Abstract
Objective
To evaluate the impact of comorbid anxiety or depressive disorders on treatment response to cognitive behavior therapy (CBT) for insomnia, behavior therapy (BT), or cognitive therapy (CT).
Method
Participants were 188 adults (117 women; M age = 47.4 years) with chronic insomnia, including 45 also presenting a comorbid anxiety or mild to moderate depressive disorder. They were randomized to BT (n = 63), CT (n = 65), or CBT (n = 60). Outcome measures were the proportion of treatment responders (decrease of ≥ 8 points on the Insomnia Severity Index; ISI) and remissions (ISI score < 8) and depression and anxiety symptoms.
Results
Proportion of treatment responders and remitters in the CBT condition was not significantly different between the subgroups with and without comorbidity. However, the proportion of responders was lower in the comorbidity subgroup compared to those without comorbidity in both the BT (34.4% vs 81.6%; p=0.007) and CT (23.6% vs 57.6%; p=0.02) alone conditions, although remission rates and pre-post ISI change scores were not. Pre to post change scores on the depression (−10.6 vs −3.9; p<0.001) and anxiety measures (−9.2 vs −2.5; p=.01) were significantly greater in the comorbidity subgroup relative to the subgroup without comorbidity but only for those treated with the full CBT; no difference was found for those treated with either BT or CT alone.
Conclusions
The presence of a comorbid anxiety or mild to moderate depressive disorder did not reduce the efficacy of CBT for insomnia, but it did for its single BT and CT components when used alone.
Keywords: Insomnia, Cognitive-Behavior Therapy, Comorbidity, Anxiety, Depression
Introduction
There is strong evidence that cognitive behavioral therapy for insomnia (CBT-I) produces clinically significant and sustained sleep improvements over time (Buysse, 2013; Morin et al., 2006; National Institutes of Health, 2005). Nonetheless, treatment response is not optimal for all patients. Of the various demographic, clinical, and contextual (e.g., type of treatment) factors that can potentially moderate treatment outcome, psychiatric comorbidity is probably the most likely factor to impact on both severity of sleep difficulties and treatment response (Roth et al., 2006).
Insomnia is commonly comorbid with several psychiatric disorders, particularly anxiety and depression. Studies indicate that sleep disturbances are more severe in the presence of comorbid conditions (Sarsour, Morin, Foley, Kalsekar, & Walsh, 2010). Insomnia is also a common residual symptom after treatment of PTSD (Zayfert & DeViva, 2004) and depression (Nierenberg et al., 2010), and its persistence may actually increase the risk of relapse. Longitudinal studies have also shown that insomnia increases the risk of anxiety and depression (Baglioni et al., 2011; Breslau, Roth, Rosenthal, & Andreski, 1996; Roth et al., 2006) although this relationship is probably bidirectional, suggesting that insomnia and comorbid conditions contribute to a vicious cycle of mutual maintenance (Harvey, 2008; Jansson-Frojmark & Lindblom, 2008; Sivertsen et al., 2012).
The few studies that have investigated the relationship between psychiatric comorbidity and response to CBT-I have yielded mixed findings. For example, in a study of 81 veterans with persistent insomnia, including 41 with comorbid psychiatric disorders (mostly PTSD, major depression, or dysthymia), there was no significant interaction between treatment response and presence or absence of comorbidity (Edinger, Olsen, et al., 2009). However, patients with comorbid disorders presented with more severe insomnia at baseline, and the magnitude of changes on several end points were smaller for patients with comorbidity relative to those with primary insomnia. An earlier clinical case replication series of 100 patients treated for insomnia at a sleep disorders clinic had previously reported that while patients with comorbid psychiatric disorders (mostly depressive and anxiety disorders) presented with more severe insomnia at baseline and at post treatment, both groups with and without comorbidity showed significant sleep improvements with insomnia treatment (Morin, Stone, McDonald, & Jones, 1994). These findings are in contrast with those of a more recent study of 60 patients also referred to a sleep clinic for insomnia treatment showing that the presence of psychiatric comorbidity (e.g., anxiety, somatoform, and major depressive disorders) was predictive of negative treatment outcome and that this relationship was not influenced by whether patients were treated or not for the coexisting psychiatric disorder (van de Laar, Pevernagie, van Mierlo, & Overeem, 2014). Given that the samples in the latter two studies were similar in terms of recruitment and comorbidity, it is unclear how to explain the discrepancies between these findings.
Other studies have examined the impact of treating insomnia on co-existing depressive symptoms and disorders. For example, two small studies of patients with comorbid insomnia and depression reported that CBT-I produced slight reductions of depressive symptoms in addition to improving sleep efficiency and sleep quality (Taylor, Lichstein, Durrence, Reidel, & Bush, 2005; Wagley, Rybarczyk, Nay, Danish, & Lund, 2013). In another study of 30 patients with comorbid insomnia and major depression, CBT-I produced significant added value to an antidepressant drug as evidenced by higher remission rates for both insomnia (50% vs. 8% for antidepressant drugs but no CBT-I) and depression (62% vs. 33%) (Manber et al., 2008).
A final set of studies evaluated the impact of CBT-I on anxiety symptoms or the impact of anxiety treatment on sleep. For example, a meta-analysis of 72 trials of CBT-I concluded that insomnia treatment had only a moderate impact on co-existing anxiety symptoms (Belleville, Cousineau, Levrier, & St-Pierre-Delorme, 2011). Conversely, in a study of 44 patients treated for generalized anxiety disorder with CBT, which targeted worry and anxiety without any attention to sleep disturbances, treatment produced a significant impact on sleep quality (Bélanger, Morin, Langlois, & Ladouceur, 2004).
Collectively, the current state of evidence suggests that insomnia often presents as a comorbid condition with anxiety and depression, and that sleep disturbances are more severe in the presence of such comorbid disorders. The few treatment studies available also suggest that CBT-I may possibly have a positive impact on comorbid depression and anxiety symptoms, although the extent of this evidence is still very preliminary. Additional research is warranted to document the effects of CBT-I in insomnia patients with and without comorbid psychiatric conditions.
The present study examined whether the presence of a comorbid anxiety or depressive disorder reduced the effect of insomnia treatment, and whether insomnia treatment had an impact on anxiety and depression symptoms and diagnoses. Data were derived from a larger study evaluating the unique contribution of behavior therapy (BT) and cognitive therapy (CT) relative to full CBT (Harvey et al., 2014), so we examined moderating effects of comorbidity within each single treatment condition. Given that CT targets processes and mechanisms (e.g., worry, dysfunctional beliefs, attentional bias) shared by insomnia and some of its comorbid disorders (i.e., anxiety and depression) (Harvey, Sharpley, Ree, Stinson, & Clark, 2007), and that BT addresses mechanisms specific to insomnia (e.g., sleep scheduling factors), it was expected that CT would be more effective than BT, and equally effective to CBT, in reducing symptoms associated with comorbid anxiety and depression.
Method
For a more detailed description of the study design, treatment protocol and participant flow in the study, see (Harvey et al., 2014).
Participants
A total of 188 participants were recruited through advertisements and referrals from health care practitioners at two sites: Laval University (Quebec City, Canada) and University of California (Berkeley). A telephone interview was completed for initial eligibility screening and eligible individuals were invited for a face-to-face diagnostic interview, using the Duke Structured Interview for Sleep Disorders (Edinger, Wyatt, et al., 2009) and the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995). They completed a sleep diary for a two-week period and two nights of in-lab polysomnography testing. Diary data were further used to confirm insomnia criteria while polysomnography was used to exclude participants presenting other sleep disorders.
Inclusion criteria were: (a) 25 years of age or older, (b) chronic insomnia defined as difficulty initiating and/or maintaining sleep, with a sleep onset latency and/or wake after sleep onset greater or equal to 30 min, and a corresponding sleep time of less than or equal to 6.5 hours per night, as ascertained by daily sleep diaries over a two-week baseline period; (c) presence of insomnia 3 nights or more per week for more than 6 months; (d) significant distress or impairment in social, occupational, or other areas of functioning as measured by a rating of at least 2 on item no. 5 or 7 on the Insomnia Severity Index (Morin, Belleville, Belanger, & Ivers, 2011). This definition represents a combination of the Research Diagnostic Criteria and the Diagnostic and Statistical Manual of Mental Disorders’ criteria (American Psychiatric Association, 2013).
Exclusion criteria were: (a) presence of a serious or unstable physical illness or neurological degenerative disease related to the onset and course of insomnia, (b) use of hypnotics and other medications known to alter sleep (patients on SSRI for at least 3 months were included), (c) evidence of another sleep disorder, or body mass index (BMI) of 35 or above, or BMI of 32 or above and reporting at least 3 symptoms of breathing-related sleep disorder, (d) irregular sleep schedules or working on night or rotating shifts within the last year, (e) current or past psychological treatment of insomnia (past 5 years), (f) lifetime diagnosis of a psychotic or bipolar disorder or more than two lifetime episodes of major depressive disorder or an untreated current major depressive disorder or alcohol or drug abuse within the past year. Of the total 188 participants, 45 (23.9%) had at least one current comorbid Axis I disorder (ranging from 1 to 4 diagnoses, M = 1.4). Most frequent disorders were generalized anxiety disorder (n = 18), specific phobia (n = 10), adjustment disorder (n= 5), dysthymia (n = 4), obsessive-compulsive disorder (n = 4), social phobia (n = 3), panic disorder (n = 3), and major depression disorder (n= 3).
Study Design
Participants were randomly assigned to one of three treatments: (a) BT (n = 63), (b) CT (n = 65), or (c) CBT (n = 60). Randomization was stratified by age (25–49 versus 50+) and presence of a comorbid psychiatric disorder. Group allocation concealment was achieved by sequentially numbered, sealed envelopes opened by the project coordinator at each study site. Outcome measurements were taken at baseline (Time 1), at the end of treatment (Time 2), and at 6-month follow-up (Time 3). All participants provided written informed consent.
Measures
The Duke Structured Interview for Sleep Disorder (DSISD; Edinger et al., 2004) is a semi-structured interview that assesses research diagnostic criteria for sleep disorders. The DSISD has good reliability and validity (Edinger, Wyatt, et al., 2009).
Structured Clinical Interview for DSM-IV (SCID; First et al., 1995) is a semi-structured interview designed to assess DSM-IV-TR diagnostic criteria for Axis I disorders. It was administered by trained psychology doctoral students and postdoctoral fellows to assess current and lifetime Axis I disorders.
Sleep Diary
Participants kept daily sleep diaries during a 2-week baseline period, the 8-week treatment phase, and for 2 weeks at the post-treatment and 6-month follow up assessments. The sleep diary has been shown to be a reliable estimate and is considered the gold standard subjective measure of sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006).
Polysomnography (PSG)
All participants underwent PSG evaluations in the sleep laboratory, including 1 screening/adaptation night, 2 baseline nights and 2 nights after the end of treatment. A standard montage was used (Rechtschaffen & Kales, 1968). Respiration (air flow, tidal volume, and oxygen saturation) and anterior tibialis EMG was also monitored during the first (screening) night to evaluate sleep apnea and periodic limb movements during sleep. All recordings were scored by experienced technicians, blind to participants’ condition, and according to standardized criteria (Rechtschaffen & Kales, 1968).
The Insomnia Severity Index (ISI; Bastien, Vallières, & Morin, 2001; Morin et al., 2011) is a 7-item scale assessing severity of sleep difficulties and associated daytime impairments (Morin et al., 2009). The total score (ranges 0–28), as well as rates of treatment responders (defined as a change of more than 7 points) and remitters (defined as a final score below 8) were the primary outcome measures for this study.
The Beck Depression Inventory II (BDI-II) (Beck, Steer, & Garbin, 1988) and the Trait part of the State-Trait Anxiety Inventory (STAI-Trait) (Spielberger, 1983) were used to assess depressive and trait-like anxiety symptoms.
Treatments
Treatments were provided in the context of eight weekly individual therapy sessions, with BT and CT sessions lasting 45–60 minutes and CBT sessions up to 75 minutes. Common treatment elements across all three arms included a generic overview of CBT within a self-management framework, the 3 P Model of Insomnia (Spielman & Glovinsky, 1991), keeping a daily sleep diary, setting treatment goals, and reviewing sleep hygiene information. The week-by-week content of sessions was published as an online supplement to the main study and can be found at: http://europepmc.org/articles/PMC4185428;jsessionid=pfBi8HL2aa2rUZPa6ZeW.12.
Behavior Therapy included a combination of usual stimulus control instructions (Bootzin, Epstein, & Wood, 1991) and sleep restriction procedures (Spielman, Saskin, & Thorpy, 1987), which involves curtailing time in bed to the actual time slept and gradually increasing it back to an optimal sleep time.
Cognitive Therapy was based on Beck’s model (Beck, 1979; Beck, Emery, & Greenberg, 1985) and sought to reverse a broad range of cognitive maintaining mechanisms including unhelpful beliefs about sleep (Morin, Blais, & Savard, 2002), sleep-related worry (Tang & Harvey, 2004), attentional bias and monitoring for sleep-related threats, and misperception of sleep (Harvey & Tang, 2012). These procedures are described elsewhere (Bélanger, Savard, & Morin, 2006; Harvey, 2002; Harvey et al., 2007; Morin & Espie, 2003). Third, CT included individually formulated experiments to challenge beliefs (Harvey et al., 2007; Perlis, Aloia, & Kuhn, 2011; Ree & Harvey, 2004).
Cognitive-Behavior Therapy consisted of a combination of both the BT and CT components. A case formulation driven approach (Harvey, 2006) was used to determine the relative time and ordering of CT vs. BT.
Therapists
All treatments were administered by licensed clinical psychologists or advanced graduate students who had attended joint training workshops with the study principal investigators (Harvey and Morin). Treatment manuals were used by therapists and ongoing joint supervision from both study sites were provided during the entire course of the study. Both workshops involved a specific focus on promoting adherence and on delivering each individual treatment with a high level of fidelity. In addition, sessions within these workshops focused on identifying specific methods to avoid contamination across the three treatments. Specifically, the therapists were instructed to gently disengage the patient’s attention from a question or tangent not allowed within the intervention being delivered and to redirect attention back to the allowable session content. Second, fidelity and contamination were major topics within the weekly supervision sessions. One hour per week was devoted to a conference call involving therapists and supervisors across both sites, while additional site-specific supervision sessions were also provided so that potential issue regarding treatment integrity and fidelity were addressed as soon as they arose.
Data Management and Analyses
Analyses for the main hypotheses were performed using an intent-to-treat approach, such that all randomized participants were included in the analyses. No data imputation was performed. Site was included in all main analyses as a fixed effect.
Primary outcome measures were ISI total scores and rates of treatment responders and remitters. To study changes on sleep, anxiety and depression symptoms within and between conditions, 3 (Groups) X 3 (Time: Pre, Post, 6-month follow up) split-plot mixed model analyses were computed to test Group, Time, and Interaction effects. Group X Time interactions were decomposed using simple effects in order to compare pre to post changes associated with each treatment condition, as well as averaged change scores between conditions. Effect sizes for temporal changes were computed as the difference between means, divided by the root mean squared error of the mixed model. The possible multiple comparison problem was addressed by computing adjusted p-values using the Hochberg and Benjamini (Hochberg & Benjamini, 1990) adaptive step-down Bonferroni method when appropriate. Since all p-values remained significant after correction, only the raw p-values for all simple effects are reported in the tables. All initial power analyses (sensitivity analysis) were computed according to procedures outlined in (Cohen, 1988) using G-Power software (Faul, Erdfelder, Lang, & Buchner, 2007) and were based on standard conditions: a two-sided alternative hypothesis, 80% power, and a type I error rate of 5%. Assuming an attrition rate of 10% during the interventions, a planned sample size of 180 participants (effective sample after attrition = 162) would give a standard power to detect a small effect size (Cohen d = 0.25) or larger on the condition (3) x time (baseline vs. post) interaction for continuous outcomes. Translated into clinically relevant units, this sample size would allow the detection of a difference of 1.21 units on the ISI between conditions. Considering that a clinically meaningful change on the ISI is estimated to be of at least 8 points (Morin et al., 2011), the projected sample size of 180 was more than adequate to detect a change of 1.21. All analyses were performed using SAS 9.3 using two-tailed 5% alpha level unless otherwise specified.
Results
There were no significant differences between treatment groups at baseline on demographic variables, medical or psychological comorbidity, insomnia duration and severity (ISI) (see Table 1). However, there was a significant difference in the BT group between those with and those without comorbidity, with the former group reporting higher ISI mean scores than the latter.
Table 1.
Sample Characteristics by Treatment Group
| CBT (n = 60) | CT (n = 65) | BT (n = 63) | Total (N = 188) | Statistic | |
|---|---|---|---|---|---|
| Gender (female) - % (n) | 53.3 (32) | 69.2 (45) | 63.5 (40) | 62.2 (117) | 3.42, p = .18 |
| Age (yrs) – M (SD) | 46.9 (11.3) | 46.7 (12.8) | 48.5(13.6) | 47.4 (12.6) | 0.40, p = .67 |
| Ethnicity (non-caucasian) - % (n) | 8.5 (5) | 9.2 (6) | 3.2 (2) | 6.9 (13) | 3.61, p = .23 |
| Education (yrs) – M (SD) | 16.7 (3.0) | 15.9 (3.4) | 15.5 (3.3) | 16.0 (3.2) | 2.00, p = .14 |
| Insomnia duration (yrs) – M (SD) | 13.8 (11.9) | 14.8 (12.9) | 14.8 (13.6) | 14.5 (12.8) | 0.11, p = .90 |
| Insomnia Severity Index – M (SD) | 17.9 (3.4) | 17.6 (3.5) | 18.3 (3.4) | 17.9 (3.4) | 0.70, p = .50 |
| Type of insomnia - % (n) | 9.53, p = .30 | ||||
| Initial | 6.7 (4) | 4.6 (3) | 6.4 (4) | 5.9 (11) | |
| Middle | 21.7 (13) | 29.2 (19) | 12.7 (8) | 21.3 (40) | |
| Late | 3.3 (2) | 9.2 (6) | 6.4 (4) | 6.4 (12) | |
| Mixed | 65.0 (39) | 56.9 (37) | 73.0 (46) | 64.9 (122) | |
| Comorbidity - % (n) | |||||
| Medical (any) | 59.3 (35) | 52.3 (34) | 69.8 (44) | 60.4 (113) | 4.16, p = .13 |
| Psychiatric (any) | 25.0 (15) | 23.1 (15) | 23.8 (15) | 23.9 (45) | 0.06, p = .97 |
| Anxiety | 18.3 (11) | 21.5 (14) | 23.8 (15) | 21.3 (40) | 0.55, p = .76 |
| Mood | 6.7 (4) | 6.2 (4) | 6.4 (4) | 6.4 (12) | 0.01, p = .99 |
| Prior medication - % (n) | |||||
| Antidepressant | 1.7 (1) | 6.2 (4) | 3.2 (2) | 3.7 (7) | 2.66, p = .26 |
| Hypnotic | 35.0 (21) | 30.8 (20) | 39.7 (25) | 35.1 (66) | 1.17, p = .57 |
| OTC | 20.0 (12) | 15.4 (10) | 20.6 (13) | 18.6 (35) | 0.69, p = .71 |
Treatment attendance, credibility, and attrition
All three treatments were rated as highly acceptable and credible and generated high expectancies for success. Overall, 94.2% of the patients attended the planned number of eight therapy sessions (M = 7.8) and attendance was not different across conditions nor according to comorbidity status or severity of anxiety or depression symptoms. Overall attrition rate was 7.5% (14/188) during treatment and 10.6% (20/188) at the 6-month follow up and it was not significantly different across treatment groups or according to presence or absence of comorbidity or severity of anxiety or depression symptoms.
Impact of comorbidity on insomnia treatment response
ISI adjusted mean scores across treatment conditions, presence of a comorbid disorder and time are displayed in Table 2, as are the percentages of treatment responders and remitters. For the mean ISI scores, a significant time effect was observed, F(2,317) = 202.97, p < .001, indicating that all six groups (3 treatment conditions x 2 comorbidity status) reduced their insomnia severity over time. The treatment condition x comorbidity status x time interaction was not significant, p = .15; nor was the comorbidity x time interaction in any of the conditions (all p’s > .20). Simple effects showed no significant differences between participants with and without a comorbid disorder both at post and FU-6. A similar pattern of results was observed regarding remission rates at post treatment and FU-6, as no significant between-group differences were observed in any of the conditions.
Table 2.
Adjusted Means and Change Scores on Sleep Measures According to Group, Comorbidity Status and Time
| Cognitive-Behavior Therapy | Cognitive Therapy | Behavior Therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time/change scores | No comorbidity (n = 45) M (SE) |
With Comorbidity (n = 15) M (SE) |
t | No comorbidity (n = 50) M (SE) |
With Comorbidity (n = 15) M (SE) |
t | No comorbidity (n = 48) M (SE) |
With Comorbidity (n = 15) M (SE) |
t |
| Insomnia Severity Index (Total ISI Score) | |||||||||
| Pre | 17.7 (0.5) | 18.4 (0.7) | 0.63, p=.43 | 17.1 (0.5) | 19.2 (1.0) | 3.43, p=.07 | 17.7 (0.5) | 20.2 (0.8) | 7.44, p<.01 |
| Post | 7.7 (0.6) | 6.6 (0.9) | 0.90, p=.34 | 8.3 (0.6) | 12.7 (1.4) | 8.70, p<.01 | 8.0 (0.6) | 11.9 (1.7) | 4.73, p=.03 |
| FU6 | 7.6 (0.7) | 7.2 (1.1) | 0.10, p=.75 | 8.3 (0.8) | 10.1 (1.6) | 1.05, p=.31 | 9.0 (0.7) | 11.4 (1.8) | 1.64, p=.20 |
| Change Pre-Post | −10.0 | −11.8 | 1.26, p=.21 | −8.8 | −6.5 | 1.43, p=.15 | −9.7 | −8.3 | 0.70, p=.48 |
| Change Pre-FU6 | −10.1 | −11.2 | 0.72, p=.47 | −8.8 | −9.1 | 0.11, p=.91 | −8.7 | −8.8 | 0.02, p=.98 |
| Percentage of Treatment Responders (reduction of at least 8 points from baseline on the ISI) | |||||||||
|
|
|||||||||
| Pre | |||||||||
|
|
|||||||||
| Post | 71.1 (7.8) | 80.8 (10.8) | 0.46, p=.50 | 57.6 (7.1) | 23.6 (9.8) | 5.68, p=.02 | 81.6 (6.1) | 34.4 (14.5) | 7.51, p<.01 |
| FU6 | 74.1 (7.4) | 73.5 (11.3) | 0.00, p=.96 | 78.4 (6.6) | 45.2 (16.3) | 3.69, p=.06 | 52.5 (9.0) | 29.3 (13.8) | 1.65, p=.20 |
| Percentage in Remission (ISI score < 8) | |||||||||
|
|
|||||||||
| Pre | |||||||||
|
|
|||||||||
| Post | 56.8 (8.1) | 73.3 (12.2) | 1.10, p=.30 | 38.4 (7.5) | 20.2 (11.1) | 1.42, p=.23 | 51.1 (7.4) | 23.1 (12.0) | 2.86, p=.09 |
| FU6 | 57.5 (7.8) | 66.9 (13.2) | 0.35, p=.55 | 60.7 (7.8) | 39.2 (13.6) | 1.77, p=.19 | 41.6 (7.6) | 39.7 (14.0) | 0.01, p=.90 |
p < .05,
p < .01,
p < .001
Note. All means (standard errors) and change scores are adjusted for site effect. Error df = 317 for ISI and 150 for ISI Response and Remission.
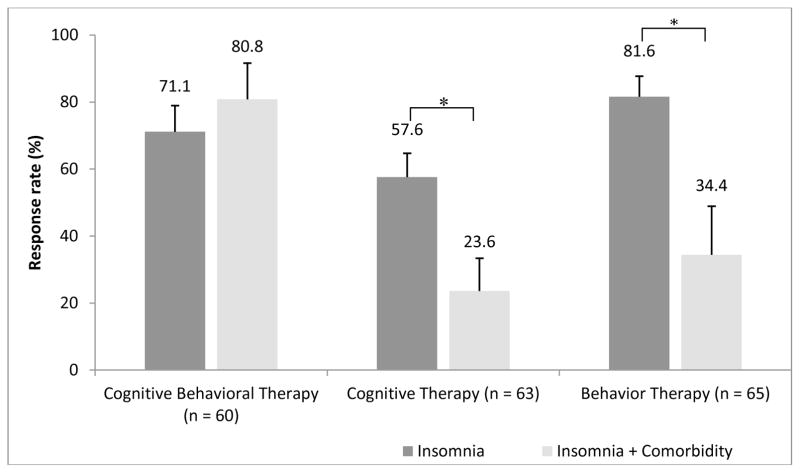
When the subgroups were compared with respect to rates of treatment responders, significant differences were observed at post treatment in the CT condition (57.6% vs. 23.6%, p=.02) and in the BT condition (81.6% vs. 34.4%, p<.01). In both conditions, there was a greater proportion of treatment responders in the subgroups without comorbidity but there was no such difference between subgroups in the CBT condition (71.1% vs. 80.8%, p=.50) (see Figure 1).
Figure 1.
Insomnia Treatment Response according to Condition and Psychiatric Comorbidity
Impact of insomnia treatment on anxiety and depression symptoms
Adjusted mean scores on the STAI and the BDI-II across treatment conditions, comorbidity status and time are displayed in Table 3. A significant time effect was observed on the STAI, F(2,306) = 21.26, p < .001, indicating that all six subgroups (3 treatment conditions x 2 comorbidity status) reduced their anxiety symptoms over time. The group x comorbidity x time interaction was not significant, p = .41. However, a significant comorbidity x time interaction was observed in the CBT condition, t(306)=2.45, p=.01. Simple effects showed a greater mean change score reported by those with a comorbid disorder than those without (M = −9.2 vs −2.5, p = .01) and this difference remained significant at the FU-6 (M = −3.0 vs. −8.1, p=.02). While mean scores between those with and without comorbidity were significantly different at baseline in all three conditions, they no longer were statistically different at post and FU-6 in the CBT condition. Such results were not observed in the CT and BT conditions. There were no significant differences on change scores between those with and without comorbidity and the mean scores remained statistically different between the subgroups.
Table 3.
Adjusted Means and Changes Scores on Anxiety and Depression Measures According to Group, Comorbidity Status and Time
| Cognitive-Behavior Therapy | Cognitive Therapy | Behavior Therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time (or change) | No comorbidity M (SE) | Comorbidy M (SE) | t | No comorbidity M (SE) | Comorbidy M (SE) | t | No comorbidity M (SE) | Comorbidy M (SE) | t |
| State-Trait Anxiety Inventory (STAI-T) | |||||||||
| Pre | 36.2 (1.0) | 44.6 (2.2) | 12.34, p<.01 | 36.0 (1.1) | 50.8 (3.0) | 21.70, p<.01 | 38.4 (1.4) | 45.6 (2.9) | 5.08, p=.02 |
| Post | 33.7 (1.2) | 35.4 (2.3) | 0.42, p=.51 | 33.1 (1.3) | 47.2 (3.9) | 11.70, p<.01 | 33.5 (1.3) | 41.6 (3.4) | 4.86, p=.03 |
| FU6 | 33.2 (1.3) | 36.5 (2.8) | 1.17, p=.28 | 32.4 (1.2) | 46.1 (4.3) | 9.74, p<.01 | 32.9 (1.4) | 39.4 (2.8 | 4.37, p=.04 |
| Change Pre-Post | −2.5 | −9.2 | 2.45, p=.01 | −2.9 | −3.6 | 0.28, p=.78 | −4.9 | −4.0 | 0.27, p=.78 |
| Change Pre-FU6 | −3.0 | −8.1 | 2.28, p=.02 | −3.6 | −4.7 | 0.34, p=.74 | −5.5 | −6.2 | 0.24, p=.81 |
| Beck Depression Inventory (BDI) | |||||||||
| Pre | 9.0 (0.6) | 14.1 (1.9) | 6.67, p=.01 | 8.3 (0.8) | 15.4 (2.5) | 7.26, p<.01 | 8.4 (0.8) | 15.1 (2.2) | 8.62, p<.01 |
| Post | 5.1 (0.6) | 3.5 (0.7) | 2.88, p=.09 | 4.1 (0.7) | 10.6 (2.9) | 4.84, p=.03 | 4.0 (0.6) | 8.4 (2.3) | 3.29, p=.07 |
| FU6 | 4.5 (0.5) | 5.5 (1.1) | 0.82, p=.37 | 4.8 (0.7) | 11.4 (3.3) | 3.91, p=.05 | 4.2 (0.6) | 8.1 (1.3) | 7.30, p<.01 |
| Change Pre-Post | −3.9 | −10.6 | 3.59, p<.01 | −4.2 | −4.8 | 0.21, p=.84 | −4.4 | −6.7 | 1.07, p=.29 |
| Change Pre-FU6 | −4.5 | −8.6 | 1.90, p=.06 | −3.5 | −4.0 | 0.18, p=.86 | −4.2 | −7.0 | 1.24, p=.22 |
p < .05,
p < .01,
p < .001
Note. All means (standard errors) and change scores are adjusted for site effect. Error df = 317 for ISI and 150 for ISI Response and Remission.
A similar result pattern was observed on the BDI-II. A significant main effect for time was observed, F(2,316) = 39.17, p < .001, but the group x comorbidity x time interaction failed to reach significance, p = .18. A significant comorbidity x time interaction was observed in the CBT condition, t(316) = 3.59, p<.001 and simple effects showed a greater pre to post mean change score in those with a comorbid disorder than in those without (M = −3.9 vs. −10.6, p<.001) but this difference failed to reach significance at FU-6 (M = −4.5 vs. −8.6, p=.06). In the CT and BT conditions, there were no significant differences on change scores between those with and without comorbidity and the mean scores between the groups remained significantly different at both post and FU-6.
Impact of insomnia treatment on presence of comorbid disorders
At post treatment, 65.7% of the comorbidity group no longer met the diagnostic criteria for the mood or anxiety disorder they presented at baseline and this rate was of 66.4% at FU-6. A significant difference was observed across treatment conditions at post treatment F(2,163) = 3.17, p = .04, with a significantly smaller remission rate in the CT condition compared with the rates observed in the two other conditions (CT, 40.1% vs 78.8%, BT and 80.3%, CBT). This difference was no longer significant at FU-6 (CT, 52.5 %; 68.2%, BT and 72.6%, CBT).
Discussion
These results were derived from a main study designed to compare the relative efficacy of BT and CT as single therapies for insomnia compared to full CBT-I (Harvey et al., 2014). In the present study, our goal was to assess if individuals with insomnia and a comorbid anxiety or depressive disorder would respond similarly as their counterparts without comorbidity to an intervention targeting insomnia. The main findings were that the presence of a comorbid anxiety or depressive disorder did not alter response to full CBT-I. Conversely, when treatment involved BT and CT as single therapies, treatment response was moderated by the presence of comorbidity.
Few studies have examined the impact of comorbidity on treatment response to CBT-I and data are inconsistent. Our results are more in line with data indicating that comorbid anxiety and depressive conditions tend to improve with interventions targeting insomnia even without specifically addressing the comordid conditions’ symptoms (Manber et al., 2008; Talbot et al., 2014), at least with respect to full CBT-I. However, this was not the case for single therapies as the proportions of responders were smaller in the comorbidity subgroups for both the BT (34.4% vs 81.6%) and CT conditions (23.6% vs 57.6%). These observations suggest that full CBT-I is more robust to the presence of comorbidity than either of its single components alone.
Also noteworthy is the greater reductions of anxiety and depression symptoms after CBT-I in the comorbidity subgroup compared to the group without. This group difference may be partly explained by a “floor effect” since anxiety and depression symptoms were already low at baseline in the subgroup without comorbidity. However, this hypothesis does not explain the magnitude of change in the CBT-I group or why significant changes would be observed in the CBT-I condition and not in the single therapies. This result was expected for BT since that intervention mainly addresses sleep scheduling behaviors, but it was not expected for CT. One of the hypotheses of the main study (Harvey et al., 2014) was that CT would have a greater impact than BT on daytime symptoms (including anxiety and depression), and be equivalent to CBT. A possible explanation is that eight sessions of CT, which is shorter than prior tests of CT for insomnia (Harvey et al., 2007) and shorter than typical CBT for depression and anxiety, may not have been sufficient to expect changes on these symptoms. Hence, it is possible that an adequate dose of CT requires more than the 8 sessions used in the current study. Nonetheless, taken together the present results, along with those from the parent study, suggest that there may be a synergetic effect between the behavioral aspects of sleep scheduling interventions and the cognitive interventions in CBT-I and that this effect may be more robust to comorbidity.
There is a widespread assumption that insomnia is a symptom of - or is secondary to -other psychiatric disorders such as depression or anxiety disorders and that the “primary” disorder’s core symptoms need to be targeted for sleep to improve. Our data suggest otherwise for CBT-I. First, the presence of a comorbid anxiety or depressive disorder does not appear to negatively impact treatment response regarding sleep outcomes, but most importantly, significant improvements on anxiety and depression measures were observed with insomnia treatment. Also, at post treatment, 65.7% of the comorbidity group no longer met the diagnostic criteria for the mood or anxiety disorder they presented with at baseline, an improvement that was maintained at FU-6. These findings are in line with the recent changes in DSM-5 diagnostic criteria of insomnia and specifically with the elimination of the distinction between primary and secondary insomnia. It would be of interest in future studies to examine whether treatment response to CBT-I varies based on whether the comorbid psychiatric condition preceded or followed the onset of insomnia. Likewise, prospective studies investigating the potential impact of early intervention for insomnia on reducing the occurrence of psychiatric disorders would be of significant interest.
Previous studies have shown that sleep difficulties, unless specifically targeted in treatment, tend to persist even if the anxiety and depression disorders’ core symptoms improved (Nierenberg et al., 2010). In showing that treating the sleep problem produces beneficial effects on comorbid depression and anxiety symptoms, the present study further highlights the importance of addressing sleep difficulties through sleep-specific interventions. However, generalization of our findings is limited. First, we had to pool disorders into one comorbidity sample in order to maximize sample size, which makes it impossible to further assess for differences by disorder types or even broader classes, i.e., depression versus anxiety disorders. Also, we included cases with mild to moderate depression; potential participants with more severe depression needing immediate treatment were excluded from this trial for clinical and ethical reasons. Individuals with a lifetime history of recurrent MDD were also excluded. Hence, the results from this study need to be replicated with a larger sample and with individuals presenting more severe psychiatric disorders and other conditions beside anxiety and depression. Such studies would allow to compare treatment response as a function of different disorders or classes of disorders and severity of symptoms. Other limitations also need to be taken into consideration. We cannot rule out that therapy session length may have had an impact on our results. CBT sessions could last up to 75 minutes in order to cover all aspects of treatment, in contrast to 50–60 minutes in BT and CT. Consequently, individuals in this condition had longer therapeutic contact and it is possible that those with comorbid anxiety or depression had more opportunities to engage in interventions targeting mechanisms common to insomnia, anxiety and depression.. Nevertheless, another plausible hypothesis for greater efficacy of full CBT-I is that experimenting with both, interventions targeting maladaptive behaviors and targeting cognitions, produces a synergetic effect between BT and CT interventions, which may enhance learning.
These results have important clinical implications as they show that comorbidity is not a contraindication to CBT-I, and that individuals may expect some relief from concurrent psychological symptoms as well. The latter is consistent with the proposal that the nature of insomnia and co-existing disorders is often bidirectional, with mutually maintaining symptoms such as anxiety and depression (Jansson-Frojmark & Lindblom, 2008; Sivertsen et al., 2012). On the other hand, those with a comorbid disorder receiving only some components of CBT-I did not fare as well as their counterparts without comorbidity. This is important in a context of budgetary constraints where there may be a temptation to restrict treatment of insomnia to its simpler therapy components. Consequently, presence of a comorbid disorder is an important clinical feature to consider when planning insomnia treatment.
Public Health Significance Statement.
This study indicates that cognitive behavioral therapy is effective for treating insomnia disorder, even when there is a co-existing anxiety disorder or a depressive disorder of mild to moderate severity.
Individuals with a co-existing anxiety disorder or mild to moderate depression disorder may also expect relief from their concurrent psychological symptoms even when treatment focuses specifically on insomnia.
The findings suggest that it is important to include both the behavioral and cognitive components of CBT to optimize treatment outcomes.
Acknowledgments
This research was supported by the National Institute of Mental Health Grant RO1MH079188 (CM, AH). Charles Morin has received research grant from Novartis.
Footnotes
No other conflict of interest to disclose from the other authors.
Trial Registration: Clinical Trial #NCT 00869934
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Publishing; 2013. DSM-5. [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, … Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for gad on insomnia symptoms. J Anxiety Disord. 2004;18(4):561–571. doi: 10.1016/S0887-6185(03)00031-8. [DOI] [PubMed] [Google Scholar]
- Bélanger L, Savard J, Morin CM. Clinical management of insomnia using cognitive therapy. Behavioral Sleep Medicine. 2006;4(3):179–198. doi: 10.1207/s15402010bsm0403_4. [DOI] [PubMed] [Google Scholar]
- Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clinical Psychology Review. 2011;31(4):638–652. doi: 10.1016/j.cpr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Epstein D, Wood JM. Stimulus Control Instructions. In: Hauri P, editor. Case Studies in Insomnia. New York: Plenum Press; 1991. pp. 19–28. [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia. Journal of the American Medical Association. 2013;309(7):706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Publishers; 1988. [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, … Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, Carney CE. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Paper presented at the 23rd Annual Meeting of the Associated Professionnal Sleep Societies; Seatle: LLC; 2009. [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition (SCID-I/P, Version 2.0) New York: Biomedics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Harvey AG. A cognitive model of insomnia. Behaviour Research and Therapy. 2002;40(8):869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Harvey AG. What about patients who can’t sleep? Case formulation for insomnia. In: Tarrier N, editor. Case Formulation in Cognitive Behaviour Therapy: The Treatement of Challenging and Complex Clinical Cases. New York: Brunner-Routledge; 2006. [Google Scholar]
- Harvey AG. Insomnia, psychiatric disorders, and the transdiagnostic perspective. Current Directions in Psychological Science. 2008;17(5):299–303. [Google Scholar]
- Harvey AG, Belanger L, Talbot L, Eidelman P, Beaulieu-Bonneau S, Fortier-Brochu E, … Morin CM. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: a randomized controlled trial. Journal of Consult and Clinical Psychology. 2014;82(4):670–683. doi: 10.1037/a0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007;45(10):2491–2501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychological bulletin. 2012;138(1):77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Jansson-Frojmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research. 2008;64(4):443–449. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behaviour Research and Therapy. 2002;40(7):741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum; 2003. [Google Scholar]
- Morin CM, Stone J, McDonald K, Jones S. Psychological management of insomnia: a clinical replication series with 100 patients. Behavior therapy. 1994;25(2):291–309. doi: 10.1016/S0005-7894(05)80289-8. [DOI] [Google Scholar]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, … Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. Journal of the American Medical Association. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep. 2005;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, … Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychological Medicine. 2010;40(1):41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Aloia M, Kuhn B. Behavioral treatment for sleep disorders: A comprehensive primer of behavioral sleep medicine. Amsterdam: Elsevier; 2011. [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Md: U.S. Dept. of Health Education and Welfare; 1968. [Google Scholar]
- Ree M, Harvey AG. Insomnia. In: Bennet-Levy J, Butler G, Fennell M, Hackman A, Muelles M, Westbrook D, editors. Oxford Guide to Behavioural Experiments in Cognitive Therapy. Oxford: Oxford University Press; 2004. pp. 287–305. [Google Scholar]
- Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biological Psychiatry. 2006;60(12):1364–1371. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Morin CM, Foley K, Kalsekar A, Walsh JK. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: insomnia severity and comorbidities. Sleep Medicine. 2010;11(1):69–74. doi: 10.1016/j.sleep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, … Overland S. The bidirectional association between depression and insomnia: the HUNT study. Psychosomatic Medicine. 2012;74(7):758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Spielman AJ, Glovinsky PB. The varied nature of insomnia. In: Hauri P, editor. Case Studies in Insomnia. New York: Plenum Press; 1991. pp. 1–15. [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, … Neylan TC. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NK, Harvey AG. Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behaviour Research and Therapy. 2004;42(1):27–39. doi: 10.1016/s0005-7967(03)00068-8. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- Wagley JN, Rybarczyk B, Nay WT, Danish S, Lund HG. Effectiveness of abbreviated CBT for insomnia in psychiatric outpatients: sleep and depression outcomes. Journal of clinical psychology. 2013;69(10):1043–1055. doi: 10.1002/jclp.21927. [DOI] [PubMed] [Google Scholar]
- Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. Journal of Traumatic Stress. 2004;17(1):69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]