95% CI 1.37–1.66). The association between black race and increased risk of AKI persisted after additional adjustment for multiple AKI-related risk factors, including chronic kidney disease, sepsis, hypertension, hypotension, length of stay, myocardial infarction, congestive heart failure, angiography, computed tomography scan, cirrhosis, admission source, payor source, hospital region, and hospital bedsize (OR 1.71; 95% CI, 1.31–2.25). Among cases of AKI, there was no racial difference in length of stay or in-hospital mortality.
Conclusions
Among hospitalized adults in the U.S. with diabetes, black race is associated with a higher risk of AKI compared to white race.
Keywords: acute kidney injury, ethnicity, race, diabetes, hospitalization, mortality, racial disparity, inequality
1 Introduction
Race is associated with disparate clinical outcomes in multiple health conditions (1). Such disparities are particularly conspicuous in diabetes, where racial minorities have higher rates of microvascular complications and mortality (2). Compared to other racial groups, blacks with diabetes make more frequent emergency department visits for hyperglycemia and hypoglycemia (3–5), are hospitalized at younger ages (1), have higher readmission rates(6), and experience more preventable complications (2). Among the complications of diabetes, the racial gap in disease incidence is widest in nephropathy; blacks are nearly four times more likely to progress to end-stage renal disease compared to their white counterparts(2).
Several factors may explain racial differences in the risk of diabetic chronic kidney disease (CKD), including genetics, modifiable cardiovascular risk factors (e.g. hypertension, hyperlipidemia), and socioeconomic status (7). Acute kidney injury (AKI) is also a well-described risk factor for CKD, which reciprocally confers an increased risk of AKI(8). As with CKD, black race is associated with an increased risk of AKI compared to non-black race (9). However, to our knowledge there are no studies that have examined the association between race and AKI specifically among hospitalized persons with diabetes, a population at theoretically increased risk for this outcome. Since both black race and diabetes are well-established risk factors for chronic kidney disease (CKD) (2), which is the strongest independent risk factor for AKI (8), we hypothesized that blacks with diabetes are at higher risk of AKI.
Considering that AKI occurs in one of every 5 hospitalizations (10), it is important to understand the risk factors for this outcome to inform preventive efforts. Among Medicare recipients, hospitalization for AKI is increasing annually and the racial disparity in AKI incidence between blacks and whites is expanding (11). Moreover, the prevalence of AKI requiring dialysis is increasing by about 10% per year, and black race has been identified as a significant risk factor for initiation of dialysis (12). AKI has significant financial and clinical repercussions, including increased length of hospital stay, higher readmission rates, risk of progression to CKD, and increased mortality (8, 13, 14).
The primary aim of this study was to explore whether there is a racial difference in the risk of acute kidney injury (AKI) among hospitalized persons with diabetes. A secondary aim of our study was to examine whether outcomes following AKI, such as length of hospitalization and mortality, differ according to race. Identification of racial differences in the risk of AKI and subsequent outcomes among individuals with diabetes could have broad implications on the quality of hospital care and resource utilization.
2 Materials and Methods
2.1 Data Source
This study used restricted cross-sectional data from the National Hospital Discharge Survey (NHDS) databases, accessed at the Research Data Center at the National Center for Health Statistics. NHDS is a national probability survey conducted annually from 1965 to 2010 of inpatients discharged from non-Federal short-stay hospitals in the United States. Hospitals with an average length of stay fewer than 30 days were included in the survey. Federal, military, Department of Veterans Affairs hospitals, institutional hospital units, and hospitals with fewer than six beds were excluded. From 2000 to 2007, the NHDS collected data from a national sample of approximately 500 hospitals annually, and from 2008–2010 sampled 239 hospitals annually (15).
The contents of the NHDS restricted database include: demographics (age, sex, race, marital status, zipcode), length of stay, discharge status (routine to home, against medical advice, transfer to short or long-term facility, alive, dead, not stated), hospital geographic region (Northeast, Midwest, South, and West), hospital bed size, hospital ownership (proprietary, church, government, nonprofit), admission type (emergency, urgent, elective, newborn), source of admission (physician referral, clinical referral, HMO referral, transfer, emergency room, court/law enforcement, other), payment source (worker’s compensation, Medicare, Medicaid, other government payments, Blue Cross/Blue Shield, HMO/PPO, other private/commercial insurance, self-pay, no charge, and other), procedure and diagnostic codes. In addition, the restricted NHDS dataset contains weighted variables, population counts of discharges within a hospital, stratum identifiers, and other survey design variables required in regression analyses to inflate estimates to the national hospitalized U.S. population.
2.2 Study Population
Diagnostic and procedure codes derived from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) were used to identify the study population of hospitalized adults with diabetes. Figure 1 summarizes the eligibility criteria for this study. Among 2.6 million discharge records from 2000–2010, included records were extracted from 420,426 discharge records of adult patients (age 18 years and older) with at least one diagnostic code for diabetes mellitus (ICD-9-CM 250.00–250.93). Among these records, records were excluded if race status was not provided (27.5%), discharge status was not provided (0.7%), or ESRD was a hospital diagnosis (ICD-9-CM 585.6) (1.5%). Given that race status other than black and white represented a minority of this discharge records (4.7%), they were excluded from the analysis. After exclusion, 276,138 records were included in the final analysis.
Figure 1.
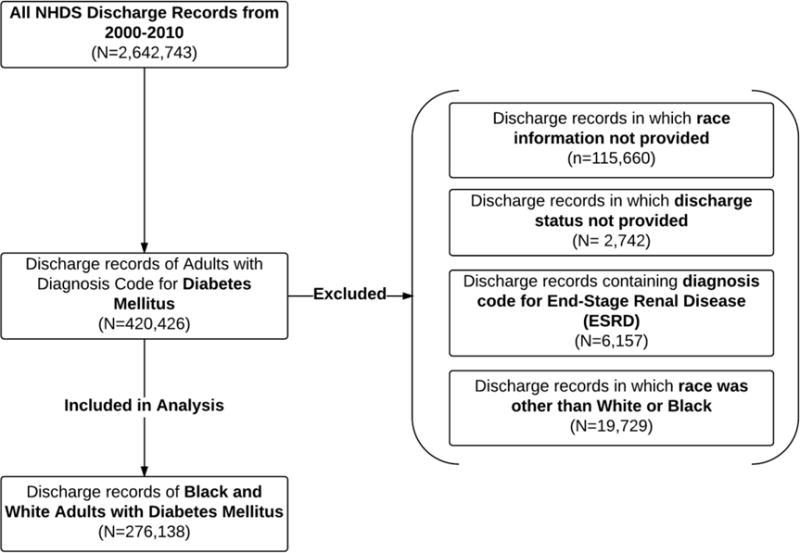
Eligibility Criteria.
2.3 Risk factors for AKI
The main variable of interest was race, which was defined as white or black based on demographic information provided in the dataset. Age was evaluated as a categorical variable with 18–24 years as the reference group, increasing by decade intervals up to ≥ 75 years. We considered the following conditions as risk factors for AKI, defined by their corresponding ICD-9-CM discharge diagnosis codes: CKD (585–585.5), hypertension (401.0, 401.1, 401.9), hypotension (458), chronic liver disease (571.0–571.9), acute myocardial infarction (410.0–410.9), congestive heart failure (428.0–428.4, 428.9), and sepsis (999.90–999.95, 038.0–038.9). Contrast-induced nephropathy was considered another possible risk factor and was evaluated using the following ICD-9-CM procedure codes: angiography (88.50–88.59), and computed tomography (87.41, 87.42, 87.03, 87.04, 88.01, 88.02, 87.71, and 88.38). Data regarding the use of intravenous contrast with computed tomography was not available.
In addition to length of stay (LOS), admission source was included as a potential indicator of patient acuity. Admission source was available for 91% of records, and was categorized as emergency department, physician/clinic, transfer, or other. Payment source information was available for 99% of records, and was categorized as Medicare, Medicaid, private insurance, or other payment. This information was included as a potential surrogate of socioeconomic status or access to health care. Other hospital-specific factors included as potential risk factors were hospital region (Northeast, Midwest, South, West), and hospital size (dichotomous variable with ≥ vs. < 300 beds).
2.4 Ascertainment of AKI
The primary outcome was documented AKI, defined by ICD-9-CM codes 584.0–584.9 ascertained from discharge records. The accuracy of ICD-9-CM codes has been previously validated(16). Data regarding admission diagnosis was only available for years 2008 to 2010 of the NHDS survey period (16.9% of all eligible records in this study); during these three years of the study period, only 286 records had an admission diagnosis of AKI. Given this limitation in the NHDS dataset, the present study was not able to distinguish cases of community-acquired AKI (i.e. hospitalization for which AKI was the reason for admission) from hospital-acquired AKI.
2.5 Mortality and Length of Hospitalization
Secondary outcomes included in-hospital mortality and length of stay. Since a direct association between AKI and mortality is well established, we analyzed mortality and length of stay exclusively among cases of AKI to determine whether there were differences in this outcome by race.
2.6 Sensitivity Analyses
2.6.1 Change in definition of AKI
During the study period, there was a change in the definition of AKI. The Acute Kidney Injury Network (AKIN) criteria for AKI were published in March, 2007 (17). By these criteria, AKI is defined as an absolute increase in serum creatinine of more than or equal to 0.3 mg/dl or an increase in serum creatinine of more than or equal to 50% (1.5-fold increase from baseline) within 48 hours, or urine output of less than 0.5 ml/kg per hour for more than six hours (17). To allow time for dissemination of the new criteria, we divided the study into pre-AKIN years (2000–2007) and AKIN years (2008–2010) to evaluate whether there were secular trends in the association between race and AKI because of evolving diagnostic criteria.
2.6.2 AKI Requiring Dialysis
Although the majority of hospitalized patients requiring dialysis have ESRD, a subset of patients with AKI may require in-hospital dialysis. In our overall analysis, we included records of patients who had AKI requiring dialysis (ICD-9 codes v45.11, v56.8, v56.0) without a concomitant diagnosis of ESRD. To evaluate whether the association between race and AKI persisted according to need for in-hospital dialysis, we performed a sensitivity analysis in which 802 records with AKI requiring dialysis were excluded.
2.6.3 Missing Race Information
Race data was missing from 27.5% of eligible records, which is consistent with historical under-reporting of race in the NHDS database.(18) To take into account possible selection bias resulting from non-random missing race data, we performed a sensitivity analysis of the outcome of AKI by reporting of racial status. Using the same inclusion criteria of our study population, we analyzed 388,401 records from 2000–2010, of which 71% had race status reported (as white or black) and 29% lacked race status information. Using multivariable logistic regression, we compared the odds of AKI among discharge records of white and black persons compared to missing race status as the reference group.
2.7 Statistical Analyses
Descriptive statistics were used to characterize the sample of discharge records from hospitalized adults with diabetes stratified by race. The Shapiro-Wilk test was used to assess normality of the data. For non-normally distributed data, median and interquartile ranges are provided. To compare differences in distributions by race, the two-sided Student t test was used for normally distributed continuous variables, the Wilcoxon rank-sum test was used for non-normally distributed continuous variables, and the χ2 test was used for categorical variables. P <0.05 was considered significant.
Logistic regression analysis was used to explore the association between AKI and race, co-existing conditions, and other covariates. Simple logistic regression was used to estimate the total effect of each covariate with AKI. Variables identified to be potentially significant at bivariate analysis (P <0.1) were analyzed further using forward and backward stepwise model selection by minimum Akaike Information Criterion (AIC). Multivariable logistic regression included known confounders and variables that were potentially significant in bivariate analysis and model selection by AIC. Three logistic regression models were used to analyze the association between AKI and other explanatory variables: Model 1 was adjusted for age and sex, Model 2 was adjusted for age, sex, and AKI-related clinical risk factors (CKD, sepsis, hypertension, hypotension, length of stay, acute myocardial infarction, congestive heart failure, angiography, computed tomography scan, and chronic liver disease). In addition to the covariates in Model 2, Model 3 was adjusted for the following sociodemographic factors: admission source, payer source, race/payer interaction, hospital region, and hospital size. Effect modification of the association between race and AKI by age, gender, and payer source were explored; there was a strong trend toward interaction between race and payer source, so the interaction term was included in Model 3.
To explore the association between race and the secondary outcome of mortality among patients with AKI, we used multivariable logistic regression, and to explore the association between race and length of stay among patients with AKI, we used multivariable linear regression. The association between mortality and race was adjusted for the same covariates as in Model 3 above; the association between length of stay and race was adjusted for the same covariates as in Model 3, excluding length of stay since this was the outcome variable).
All effect estimates in the regression analyses were weighted to allow inflation to national estimates based on the weight variable and other restricted survey design variables provided by the NHDS. Since hospital admissions for individual patients cannot be linked, it is important to note that inferences drawn from this analysis relate to samples of hospital discharges rather than individual patients. Of note, given the large sample size of this study, observed differences in estimated effects of even small magnitude would be expected to achieve statistical significance.
All statistical analyses were performed with Stata Statistical Software: Release 13.1. College Station, Tx: Stata Corp, LP.
3 RESULTS
3.1 Patient Characteristics
Detailed patient characteristics stratified by race are presented in Table 1. There were several notable racial differences in demographics and AKI-related risk factors. Compared to whites, blacks were more likely to be female (60.4% vs. 52.7%, P<0.001) and were younger (median age 61 vs. 68 years, P<0.001). Blacks were more likely to have had comorbid conditions that increase the risk of AKI, including sepsis (2.7% vs. 2.2%, P<0.001), hypertension (50.5% vs. 46.2%, P<0.001), and chronic kidney disease (5.0% vs. 4.0%, P<0.001). In contrast, whites were more likely to have had hypotension (1.8 % vs. 1.5%, P <0.001), acute myocardial infarction (4.4% vs. 2.5%, P <0.001), and chronic liver disease (1.7% vs. 1.1%, P <0.001). Although blacks were more likely to have undergone computed tomography scan (3.1% vs. 2.8%, P <0.001), whites were more likely to have had an angiography procedure (5.8% vs. 4.3%, P <0.001).
Table 1.
Characteristics of Discharge Records (n=276,138) of Hospitalized Adults with Diabetes Mellitus, 2000–2010.
| Variable | Black (N=72,957) | White (N=203,181) | P-value |
|---|---|---|---|
| Primary Outcome | |||
| Acute kidney injury, N (%) | 4, 368 (6.0) | 9,380 (4.6) | <0.001 |
| Demographics | |||
| Female (%) | 60.4 | 52.7 | <0.001 |
| Median age (IQR), years | 61 (49, 72) | 68 (56,78) | <0.001 |
| Discharge Status | |||
| Median LOS (IQR), days | 4 (2,7) | 4 (2,6) | <0.001 |
| Transfer to Short-Term Facility (%) | 3.0 | 4.2 | <0.001 |
| Transfer to Long-Term Facility (%) | 10.5 | 13.2 | <0.001 |
| In-hospital Death (%) | 1.6 | 1.9 | <0.001 |
| Hospital Region | |||
| Northeast (%) | 22.4 | 29.5 | <0.001 |
| Midwest (%) | 19.3 | 20.0 | <0.001 |
| South (%) | 52.6 | 40.2 | <0.001 |
| West (%) | 5.7 | 10.3 | <0.001 |
| Hospital Size | |||
| <300 beds (%) | 53.1 | 60.3 | <0.001 |
| ≥300 beds (%) | 46.9 | 39.7 | <0.001 |
| Admission Source | |||
| Emergency Room (%) | 73.6 | 63.0 | <0.001 |
| Clinic (%) | 19.2 | 27.3 | <0.001 |
| Transfer (%) | 5.6 | 7.4 | <0.001 |
| Other (%) | 1.6 | 2.3 | <0.001 |
| Payment Source | |||
| Medicare (%) | 51.9 | 60.4 | <0.001 |
| Medicaid (%) | 16.6 | 7.0 | <0.001 |
| Private Insurance (%) | 23.4 | 26.6 | <0.001 |
| Other (%) | 8.1 | 5.9 | <0.001 |
| AKI-Related Risk Factors | |||
| Sepsis (%) | 2.7 | 2.2 | <0.001 |
| Congestive heart failure (%) | 19.1 | 19.3 | 0.240 |
| Hypotension (%) | 1.5 | 1.8 | <0.001 |
| Hypertension (%) | 50.5 | 46.2 | <0.001 |
| Acute myocardial infarction (%) | 2.5 | 4.4 | <0.001 |
| Chronic kidney disease (%) | 5.0 | 4.0 | <0.001 |
| Chronic liver disease (%) | 1.1 | 1.7 | <0.001 |
| Computed tomography scan (%) | 3.1 | 2.8 | <0.001 |
| Angiography (%) | 4.3 | 5.8 | <0.001 |
IQR= interquartile range.
The median length of stay (4 days) was similar between blacks and whites, although given the large sample size of the study and differences in the inter-quartile range (5 days for blacks and 4 days for whites), there was a statistically significant difference between the groups (P<0.001). Compared to whites, blacks were significantly less likely to be transferred to short-term (3.0% vs. 4.2%) and long-term facilities (10.5% vs. 13.2%) (P<0.001). Overall, in-hospital mortality was higher in whites compared to blacks (1.9% vs. 1.6%, P <0.001). Blacks were more likely to have been admitted to the hospital from the emergency room (73.6% vs. 63.0%, P<0.001) and were more likely to be recipients of Medicaid (16.6% vs. 7.0%, P <0.001). Compared to whites, blacks represented the majority of hospitalized patients in southern hospitals (52.6% vs. 40.2%, P<0.001), and the minority in other U.S. regions. The proportion of black patients hospitalized in larger hospitals (bed size ≥ 300 beds) was greater than whites (46.9% vs. 39.7%, P<0.001).
3.2 Primary Outcome: AKI
The overall period prevalence of AKI was 5.0% in this population of hospitalized adults with diabetes mellitus, and the period prevalence differed significantly by race: 5.9% in blacks vs. 4.6% in whites (P<0.001). Compared to white race, black race was associated with a 1.40 (95% CI 1.27–1.54) higher unadjusted odds of AKI. Table 2 shows the adjusted odds ratios of AKI in the three logistic regression models. After adjustment for age category and sex (Model 1), black race was associated with 1.51 (95% CI 1.37–1.67) higher odds of AKI compared to white race. After additional adjustment for AKI-related clinical risk factors (Model 2), black race was associated with a similar odds of AKI (OR 1.48; 95% CI 1.35–1.63). After additional adjustment for demographic variables, the association between black race and AKI increased to 1.71 (95% CI 1.31–2.25).
Table 2.
Adjusted Odds Ratiosa (OR) of AKI in Hospitalized Adults with Diabetes Mellitus, 2000–2010.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | B | SE | eB [OR (95% CI)] | B | SE | eB [OR (95% CI)] | B | SE | eB [OR (95% CI)] |
| Race (B vs. W) | 0.41 | 0.05 | 1.51*** (1.37–1.67) | 0.39 | 0.05 | 1.48*** (1.35–1.63) | 0.54 | 0.14 | 1.71 (1.31–2.25)*** |
|
| |||||||||
| Age (years) | |||||||||
| 18–24 | – | – | 1.00 (ref) | – | – | 1.00 (ref) | – | – | 1.00 (ref) |
| 25–34 | 0.06 | 0.23 | 1.06 (0.67–1.68) | 0.07 | 0.24 | 1.07 (0.66–1.73) | 0.10 | 0.26 | 1.11 (0.67–1.83) |
| 35–44 | 0.06 | 0.19 | 1.06 (0.72–1.54) | 0.13 | 0.20 | 1.14 (0.77–1.68) | 0.19 | 0.21 | 1.22 (0.80–1.85) |
| 45–54 | 0.03 | 0.20 | 1.03 (0.69–1.52) | 0.17 | 0.21 | 1.18 (0.78–1.78) | 0.23 | 0.22 | 1.26 (0.81–1.95) |
| 55–64 | 0.32 | 0.20 | 1.37 (0.93–2.03) | 0.43 | 0.21 | 1.53 (1.02–2.30) | 0.51 | 0.22 | 1.67 (1.08–2.59)* |
| 65–74 | 0.27 | 0.18 | 1.30 (0.91–1.87) | 0.33 | 0.19 | 1.39 (0.95–2.01) | 0.46 | 0.21 | 1.59 (1.05–2.40)* |
| ≥ 75 | 0.46 | 0.19 | 1.58 (1.09–2.29)* | 0.42 | 0.20 | 1.52 (1.0.3–2.23)* | 0.54 | 0.22 | 1.72 (1.11–2.64)* |
|
| |||||||||
| Sex (F vs. M) | 0.23 | 0.04 | 1.26 (1.17–1.36)*** | 0.16 | 0.04 | 1.18 (1.09–1.27)*** | 0.15 | 0.04 | 1.17 (1.08–1.26)*** |
|
| |||||||||
| AKI-Risk | |||||||||
| Factors: | |||||||||
| CKD | – | – | – | 1.26 | 0.06 | 3.53 (3.16–3.94)*** | 1.21 | 0.06 | 3.34 (3.00–3.73)*** |
| Sepsis | – | – | – | 0.98 | 0.07 | 2.66 (2.31–3.06)*** | 0.96 | 0.07 | 2.60 (2.25–3.01)*** |
| Hypertension | – | – | – | −1.01 | 0.05 | 0.36 (0.33–0.40)*** | −1.02 | 0.05 | 0.36 (0.32–0.40)*** |
| Hypotension | – | – | – | 0.75 | 0.09 | 2.13 (1.78–2.55)*** | 0.73 | 0.10 | 2.08 (1.72–2.51)*** |
| LOS (days) | – | – | – | 0.03 | 0.00 | 1.03 (1.02–1.04)*** | 0.03 | 0.00 | 1.03 (1.02–1.03)*** |
| AMI | – | – | – | 0.25 | 0.08 | 1.29 (1.10–1.50)* | 0.19 | 0.08 | 1.21 (1.03–1.43)* |
| CHF | – | – | – | 0.21 | 0.04 | 1.23 (1.14–1.33)*** | 0.21 | 0.04 | 1.23 (1.13–1.33)*** |
| Angiography | – | – | – | −0.65 | 0.10 | 0.52 (0.43–0.63)*** | −0.70 | 0.10 | 0.50 (0.41–0.60)*** |
| CLD | – | – | – | −0.09 | 0.15 | 0.92 (0.69–1.22) | −0.11 | 0.15 | 0.90 (0.67–1.21) |
| CT scan | – | – | – | −0.25 | 0.12 | 0.78 (0.62–0.98)* | −0.37 | 0.13 | 0.69 (0.54–0.89)** |
|
| |||||||||
| Admission Source: | |||||||||
| ER (ref) | – | – | – | – | – | – | – | – | 1.00 (ref) |
| Clinic | – | – | – | – | – | – | −0.32 | 0.06 | 0.73(0.65–0.82)*** |
| Transfer | – | – | – | – | – | – | 0.44 | 0.07 | 1.56 (1.36–1.78)*** |
| Other | – | – | – | – | – | – | −0.09 | 0.18 | 0.92 (0.65–1.30) |
|
| |||||||||
| Payor Source: | |||||||||
| Medicare | – | – | – | – | – | – | – | – | 1.00 (ref) |
| Medicaid | – | – | – | – | – | – | 0.14 | 0.09 | 1.16 (0.97–1.38) |
| Private | – | – | – | – | – | – | −0.06 | 0.07 | 0.94 (0.83–1.08) |
| Other | – | – | – | – | – | – | 0.08 | 0.10 | 1.08 (0.90–1.30) |
|
| |||||||||
| Race/Payor Interaction† | |||||||||
| Medicare | – | – | – | – | – | – | −0.29 | 0.15 | 0.75 (0.56–1.00) |
| Medicaid | – | – | – | – | – | – | −0.23 | 0.20 | 0.79 (0.54–1.17) |
| Private | – | – | – | – | – | – | −0.04 | 0.16 | 0.96 (0.70–1.31) |
| Other‡ | – | – | – | ||||||
|
| |||||||||
| Region: | |||||||||
| Northeast | – | – | – | – | – | – | – | – | 1.00 (ref) |
| Midwest | – | – | – | – | – | – | −0.04 | 0.07 | 0.96 (0.84–1.11) |
| South | – | – | – | – | – | – | 0.13 | 0.06 | 1.14 (1.00–1.29) |
| West | – | – | – | – | – | – | 0.09 | 0.08 | 1.09 (0.93–1.28) |
|
| |||||||||
| Hospital size | |||||||||
| (≥ 300 vs. <300 beds) | – | – | – | – | – | – | 0.29 | 0.05 | 1.34 (1.21–1.49)*** |
|
| |||||||||
| Constant | −3.39 | 0.19 | – | −3.43 | 0.20 | – | −3.58 | 0.22 | – |
eB= exponentiated beta (odds ratio).
Analysis based on 276,138 discharge records, among which 4,368 blacks and 9,380 whites had AKI. Odds ratios are inflated to national estimates (population size 408,647,876) based on survey design variables provided by the National Hospital Discharge Survey.
B= Blacks, W= Whites (ref); F = Female; M= Male (ref); Ref= reference group for comparisons of categorical variables. LOS= length of stay. AMI= acute myocardial infarction. CLD= chronic liver disease. CT= computed tomography. ER= emergency room.
Bolded values indicate significant associations.
P<0.05;
P <0.01;
P <0.001.
Blacks with vs. without specified insurance.
Omitted from model because of collinearity.
There were several notable predictors of AKI in the logistic regression models. In Model 1 (age/sex adjustment), only the highest age group (≥ 75 years) was associated with a higher odds of AKI compared to the lowest age group (P<0.05), after adjustment for race and sex. Similarly, females had a higher odds of AKI compared to males, after adjustment for race and age category (P<0.001). These age and sex associations persisted in all three models. Among the AKI-related clinical risk factors, the strongest predictor of AKI was CKD, which was independently associated with a 3.5 fold higher odds of AKI (P<0.001). Other strong positive predictors of AKI included sepsis, hypotension, and congestive heart failure, while hypertension, angiography, and CT scan were paradoxically associated with lower odds of AKI in both Models 2 and 3.
The source of admission and hospital size were both independently associated with AKI. Compared to admission from the ER, there was a lower odds of AKI among patients admitted from a clinic (0.73; 95% 0.65–0.82) and a higher odds of AKI among patients transferred from a nursing facility or outside hospital (95% CI; 1.36–1.78). The odds of AKI was 34% higher (95% CI, 1.21–1.49) among larger compared to smaller hospitals. While payer source itself was not associated with AKI, there was a strong trend toward interaction between race and payer source. For example, among blacks insured by Medicare vs. other payer source, the odds of AKI was 0.75 (95% CI 0.56–1.00) (P=0.05).
3.3 Secondary Outcomes: Mortality and Length of Stay
During the study period of 2000–2010, the overall mortality rate in our study population was 1.8%. The mortality rate was three times higher among discharge records containing a diagnosis of AKI (5.9%). Although the odds of AKI was higher among discharge records of black patients, there was no racial difference in mortality among records of hospitalized adults with diabetes and AKI (Table 3). From 2000–2010, among 13,748 records of patients with diabetes and AKI, the adjusted odds of mortality comparing blacks to whites was 1.28 (95% CI 0.44–3.71). We did not observe any secular trends in mortality before or after implementation of the AKIN criteria for the diagnosis of AKI. Similarly, there were no significant differences in the adjusted median length of stay between blacks and whites with diabetes and AKI. In the overall study period, the median (IQR) length of stay was 5 (3, 9) days for both whites and blacks (P=0.79).
Table 3.
Adjusted odds ratio (OR) of Mortality and Median Length of Stay in Adults with Diabetes and AKI by Race
| NHDS Survey Period | N† | Race | Adjusted OR* of In-Hospital Mortality (95% CI) | Median (IQR) LOS, days | P-value for difference in LOS** |
|---|---|---|---|---|---|
| 2000–2007 (Pre-AKIN) | 6,531 3,292 |
White Black |
1.00 (ref) 1.59 (0.52–4.83) |
6 (3, 10) 6 (3, 10) |
0.92 |
| 2008–2010 (AKIN) | 2,849 1,076 |
White Black |
1.00 (ref) 0.35 (0.04–3.47) |
5 (3,7) 5 (3,8) |
0.71 |
| 2000–2010 (Overall) | 9,380 4,368 |
White Black |
1.00 (ref) 1.28 (0.44–3.71) |
5 (3, 9) 5 (3, 9) |
0.79 |
Number of discharge records among adults with diabetes mellitus in which AKI was a hospital diagnosis, End-stage renal disease was not a hospital diagnosis, and discharge status information was available. IQR= interquartile range. AKIN= Acute Kidney Injury Network criteria for definition of AKI.
Adjusted for age category, sex, CKD, sepsis, hypertension, hypotension, length of stay (LOS), acute myocardial infarction, congestive heart failure, chronic liver disease, computed tomography scan, angiography, admission source, payor source, race/payor interaction, hospital region, and hospital size.
After adjustment for age category, sex, CKD, sepsis, hypertension, hypotension, acute myocardial infarction, congestive heart failure, chronic liver disease, computed tomography scan, angiography, admission source, payor source, race/payor interaction, hospital region, and hospital size.
3.4 Sensitivity Analyses
Table 4 summarizes the results of our sensitivity analyses.
Table 4.
Sensitivity Analyses: Odds Ratio (OR) of AKI in Hospitalized Adults with Diabetes Mellitus According to Varying Inclusion Criteria.
| Inclusion Criteria | Study Period | N† | Race | Unadjusted OR (95% CI) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Pre-AKIN Definition of AKI | 2000–2007 | 168,378 63,409 |
White Black |
1.00 (ref) 1.39 (1.27–1.52) |
1.00 (ref) 1.48 (1.36–1.63) |
1.00 (ref) 1.48 (1.36–1.63) |
1.00 (ref) 2.05 (1.49–2.81) |
| AKIN Definition of AKI | 2008–2010 | 34,803 10,548 |
White Black |
1.00 (ref) 1.39 (1.19–1.63) |
1.00 (ref) 1.52 (1.30–1.79) |
1.00 (ref) 1.48 (1.26–1.74) |
1.00 (ref) 1.39 (0.89–2.14) |
| Excluded AKI requiring in-hospital dialysis | 2000–2010 | 202,753 72,583 |
White Black |
1.00 (ref) 1.41 (1.28–1.54) |
1.00 (ref) 1.52 (1.37–1.67) |
1.00 (ref) 1.49 (1.35–1.64) |
1.00 (ref) 1.72 (1.31–2.25) |
| Included records missing race status | 2000–2010 | 112,263 203,181 72,957 |
Missing White Black |
1.00 (ref) 1.10 (1.00–1.22) 1.54 (1.37–1.74) |
1.00 (ref) 1.08 (0.98–1.20) 1.63 (1.43–1.84) |
1.00 (ref) 1.06 (0.96–1.18) 1.57 (1.39–1.78) |
1.00 (ref) 1.09 (0.97–1.22) 1.38 (1.19–1.59) |
Number of discharge records of adults with diabetes in which discharge status information was available and End-Stage Renal Disease was not a hospital diagnosis. AKIN= acute kidney injury network. AKI= acute kidney injury. Ref= reference group.
3.4.1 Pre-AKIN (2000–2007) and AKIN (2008–2010) study periods
The association between black race and AKI was similar in both unadjusted and adjusted models before and after implementation of the AKIN criteria. In the fully-adjusted model 3, there was a trend towards higher odds of AKI among blacks before compared to after implementation of the AKIN criteria (2.05; 95% CI 1.49–2.81 vs. 1.39; 95% CI 0.89–2.14).
3.4.2 AKI not requiring dialysis
Given the small number of records containing diagnosis codes for both AKI and dialysis (n=802), as expected we did not observe any significant differences in the unadjusted or adjusted odds of AKI when restricting the analysis to hospital records of patients with non-dialysis requiring AKI. The effect estimates for AKI in this subgroup were essentially identical to those in the overall analysis shown in Table 2.
3.4.3 Missing vs. reported race status
When comparing the odds of AKI among discharge records of whites and blacks, respectively, to those with missing race data, we observed no differences in the odds of AKI among records from whites compared to those with missing race data. In contrast, blacks had higher unadjusted and adjusted odds of AKI compared to records with missing race data. The odds ratios from this analysis were similar in the age-sex adjusted models (Model 1) and after additional adjustment for AKI-related clinical risk factors (Model 2) compared to the overall analysis in Table 2; however, the odds ratio from the fully-adjusted Model 3 was attenuated in this analysis compared to the overall analysis (1.38 vs. 1.71).
4 DISCUSSION
In our study of a nationally representative sample of the hospitalized U.S. population, we found that black race was independently associated with a higher odds of AKI compared to white race among discharge records of adults with diabetes; however, we did not observe any racial differences in mortality or length of stay associated with AKI. The findings of the present study, which focused on a patient population at high-risk for this outcome, contribute to a growing body of evidence that black race is a risk factor for AKI.
Our results align with an earlier study that used the same data source (NHDS data from the year 2001) (16). In that study, which was not restricted to a diabetic patient population, black race was associated with a 1.29 (95% CI, 1.28–1.30) fold greater unadjusted odds of AKI compared to white race (16). This effect estimate is similar to the unadjusted odds ratio of AKI comparing blacks vs. whites in our study (1.40; 95% CI 1.27–1.54). Despite a change in the diagnostic criteria for AKI during the study period, we did not observe secular variation in racial differences of AKI risk before and after implementation of the diagnostic criteria.
The largest prospective study to explore racial differences in AKI risk was the Atherosclerosis Risk in Communities Study (ARIC) study, which was conducted in a community-based population(9). In this study of 10,588 middle-aged black and white adults, blacks were nearly twice as likely to have diabetes and had a 30% greater risk of AKI than whites during a 13-year follow-up.(9) Few studies have explored AKI as an outcome specifically among hospitalized patients. In a prospective study of 400 critically ill trauma patients, black race was associated with an increased risk of AKI in comparison to non-black race when adjusted for other risk factors (RR: 1.86; 95% CI, 1.08–3.18) (19). In another large retrospective study of 45,655 intensive care unit patients, blacks were at higher risk of AKI in the 18–54 year old age group only, with an odds ratio of 1.22 (95% CI 1.07–1.38) compared to whites(20).
Several studies have investigated the effect of AKI on other outcomes. In a prospective study of 3,679 adults with diabetes mellitus in the Veterans Affairs health system, AKI was associated with a 3.56 (95% CI, 2.76 to 4.61) increased risk of stage 4 CKD in persons with diabetes (21). In this study, black race was not independently associated with an increased risk of progression to CKD following AKI when compared to non-black race (21). While we were not able to assess incident CKD following AKI in our study, we observed a higher prevalence of underlying CKD in blacks compared to whites. Since one in four hospitalized patients in the United States has diabetes (6, 22) and one in five has AKI (10), racial differences in the risk of AKI during hospitalization may have implications on other clinical outcomes during hospitalization (e.g. risk of insulin-associated hypoglycemia from reduced renal clearance of insulin).
In this study, we did not observe a mortality difference in blacks and whites with diabetes and AKI. Our findings are consistent with prior studies. In critically ill trauma patients, development of AKI conferred a 4-fold increased risk of mortality, however mortality did not differ between black and non-black patients (19). Similarly, another prospective study of 863 intensive care unit patients found no racial difference in mortality among patients with AKI (23). The same findings have been observed in the general hospitalized population (24). In an earlier study of the 2001 NHDS cohort, AKI was associated with increased mortality, prolonged length of stay, and greater requirement for post-hospitalization care (16). Although this study showed a longer length of stay in blacks compared to whites with AKI, it did not report racial differences in mortality as an outcome (16). AKI is associated with a significant increase in health care expenditures attributed to diagnostic testing and monitoring, longer ICU and hospital stays, increased readmissions, and the need for acute renal replacement therapy (14). We did not find a racial difference in hospital length of stay among those with AKI, but we were not able to assess the likelihood of readmission.
Several factors have been postulated to explain the increased risk of nephropathy in black persons with diabetes, including biologic factors or responses, individual risk behaviors, and physical, social and healthcare contexts (2). In the ARIC study, the elevated risk of AKI among blacks was attenuated after adjustment for differences in income and/or insurance status, suggesting that socioeconomic factors and access to healthcare may be mediating the racial disparity in AKI (9). In another large cross-sectional study, lower income was found to be associated more strongly with albuminuria in blacks than whites (25). Since albuminuria is strongly associated with AKI, racial differences in pathologic albuminuria may partly explain the excess risk of AKI in blacks compared to whites (26). Although we were able to adjust for comorbid conditions that increase the risk of AKI and a surrogate indicator of socioeconomic status (i.e. payment source), we did not have laboratory data to evaluate the effect of albuminuria. For reasons that are not clear, we that female sex was associated with a higher odds of AKI, which has previously been observed and may be related to differences in baseline health.(27)
We recognize several limitations in this study. Ascertainment of the diagnosis of AKI and related risk factors relied on ICD-9 codes, which are prone to misclassification if inaccurately or incompletely documented by healthcare providers. A previous study validated the AKI diagnostic coding of the NHDS dataset and found positive and negative predictive values of 87.6% and 90.1%, respectively; however, the sensitivity of diagnostic coding for AKI is quite low (~20%).(16)
Previous studies that used laboratory confirmation reported a higher incidence of AKI when compared to studies that relied on administrative data (10, 16, 28). Without laboratory data, we were not able to assess stages of AKI, CKD, or albuminuria. Although we adjusted for many known risk factors for AKI, it is possible that we have not accounted for all confounders and we were not able to control for disease severity or diabetes duration. Nephrogenic medications and intravenous contrast are known risk factors for AKI, but were not analyzed due to database limitations. Since there were no unique patient identifiers, we could not control for recurrent hospitalizations on the same individual. Furthermore, due to limitations in this dataset, we were not able to distinguish between community-acquired and hospital-acquired AKI.
Another limitation that requires mention is the underreporting of race in our data source, which could have resulted in selection bias. A previous study investigated the influence of missing race data in the NHDS dataset and found a 7% higher proportion of whites in hospitals that do not report race compared to those that do(18). This fact is consistent with the findings from our sensitivity analysis, in which we noted similarities in the odds of AKI comparing records of whites to those with missing race data, likely explained by the greater proportion of whites in these hospitals and their lower risk of AKI compared to blacks; however, given the large proportion of records missing race data, the race-specific odds of AKI reported in this study should be interpreted with some caution.
Our study has several strengths. To our knowledge, this is the first study to specifically investigate the association of race and AKI in hospitalized patients with diabetes. We used a robust nationally representative dataset over a 10-year study duration. By restricting our study to patients with diabetes – a strong risk factor for AKI – we reduced the potential for confounding by this variable, and carefully accounted for additional known and potential confounders. In addition, we attempted to account for secular changes in the diagnosis of AKI by stratifying study periods according to publication date of the AKIN criteria.
In summary, we showed here that black race is associated with an increased risk of AKI compared to white race in a large sample of hospitalized U.S. adults with diabetes, but there were no racial differences in mortality or hospital length of stay following AKI. We suspect that the racial difference observed in this study is most likely explained by differences in outcome ascertainment (i.e. diagnostic coding) for AKI by race. Blacks with diabetes have a higher prevalence of CKD compared to their white counterparts, and it is possible that inaccurate diagnostic coding for CKD status pre-admission may result in residual confounding by race. These questions could be addressed by a large multi-center prospective study that uses laboratory data to confirm the diagnosis of AKI, to distinguish community and hospital-acquired AKI, and to established baseline CKD status through measurement of urine albumin and attainment of pre-admission laboratory data when possible. Although the excess risk of AKI among blacks with diabetes remains small (risk difference of 1.4% in this study), this risk difference may nonetheless carry important financial and clinical consequences. A clearer understanding of the setting and causative factors of AKI would facilitate the development of strategies to reduce this racial gap.
Acknowledgments
Funding This research projected was supported by NIH grants 5KL2TR001077-02 (NM), T32 DK062707 (MG), NHLBI Career Development Award 1K01HL108832-01 (CH), and K23DK094975 (RG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors wish to acknowledge the late Dr. Frederick L. Brancati for his guidance and invaluable insights on this study, and Dr. Jian Tian of the Research Data Center at the National Center for Health Statistics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions: N.M. analyzed and interpreted the data and wrote the manuscript. M.G. researched data and contributed data management, data analysis, data interpretation, and manuscript writing. C.H., H.Y., and R.G. contributed to study design, data interpretation, and manuscript writing.
References
- 1.Biello KB, Rawlings J, Carroll-Scott A, Browne R, Ickovics JR. Racial disparities in age at preventable hospitalization among U.S. Adults. American journal of preventive medicine. 2010;38(1):54–60. doi: 10.1016/j.amepre.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. The Journal of clinical endocrinology and metabolism. 2012;97(9):E1579–639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menchine MD, Wiechmann W, Peters AL, Arora S. Trends in diabetes-related visits to US EDs from 1997 to 2007. The American journal of emergency medicine. 2012;30(5):754–8. doi: 10.1016/j.ajem.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Ginde AA, Espinola JA, Camargo CA., Jr Trends and disparities in U.S. emergency department visits for hypoglycemia, 1993–2005. Diabetes care. 2008;31(3):511–3. doi: 10.2337/dc07-1790. [DOI] [PubMed] [Google Scholar]
- 5.Ginde AA, Pelletier AJ, Camargo CA., Jr National study of U.S. emergency department visits with diabetic ketoacidosis, 1993–2003. Diabetes care. 2006;29(9):2117–9. doi: 10.2337/dc06-0627. [DOI] [PubMed] [Google Scholar]
- 6.Jiang HJ, Stryer D, Friedman B, Andrews R. Multiple hospitalizations for patients with diabetes. Diabetes care. 2003;26(5):1421–6. doi: 10.2337/diacare.26.5.1421. [DOI] [PubMed] [Google Scholar]
- 7.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Current opinion in nephrology and hypertension. 2014;23(3):298–305. doi: 10.1097/01.mnh.0000444822.25991.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. The New England journal of medicine. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, et al. Explaining the racial difference in AKI incidence. Journal of the American Society of Nephrology: JASN. 2014;25(8):1834–41. doi: 10.1681/ASN.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. American journal of nephrology. 2012;35(4):349–55. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes technology & therapeutics. 2007;9(3):232–40. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 12.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. Journal of the American Society of Nephrology: JASN. 2013;24(1):37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-Acquired Acute Kidney Injury and Hospital Readmission: A Cohort Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014 doi: 10.1053/j.ajkd.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews Nephrology. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 15.Wanderer JP, Sandberg WS, Ehrenfeld JM. Real-time alerts and reminders using information systems. Anesthesiology clinics. 2011;29(3):389–96. doi: 10.1016/j.anclin.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clinical journal of the American Society of Nephrology: CJASN. 2006;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak LJ. Underreporting of race in the National Hospital Discharge Survey. Advance data. 1995;(265):1–12. [PubMed] [Google Scholar]
- 19.Shashaty MG, Meyer NJ, Localio AR, Gallop R, Bellamy SL, Holena DN, et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. Journal of critical care. 2012;27(5):496–504. doi: 10.1016/j.jcrc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(6):860–9. doi: 10.1053/j.ajkd.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(11):2567–72. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. The Journal of clinical endocrinology and metabolism. 2002;87(3):978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 23.Allegretti AS, Steele DJ, David-Kasdan JA, Bajwa E, Niles JL, Bhan I. Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: a cohort study. Crit Care. 2013;17(3):R109. doi: 10.1186/cc12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham KA, Thompson EB, Bodger K, Pearson M. Inequalities in outcomes of acute kidney injury in England. QJM: monthly journal of the Association of Physicians. 2012;105(8):729–40. doi: 10.1093/qjmed/hcs037. [DOI] [PubMed] [Google Scholar]
- 25.Crews DC, McClellan WM, Shoham DA, Gao L, Warnock DG, Judd S, et al. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60(5):779–86. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. Journal of the American Society of Nephrology: JASN. 2010;21(10):1757–64. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Renal failure. 2008;30(6):581–9. doi: 10.1080/08860220802134649. [DOI] [PubMed] [Google Scholar]
- 28.Waikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Current opinion in nephrology and hypertension. 2007;16(3):227–36. doi: 10.1097/MNH.0b013e3280dd8c35. [DOI] [PMC free article] [PubMed] [Google Scholar]


