Abstract
Background
The effectiveness of preemptive treatment (PET) for CMV in recipients of ex vivo T-cell depleted (TCD) hematopoietic cell transplantation (HCT) by CD34+ selection is not well defined.
Methods
We analyzed 213 adults who received TCD-HCT at our institution from June 2010 through May 2014. Patients were monitored by a CMV quantitative PCR assay if recipient (R) or donor (D) were CMV seropositive.
Results
CMV viremia occurred early (median 27 days post HCT) in 91 of 213 (42.7%) patients for a180-day cumulative incidence of 84.5%, 61.8%, and 0 for R+/D+, R+/D−, R−/D+ patients, respectively. CMV disease occurred in 5% of patients. In Cox regression analysis, R+/D+ status was associated with increased risk for CMV viremia compared to R+/D− (hazard ratio, HR: 1.79, 95% confidence interval, CI: 1.16–2.76, P=0.01) while matched unrelated donor allograft was associated with decreased risk (HR: 0.62, 95% CI: 0.39–0.97, P=0.04). Of 91 patients with CMV viremia, 52 (57%) had persistent viremia (>28 days duration). Time lag from detection of CMV viremia to PET was associated with incremental risk for persistent viremia (HR: 1.09, 95%CI: 1.01–1.18; P=0.03). Overall, 166 of 213 (77.9%) patients were alive one-year post-HCT with no difference between patients with and without CMV viremia or among the different CMV serostatus pairs (P=NS).
Conclusions
CMV viremia occurred in 70% of R+ TCD-HCT. Delay in PET initiation was associated with persistent viremia. With PET, CMV R/D serostatus did not adversely impact survival in TCD-HCT on one-year survival in the present cohort.
Keywords: Cytomegalovirus, CMV viremia, epidemiology, hematopoietic cell transplant, T-cell depletion
Introduction
Cytomegalovirus (CMV) infection, a common complication of allogeneic hematopoietic cell transplantation (HCT), is associated with substantial morbidity and mortality. The CMV serostatus of donor (D) and recipients (R) is a major risk determinant of post-transplant CMV infection, with recipient seropositivity (R+) conferring the greatest risk1. Although CMV R−/D− HCTs confer the lowest risk for CMV infection and associated complications post-HCT, the impact of other CMV serostatus R/D combinations on clinical outcomes remains controversial1. A large number of other variables, including conditioning regimens, graft source and manipulation, or R/D matching may hinder our ability to fully understand the effect of R/D CMV serostatus on CMV infection and survival post-HCT 1–5. The impact of CMV sero-concordance is even less clear in the setting of ex-vivo T-cell depletion (TCD).
T-cell depletion by CD34+ selection effectively reduces rates of graft-versus-host-disease (GvHD), but is also associated with delayed immune recovery and higher rates of CMV infection compared to unmodified HCT 6–10. Previously, we have reported an incidence of 58% for CMV antigenemia among R+ HCT recipients who received TCD allografts using the Isolex system in conjunction with sheep red blood cell rosetting7. Since 2010, we have used the CliniMACS® CD34 Cell Reagent system (Miltenyi Biotec, Gladbach, Germany) for TCD, which achieves a 5-log10 reduction of T-cells in the allograft directly compared to 3–5-log10 with the Isolex system.
We performed a retrospective observational singe-institution cohort study to describe the incidence, timing, risk factors and outcomes of CMV viremia after TCD-HCT by the recently approved by the United States Food and Drug Administration (US-FDA) method for CD34+ selection.
Methods
The study was reviewed by the Memorial Sloan Kettering (MSK) Institutional Review Board (IRB) and was granted a waiver of authorization (WA0020-15). A total of 228 consecutive adults with acute leukemia, chronic myeloid leukemia, myelodysplastic syndrome, and myeloproliferative disease who underwent their first TCD-HCT at MSK between June 9, 2010 and May 31, 2014 were included in the study. Patients were followed until death, second HCT or July 1, 2015, whichever occurred first. Data was extracted from medical records and hospital and research databases.
Graft manipulation and conditioning regimens
Ex vivo TCD was performed by the US-FDA approved CliniMACS® CD34+ Reagent System (Miltenyi, Biotec, Gladbach, Germany). Patients received TCD grafts after conditioning with one of the following preparative regimens: busulfan/melphalan/fludarabine, total body irradiation/thio-TEPA/cyclophosphamide, and clofarabine/thio-TEPA/melphalan.
Supportive care
Antibacterial and antifungal prophylaxis (including prophylaxis for Pneumocystis jiroveci) were administered as per institutional guidelines and as previously described 11, 12. All patients received acyclovir 250 mg/m2 intravenously (IV) every 8 hours or 400mg orally twice daily if they were able to take oral medications. Patients were given acyclovir for minimum of one year from the day of admission for transplant13 until a documented response to revaccination with killed vaccines. Immunization series with killed vaccines was commenced at 1 year if patients met the following immunologic milestones: CD4 T cells >200 cells/μL, CD19 B cells > 50 cells/μL, IgG > 500 mg/dl, measured at > 8 weeks after last dose of IVIG and > 6 months after last dose of Rituximab. A protective antibody response was required for at least diphtheria, tetanus and inactivated polio vaccines. Standard commercial assays were used to assess protective immunity. Tetanus antitoxin assay was performed by Quest diagnostics and value > 0.15 IU/ml was considered as protective. Diphtheria immune status and serum neutralizing antibodies for polio virus types I-III were performed by Focus Diagnostics. A value ≥ 0.01 IU/mL was considered protective for diphtheria and a titer ≥ 1:8 was considered protective for polio virus types I,II and III. Patients who demonstrated protective immunity to tetanus, diphtheria and polio vaccines were checked for immunity to varicella zoster virus by Vitek Immunodiagnostic Assay System. Varicella IgG ≥ 0.90 was considered as protective. Acyclovir was discontinued if patients had protective immunity to varicella. Patients lacking protective immunity to varicella were immunized with the varicella vaccine and continued acyclovir prophylaxis until protective immunity to varicella was documented14.
CMV management
Patients with CMV serostatus of R+/D+, R+/D− and R−/D+ were considered at risk for CMV and were monitored by CMV quantitative PCR (qPCR) at least weekly from day +14 until day +100, every 2 weeks until day +180 post-HCT and thereafter as clinically indicated. CMV R−/D− patients were not routinely monitored for CMV; however CMV qPCR was ordered as clinically indicated and at the discretion of the treating physician. Preemptive therapy (PET) was initiated at the discretion of the treating physician as per institutional guidelines. Briefly, CMV treatment was considered for CMV-viral load (VL) ≥1,000 copies/mL in whole blood or ≥300 IU/mL in plasma or with even lower quantifiable CMV-VL provided a rising trend from baseline CMV-VL was observed on consecutive measurements. Induction CMV treatment doses were administered for 2–3 weeks or until CMV-VL was <1,000 copies/mL in whole blood or < 300 IU/mL in plasma on ≥2 consecutive measurements (whichever was longer), followed by maintenance doses for at least 4–6 weeks. CMV resistance testing was at the discretion of the treating physician. Induction and maintenance doses for IV ganciclovir, valganciclovir and foscarnet were administered and dose adjusted for renal impairment as per package insert recommendations. Valganciclovir (or ganciclovir) was the preferred first line therapy. Foscarnet was used preferentially in patients with cytopenias (particularly prior to engraftment) or other contraindications to ganciclovir.
Laboratory methods
CMV IgG levels were determined using an automated semi-quantitative enzyme-linked fluorescent immunoassay (VIDAS, Biomerieux Inc., NC, USA). Values > 6 IU/mL were considered positive. Prior to March 17, 2013, CMV qPCR on whole blood sample was performed by using the Roche Molecular Diagnostics Assay (Roche Diagnostics, IN, USA), with a quantitation range of 500–1,000,000 copies/mL. Since March 18, 2013, CMV-VL was determined in plasma samples using the Cobas Ampliprep/Cobas Taqman CMV assay (Roche Molecular Diagnostics, NJ, USA), with a quantitation range of 137–9,100,000 IU/mL 15. Genotypic resistance testing was performed by nucleotide sequence analysis of the UL97 and UL54 genes (Viracor-IBT Laboratories, Lee’s Summit, Mo,USA).
Definitions
CMV viremia was defined as ≥1 CMV qPCR >500 copies/ml for whole blood or >137 IU/mL for plasma. Persistent CMV viremia was defined as consecutively positive CMV qPCR for >28 days despite appropriate CMV treatment. Maximum CMV-VL was defined as the highest CMV qPCR value by day +180 post-HCT or infusion of CMV specific T- cells, whichever occurred first. CMV disease was diagnosed using standard definitions 16. GvHD diagnosis and grading were based on consensus guidelines 17.
Statistical analysis
The incidence for CMV viremia and disease was estimated by cumulative incidence analysis, with second transplant, relapse, death, and last follow-up before the event of interest treated as competing risks. Categorical and continuous variables were compared using the chi-square and Mann-Whitney rank-sum tests, respectively. Risk factor analysis was performed in the population at risk for CMV to define risk factors associated with CMV viremia, persistent viremia and overall survival. Demographics, HCT-characteristics, R/D CMV serostatus and time from CMV viremia to initiation of antiviral treatment were examined as independent variables, in univariate and multivariate analyses. Acute GVHD (aGVHD) diagnosed prior to the event (CMV reactivation and persistent CMV viremia) was included as an independent predictor. In the model for overall survival, aGVHD development during the first 100 days post-SCT was used as an independent variable. Hazards ratio (HR) and 95% confidence intervals (CI) were obtained using time-dependent Cox proportional hazard regression models. Forward stepwise selection was used to identify variables to be included in the multivariate models based on statistical significance (P< 0.3 to enter and P<0.1 to stay in the final model). Overall survival, which was defined as the time from HCT until death from any cause or last follow-up, was estimated using the Kaplan-Meier method. Log-rank test was used in time-to-event analyses. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). All reported P values are two-sided. A P value < 0.05 was considered statistically significant.
Results
Of 228 patients, 15 patients were excluded from the analyses because they received CMV-active antivirals for indications other than CMV (N=8) or were enrolled in randomized studies of CMV prevention (N=7). Thus, the study cohort consists of 213 TCD-HCT recipients, including 147 patients at risk for CMV (68 R+/D+, 55 R+/D−, 24 R−/D+) and 66 R−/D− patients. Table 1 shows the baseline characteristics of the cohort.
Table 1.
Baseline patient characteristics of 213 hematopoietic cell transplant (HCT) recipients of T-cell depleted allografts.
| Characteristic | Entire cohort N (%) Total N=213 |
R+/D+ N (%) Total N=68 |
R+/D− N (%) Total N=55 |
R−/D+ N (%) Total N=24 |
R−/D− N (%) Total N=66 |
|---|---|---|---|---|---|
| Age | |||||
| Median (range), years | 57.0 (19.6–73.0) | 59.7 (22.5–73.0) | 53.7 (19.6–70.8) | 57.3 (25.3–72.1) | 57.7 (25.8–70.5) |
| Gender | |||||
| Female | 91 (43) | 32 (47) | 25 (45) | 8 (33) | 26 (39) |
| Male | 122 (57) | 36 (53) | 30 (55) | 16 (67) | 40 (61) |
| Ethnicity | |||||
| Caucasian | 186 (88) | 50 (74) | 50 (90) | 22 (92) | 64 (97) |
| African American | 9 (4) | 5 (7) | 1 (2) | 1 (4) | 2 (3) |
| Asian | 11 (5) | 9 (13) | 1 (2) | 1 (4) | 0 |
| Other/Unknown | 7 (3) | 4 (6) | 3 (6) | 0 | 0 |
| Underlying disease | |||||
| AML | 105 (49) | 32 (47) | 33 (60) | 10 (42) | 30 (45) |
| ALL | 24 (11) | 9 (13) | 6 (11) | 2 (8) | 7 (11) |
| CML | 11 (5) | 5 (7) | 3 (5) | 1 (4) | 2 (3) |
| MDS | 61 (29) | 16 (24) | 12 (22) | 10 (42) | 23 (35) |
| MPFD | 12 (6) | 6 (9) | 1 (2) | 1 (4) | 4 (6) |
| Conditioning regimen1 | |||||
| Bu/Flu/Mel | 142 (66) | 44 (65) | 31 (56) | 16 (67) | 51 (77) |
| TBI/Thio-TEPA/CYT | 62 (29) | 20 (29) | 22 (40) | 6 (25) | 14 (21) |
| Clo /Thio-TEPA/Mel | 9 (5) | 3 (4) | 2 (4) | 2 (8) | 1 (2) |
| Donor type | |||||
| Matched Related | 76 (36) | 26 (38) | 22 (40) | 5 (21) | 23 (35) |
| Matched Unrelated | 94 (44) | 31 (46) | 24 (44) | 10 (42) | 29 (44) |
| Mismatched2 | 43 (20) | 11 (16) | 9 (16) | 9 (37) | 14 (21) |
R: Recipient, D: Donor, CMV: Cytomegalovirus, AML: Acute Myeloid Leukemia, ALL: Acute Lymphoblastic Leukemia, CML: Chronic Myeloid Leukemia, MDS: Myelodysplastic Syndrome, MPFD: Myeloproliferative Disorder.
Bu/Flu/Mel: Busulfan/Fludarabine/Melphalan, TBI/Thio-TEPA/CYT: TBI/Thio-TEPA/Cyclophosphamide, Clo/Thio-TEPA/Mel: Clofarabine/Thio-TEPA/Melphalan
Mismatched related or unrelated HCT recipients.
CMV viremia monitoring, incidence and timing
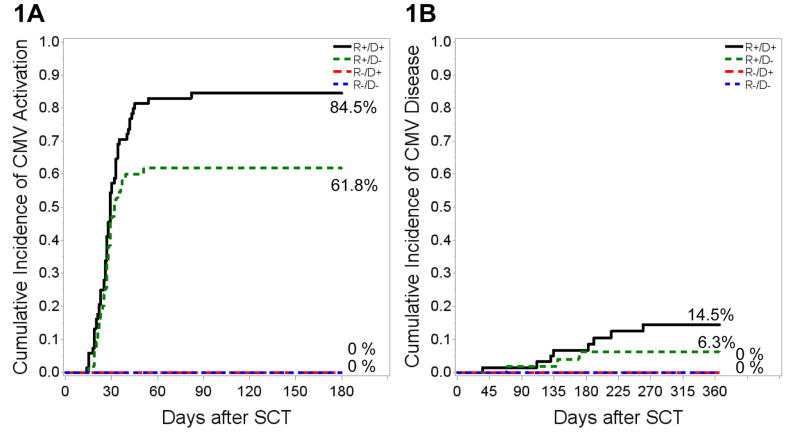
A total of 4,750 CMV-PCR tests were performed for 213 patients during the study period. The median number of tests was similar for R+/D+, R+/D− and R−/D+ (28, interquartile range, IQR: 23–36, 25, IQR: 18–32, and 21, IQR: 18–26, respectively). In addition, 748 tests were performed in CMV R−/D− patients (median10, IQR: 4–17). Ninety-one of 213 (42.7%) patients developed CMV viremia. The cumulative incidence of CMV viremia by day +180 was 84.5% for R+/D+ and 61.8% for R+/D− patients (P<0.05) (Figure 1A); none of the R−/D+ and R−/D− patients developed CMV viremia. CMV viremia occurred at a median of 27 days post-HCT (range 14–82; IQR: 22–33), with similar onset between R+/D+ (median: 28 days; range: 14–82; IQR: 23–33) and R+/D− patients (median: 27 days; range: 15–51; IQR: 22–30; P=0.58). The maximum CMV-VL was similar between R+/D+ and R+/D− HCT recipients (P=0.37) with a median CMV-VL of 3.9 (log10) copies/mL (range: 2.9–6.1).
Figure 1. Cumulative Incidence of CMV viremia (A) and CMV disease (B) post-HCT for CMV R+/D+, R+/D−, R−/D+ and R−/D− transplant recipients.
(A) The 180-day incidence of CMV viremia is shown by CMV R/D serostatus. R+/D+ patients had higher 180-day incidence compared to R+/D− (P=0.02); none of the R−/D+ and R−/D− patients developed CMV viremia. (B) The 365-day cumulative incidence of CMV disease is shown by CMV R/D serostatus. One-year post-HCT incidence of CMV disease was similar for R+/D+ and R+/D− (P=0.19). CMV: Cytomegalovirus, HCT: Hematopoietic cell transplant, R: Recipient, D: Donor, +/−: Positive/Negative CMV serostatus.
Risk factors for CMV viremia
Among 147 patients at risk, 91 (61.9%) developed CMV viremia. Patient and transplant characteristics were examined in univariate and multivariate models to identify risk factors for CMV viremia (Table 2). In multivariate analyses, R+/D+ status was associated with higher risk for CMV viremia (HR: 1.79, 95% CI: 1.16–2.76; P=0.01). In contrast, matched unrelated donor allograft was associated with lower risk for CMV viremia (HR: 0.62, 85% CI: 0.39–0.97, P=0.04). Asian ethnicity was a risk factor in univariate analysis (HR: 2.26, 95% CI: 1.16–4.42; P=0.02), while further examination showed that Asian ethnicity contained more matched related donors and R+.
Table 2.
Risk factors for CMV viremia among 147 patients at risk for CMV1.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age per year | 1.01 (0.99–1.02) | 0.39 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 0.92 (0.61–1.39) | 0.70 | ||
| Ethnicity | 0.09 | |||
| White | Reference | |||
| African American | 1.72 (0.75–3.98) | 0.20 | ||
| Asian | 2.26 (1.16–4.42) | 0.02 | ||
| Unknown | 1.76 (0.76–4.06) | 0.18 | ||
| CMV serostatus | ||||
| R+/D− | Reference | Reference | ||
| R+/D+ | 1.63 (1.06–2.49) | 0.03 | 1.79 (1.16–2.76) | 0.01 |
| R−/D+ | NA | NA | ||
| Underlying disease | ||||
| Acute leukemia | Reference | |||
| CML | 0.77 (0.33–1.79) | 0.55 | ||
| MDS | 0.76 (0.47–1.23) | 0.27 | ||
| MPFD | 1.13 (0.49–2.62) | 0.78 | ||
| Donor type | ||||
| Matched Related | Reference | Reference | ||
| Matched Unrelated | 0.62 (0.40–0.98) | 0.04 | 0.62 (0.39–0.97) | 0.04 |
| Mismatched2 | 0.70 (0.39–1.25) | 0.22 | 1.21 (0.68–2.18) | 0.52 |
| Acute GvHD | ||||
| Grade<2 | Reference | |||
| Grade≥2 | 1.25 (0.39–3.95) | 0.71 | ||
HR: Hazard Ratio, CI: Confidence Interval, CMV: Cytomegalovirus, NA: Not Applicable, R: Recipient, D: Donor, (+): CMV seropositive, (−): CMV seronegative, CML: Chronic Myeloid Leukemia, MDS: Myelodysplastic Syndrome, MPFD: Myeloproliferative Disorder, GvHD: Graft versus Host Disease.
147 patients at risk for CMV included 68 R+/D+, 55 R+/D−, and 24 R−/D+ transplant recipients.
Mismatched related or unrelated HCT recipients.
CMV treatment
Among 88 of 91 (96.7%) patients with CMV viremia who received PET, the initial antiviral was ganciclovir/valganciclovir in 73 (83%), foscarnet in 13 (14.7%), and cidofovir in 2 (2.3%) patients. Three patients had transient low grade CMV viremia with spontaneous resolution and were not treated. During treatment, 13 of 73 (17.8%) patients who initially received ganciclovir/valganciclovir were changed to foscarnet and 4 of 13 (30.8%) patients who initially received foscarnet were changed to valganciclovir due to count recovery, treatment-associated toxicity, or lack of virologic response. Excluding 2 patients treated with cidofovir, induction treatment was administered to 79 of 86 (92%) patients: 67/79 (85%) patients were started on induction and 12/79 (15%) were initially started on maintenance dosing and eventually escalated to induction dose due to rising CMV-VL (8 and 4 patients were escalated to induction within 2 weeks or 4 weeks of starting maintenance treatment, respectively). Induction treatment duration for 67 patients who were started on induction doses was at a median of 22 (IQR: 13–31) days. The duration of induction treatment for 12 patients who were initially started on maintenance dosing and eventually escalated to induction dose was at a median of 19 (IQR: 7–23) days. Overall, 15 of 88 (17%) patients required dose modifications for treatment-induced toxicities and/or intolerance to standard doses.
Sixteen (18.2%) patients received investigational agents as second (N=8) or third-line (N=8) therapy for persistent CMV viremia and/or intolerance to primary treatment: 10 patients received brincidofovir (ClinicalTrials.gov, NCT01143181) and 6 patients received maribavir (ClinicalTrials.gov, NCT01611974). In addition, 13 patients received donor or third party-derived CMV-specific cytotoxic T-cell lines through MSK-IRB approved protocols (ClinicalTrials.gov, NCT01646645 and NCT02136797).
Persistent CMV viremia
Of 91 viremic patients, 52 (42%) had persistent viremia. The patients with persistent viremia demonstrated higher maximum CMV-VL (median log10: 4.1copies/mL; range: 3.3–6.1; IQR: 3.8–4.7) compared to those with non-persistent viremia (median log10: 3.6 copies/mL; range: 2.9–4.3; IQR: 3.4–3.9; P<0.0001). In multivariate analyses, longer interval from quantifiable CMV-VL to treatment initiation (HR: 1.09, 95% CI: 1.01–1.18; P<0.05) and mismatched donor allograft (HR: 2.26, 95% CI: 1.09–4.69; P<0.05) were significant predictors of persistent CMV viremia among preemptively treated CMV viremic patients (Table 3). Myelodysplastic syndrome was associated with a reduced risk for persistent viremia (HR: 0.37, 95% CI: 0.19–0.73; P<0.01).
Table 3.
Risk factors for persistent CMV viremia among 88 with CMV viremia who received preemptive treatment for CMV reactivation.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, per year | 0.99 (0.97–1.01) | 0.31 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 1.17 (0.67–2.02) | 0.59 | ||
| Ethnicity | ||||
| Caucasian | Reference | |||
| African American | 0.88 (0.31–2.50) | 0.94 | ||
| Asian | 1.07 (0.45–2.55) | 0.84 | ||
| Unknown | 1.50 (0.58–3.84) | |||
| CMV donor serostatus | ||||
| R+/D− | Reference | |||
| R+/D+ | 0.98 (0.55–1.74) | 0.94 | ||
| Underlying disease | 0.02 | |||
| Acute leukemia | Reference | Reference | ||
| CML | NA | NA | NA | NA |
| MDS | 0.38 (0.20–0.75) | 0.005 | 0.37 (0.19–0.73) | 0.004 |
| MPFD | 0.32 (0.08–1.33) | 0.12 | 0.27 (0.06–1.18) | 0.081 |
| Donor type | ||||
| Matched Related | Reference | Reference | ||
| Matched Unrelated | 0.98 (0.52–1.85) | 0.96 | 1.04 (0.54–1.99) | 0.92 |
| Mismatched1 | 1.92 (0.95–3.88) | 0.07 | 2.26 (1.09–4.69) | 0.03 |
| CMV treatment onset2 | 1.04 (0.97–1.10) | 0.26 | 1.09 (1.01–1.18) | 0.03 |
| Acute GvHD | ||||
| Grade<2 | Reference | |||
| Grade≥2 | 1.67 (0.66–4.24) | 0.28 | ||
HR: Hazard Ratio, CI: Confidence Interval, CMV: Cytomegalovirus, NA: Not Applicable, CML: Chronic Myeloid Leukemia, MDS: Myelodysplastic Syndrome, MPFD: Myeloproliferative Disorder, GvHD: Graft versus Host Disease.
Mismatched related or unrelated HCT recipients.
Calculated as days from first positive CMV PCR to treatment initiation.
CMV disease
Among 91 patients with CMV viremia, 11 (12.1%) developed CMV disease at a median of 141 days (range 35–259, IQR: 111–191) post-HCT. Rates of CMV disease were similar between R+/D+ (14.5%) and R+/D− (6.3%) HCT recipients (P=0.19; Figure 1B). CMV disease involved the gastrointestinal (GI) tract (N=5), retina (N=3), and lungs (N=3); 2 patients had disseminated disease. Persistent viremia preceded CMV disease in 10 (91%) patients: 10/52 (19%) patients with persistent viremia developed CMV disease compared to 1/39 (3%) patients with non-persistent viremia (P=0.02). Among 10 patients with CMV disease tested for CMV resistance, 6 (60%) were found to harbor known CMV resistance associated mutations.
Survival
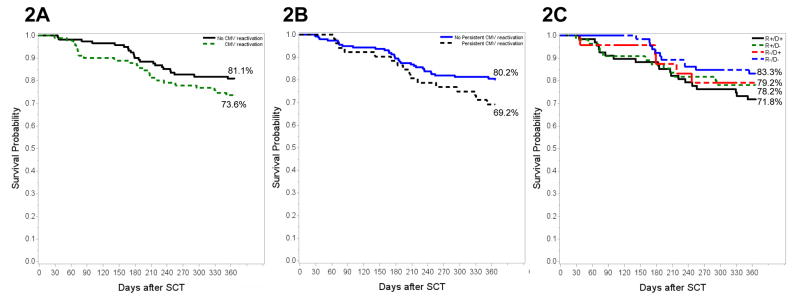
A total of 166 patients (77.9% of 213) were alive one-year post-HCT. No significant difference in overall survival was found between patients with (67/91, 73.6%) and without CMV viremia (99/122, 81.1%; P=0.18) (Figure 2A). Similarly, no significant difference was found in overall survival between patients with persistent CMV viremia (69.2 %, 36/52) versus non persistent viremia (80.2%, 130/161; P=0.11) (Figure 2B). Overall survival was 71.8% (49/68), 78.2% (43/55), 79.2% (19/24) and 83.3% (55/66) for R+/D+, R+/D−, R−/D+ and R−/D− patients, respectively (Figure 2C). In multivariate analysis, ≥2 grade aGvHD was the only significant mortality predictor (HR: 2.77, 95% CI: 1.17–6.56; P=0.02). When controlled for variables listed in Table 4, CMV disease was associated with increased mortality but did not reach statistical significance (HR: 2.34, 95%CI: 0.88–6.25; P=0.09).
Figure 2. Kaplan-Meier analysis of overall survival (OS) at 365 days for the entire cohort (N=213): (A) patients with CMV viremia versus no viremia, (B) CMV persistent viremia versus no persistent viremia and (C) CMV recipient (R)/Donor (D) serostatus.
All 213 patients were included in each survival analysis. 365-day OS was compared by the log rank test between (A) patients with (solid line) versus without CMV viremia (dotted line) (B) Persistent viremia (solid line) versus non persistent viremia (dotted line) and (C) By CMV R/D serostatus. Differences of OS between different groups were not statistically significant.
Table 4.
Mortality predictors among all transplant recipients included in the cohort (n=213).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, per year | 1.00 (0.98–1.03) | 0.74 | ||
| Gender | ||||
| Female | 1 | |||
| Male | 0.92 (0.52–1.63) | 0.77 | ||
| CMV donor serostatus | 0.46 | |||
| R−/D− | 1 | |||
| R−/D+ | 1.30 (0.45–3.75) | 0.62 | ||
| R+/D− | 1.39 (0.61–3.16) | 0.43 | ||
| R+/D+ | 1.82 (0.87–3.83) | 0.11 | ||
| Ethnicity | 0.21 | |||
| Caucasian | 1 | |||
| African American | 2.35 (0.84–6.58) | 0.10 | ||
| Asian | 0.87 (0.21–3.59) | 0.84 | ||
| Unknown | 2.41 (0.74–7.82) | 0.14 | ||
| Caucasian | ||||
| Underlying disease | 0.46 | |||
| Acute leukemia | 1 | |||
| CML | 0.32 (0.04–2.33) | 0.26 | ||
| MDS | 0.85 (0.44–1.61) | 0.61 | ||
| MPFD | 0.31 (0.04–2.28) | 0.25 | ||
| Donor type | 0.03 | |||
| Matched Related | 1 | |||
| Matched Unrelated | 0.88 (0.44–1.79) | 0.73 | ||
| Mismatched1 | 2.12 (1.05–4.28) | 0.04 | ||
| CMV reactivation | 0.57 | |||
| No reactivation | 1 | |||
| Activation, not-persistent | 0.94 (0.35–2.51) | 0.91 | ||
| Activation, persistent | 1.41 (0.60–3.29) | 0.43 | ||
| CMV disease | 0.09 | |||
| No | 1 | 1 | ||
| Yes | 2.22 (0.88–5.61) | 0.09 | 2.34 (0.88–6.25) | 0.09 |
| Acute GvHD | 0.01 | |||
| Grade<2 | 1 | 1 | ||
| Grade≥2 | 2.44 (1.21–4.91) | 0.01 | 2.77 (1.17–6.56) | 0.02 |
HR: Hazard Ratio, CI: Confidence Interval, CMV: Cytomegalovirus, R: Recipient, D: Donor, (+): CMV seropositive, (−): CMV seronegative, CML: Chronic Myeloid Leukemia, MDS: Myelodysplastic Syndrome, MPFD: Myeloproliferative Disorder, GvHD: Graft versus Host Disease.
Mismatched related or unrelated HCT recipients.
Discussion
We describe the epidemiology and outcomes of CMV infection in adult HCT recipients of CD34+ selected allografts. This is, to our knowledge, the largest cohort of TCD-HCT recipients using the currently US-FDA approved system for CD34+ selection and contemporary molecular assays for CMV monitoring. CMV reactivation was exclusively observed among R+ patients and during the first 3 months post-HCT and was associated with CMV R/D serostatus and grafts from matched unrelated donors. CMV infection did not impact one year overall survival.
Cumulative incidence of CMV viremia was reached at 82 days post-HCT, with 75% of the CMV viremic patients developing viremia within 30 days post-HCT. The first CMV PCR test of 210 (99%) patients was negative. This supports that starting monitoring 14 days post-HCT was adequate to detect the onset of CMV viremia. Consistent with prior reports in recipients of TCD-HCT using different methods for TCD, we observed that almost two-thirds of R+ HCT recipients developed CMV viremia post-HCT 7, 18–20. In contrast, none of the R- patients had CMV infection. Among R+ patients, CMV viremia was significantly more frequent among R+/D+ when compared to R+/D− patients. Furthermore, R+/D+ was identified as an independent predictor of CMV reactivation. A similar trend for higher rates of CMV viremia among R+/D+ compared to R+/D− was observed in a prior cohort of TCD-HCT recipients from our institution monitored by CMV antigenemia21.
The impact of donor CMV serostatus on post-HCT CMV viremia has been analyzed in registry studies, mainly of unmodified HCT1–5. In unmodified HCT, D+ has been associated with higher rates of CMV reactivation than D- among R+ patients, albeit with lower CMV-VL and shorter duration of CMV viremia22. Higher rates of reactivation among R+/D+ patients are hypothesized to be due to a small contribution of infection from D+ allografts. A recent study in recipients of reduced-intensity conditioning HCT with grafts post-in-vivo TCD with alemtuzumab showed that R+/D− patients had fewer CMV related events and higher levels of recipient chimerism than R+/D+ recipients. Furthermore, CMV specific T-cells in R+/D− recipients were exclusively of recipient origin 23. In our cohort, we also observed that R+/D+ patients had significantly higher CMV reactivation, numerically higher CMV persistent viremia, and worse one-year overall survival outcomes than R+/D− patients. Latently CMV infected monocytes and macrophages present in unmodified D+ allografts are the major reservoir for CMV transmission. Pergam et al reported a correlation between total nucleated cell count in the allograft and CMV transmission from D+ to R− recipients24. However, in our cohort none of the R- patients, including those that received grafts from D+ , developed CMV infection, suggesting that infection may not be associated with D+ grafts in the CD34+ selection setting. While CD34+ cells are known to harbor latent CMV, in very low frequencies they do not support lytic infection in the absence of myeloid differentiation25, 26. We postulate that CD34+ selection may result in stringent depletion of latently infected donor cells from D+ allografts.
The associations between R+/D+ serostatus with Asian ethnicity and matched related grafts may contribute to the importance of D+ on post-HCT CMV reactivation observed in our cohort. Consistent with prior reports of higher rates of CMV infection among Asian patients post-HCT27, 28, Asian ethnicity was the strongest independent predictor for CMV reactivation in univariate analyses. This potential association between Asian ethnicity and R+/D+ status may partially explain why R+/D+ patients had higher CMV viremia rate. Similarly, matched related donor grafts were more common among patients of Asian ethnicity while matched unrelated donor grafts were more common among Caucasian patients. This may explain why TCD-HCT recipients of matched unrelated grafts had lower CMV viremia rate. Allograft from a mismatched or unrelated donor has been associated with higher rates of CMV viremia previously19 and was associated with persistent viremia in our cohort. These observations should be studied in larger cohorts of more ethnically diverse HCT recipients.
One-year overall survival did not significantly differ between patients with and without CMV viremia, between patients with and without persistent CMV viremia, or among the four R/D CMV serostatus pairs. While the mortality of patients with CMV disease was higher compared to that of patients without CMV disease, the difference was not significant. These findings suggest that CMV viremia did not confer a survival disadvantage at 1-year in the present cohort of TCD-HCT recipients monitored by sensitive molecular assays and early initiation of PET. Our results have to be interpreted with caution as longer follow-up may be necessary to discern potential differences in survival among the different groups.
The intensive CMV-VL monitoring on all high-risk patients even bi-weekly in the early peri-transplant period followed by early PET initiation appeared to be an effective approach in preventing CMV disease in most cases. Notably, CMV disease was a relatively late event, predominately seen in patients with persistent CMV viremia. Importantly, a longer time interval between viremia onset and PET initiation was an independent predictor for persistent viremia. On average, the risk for persistent viremia increased by 9% for each day of delayed antiviral treatment. The above further supports the importance of prompt initiation of appropriately dosed CMV treatment. Our data also demonstrate that persistent CMV replication in the presence of currently available CMV antivirals is quite common; occurring in approximately 40% of TCD-HCT recipients with CMV viremia. The need for prolonged pharmacologic suppression of CMV viremia poses a major hurdle in the management of TCD-HCT recipients and currently addressed in ongoing studies of vaccination or adoptive T-cell therapy to restore immunity to CMV in this patient population29.
Our study has several limitations, inherent to its observational nature and sample size. First, due to the heterogeneity in the management of CMV infection, we could not perform detailed analyses to evaluate the impact of initial choice of antiviral, dosing regimen and duration nor changes in type of antivirals including investigational agents or dosing changes that occurred during treatment. The immunomodulatory effects of persistent CMV viremia and its potential contribution to a potentiated state of immunosuppression were beyond the scope of our study, but these are important issues to consider when choosing an optimal strategy.
In summary, CMV R+ recipients of TCD-HCT allografts had frequent, early, and prolonged CMV reactivation. Early and frequent surveillance in conjunction with aggressive PET was effective in preventing CMV disease in TCD-HCT. Additional controlled studies focusing specifically on TCD-HCT recipients are required to elucidate the importance of D+ on CMV infection post-TCD-HCT.
Highlights.
CMV viremia occurs common and early among R+ TCD-HCT recipients.
Persistent viral replication despite antiviral treatment highlights the need for newer effective and safe CMV therapies.
CMV R/D serostatus did not have a significant impact in 1-year overall survival.
Acknowledgments
This research was funded in part through the National Cancer Institute at the National Institutes of Health Cancer Center Support Grant P30 CA008748.
Footnotes
Financial Disclosure Statement
D. N. has received research grants from Pfizer, has served as a consultant or advisor r for Roche and Astellas; and is currently employed by Roche. M. P. has served as an advisory board member for Astellas and Merck. G. P. has received research grants and or served as advisor for Astellas Chimerix, Merck, and Viropharma . All other authors report no potential conflicts.
Authorship contributions
Y.-T. H., D.N., and G.A. P. designed research; Y.-T. H., J.F., S.J.K., M.M., and D.C. collected the data; Y.-T. H. performed analyses; Y.-T. H., D.N., and G.A. P. prepared the figures/tables and wrote the manuscript with input from M.-A. P., H. C.-M., S.A.G., E.P., A.A. J.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 2.Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: Evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102:4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59:473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 6.Kroger N, Zabelina T, Kruger W, et al. Patient cytomegalovirus seropositivity with or without reactivation is the most important prognostic factor for survival and treatment-related mortality in stem cell transplantation from unrelated donors using pretransplant in vivo T-cell depletion with anti-thymocyte globulin. British journal of haematology. 2001;113:1060–1071. doi: 10.1046/j.1365-2141.2001.02849.x. [DOI] [PubMed] [Google Scholar]
- 7.Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2007;9:286–294. doi: 10.1111/j.1399-3062.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 8.Martino R, Rovira M, Carreras E, et al. Severe infections after allogeneic peripheral blood stem cell transplantation: a matched-pair comparison of unmanipulated and CD34+ cell-selected transplantation. Haematologica. 2001;86:1075–1086. [PubMed] [Google Scholar]
- 9.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.van Burik JA, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo SK, Xiao K, Huang YT, et al. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: a 12-year single institution study. The Journal of infection. 2014;69:341–351. doi: 10.1016/j.jinf.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow 16 transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forlenza CJ, Small TN. Live (vaccines) from New York. Bone marrow transplantation. 2013;48:749– 754. doi: 10.1038/bmt.2012.141. [DOI] [PubMed] [Google Scholar]
- 15.Babady NE, Cheng C, Cumberbatch E, Stiles J, Papanicolaou G, Tang YW. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. Journal of clinical microbiology. 2015;53:1252–1257. doi: 10.1128/JCM.03435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transplant Infectious Disease. 2010;12:322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematology/oncology clinics of North America. 2011;25:151–169. doi: 10.1016/j.hoc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lilleri D, Gerna G, Fornara C, et al. Human cytomegalovirus-specific T cell reconstitution in young patients receiving T cell-depleted, allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2009;199:829–836. doi: 10.1086/597123. [DOI] [PubMed] [Google Scholar]
- 21.Xiao K, Jongwutiwes U, Chung D, Jakubowski AA, Papanicolaou G. Incidence and Kinetics of CMV Infection After T-Cell Depleted and Unmodified Allogeneic Hematopoietic Stem Cell Transplantation: A 10-Year Experience at Memorial Sloan-Kettering Cancer Center. Biol Blood Marrow Tr. 2013;19:S128–S128. [Google Scholar]
- 22.Pietersma FL, van Dorp S, Minnema MC, et al. Influence of donor cytomegalovirus (CMV) status on severity of viral reactivation after allogeneic stem cell transplantation in CMV-seropositive recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:e144–148. doi: 10.1093/cid/cir002. [DOI] [PubMed] [Google Scholar]
- 23.Sellar RS, Vargas FA, Henry JY, et al. CMV promotes recipient T-cell immunity following reducedintensity T-cell-depleted HSCT, significantly modulating chimerism status. Blood. 2015;125:731–739. doi: 10.1182/blood-2014-07-589150. [DOI] [PubMed] [Google Scholar]
- 24.Pergam SA, Xie H, Sandhu R, et al. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1391–1400. doi: 10.1016/j.bbmt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeves MB, Sinclair JH. Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. Journal of General Virology. 2010;91:599–604. doi: 10.1099/vir.0.015602-0. [DOI] [PubMed] [Google Scholar]
- 26.Saffert RT, Penkert RR, Kalejta RF. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. Journal of virology. 2010;84:5594–5604. doi: 10.1128/JVI.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asano-Mori Y, Oshima K, Sakata-Yanagimoto M, et al. High-grade cytomegalovirus antigenemia after hematopoietic stem cell transplantation. Bone marrow transplantation. 2005;36:813–819. doi: 10.1038/sj.bmt.1705134. [DOI] [PubMed] [Google Scholar]
- 28.Han XY. Epidemiologic analysis of reactivated cytomegalovirus antigenemia in patients with cancer. Journal of clinical microbiology. 2007;45:1126–1132. doi: 10.1128/JCM.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeckh M, Murphy WJ, Peggs KS. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21:24–29. doi: 10.1016/j.bbmt.2014.11.002. [DOI] [PubMed] [Google Scholar]