Abstract
Older adults are more likely than younger adults to experience stress when confronted with cognitive challenges. However, little is known about individual differences that might explain why some older adults exhibit stronger stress responses than others. We examined the interplay of two social-cognitive factors to explain older adults’ cortisol reactivity: (1) subjective social status and (2) essentialist beliefs about cognitive aging. We hypothesized that depending on whether older adults believe that aging-related cognitive decline is inevitable vs. modifiable, low subjective social status should lead to stronger or weaker cortisol reactivity. Using longitudinal data, we assessed the impact of cognitive challenges on stress reactivity in a sample of older adults (N = 389; 61 to 86 years). As predicted, regression analyses confirmed that 44 minutes after cognitively challenging tasks, older adults exhibited a significantly different cortisol reactivity depending on their subjective social status and their essentialist beliefs about cognitive aging. Specifically, older adults with low subjective social status and high essentialist beliefs showed a significantly elevated cortisol reactivity. We discuss the role of essentialist beliefs about cognitive aging to predict when and why high vs. low subjective social status leads to stress responses in older adults.
Keywords: aging, subjective social status, essentialism, stress, cortisol, challenge
Research shows that with increasing age people are more likely to experience stress when completing tasks that require cognitive skills (Gruenewald & Seeman, 2010; Hess, 2006; Neupert, Miller, & Lachman, 2006). This increased susceptibility to stress can have a profound negative impact on a variety of health outcomes. At this point, little is known about how psychosocial factors affect older adults’ stress reactivity. Therefore, we examined two important social-cognitive factors that may affect older adults’ susceptibility to stress when confronted with cognitively challenging tasks: (1) subjective social status and (2) essentialist beliefs about cognitive aging.
Stress reactivity is associated with the release of cortisol, a hormone of the hypothalamic-pituitary-adrenal (HPA) system. Cortisol is the primary hormone released when an individual is confronted with a challenge. Cortisol reactivity is the deviation from a person’s baseline cortisol level in response to a challenge and has been described as a “double-edged sword” (Sapolsky, 2004). On the one hand, a cortisol release in response to challenges can be adaptive in order “to provide the metabolic resources to deal with the demands of the situation” (Dickerson & Zoccola, 2013, p. 144). On the other hand, however, cortisol can have negative consequences for physiological functioning and health (Dickerson, Gruenewald, & Kemeny, 2009; Lupien et al., 1998; McEwen, 1998). In later adulthood, high levels of cortisol have been shown to predict poor cognitive performance (Lee et al., 2007; Li et al., 2006; Lupien et al., 1998). Moreover, compared to younger adults, older adults elicit stronger cortisol reactivity and show a longer recovery period after challenges (Gotthardt et al., 1995; Neupert et al., 2006; Otte et al., 2005; Seeman & Robins, 1994; Steptoe, Kunz-Ebrecht, Wright, & Feldman 2005). This heightened cortisol response might have detrimental consequences for older adults’ morbidity and mortality (Steptoe & Kivimäki, 2012). Therefore, it is crucial to understand the potential psychosocial factors that determine older adults’ cortisol reactivity to challenges.
One psychosocial factor that has been commonly studied in the context of stress responses is subjective social status (SSS), defined as the self-perceived rank that a person has in the social hierarchy (Abbott et al., 2003; Dickerson & Kemeny, 2004; Sapolsky, 2004). Importantly, SSS differs from objective indicators of social status such as socio-economic status (SES) in that it captures an individual’s perceived standing in the hierarchy rather than his or her actual standing (Anderson, Kraus, Galinsky, & Keltner, 2012; Jackman & Jackman, 1973). There is ample evidence that SSS is a more reliable predictor of health than objective social status indicators such as SES (Adler, Epel, Castellazzo, & Ickovics, 2000; Demakakos, Nazroo, Breeze, & Marmot, 2008; Operario, Adler, & Williams, 2004; Singh-Manoux, Marmot, & Adler, 2005). Moreover, it has been shown that SSS can affect psychological and physiological functioning such as anxiety, stress, and cardiovascular responses above and beyond the effects of SES (Ghaed & Gallo, 2007).
A number of studies show that when individuals feel that they have low SSS, that is, a sense of being inferior relative to others, they are more susceptible to stress and illnesses (Cohen et al., 2008; Derry et al., 2013; Dickerson & Kemeny, 2004). For example, Cohen and colleagues (2008) found that people with low SSS are more likely to contract an infection (i.e., common cold). In addition, an experimental study by Derry and colleagues (2013) showed that people with low SSS exhibited greater physiological (i.e., interleukin-6) and psychological responses (i.e., perceived threat) following a stress test. Moreover, it has been shown that threats to the social self lead to higher post-stressor and recovery cortisol levels (Gruenewald, Kemeny, Aziz, & Fahey, 2004). Finally, Dickerson and Kemeny (2004) concluded on the basis of their meta-analysis that threats to social status consistently resulted in stronger cortisol reactivity as well longer recovery periods.
Older adults may be more prone to feel that they have low SSS due to profound social changes (e.g., transitioning from work to retirement) and the increasing salience of negative age stereotypes. Despite the importance of SSS in old age, there is a surprising scarcity of research looking at how SSS may affect stress reactivity in older adults. One study has examined the effects of SSS on stress markers (i.e., C-reactive protein) in older adults and found no relationship between the two (Demakakos et al., 2008). However, this study used a correlational design and did not look at how SSS might predict stress responses such as cortisol reactivity in response to challenges. Another study has shown that lower SSS is associated with more general stress in older adults as indicated by disrupted cortisol responses to awakening (Wright & Steptoe, 2005). Finally, it has been found that objective social status (i.e., SES) did not affect cortisol reactivity of older adults in response to cognitive challenges (Steptoe, et al., 2005).
We argue that SSS might be a particularly relevant predictor of stress reactivity in older adults, as later adulthood is associated with a loss of social status and cognitive declines (Weiss, Sassenberg, & Freund, 2013). Negative stereotypes about old age that suggest that older adults are incompetent, slow, and inflexible have been shown to impair the performance of older adults on relevant tasks (e.g., Hess, 2006). Thus, challenges that demand cognitive skills might be particularly threatening for older adults. Moreover, when older adults feel socially devalued as indicated by low levels of SSS, they may be even more likely to experience increased stress when engaging in cognitively challenging tasks.
However, we argue that the impact of low SSS on stress reactivity depends on a second factor: older adults’ essentialist beliefs about cognitive aging (EBCA). Specifically, in the current research we look at EBCA as a moderating factor, as research has shown that people’s beliefs influence how they make sense of their experiences and respond to aging-related challenges (Lachman, 2006; Tomaka & Blascovich, 1994; Weiss et al., 2013).
People do not only differ in their perceptions of their social status, but also in the extent to which they view aging as inevitably associated with cognitive decline (e.g., Levy, 2009; Rodin, 1986). These views have been described by Lachman (2000) as “a constellation of beliefs about the perceived change (decline) in abilities, lack of control over the decline, and limited potential for improvement” (p. 107). Based on this theorizing, we define EBCA as views that define aging as an intrinsic and inevitable process associated with cognitive decline. For example, when EBCA are high, people feel that aging-related changes in cognitive functioning are fixed and that there is not much one can do about aging-related decline. In contrast, when EBCA are low, people feel that aging-related changes in cognitive functioning are modifiable and not set in stone. Research shows that fixed beliefs about cognitive functioning predict lower levels of memory performance in later adulthood (Plaks & Chasteen, 2013). Another recent study shows that essentialist beliefs about aging are linked to the perception that aging-related changes are threatening, thereby impacting people’s outlook of their future (Weiss, Job, Mathias, Grah, & Freund, in press).
In the current study, we were particularly interested in exploring the intertwined effects of SSS and EBCA on older adults’ cortisol reactivity in response to cognitive challenge. We propose that the effect of SSS on cortisol reactivity depends on individuals’ beliefs about the nature of aging. We argue that having low SSS may not automatically lead to higher cortisol reactivity. Rather, we suggest that EBCA determine how older adults deal with the potential threat of having low SSS. If older adults with low SSS believe that cognitive abilities inevitably decline with age, they are likely to experience stress because they might feel that they have little control over the situation (cf. Sapolsky, 2004). By contrast, those with low SSS who feel that they nevertheless can influence aging-related cognitive decline should feel less threatened by a cognitive challenge and hence experience less stress. In other words, despite the detrimental effects of low social status, non-essentialist beliefs might serve as a buffer. Accordingly, older adults who reject EBCA feel that they can actively shape their own aging process, and may thus counteract stress responses when confronted with cognitive challenges.
Based on this theorizing, we hypothesized that older adults with low rather than high SSS should exhibit stronger cortisol reactivity when faced with cognitively challenging tasks if they hold strong EBCA. In contrast, we predicted that the effect of low SSS on cortisol reactivity can be buffered for older adults who reject EBCA. To test this hypothesis, we investigated the interplay of SSS and EBCA on cortisol reactivity to cognitively challenging tasks in later adulthood.
Method
Participants and Design
We used data from the National Survey of Midlife Development in the US (MIDUS; Brim, Ryff, & Kessler, 2004). The MIDUS study is a national random digit dial sample of non-institutionalized, English-speaking adults living in the United States. The MIDUS study consists of two waves: MIDUS 1 (1995–96) and MIDUS 2 (2004–2006). Additionally, a subsample of the MIDUS group participated in the Biomarker Project conducted between 2004 and 2009 (see Love, Seeman, Weinstein, & Ryff, 2010). The current study consists of data from MIDUS 2 and the Biomarker Study. We included adults who were 60 years and older (N = 389; M = 70.80, SD = 6.51, age range: 61 to 86 years; 46.4% male) and participated in MIDUS 2 as well as in the Biomarker Study. Specifically, we included those older adults who completed the social-cognitive measures in MIDUS 2 (i.e., SSS and EBCA), the Psychophysiology Protocol of the Biomarker Study, and had saliva cortisol data available.
Subjective social status (SSS)
As part of the MIDUS 2 study, subjective social status was assessed using a status ladder also known as the MacArthur Scale of Subjective Social Status (Adler et al., 2000). Participants were asked to assess their social standing on a ladder ranging from 1 (low status) to 10 (high status). This social status measure is a well-validated measure to assess subjective social status (e.g., Cundiff, Smith, Uchino, & Berg, 2013).
Essentialist beliefs about cognitive aging (EBCA)
We used three items of the Personality in Intellectual Aging Contexts scale (MIDUS 2; Lachman, 1986; Lachman, Baltes, Nesselroade, & Willis, 1982) that assessed beliefs concerning aging-related changes with a focus on general cognitive ability. The three items (“It’s inevitable that my intellectual functioning will decline as I get older”, “The older I get, the harder it is to think clearly”, “My mental capacity (sharpness) is bound to decline”) were averaged to create a scale (Cronbach’s Alpha = .78) with higher values representing higher levels of essentialist beliefs about cognitive aging. The scale was anchored from 1 (strongly agree) to 7 (strongly disagree).
Cognitive challenge tasks
The psychophysiology protocol of the Biomarker Study included cognitive challenge tasks. The cognitive challenge tasks consisted of two 6-min tasks that were each followed by a 6-min recovery period (Love et al., 2010). Specifically, to induce cognitive challenge, participants completed a mental arithmetic task and a Stroop color-word matching task. The order of the two tasks was randomized. The psychophysiology protocol was administered in the morning.
Cortisol reactivity
Saliva samples were collected during the psychophysiology protocol of the Biomarker Study (Love et al., 2010). Saliva cortisol was assessed at four time points (T0 - baseline, T1 – immediately after the second cognitive challenge task, T2 – 14 min after the cognitive challenge tasks, and T3 – 44 min after the cognitive challenge tasks). At the designated time respondents placed the cotton swab of the Salivette® in their mouth and chewed it until saturated. The salivettes were stored in a −80° F freezer. Cortisol is reported in nanomoles per liter (nmol/l) and was log transformed for analyses.
Control variables
We used age, gender, subjective health, level of education, and BMI as control variables, as prior research has revealed that these variables are associated with cortisol responses (Neupert et al., 2006; Otte et al., 2005; Wright & Steptoe, 2005). In addition, we included cognitive functioning as a further covariate as older adults with better functioning might show a decreased stress response to cognitively challenging tasks. Cognitive functioning included episodic memory and executive functioning that were assessed with the Brief Test of Adult Cognition by Telephone (BTACT; Tun & Lachman, 2008). Moreover, research on the diurnal cortisol rhythm indicates that cortisol peaks at 30 minutes after awakening and shows a subsequent decline over the remainder of the day (Almeida, Piazza, & Stawski, 2009). This pattern has also been found in older adults, although there tends to be more variability in this group (Almeida et al., 2009; Ice, Katz-Stein, Himes, & Kayne, 2004). In the current study, cortisol was assessed in the morning (see Love et al., 2010). To control for diurnal cortisol rhythms, we included the time at which the psychophysiology protocol was administered as a covariate in our analyses. In addition, to control for potential effects of a recent meal on cortisol, we also included the number of hours since breakfast as a covariate.
Analytic method
We used multiple regression models to assess the main and interaction effects of SSS and EBCA on cortisol reactivity, including covariates. We used cortisol reactivity as the dependent variable, that is, the deviation from a person’s baseline cortisol in response to a challenge by predicting cortisol at T3 and controlling for baseline cortisol at T0. As the peak concentrations of salivary cortisol occur not before 20–40 minutes after a stressor (Dickerson & Kemeny, 2004), our main analyses focuses on the cortisol measure at T3, that is, 44 min after the cognitive challenge tasks, in order to detect time-lagged responses to cognitive challenge. Cortisol reactivity thus refers to a change in cortisol from baseline to 44 min after the cognitive challenge tasks. In addition to that, we analyzed the area under the curve with respect to increase/decrease (AUCI) including all four cortisol assessments (T0, T1, T2, and T3; see Fekedulegn et al., 2007; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). AUCI is an aggregated index based on repeated measurements of cortisol with reference to the baseline measurement focusing on change over time. Given that cortisol reactivity in our study includes both an increase and decrease after baseline, AUCI should be considered as an index of increase or decrease rather than an area (Pruessner et al., 2003). Before we entered cortisol as the dependent variable in our model, the data were log-transformed to normalize the skewed distribution. SSS and EBCA were entered as continuous predictors.
Results
Table 1 shows the means, standard deviations, and bivariate correlations between all variables. In line with previous research, bivariate correlations revealed higher levels of cortisol across all measurement points for men and higher levels of cortisol for participants who were older and reported lower subjective health (Gruenewald & Seeman, 2010; Otte et al., 2005). Because cortisol levels decline from awakening across the day and after breakfast (e.g., Almeida et al., 2009), session start time and time since breakfast appeared to be negatively associated with cortisol.
Table 1.
Means, Standard Deviations, and Bivariate Correlations for Variables Included in the Study.
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 70.80 | 6.51 | _ | |||||||||||
| 2. Women | 53.6% | - | −.05 | _ | ||||||||||
| 3. Education | 7.39 | 2.57 | .02 | −.10 | _ | |||||||||
| 4. SH | 3.68 | .92 | −.05 | .03 | .16** | _ | ||||||||
| 5. BMI | 29.22 | 5.56 | −.21*** | −.03 | −.06 | −.07 | _ | |||||||
| 6. Cognition | 67.33 | 15.39 | −.26*** | −.03 | .31*** | .21*** | −.004 | _ | ||||||
| 7. TOP | 8.30 | .69 | .005 | .03 | −.02 | −.007 | .05 | −.05 | _ | |||||
| 8. HSM | 1.19 | .70 | .009 | −.008 | −.03 | −.02 | .07 | −.05 | .87*** | _ | ||||
| 9. SSS | 6.96 | 1.52 | .004 | −.09 | .18** | .03 | .05 | .02 | .07 | .04 | _ | |||
| 10. EBCA | 4.15 | 1.48 | .08 | −.05 | −.15** | −.13 | .02 | −.17*** | .04 | .05 | −.15** | _ | ||
| 11. Cor T0 | 2.37 | .58 | .22*** | −.15** | .006 | −.12* | −.07 | −.04 | −.34*** | −.25*** | .06 | .02 | _ | |
| 12. Cor T3 | 2.16 | .57 | .16** | −.28*** | −.06 | −.17** | −.05 | .03 | −.28*** | −.22*** | .01 | .05 | .57*** | _ |
| 13. AUCI | −1.50 | 39.26 | −.15** | −.15** | .01 | .01 | −.01 | .16** | .04 | .01 | −.002 | −.03 | −.43*** | .37*** |
Note. AUCI = area under the curve with regard to increase/decrease;
p < .05,
p < .01,
p < .001
SH = Subjective health, TOP = Time of protocol, HSM = Hours since meal, Cor T0 = Cortisol T0 (log), Cor T3 = Cortisol T3 (log)
To test our hypothesis, we computed a multiple regression model in three steps predicting cortisol assessed at 44 minutes after the cognitive challenge tasks. In each step, we controlled for cortisol levels at baseline (T0). First, no significant main effects of SSS and EBCA appeared. Second, as predicted, the analyses revealed a significant interaction effect of SSS and EBCA (B = −.03; SE = .01, p = .02, see Table 2), F(4, 322) = 5.17, p = .02. Third, the interaction effect remained significant (B = −.02; SE = .01, p = .02, see Table 2) after the inclusion of covariates (chronological age, gender, education, subjective health, BMI, cognitive functioning, session start time, time of last meal).
Table 2.
Multiple Regression Analyses Predicting Cortisol Reactivity
| Cortisol T3 | |||
|---|---|---|---|
|
| |||
| Main Effects | B | SE B | t |
| Cortisol T0 | .54 | .04 | 12.23*** |
| SSS | −.01 | .02 | −.31 |
| EBCA | .01 | .02 | .39 |
| R2 | .32*** | ||
|
| |||
| Main & Interaction Effects | B | SE B | t |
|
| |||
| Cortisol T0 | .54 | .04 | 12.39*** |
| SSS | .09 | .05 | 2.01* |
| EBCA | .18 | .08 | 2.31* |
| SSS* EBCA | −.03 | .01 | −2.27* |
| R2 | .33*** | ||
| ΔR2 | .01* | ||
|
| |||
| Covariates, Main Effects, & Interaction Effects | B | SE B | t |
|
| |||
| Cortisol T0 | .47 | .05 | 9.67*** |
| Age | .001 | .004 | −.08 |
| Gender | −.22 | .05 | −4.44*** |
| Education | −.01 | .01 | −1.33 |
| SH | −.06 | .03 | −2.23* |
| BMI | .002 | .005 | .48 |
| Cognition | .001 | .002 | −.07 |
| TOP | −.05 | .07 | −.73 |
| HSM | −.02 | .07 | −.35 |
| SSS | .09 | .05 | 2.00* |
| EBCA | .17 | .08 | 2.15* |
| SSS* EBCA | −.02 | .01 | −2.28* |
| R2 | .40*** | ||
| ΔR2 | .07*** | ||
Note.
p < .05,
p < .01,
p < .001
SH = Subjective health, TOP = Time of protocol, HSM = Hours since meal
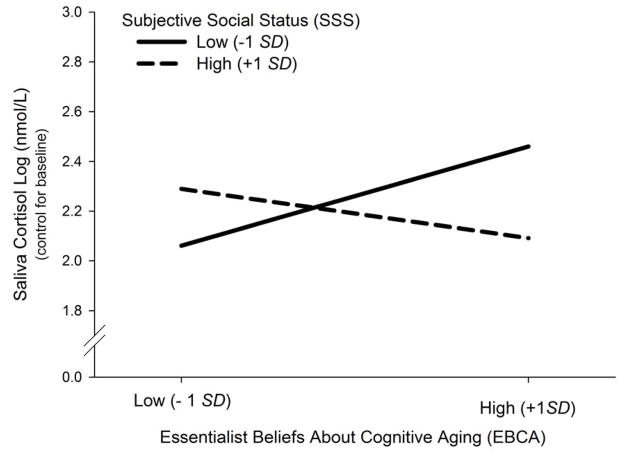
Simple slope analyses confirmed that older adults who perceived themselves as having a low social status exhibited a stronger cortisol reactivity when they endorsed rather than rejected essentialist beliefs about cognitive aging (B = .05, SE = .03; p = .05). In addition, older adults with high EBCA showed a stronger cortisol reactivity when they reported low rather than high SSS (B = −.05, SE = .02; p = .04). Note, that the slope for high SSS is not significantly different under low and high EBCA (B = −.02, SE = .02, p = .23). Figure 1 depicts this interaction effect. Taken together, these analyses reveal that older adults with low SSS (− 1 SD) and high EBCA (+ 1 SD) showed a stronger cortisol reactivity than those with low SSS and low EBCA (− 1 SD).
Figure 1.
The interaction effect of subjective social status (SSS) and essentialist beliefs about cognitive aging (EBCA) on cortisol reactivity to cognitive challenge (controlling for baseline cortisol). Older adults with high EBCA (+ 1 SD) showed a stronger cortisol reactivity when they had low SSS (− 1 SD).
Finally, in order to show that SSS and EBCA affect all repeated measurements of cortisol after baseline with regard to change over time, we computed a multivariate regression model predicting AUCI. As predicted, we found an interaction effect of SSS and EBCA on AUCI as an index of cortisol increase/decrease after baseline (B = −2.00; SE = .98, p = .04), F(1, 298) = 4.19, p = .04 (see Supplementary Material, Table 3).
In summary, these results support our hypothesis that EBCA functions as a moderator in the relationship between SSS and cortisol reactivity to cognitive challenges. Specifically, depending on their degree of SSS and EBCA, older adults showed significantly weaker or stronger stress responses1.
Discussion
The current research demonstrates that the interplay of subjective social status and essentialist beliefs about cognitive aging predicts older adults’ cortisol reactivity to cognitively challenging tasks. In line with our hypothesis, we found an interaction effect of SSS and EBCA on cortisol reactivity: older adults with low SSS who endorsed EBCA exhibited a stronger cortisol reactivity to cognitive challenges as compared to those who rejected an essentialist view of cognitive aging. Specifically, we found that this interaction predicted higher cortisol reactivity 44 minutes after the challenging tasks. Thus, older adults who perceived themselves as having low SSS and thought that their cognitive functions are inevitably bound to decline showed a heightened stress response. In contrast, older adults who perceived themselves as having low SSS and believed that their cognitive functions are still modifiable with advancing age showed significantly lower levels of cortisol reactivity.
We suggest that the current results might extend the social self-preservation theory as outlined by Dickerson and Kemeny (2004). The authors argue that when individuals feel that their social self is threatened, they experience stress. Specifically, threats to social status, respect, and acceptance can activate the HPA system causing the release of cortisol. Against this background, the current results provide additional insight into the processes that moderate this relationship. When older adults feel that they have low social status, they experience stress while performing a cognitive challenge - but only when they also endorse essentialist beliefs about cognitive aging. In other words, only those older adults who perceive themselves as having low social status and feel that their cognitive abilities are non-modifiable and declining with age showed a stronger cortisol reactivity. We see this as an important extension of Dickerson and Kemeny’s model, as having low SSS may not automatically be perceived as a threat to one’s self-concept and lead to greater stress responses in older adults. It is their beliefs about the nature of aging that seem to make a difference. Possibly, older adults who perceive aging-related changes in cognition as malleable and less inevitable may perceive cognitive challenges as less threatening. Because they feel that changes that occur with aging are modifiable, they may be able to counteract stress responses when confronted with challenges.
In this regard, the present findings also contribute to the growing literature demonstrating the influence of aging-related attitudes on older adults’ physiological well-being (Levy, Hausdorff, Hencke, & Wei, 2000). In fact, the current findings shed more light on why some individuals are more susceptible to stress in response to cognitive challenge in later adulthood and the consequences for a variety of health outcomes (Gruenewald & Seeman, 2010; Neupert et al., 2006). For example, elevated cortisol reactivity to cognitive challenge in old age might be one physiological mechanism through which EBCA affects cognitive decline, cardiovascular disease, and Alzheimer’s disease-related pathological changes in the brain (Levy, et al., 2015; Lupien, McEwen, Gunnar, & Helm, 2009).
Stereotype embodiment theory suggests that age stereotypes can be internalized and, thus, often operate on an implicit level affecting older adults’ self-concept, physical functioning, and health (Levy, 2009). A body of research provides empirical evidence for the short- and long-term effects of age stereotypes (e.g., Levy & Leifheit-Limson, 2009; Levy et al., 2015). Against this background, it might be likely that for older adults who feel that they have a lower social status and endorse EBCA, negative age stereotypes might be particularly harmful. Given that these older adults feel that they occupy a lower rank in the social hierarchy and feel that their aging-related decline is inevitable, they might be particular vulnerable to adopt a negative age-stereotypical self-image. Specifically, EBCA imply that changes in cognitive functioning are irreversible which may lead to increased stereotypical thinking and a greater internalization of age stereotypes. Thus, EBCA may perpetuate negative age stereotypes, as these beliefs construe losses as inevitable and, thus, diminish ways to mitigate the impact of negative age stereotypes (Weiss et al., 2013).
On the basis of the findings, it seems reasonable to assume that stronger EBCA may undermine older adults’ “ability to make active responses during the occurrence of an aversive stimulus” (Rodin, 1983; p. 157). Thus, in the face of aging-related challenges, older adults with low SSS and strong EBCA may view aging-related declines in cognitive functioning as uncontrollable and, therefore, suffer from stress. As older adults may be particularly prone to develop illnesses when they are chronically exposed to elevated cortisol levels, interventions that aim at addressing and changing essentialist beliefs about aging could help to promote the health of older populations.
The current findings are in line with previous research on psychosocial factors and stress which show that cortisol responses can be influenced by people’s control beliefs, optimism, or self-esteem (Dickerson and Kemeny, 2004; Rodin, 1983; Taylor et al., 2008; Wrosch, Miller, & Schulz, 2009). However, the current study shows that different social-cognitive factors often work in concert: Older adults with low SSS who believe that cognitive abilities inevitably decline with age experience more stress than their low EBCA counterparts when confronted with challenging tasks.
The present study shows that SSS and EBCA contribute to the experience of stress. However, the pathways by which beliefs about cognitive aging and SSS may increase the vulnerability to stress remain unclear. For example, EBCA might be associated with the adoption of maladaptive coping behaviors and, thus, with the repeated activation and dysregulation of the HPA axis (McEwen, 1998). Specifically, low social status has been shown to be associated with poorer mental and physical health (e.g., Cohen et al., 2008; Derry et al., 2013; Singh-Manoux, Marmot, & Adler, 2003). Thus, EBCA may exacerbate the effects of low SSS by stimulating a stronger stress reactivity, because older adults who believe that aging related changes are irreversible may feel that they lack the psychological resources to cope with aging-related challenges. This perceived lack of resources might make them more prone to adopt maladaptive strategies (e.g., unhealthy behaviors such as drinking or smoking) to cope with challenges (Hobfoll, 1989). In contrast, older adults with non-EBCA might be able to mitigate the negative consequences of low SSS and mobilize psychological resources and hence feel less threatened by cognitive challenges.
This study has some limitations. The first is related to the fact that we focused on acute stress reactivity. Our results revealed an interaction effect of SSS and EBCA not only on cortisol reactivity at T3 but also on AUCI – an index of cortisol change after baseline including all measurements of cortisol. However, given that the T1 and T2 cortisol measurement were assessed shortly after the cognitive challenge tasks, the interpretability might be limited because at that time stress reactivity is hardly detectable (Dickerson & Kemeny, 2004). Thus, the predicted changes in AUCI might be driven by change from baseline to T3 rather than before. To determine whether individuals experience (mal)adaptive stress responses, it is important to examine longitudinal patterns of cortisol release. Specifically, examining prolonged cortisol responses is important, as research has shown that longer cortisol releases are particularly harmful for health (Roy, Steptoe, & Kirschbaum, 1998; Sapolsky, 2004). Thus, when analyzing stress responses, it is not only important to consider stress reactivity but also look at the trajectory of recovery, because failure to return to baseline levels after a stressful event can lead to chronically elevated cortisol levels. In the current study, cortisol responses were only assessed about 44 min after the cognitively challenging tasks. Future research needs to examine long-term effects of the interplay of status and essentialist beliefs on older adults’ responses to challenge. For example, future studies may include multiple assessments of cortisol (i.e., 60 min or longer after a stressor) in order to capture the recovery process (see Dickerson & Kemeny, 2004). Another limitation of the current study is the selective nature of our longitudinal subsample of the initial MIDUS national sample. A final limitation is the conceptualization and assessment of SSS and EBCA as individual difference variables. Future research clearly needs to experimentally induce high vs. low SSS and activate EBCA to confirm the causal role of these variables.
In terms of practical implications, we argue that the results may help to guide interventions and health programs to improve individuals’ health and adaptive capacity for dealing with aging-related challenges in daily life. For example, possible interventions could target older adults’ essentialist beliefs about cognitive aging by demonstrating the potential for positive cognitive plasticity in all ages across the life span. In this regard, it might also be worthwhile to examine the extent to which essentialist beliefs can be modified by promoting novel positive experiences in the context of cognitive challenges.
To summarize, the present research demonstrates that the effect of older adults’ SSS on their stress response to cognitive challenges depends on their essentialist beliefs about cognitive aging. Specifically, low SSS led to a stronger stress response as indicated by higher cortisol reactivity when older adults believed that aging-related cognitive decline is inevitable as opposed to modifiable. These findings underscore the idea that gaining more insight into the social-cognitive antecedents of stress in later adulthood is crucial for identifying the conditions of healthy aging. Identifying the psychological mechanisms that buffer stress responses of older adults to aging-related challenging tasks can enlarge our understanding of the protective factors that help to maintain physiological functioning and psychological well-being in later adulthood.
Supplementary Material
Acknowledgments
The MIDUS II study was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation. The MIDUS study was further supported by the following grants M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program and UL1TR000427 (UW) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health.
Footnotes
After each challenge task, participants were asked for a stress rating on the scale from 1 to 10 (1 = ‘not stressed at all’ to 10 = ‘extremely stressed’). We found no significant main or interaction effects of SSS and EBCA on self-reported stress.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43:67–82. doi: 10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19:586–592. doi: 10.1037/0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychology and Aging. 2009;24:819–827. doi: 10.1037/a0017910. doi: http://dx.doi.org/10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Kraus MW, Galinsky AD, Keltner D. The local-ladder effect: social status and subjective well-being. Psychological Science. 2012;23:764–71. doi: 10.1177/0956797611434537. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. How healthy are we?: A national study of well-being at midlife. University of Chicago Press; 2004. [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychology. 2008;27:268–74. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- Cundiff JM, Smith TW, Uchino BN, Berg CA. Subjective social status: construct validity and associations with psychosocial vulnerability and self-rated health. International Journal of Behavioral Medicine. 2013;20:148–158. doi: 10.1007/s12529-011-9206-1. [DOI] [PubMed] [Google Scholar]
- Demakakos P, Nazroo J, Breeze E, Marmot M. Socioeconomic status and health: the role of subjective social status. Social Science & Medicine. 2008;67:330–340. doi: 10.1016/j.socscimed.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–85. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Psychobiological responses to social self threat: Functional or detrimental? Self and Identity. 2009;8:270–285. doi: 10.1080/15298860802505186. [DOI] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Zoccola PM. Cortisol responses to social exclusion. In: DeWall CN, editor. The Oxford Handbook of Social Exclusion. New York: Oxford University Press; 2013. pp. 143–151. [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Ghaed SG, Gallo LC. Subjective social status, objective socioeconomic status, and cardiovascular risk in women. Health Psychology. 2007;26:668–674. doi: 10.1037/0278-6133.26.6.668. doi: http://dx.doi.org/10.1037/0278-6133.26.6.668. [DOI] [PubMed] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. The American Journal of Physiology. 1995;268:R865–873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66:915–24. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE. Stress and aging: A biological double jeopardy? Annual Review of Gerontology and Geriatrics. 2010;30:155–177. doi: 10.1891/0198-8794.30.155. [DOI] [Google Scholar]
- Hess TM. Attitudes toward aging and their effects on behavior. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 6. San Diego: Academic Press; 2006. pp. 379–406. [Google Scholar]
- Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. American Psychologist. 1989;44:513–524. doi: 10.1037//0003-066x.44.3.513. doi: http://dx.doi.org/10.1037/0003-066X.44.3.513. [DOI] [PubMed] [Google Scholar]
- Ice GH, Katz-Stein A, Himes J, Kane RL. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology. 2004;29:355–370. doi: 10.1016/S0306-4530(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Jackman MR, Jackman RW. An interpretation of the relation between objective and subjective social status. American Sociological Review. 1973:569–582. http://www.jstor.org/stable/2094408. [PubMed]
- Lachman ME. Locus of control in aging research: A case for multidimensional and domain-specific assessment. Psychology and Aging. 1986;1:34–40. doi: 10.1037/0882-7974.1.1.34. [DOI] [PubMed] [Google Scholar]
- Lachman ME. Promoting a sense of control over memory aging. In: Hill RD, Bäckman L, Stigsdotter-Neely A, editors. Cognitive rehabilitation in old age. New York: Oxford University Press; 2000. pp. 106–120. [Google Scholar]
- Lachman ME. Perceived Control Over Aging-Related Declines: Adaptive Beliefs and Behaviors. Current Directions in Psychological Science. 2006;15:282–286. doi: 10.1111/j.1467-8721.2006.00453.x. [DOI] [Google Scholar]
- Lachman ME, Baltes P, Nesselroade JR, Willis SL. Examination of personality-ability relationships in the elderly: The role of the contextual (interface) assessment mode. Journal of Research in Personality. 1982;16:485–501. doi: 10.1016/0092-6566(82)90007-1. [DOI] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry. 2007;64:810–8. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Levy B. Stereotype Embodiment: A psychosocial approach to aging. Current Directions in Psychological Science. 2009;18:332–336. doi: 10.1111/j.1467-8721.2009.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Ferrucci L, Zonderman AB, Slade MD, Troncoso J, Resnick SM. A Culture–Brain Link: Negative Age Stereotypes Predict Alzheimer’s Disease Biomarkers. Psychology and Aging. 2015 Dec 7; doi: 10.1037/pag0000062. Advance online publication. http://dx.doi.org/10.1037/pag0000062. [DOI] [PMC free article] [PubMed]
- Levy BR, Hausdorff JM, Hencke R, Wei JY. Reducing Cardiovascular Stress With Positive Self-Stereotypes of Aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2000;55:P205–P213. doi: 10.1093/geronb/55.4.P205. [DOI] [PubMed] [Google Scholar]
- Levy BR, Leifheit-Limson E. The stereotype-matching effect: Greater influence on functioning when age stereotypes correspond to outcomes. Psychology and Aging. 2009;24:230–233. doi: 10.1037/a0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, … Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiology of Aging. 2006;27:1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–80. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, De Leon M, De Santi S, Convit A, Tarshish C, Thakur M, Mcewen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Miller LMS, Lachman ME. Physiological reactivity to cognitive stressors: variations by age and socioeconomic status. International Journal of Aging and Human Development. 2006;62:221–235. doi: 10.2190/17DU-21AA-5HUK-7UFG. [DOI] [PubMed] [Google Scholar]
- Operario D, Adler NE, Williams DR. Subjective social status: Reliability and predictive utility for global health. Psychology & Health. 2004;19:237–246. doi: http://dx.doi.org/10.1080/08870440310001638098. [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Plaks JE, Chasteen AL. Entity versus incremental theories predict older adults’ memory performance. Psychology and Aging. 2013;28:948–957. doi: 10.1037/a0034348. doi: http://dx.doi.org/10.1037/a0034348. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rodin J. Aging and health: effects of the sense of control. Science. 1986;233:1271–1276. doi: 10.1126/science.3749877. [DOI] [PubMed] [Google Scholar]
- Rodin J. Behavioral medicine: beneficial effects of self control training in aging. Applied Psychology. 1983;32:153–181. doi: 10.1111/j.1464-0597.1983.tb00901.x. [DOI] [Google Scholar]
- Roy MP, Steptoe A, Kirschbaum C. Life events and social support as moderators of individual differences in cardiovascular and cortisol reactivity. Journal of Personality and Social Psychology. 1998;75:1273–81. doi: 10.1037/0022-3514.75.5.1273. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Social Status and Health in Humans and Other Animals. Annual Review of Anthropology. 2004;33:393–418. doi: 10.1146/annurev.anthro.33.070203.144000. [DOI] [Google Scholar]
- Seeman TE, Robbins RJ. Aging and Hypothalamic-Pituitary-Adrenal Response to Challenge in Humans. Endocrine reviews. 1994;15:233–260. doi: 10.1210/edrv-15-2-233. doi: http://dx.doi.org/10.1210/edrv-15-2-233#sthash.GBX9aUPm.dpuf. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Social science & medicine. 2003;56:1321–1333. doi: 10.1016/S0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. doi: http://dx.doi.org/10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht SR, Wright C, Feldman PJ. Socioeconomic position and cardiovascular and neuroendocrine responses following cognitive challenge in old age. Biological Psychology. 2005;69:149–166. doi: 10.1016/j.biopsycho.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J. Effects of justice beliefs on cognitive appraisal of and subjective physiological, and behavioral responses to potential stress. Journal of Personality and Social Psychology. 1994;67:732–740. doi: 10.1037/0022-3514.67.4.732. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Developmental Psychology. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Job V, Mathias M, Grah S, Freund AM. The end is (not) near: Aging, essentialism, and future time perspective. Developmental Psychology. doi: 10.1037/dev0000115. in press. [DOI] [PubMed] [Google Scholar]
- Weiss D, Sassenberg K, Freund AM. When feeling different pays off: how older adults can counteract negative age-related information. Psychology and Aging. 2013;28:1140–6. doi: 10.1037/a0033811. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–90. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Schulz R. Cortisol secretion and functional disabilities in old age: importance of using adaptive control strategies. Psychosomatic Medicine. 2009;71:996–1003. doi: 10.1097/PSY.0b013e3181ba6cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.