Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) can induce long term remissions in chemosensitive relapsed follicular lymphoma (FL). The BMT CTN conducted a multicenter phase 2 trial to examine the efficacy of alloHCT using reduced intensity conditioning (RIC) with rituximab (RTX) in multiply relapsed, chemosensitive FL. The primary endpoint was 2 year progression-free survival (PFS). The conditioning regimen consisted of fludarabine, cyclophosphamide and high-dose RTX (FCR) in which 3 of the 4 doses of RTX were administered at a dose of 1 gm/m2. Graft vs host disease (GvHD) prophylaxis was with tacrolimus and methotrexate. Sixty five patients were enrolled and 62 were evaluable. Median age was 55 years (range, 29-74). This group was heavily pre-treated: 77% had received ≥ 3 prior regimens, 32% had received ≥ 5 prior regimens and 11% had received prior autologous HCT. Donors were HLA-matched siblings (n=33) or HLA-matched unrelated adults (n=29). No graft failures occurred. The overall response rate after HCT was 94% with 90% in complete remission (CR), including 24 patients not in CR before alloHCT. With a median follow-up of 47 months (range, 30-73), 3 year PFS and overall survival rates were 71% (95% confidence interval: 58%-81%) and 82% (70%-90%) respectively. Three year cumulative incidences of relapse/progression and non-relapse mortality were 13% and 16%, respectively. Two year cumulative incidences of grade 2-4 and grade 3-4 acute GvHD were 27% and 10%, respectively and extensive chronic GvHD incidence was 55%. Serum RTX concentrations peaked at day +28 and remained detectable as late as 1 year in 59% of patients with available data. In conclusion, alloHCT with FCR conditioning confers high CR rates, a low incidence of relapse/progression and excellent survival probabilities in heavily pretreated FL patients.
INTRODUCTION
Follicular lymphoma (FL) affects approximately 15,000 patients per year in the United States. The median age at diagnosis is 60 years with incidence increasing with age1. When treatment is indicated, RTX-based regimens are the standard of care and many achieve remission 2-4. The disease course is typically indolent and the median survival is 14-15 years from diagnosis. However, FL is incurable with standard therapy. Patients with poor risk features such as short remission duration after initial chemotherapy (<2-3 years) have a dramatically shorter expected survival 4. High dose chemotherapy with autologous HCT can confer long term remissions, especially if a patient is not heavily pretreated prior to HCT 5-7. However, allogeneic hematopoietic stem cell transplantation (alloHCT) is the only known curative modality for FL.
Earlier studies offering alloHCT with a myeloablative conditioning regimen demonstrated a significantly lower risk of relapse compared with autologous HCT but high treatment-related mortality (TRM) mitigated the benefit of this approach 8, 9. AlloHCT using a reduced intensity conditioning (RIC) regimen became widely utilized in FL patients. RIC affords a moderate degree of cytoreduction but also relies on potent immune-mediated graft-vs-lymphoma effects to eradicate minimal residual disease. Results from several retrospective and prospective RIC alloHCT trials have yielded event-free survivals ranging from 43%-75% and all demonstrate plateaus in relapse/progression5, 6, 10-15.
Previously, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a prospective “biologic assignment” trial in which chemosensitive FL patients beyond first response underwent either autologous HCT (autoHCT) or RIC alloHCT16. Patients with an available matched sibling donor were allocated to alloHCT and those without to autoHCT followed by maintenance therapy with RTX. Unfortunately, the trial closed prematurely due to slow accrual with only 30 patients enrolled; 22 patients underwent autoHCT and 8 patients underwent alloHCT. With a median follow-up of 36 months, the PFS and OS for the autoHCT recipients were 63% and 73%, respectively and 86% vs 100% in the allogeneic group. Although the sample size was small from this study, results were encouraging as only 3 relapses were seen in the autologous arm vs 1 relapse in the allogeneic arm. The preparative regimen for alloHCT in that trial was modeled after the FCR (fludarabine, cyclophosphamide, RTX) conditioning regimen introduced by investigators at the M.D. Anderson Cancer Center. Using this regimen, the most encouraging results to date were reported in this single institution trial in 2012. The 11 year event-free and overall survival rates in 47 patients were 72% and 78%, respectively, with only 3 relapses reported.17. Based on this experience, the BMT CTN embarked on a prospective phase 2 trial in the multicenter setting utilizing the same FCR regimen.
METHODS
Patients
Individuals 75 years of age and younger with histologically confirmed (grade I or II REAL or WHO grades 1, 2, or 3a FL in first or subsequent partial remission (PR) or second or subsequent complete remission (CR) were eligible for enrollment 18-20. If not in CR, response criteria compared to the most recent regimen required either 1) stable disease if all lymph node masses were ≤ 3 cm and unchanged (or smaller); or 2) chemotherapy sensitivity defined as reduction of all lymph node masses to ≤ 3 cm in axial diameter or, if larger than 3 cm, a minimum of 50% reduction in estimated nodal diameters. Patients with documented evidence of transformation were excluded. There was no restriction on number of lines of prior therapy except that patients could not have received prior alloHCT. Prior autologous HCT was permitted.
Other eligibility criteria included adequate organ function defined as a cardiac ejection fraction of 45% or greater; total bilirubin less than twice the upper limit of normal (ULN); aspartate (AST) and alanine (ALT) serum transaminases less than thrice ULN; a creatinine clearance of at least 40mL/min; and diffusion capacity of carbon monoxide, forced expiratory volume in 1 minute, and forced vital capacity all more than 50% of normal after adjustment for hemoglobin. Patients were seronegative for human immunodeficiency virus (HIV) and could not have evidence of active hepatitis B (HBV) or hepatitis C (HCV) by serology and/or viremia. Patients with uncontrolled infections, defined as progressing on appropriate antimicrobial therapy, were ineligible. Patients with prior malignancy expect resected basal cell carcinoma or treated cervical carcinoma in situ or those women pregnant or breastfeeding were excluded.
Donor eligibility included either 1) fully matched (6/6 allele) related donors based on high resolution typing at HLA-DRβ1 and intermediate resolution typing at HLA-A and –B typing; or 2) fully matched (8/8 allele) unrelated donors based on high resolution typing at HLA-A, -B, -C, and -DRβ1. Donors met medical eligibility of the transplant center (related) or National Marrow Donor Program (NMDP). Related donors were excluded if they had evidence of infection with HIV, HBV, HCV or had prior malignancy other than treated basal cell or carcinoma in situ of the cervix. Identical twins were not permitted.
Study Design and Treatment
Design
This multi-center, single-arm, phase II trial was designed to confirm the efficacy of a previously published RIC regimen followed by alloHCT 17. The protocol and informed consents were approved by the Protocol Review Committee and Data and Safety Monitoring Board, each independently appointed by the National Heart Lung and Blood Institute (NHLBI) and the Institutional Review Boards of all participating institutions. The study was led by the NHLBI Blood and Marrow Transplant Clinical Trials Network in collaboration with the Eastern Cooperative Oncology Group and the Southwest Oncology Group. All patients signed informed consents in accordance with the Declaration of Helsinki. The study is registered at http://www.clinicaltrials.gov as NCT00912223.
Conditioning
All patients received pre-transplant conditioning with RTX (Genentech, San Francisco, USA) at 375mg/m2 on day −13 and at 1000mg/m2 on day −6, day +1, and day +8. Patients received fludarabine intravenously (IV) at 30mg/m2/day and cyclophosphamide IV 750mg/m2/day on day −5, −4, and −3. Peripheral blood progenitor cells (PBPC) were infused on day 0. All donor cells were mobilized with G-CSF per institutional or NMDP guidelines to obtain a minimum PBPC graft of 2.0 × 106 CD34+cells/kg with a goal collection of at least 5.0 × 106 CD34+ cells/kg. If less than 1.0 × 106 CD34+cells/kg were collected after three leukaphereses, the patient was managed at the discretion of the treating physician.
Graft-versus-Host Disease (GVHD) Prophylaxis
Oral tacrolimus (target trough 5 – 15 ng/mL) and IV methotrexate 5 mg/m2/day on days +1, +3, and +6 were administered for all patients receiving a related donor transplant. Unrelated donor (URD) recipients received an additional dose of methotrexate of 5 mg/m2 on day +11. No patients received anti-thymocyte globulin. Tacrolimus taper was to start at day +180 in the absence of GVHD and the rate of taper was determined by the treating physician.
Supportive Care
All patients received antimicrobial prophylaxis and blood product support in concordance with the BMT CTN Manual of Procedures. Use of hematopoietic growth factors following HCT and immunizations were administered per institutional guidelines.
Follow-up and Disease Response
Disease response was assessed based on standard criteria 20. Within 4 weeks prior to the initiation of the conditioning regimen, all patients had disease staging including a bone marrow biopsy and aspirate, quantitative polymerase chain reaction (PCR) for assessment of t(14;18) in the peripheral blood, and imaging. Imaging included computed tomography (CT) of the chest, abdomen, and pelvis and a CT of the neck if prior evidence of neck disease. 18F-FDG positron emission tomography (PET) was recommended only if patients had known FDG-avid disease previously. All patients were restaged at 3 months, 12 months, and 24 months following HCT. Restaging included the same studies as prior to HCT with the exception that bone marrow biopsy was only required if patients had prior marrow involvement and peripheral blood quantitative PCR was only necessary in those patients with documented prior t(14;18).
Additional assessments following HCT included immune reconstitution studies by flow cytometry and quantitative immunoglobulin concentrations at 3 months, 6 months, and 1 year; peripheral blood chimerism analysis at 4 weeks, 8 weeks, 3 months, 6 months, and 1 year. Serum RTX concentrations were obtained in all patients prior to HCT as well as 4 weeks, 3 months, 6 months, and 1 year after HCT. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 at 4 weeks, 8 weeks, 3 months, 6 months, 1 year and 2 years after HCT. GVHD was monitored and assessed weekly from day 0 through 14 weeks post-HCT and then at 6 months, 1 year, and 2 years following transplantation. Acute GVHD and chronic GVHD were scored using consensus criteria 21, 22
Quality of Life Measures
Health Related Quality of Life was measured utilizing the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT –BMT) self- report, transplant specific questionnaire and the Short Form-36 (SF-36), a patient reported survey of health status. The FACT-BMT survey consists of questions linked to four subscales: physical well-being, social/family well-being, emotional well-being and functional well-being. The FACT-BMT Total is the grand total of all items in the FACT-G and BMT modules and was used as the outcome measure in summarizing the FACT-BMT data23. Standardized effect sizes were computed for each subscale and for the total by comparing the 2-year measurement to the baseline. The SF-36 assessed the health QOL with eight components: physical functioning, role limitations-physical, body pain, general health, vitality, social functioning, role limitations-emotional and mental health24. These domains can be aggregated into the physical component summary and the mental component summary as weight sums of the domain scores. All assessments were conducted prior to the initiation of conditioning therapy and at 2 years post-HCT. The questionnaires were scored according to standard procedures.
Statistical Analysis
All data were reviewed by an Endpoint Review Committee consisting of physicians on the protocol team or from the highest accruing centers. All study data for primary and secondary endpoints were reviewed and adjudicated in a blinded manner. The data cut-off was February 15, 2016. Using the historical 2-year PFS of 55% following autologous HCT, the study required enrollment of 65 patients to provide 80% power, with a two-sided α of 0.05, to reject the null hypothesis if the true PFS following the intervention was ≤73%.
The primary endpoint of the trial was 2-year PFS. Patients were considered to meet the primary endpoint if they died, experienced relapse/progression or received any therapy not specified in the protocol to prevent or treat relapse/progression, to induce a tumor response, or for mixed chimerism (i.e. donor lymphocyte infusion). The probability of PFS and overall survival (OS) were estimated by the Kaplan-Meier product limit estimator and the confidence interval calculated 25. The cumulative incidence of progression/relapse, acute GVHD, chronic GVHD and treatment related mortality (TRM) were determined using a competing risks analysis with death (for GVHD) or relapse/progression (for TRM) as the competing risk 26. Descriptive statistics were utilized to assess frequency and proportions of patients obtaining CR/PR, primary and secondary graft failure, toxicities, infections, and immunologic reconstitution. Neutrophil and platelet recovery were assessed using the cumulative incidence function with death as a competing risk. Neutrophil recovery was determined as the first of 3 consecutive days with an absolute neutrophil count (ANC) >500/μL. Platelet recovery was determined as the first of 3 consecutive days with platelet count >20,000/μL with no platelet transfusions in the prior 7 days. Primary graft failure was defined as donor peripheral blood T-cell chimerism <5% at day +30 after HCT. Secondary graft failure was defined as documented donor T-cell chimerism followed by loss of graft defined as <5% donor T-cells in a peripheral blood chimerism analysis. Correlation of RTX concentrations with development of acute GVHD, chronic GVHD and relapse were analyzed using a Cox regression model and time dependent covariates to assess the effect of RTX concentrations. Association between RTX concentrations and immune recovery were explored using correlation analysis. The statistical analyses were performed with SAS software, version 9.3 (SAS Institute). The cumulative incidence analyses were performed with the use of R software, version 2.15.1.
Results
Patient Characteristics
Sixty-five patients from 21 transplant centers were enrolled and transplanted between April 2009 and November 2012. Three patients were deemed not evaluable due to consent withdrawal (n=1), not transplanted (n = 1), and not eligible due to transformed disease (n = 1). Therefore, 62 patients were included in the primary analysis. Table 1 shows the characteristics of the patients and donors. Thirty-three (53%) received grafts from related and 29 (47%) from unrelated donors. The median age of all patients at the time of transplant was 55 (range, 29 – 74) years. The median time from diagnosis to alloHCT was 4.8 years (range, 1.0 - 23.8 years). All patients had pathologically confirmed follicular lymphoma without evidence of transformation [REAL 1, n = 8; REAL II, n = 6; WHO 1, n = 13; WHO 2, n = 23; WHO 3a, n = 12] with most patients having WHO 2 or WHO 3a disease. The patients were generally heavily pre-treated with 32 (52%) receiving 4 or more prior treatment regimens for lymphoma and only 6 (10%) patients in a first partial remission; 7 (11%) patients had previously received high dose chemotherapy and autologous HCT. Thirty-five (57%) recipients were CMV seropositive. The median follow-up of survivors was 47 (range, 30 – 73) months.
Table 1.
Baseline characteristics of evaluable patients at the time of transplantation
| Variable | Frequency (%) |
|---|---|
| Gender, male | 39 (63%) |
| Ethnicity | |
| Not Hispanic/Latino | 58 (64%) |
| Hispanic | 4 (6%) |
| Karnofsky performance status | |
| 100% | 29 (47%) |
| 90% | 28 (45%) |
| 80% | 5 (8%) |
| Comorbidity Index Score | |
| 0 | 31(50%) |
| 1-2 | 16 (26%) |
| > 3 | 15 (24%) |
| Disease status | |
| Second or subsequent CR2 | 32 (52%) |
| PR 1 | 6 (10%) |
| Greater than/equal to PR2 | 16 (26%) |
| Stable disease | 2 (1%) |
| Number of Prior Chemotherapy Regimens | |
| 2 | 14 (23%) |
| 3 | 16 (26%) |
| 4 | 12 (19%) |
| ≥ 5 | 20 (32%) |
| Donor Type (HLA match) | |
| Matched Related (6/6 allele) | 33 (53%) |
| Matched Unrelated (8/8 allele) | 29 (47%) |
Engraftment and Chimerism
Early neutrophil recovery was seen in all patients with a median time to neutrophil engraftment of 13 (range, 8 - 48) days and a day 30 cumulative incidence of neutrophil engraftment of 98% (94 – 100%). Three patients did not drop their ANC below 500/mm3. Grade 3 – 4 neutropenia occurred both early and late, with 21% of patients experiencing late neutropenia between 6 months and 1 year post-transplant, although no severe neutropenia beyond 1 year was reported and neutropenia completely resolved in all patients by 2 years post-HCT. By day 30, the median donor peripheral T-cell chimerism was 94% (range, 25 - 100) and improved to 96% (range, 25 - 100) by day 100. This was similar for patients receiving related [day 30: 88% (20 – 100); day 100: 95% (25 – 100)] and unrelated [day 30: 95% (44 – 100); day 100: 96 (69 – 100)] donor grafts. No patients experienced primary or secondary graft failure.
Survival
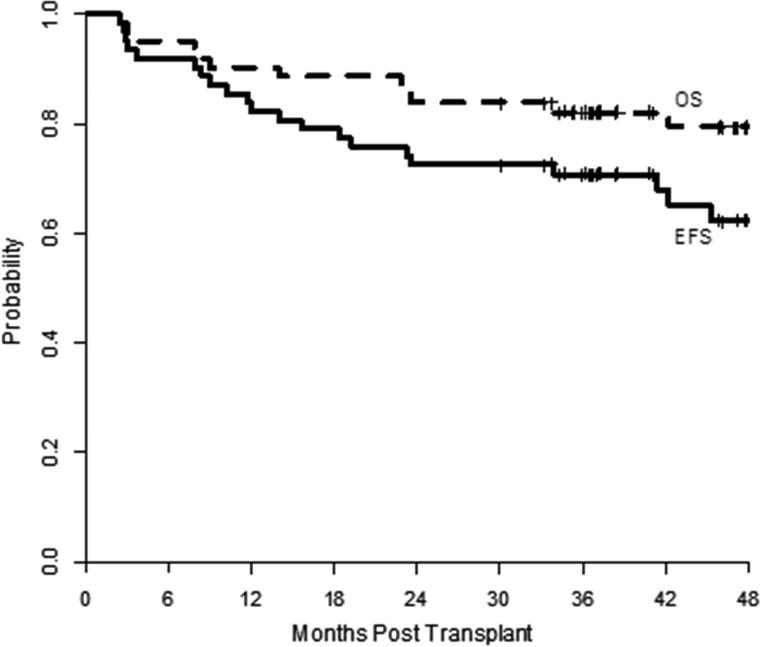
At a median follow-up of 47 months, the primary endpoint of 2 year PFS was 73% (95% confidence interval [CI]: 60 – 82%), Figure 1. Follow-up beyond 2 years was available for 52 patients and two patients died beyond 2 years after HCT. Three patients relapsed beyond 2 years after HCT and the estimated 3-year PFS is 71% (95% CI: 58 – 81%). The 2 year and 3 year OS were 84% (95% CI: 72 – 91%) and 82% (95% CI: 70 – 90%), Figure 1. When analyzing survival by donor type, the MRD recipients showed a superior 2-year OS compared to the URD recipients, 94% (95% CI: 78%, 98%) vs 72% (95% CI: 52%, 85%), p=.02, although the difference in 2-year PFS was not significant, 76% (95% CI: 57%, 87%) vs 69% (95% CI: 49% vs 83%), p= 0.55.
Figure 1.
Progression-free (solid line) and overall survival (dashed line).
Response to Transplantation
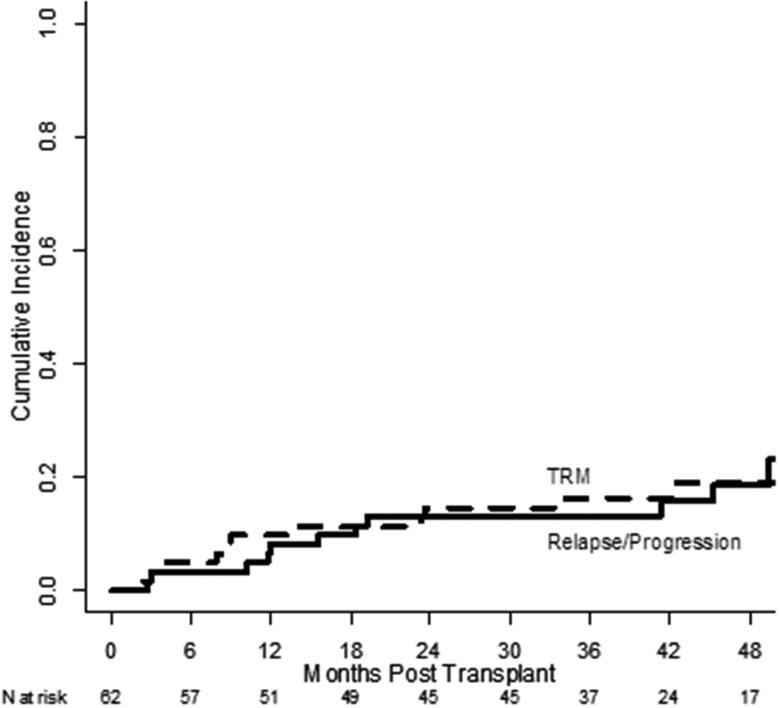
Disease status at the time of transplant is shown in Table 1. At baseline, 30 patients had a PET scan to determine disease status with 17 assessed as CR and 13 as not in CR. Among 24 patients not in CR at the time of transplant, only 6 did not obtain a CR as the best response post-transplant. Two of these patients were not evaluated due to death from infection on day 74 and death from acute GVHD on day 111. Two patients remained in PR and 2 patients experienced disease progression early both at 3 months after HCT. Overall, the 3-year cumulative incidence of relapse/progression was 13% (95% CI: 6 – 23%), Figure 2. Among patients transplanted in CR, the 2-year cumulative incidence of relapse/progression was lower compared to the patients transplanted in PR although this was not statistically significant, 6% (95% CI: 1% vs 18%) vs 21% (95% CI: 9%, 38%) , p=0.08, respectively. However, the patients in CR at time of HCT showed a significantly superior 2-year PFS and OS compared to the PR patients. The PFS for the CR patients and PR patients were 88% (95% CI: 70%, 95%) vs 57% (95% CI: 37%, 73%), p=.01, respectively and for OS, 94% (95% CI: 77%, 98%) vs 75% (95% CI: 55%, 87%), p=.04, respectively.
Figure 2.
Cumulative incidences of relapse/progression (solid line) and non-relapse mortality (dashed line)
Graft-versus-Host Disease
Acute GVHD occurred in 17 patients by day 100. No patients developed grade IV acute GVHD and only 6 patients developed grade III acute GVHD. The cumulative incidence of grade II – IV acute GVHD at day 100 was 27% (95% CI: 16 – 39%). Grade II-IV acute GVHD occurred in 18% (95% CI: 7 – 33%) of patients receiving MRD and 38% (21 – 55%) for MUD recipients; however, grade III/IV acute GVHD was similar [MRD: 9% (2 – 22%); MUD: 10% (3 – 25%)]. Chronic GVHD occurred in 37 patients, 35 of whom had extensive chronic GVHD. Chronic GVHD severity was mild in 7 (3 MRD, 4 URD) patients, moderate in 23 (12 MRD, 11 URD) patients, and severe in 7 (3 MRD, 4 MUD) patients. The cumulative incidence of any cGVHD at 2 years was 61% (95% CI: 48 – 74%). When analyzed by donor, the cumulative incidences of cGVHD for MRD recipients was 55% (95%CI: 36.0%, 70.4%) and 66% (95%CI: 44.4%, 80.2%) for URD recipients.
Toxicity
Following HCT, 50 patients experienced at least one grade 3 – 5 toxicity during the first 2 years. The most common toxicity was grade 3 – 4 neutropenia, occurring in 77% of patients. Other toxicities occurring in more than 10% of patients during the first two years included abnormal liver tests (n = 13) including grade 3 – 4 alanine aminotransferase (ALT) elevations in 7 patients and events of grade 3 – 4 hypoxia in 9 patients including 4 with pneumonitis developing beyond 2 months after alloHCT. Three patients developed unexpected, grade 3 – 5 adverse events including one patient with appendicitis at day +181 and two patients who developed deep venous thrombosis at day +28 and day +51, respectively. Overall, the 3-year TRM was 16% (95% CI: 8 – 27%, Figure 2). Ten patients died by two years after alloHCT due to the following causes: relapse (n =1), GVHD (acute, n=3; chronic, n = 3), infection (n=2), and one patient who died unexpectedly at home in whom the cause was not determined. Beyond 2 years, two additional patients have died due to infection at 34 months (n=1) and interstitial pulmonary fibrosis at 42 months (n=1). Donor type significantly affected TRM as the incidence of TRM at 2 years among the MRD vs URD recipients were 3% (95% CI: 0.2%- 15%) vs 28% (95% CI: 13% - 45%). Notably, for the 6 patients with GVHD as the COD, all 3 patients with aGVHD and 2 of the patients with cGVHD were recipients of URD transplants.
Immune Recovery
Quantitative immunoglobulin levels and peripheral blood flow cytometry for lymphocyte subset analyses were obtained at baseline, day 100, day 180, and 1 year from transplant. At baseline, patients had an absence of CD19+ and CD20+ cells [CD19+, n = 53: median 0 cells/μL (range, 0 – 300); CD20+, n = 37: median 0 cells/μL (range, 0 – 270)] and an associated mild hypogammaglobulinemia [IgG, n = 60: median 565 mg/dL (range 126 – 1470); IgA, n = 60: median 92 mg/dL (range, 7 – 450); IgM, n = 60: median 25 mg/dL (range, 4 – 111). Notably, this did not improve over time with continued near absence of CD19+ and CD20+ cells at 1 year [CD19+, n = 45: median 15 cells/μL (range, 0 – 400); CD20+, n = 23: median 0 cells/μL (range, 0 – 400)]. This translated into long-term mild hypogammaglobulinemia with a median IgG level of 431 mg/dL (range, 79 – 1430; n = 53) at 1 year after transplant.
T-cell subsets of CD4 and CD8 were lowest at baseline [CD4, n = 53: median 250 cells/μL (range, 29 – 1350) and day 100 [CD8, n = 57: median 213 cells/μL (range, 36 – 2117)], respectively. However, by day 180, the CD4 count (n = 50) had dramatically improved to a median 317 cells/μL (range, 10 – 782). Similarly, the CD8 count (n = 50) improved [median 262 cells/μL (range, 24 – 1698)].
Impact of High Dose RTX on Outcomes
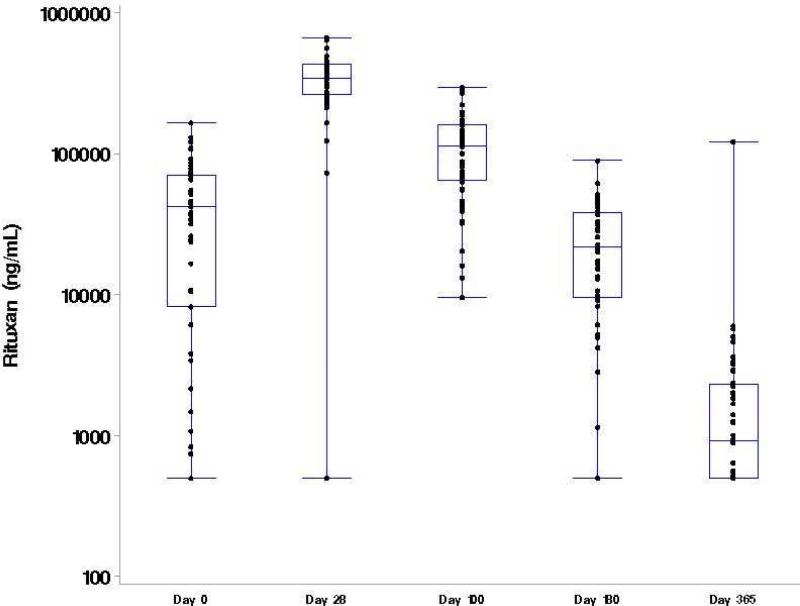
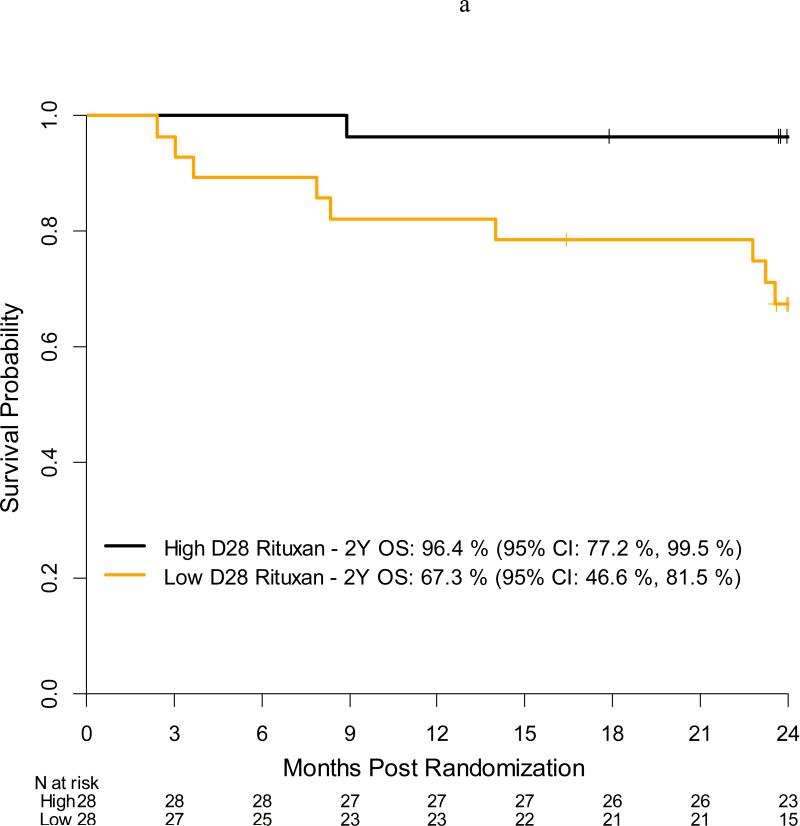
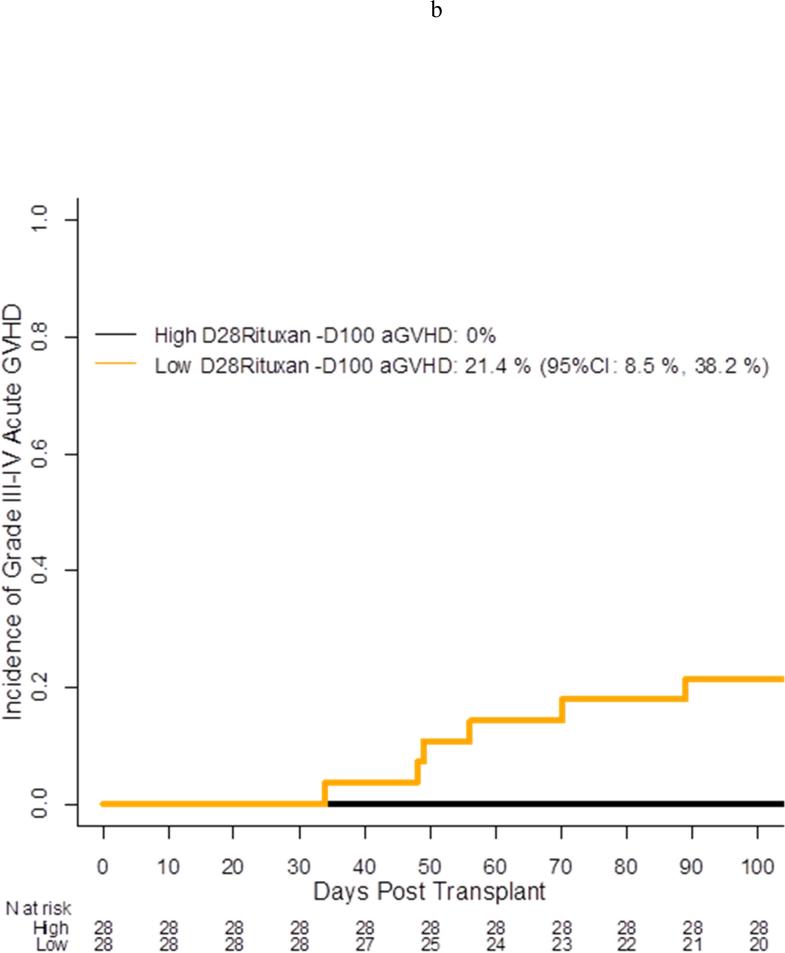
Per protocol, all patients received the first 3 doses of RTX (day −13, day −6, day +1) and only 2 patients missed the fourth dose of RTX on day +8. Serum RTX concentrations were collected at baseline (n=57), day +28 (n=56), day +100 (n=55), day +180 (n=53), and day +365 (n=44) for a compliance rate of 92%, 90%, 90%, 90%, and 80% at the assigned time points. At baseline, the median RTX concentration was 42,100 ng/mL (range, <500 – 70,200 ng/mL) with 6 patients having undetectable concentrations of RTX (Figure 3). As expected, RTX concentrations peaked at day +28 [median 341,000 ng/mL (range, <500 – 670,000 ng/mL)] with only 1 patient having undetectable concentrations. By day +365, 28 (64%) patients still had detectable RTX concentrations at a median level of 920 ng/mL (range, <500 – 122,000 ng/mL). RTX concentrations did not differ based on donor type (data not shown). For patients in CR at the time of transplant, the median RTX concentration at baseline was 53,800 ng/mL (range <500 – 166,000 ng/mL) compared to only 29,000 ng/mL (<500 – 110,000 ng/mL) for non-CR patients; however, by day +28 and all subsequent assessments, the median RTX concentrations were nearly identical [day 28: CR = 345,000 ng/mL (range, 73,400 – 670,000); non-CR = 341,000 (range, <500 – 564,000)] (data not shown for other assessments). When analyzing median RTX concentrations using landmark analysis, OS was significantly higher among patients who had a higher median serum concentration vs a lower serum concentration at day +28 with a 2 year OS of 96% (95% CI: 77%, 100%) vs 67%, (95% CI: 47% vs 82%), p=.01, respectively (Figure 4a). In univariate analysis treating RTX as a continuous variable, patients who developed Grade III/IV acute GVHD (n = 6) and patients who died (n =10) were more likely to have a lower day +28 RTX concentration [aGVHD grade III/IV yes, median: 272,500 ng/mL vs no: 351,000 ng/mL, p = 0.03; death yes, median: 253,093 ng/mL vs no: 353,000 ng/mL, p = 0.009)]. These results were consistent when categorizing RTX as high or low based on the median value of the cohort at day +28 (Figure 4b). There was no impact of RTX concentrations on the development of chronic GVHD or on relapse or progression of lymphoma (data not shown).
Figure 3.
Serum RTX concentrations over time
Figure 4a.
Acute grade 3-4 GVHD incidence by day +28 serum Rituxan level (high vs low)
Figure 4b.
Overall survival by day +28 serum Rituxan level (high vs low)
Quality of Life Measures
The results from the SF-36 instrument showed no statistically significant difference in SF-36 score between the baseline and 2 year timepoints except that the raw SF-36 ‘health transition’ item did show improvement from a mean score of 3 at baseline to 2 at 2-years (with 2 indicating that general health somewhat better now than one year ago, 3 indicating that general health about the same as one year ago). The FACT-BMT scores at baseline and at 2 years did not show a significant difference at years (data not shown)
DISCUSSION
We report the results of the largest prospective series of FL patients who underwent RIC alloHCT using a uniform conditioning regimen with a T-cell replete graft. Sixty five patients, of which 62 were evaluable, received the FCR regimen incorporating high dose RTX. The 2 year PFS and OS rates of 74% and 84% were notable considering that this was a heavily pre-treated patient population. Nearly 80% of the patients had received 3 prior regimens with one third of the patients having received 5 prior regimens and 11% receiving a prior autologous HCT. More importantly, these excellent outcomes were maintained with longer follow-up. This was a well-tolerated regimen that can be administered in the outpatient setting. Our multi-center results are similar to the outcomes from the M.D. Anderson group who reported an 11 year PFS and OS of 72% and 78% in 47 patients who underwent RIC alloHCT (all but one from a related donor) with the same FCR regimen 14. Several prospective trials of FL patients undergoing RIC alloHCT, also using fludarabine-based, regimens show median disease free survivals or EFS ranging from 43% −75% and OS ranging from 52% −81%5, 6, 10-15. As with our study, most of these prior studies also included patients who had relapsed after a prior autologous HCT. In all studies, chemosensitivity at the time of HCT was the most consistent predictor of outcome. All of these trials also demonstrated plateaus in the relapse curves which were strongly suggestive of cure.
The low relapse incidence of 13% in this study was notable and partly attributed to the graft vs lymphoma effect mediated by donor T cells and to the lack of potential graft contamination of tumor cells in autologous grafts. Another possible contributor to the low relapse rate was the high dose of RTX administered. Patients received a total of 3.375 gms/m2. Prior reports found an association between dose and serum RTX concentration as well as an association between serum RTX concentration and anti-tumor response in B-cell lymphoma27, 28. The serum half-life of RTX increases with subsequent infusions due to the elimination of circulating CD20+ B cells which clear serum antibody with the initial RTX infusions. Antibody clearance is also reduced due to saturation or reduction of involved nodal sites. In the current study, pharmacokinetic analyses revealed high serum concentrations at day +28 in the majority of patients for whom data were available, with 23 patients showing detectable concentrations up to 1 year post HCT. When analyzing median RTX concentrations using landmark analysis, OS was significantly higher among patients who had a higher (>median) serum concentration vs a lower serum concentration at day +28 (Kaplan-Meier estimator 96% vs 67%, respectively, p=.01). The improved OS associated with a higher day+28 RTX concentration could be attributed to the significantly lower risk of grade 3-4 acute GVHD seen in this specific subgroup. Acute and chronic GVHD were the leading causes of death in this study, and predominated in recipients of URD contributing to the higher TRM following URD transplant. Therefore, further improvement in outcomes may be achieved with efforts, such as the use of ATG, to decrease GVHD29.
Other direct effects of the high dose RTX were hypogammaglobulinemia and late neutropenia with the neutropenia resolving in all patients by 2 year post-HCT. We did not observe an increase in infection related deaths despite the observed neutropenia. T cell subsets, CD4+ and CD8+, remained within normal range throughout the transplant course. In contrast, peripheral blood B lymphocyte recovery was considerably impaired. At the start of HCT, CD19 and CD20+ subsets were undetectable which was attributed to prior RTX administration in all patients. However, at 1 year post HCT, CD19+ B cells showed minimal recovery while CD20+ B cells remained undetectable. Despite the impaired B cell immune reconstitution, an excess of opportunistic infections was not seen.
With the prolonged B cell depletion in the current study, we had originally hypothesized that acute or chronic GVHD might be reduced as recent data have implicated B cells in the pathogenesis of acute and chronic GVHD. B cell depletion therapy with RTX has reported efficacy in both the prevention and treatment of chronic GVHD, including steroid-refractory chronic GVHD30-33. We observed a correlation with RTX concentration at day +28 with a significantly lower incidence of grade 3 and no grade 4 acute GVHD in patients who had a high RTX concentration compared to patients who had lower concentration. However, the incidence of chronic GVHD was not impacted as we observed an incidence comparable to those reported in other studies with RIC allogeneic HCT in similarly aged patients. The lack of impact of RTX on the incidence of chronic GVHD may be explained by the complex pathophysiology of chronic GVHD which requires a coordinated response from both B and T lymphocytes. Direct comparison of chronic GVHD incidences between different studies is also challenging due to the heterogeneity among chronic GVHD criteria and grading in the literature. Additionally, despite our observed incidence of chronic GVHD in 55% of patients, their self-reported QOL was unchanged from prior to HCT compared to 2 years after HCT.
An increasing body of data confirms the efficacy of non-myeloablative or RIC alloHCT leading to less enthusiasm for myeloablative regimens in this patient population. Two large registry studies from the Center for International Blood and Marrow Transplant Research (CIBMTR) and the European Group for Blood and Marrow Transplantation (EBMT) directly compared the outcomes of FL patients who underwent alloHCT either with a RIC or myeloablative regimen. The CIBMTR analyses reported that both PFS and OS were similar regardless of intensity of conditioning regimen although progression was significantly higher in the RIC group and TRM higher in the myeloablative group34. In contrast, in the EBMT study, the RIC recipients had improved PFS and OS compared to the ablative regimens with no difference in relapse rates between the two groups35. Both studies demonstrated that chemosensitivity rather than conditioning intensity was the most reliable predictor of outcome.
For FL patients with relapsed/refractory disease, autologous HCT (autoHCT) represents one of numerous other therapeutic options. In our study, 7 patients had previously received autologous HCT. While there has only been one prospective trial comparing autoHCT with RIC alloHCT for relapsed FL patients, this study closed prematurely due to poor accrual16. Retrospective and prospective studies with autologous HCT have yielded 5 year PFS ranging from 40%-60%6, 7, 15, 36, 37. Retrospective comparisons of autologous and allogeneic HCT for relapsed/refractory FL include a report from the National Comprehensive Cancer Network (NCCN) comparing the outcomes of 135 FL patients who received an autologous HCT to 49 patients who underwent either myeloablative or RIC allogeneic HCT5. Patients receiving alloHCT had lower relapse/progression than autoHCT recipients (16% vs 32%, p=0.03) but OS was improved in the autoHCT recipients (87% vs 61%, p <0.009). This disparity was ascribed to the difference in TRM between the groups (autoHCT 3% vs alloHCT 42%, p<0.0001). However, the alloHCT group had a higher proportion of patients with resistant disease. Multivariate analyses for the autologous cohort identified age > 60 years old and having > 3 prior regimens as adverse factors for survival which is consistent with other reports7,38. The EBMT published a retrospective analyses comparing autologous HCT (n=726) to RIC alloHCT for relapsed FL 15. Similar to the NCCN report, relapse/progression was higher following autoHCT (RR 3.1, p<0.001) and 1 year TRM was higher following alloHCT (RR 4.0, p<0.001); however, OS was similar between the two groups. Interestingly, while the median time to relapse was 13 months (range .6 -107 months) following autoHCT and 5.4 months (range 0.1 -76 months) after alloHCT, the alloHCT group demonstrated a plateau in relapse/progression at 24 months. There was no plateau following autoHCT.
In summary, this trial shows that RIC alloHCT using the FCR regimen in a multicenter setting yields high response rates with durable long term remissions for patients with relapsed, chemosensitive FL. Numerous therapeutic options are available to FL patients but alloHCT remains the only curative strategy; these data suggest that it can be offered with acceptable safety to patients who fail other therapies. The optimal timing of alloHCT versus other FL therapies still remains to be determined.
Highlights.
AlloHCT with high dose RTX/Flu/Cy provides excellent PFS for high risk FL
RTX remains detectable at 1 year from HCT in more than 50% of patients
Timing of AlloHCT to optimize outcomes requires further study
Acknowledgments
The following institutions enrolled at least one patient on this trial: City of Hope National Medical Center, DFCI/Brigham& Women's Hospital, DFCI/Massachusetts General Hospital, H. Lee Moffitt Cancer Center, Medical College of Wisconsin, Ohio State/Arthur G. James Cancer Hospital, Rush University Medical Center, Stanford Hospital and Clinics, University of California San Diego Medical Center, University Hospitals of Cleveland/Case Western, University of California Davis Medical Center, University of Florida College of Medicine, University of Minnesota, University of Nebraska Medical Center, University of North Carolina at Chapel Hill, University of Oklahoma Medical Center, University of Texas/MD Anderson Cancer Research Center, University of Wisconsin Hospital and Clinics, Vanderbilt University Medical Center, Wake Forest University Health Sciences, and West Virginia University Hospital.
Support for this study was provided by grant#U10HL069294 from the National Heart, Lung and Blood Institute and the National Cancer Institute, along with contributions by Genentech, Inc. and funding by the Alliance for Clinical Trials in Oncology, the ECOG-ACRIN Cancer Research Group (CA 180820, CA 180853, CA 180799) and SWOG (U10CA180888). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above mentioned parties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For the Blood and Marrow Transplant Clinical Trials Network (BMT CTN)
REFERENCES
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 4.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. American journal of hematology. 2014;89:429–436. doi: 10.1002/ajh.23674. [DOI] [PubMed] [Google Scholar]
- 5.Evens AM, Vanderplas A, LaCasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119:3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- 6.Kothari J, Peggs KS, Bird A, et al. Autologous stem cell transplantation for follicular lymphoma is of most benefit early in the disease course and can result in durable remissions, irrespective of prior rituximab exposure. British journal of haematology. 2014;165:334–340. doi: 10.1111/bjh.12741. [DOI] [PubMed] [Google Scholar]
- 7.Vose JM, Bierman PJ, Loberiza FR, et al. Long-term outcomes of autologous stem cell transplantation for follicular non-Hodgkin lymphoma: effect of histological grade and Follicular International Prognostic Index. Biol Blood Marrow Transplant. 2008;14:36–42. doi: 10.1016/j.bbmt.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 8.van Besien K, Loberiza FR, Jr., Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 9.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 10.Thomson KJ, Morris EC, Milligan D, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 11.Shea T, Johnson J, Westervelt P, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, Saliba RM, Erwin WD, et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012 doi: 10.1182/blood-2012-03-417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson SP, Canals C, Luang JJ, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 16.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow SH. International Agency for Research on Cancer, and Who Health Organization., WHO classification of tumours of hematopoietic and lymphoid tissues. 4th ed. World Health Organization classification of tumours. International Agency for Research on Cancer; Lyon, France: 2008. p. 439. [Google Scholar]
- 19.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 20.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in medicine. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Berinstein NL, Grillo-Lopez AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 28.Tobinai K, Kobayashi Y, Narabayashi M, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDECC2B8 Study Group. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9:527–534. doi: 10.1023/a:1008265313133. [DOI] [PubMed] [Google Scholar]
- 29.Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:959–970. doi: 10.1016/j.bbmt.2014.11.676. [DOI] [PubMed] [Google Scholar]
- 30.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005–1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Kamble R, Oholendt M, Carrum G. Rituximab responsive refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:1201–1202. doi: 10.1016/j.bbmt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avivi I, Montoto S, Canals C, et al. Matched unrelated donor stem cell transplant in 131 patients with follicular lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. British journal of haematology. 2009;147:719–728. doi: 10.1111/j.1365-2141.2009.07905.x. [DOI] [PubMed] [Google Scholar]
- 36.Arcaini L, Morello L, Tucci A, et al. Autologous stem cell transplantation with in vivo purged progenitor cells shows long-term efficacy in relapsed/refractory follicular lymphoma. American journal of hematology. 2015;90:230–234. doi: 10.1002/ajh.23919. [DOI] [PubMed] [Google Scholar]
- 37.Pettengell R, Schmitz N, Gisselbrecht C, et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:1624–1630. doi: 10.1200/JCO.2012.47.1862. [DOI] [PubMed] [Google Scholar]
- 38.Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol. 2007;25:2554–2559. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]