Abstract
Objective
Patients with metastatic gastroesophageal adenocarcinoma (MGEAC) have a poor but heterogeneous clinical course. Some patients have an unusually favorable outcome. We sought to identify clinical variables associated with with more favorable outcomes.
Methods
Of 246 patients with MGEAC, we identified 64 who received systemic therapy and eventually received local consolidation therapy. Univariate and multivariate Cox regression models were used and a nomogram was developed.
Results
Among these 64 patients, 61% had received with consolidation chemoradiation (CRT) with doses of 50–55Gy and did not have surgery (78%). The median follow-up time of survivors was 3.9 years and median overall survival (OS) from CRT start was 1.5 years (95% CI, 1.2–2.2 years). Surgery (as local consolidation) was an independent prognosticator for longer OS in the multivariate analysis (p=0.02). The 5-year OS rate was 25% (SE=6%). The contributors to the nomogram were longer duration of systemic therapy pre-CRT and the type of local therapy.
Conclusions
Our data suggest that a subset of patients with MGEAC have an excellent prognosis (OS >5 years), however, such patients need to be identified during their clinical course so that local consolidation (CRT, surgery, or both) may be offered.
Keywords: Metastatic gastroesophageal adenocarcinoma, Consolidative local therapy, Systemic therapy, Overall survival
Introduction
In 2015, the American Cancer Society estimated that the number of new cases and deaths from esophageal cancer will be ~16,980 and 15,590 respectively and those from gastric cancer will be ~24,590 and 10,720, respectively in the United States [1, 2]. The 5-year overall survival (OS) rate of patients with distant metastasis, however, is only 4% [1,2, 3] Nevertheless, some patients with metastatic gastroesophageal adenocarcinoma (MGEAC) survive beyond 5 years.
Standard of care therapy for patients with MGEAC is palliative systemic therapy combined with best supportive care [4, 5]. This approach has considerable limitations but it can produce dramatic tumor regression in some patients [6, 7]. Whether such patients should receive consolidative local therapies (such as chemoradiation and/or surgery) is unclear. It may be much less important to know if local therapy is technically feasible in MGEAC patients at some point in their clinical course, but more important question is to determine when it local therapy should be recommended so that patients derive the highest benefits from it. Currently, there is no established algorithm and the published literature does not provide clear guidance. First principles would dictate that local therapy in patients with MGEAC should be delayed because if it is offered early (e.g., within 6 months of the start of systemic therapy), there remains a considerable risk of the manifestation of additional metastatic disease.
The purpose of our study is to identify clinical features that may help select patients who are likely to benefit from eventual local therapy. We also established a nomogram to make it potentially a practical endeavor [8].
Methods and Patients
Patients
From our database in the Department of GI Medical Oncology at UT MD Anderson Cancer Center (UTMDACC), we identified 246 consecutive patients with MGEAC between 2003 and 2014. Of these, 64 had received “consolidation” local therapy and were the subjects of this analysis. All patients had adenocarcinoma of the esophagus, gastroesophageal junction, or stomach and each patient had documented metastases (stage IV). All patients were staged by standard methods and stages were assigned by the American Joint Committee on Cancer (AJCC) 6th edition[9]. Prior to receiving any local therapy, each patient was discussed in the multidisciplinary conference to develop a consensus. Some patients were discussed more than once. The Institutional Review Board of the UTMDACC approved this analysis.
Treatment and follow up
All 64 patients received systemic therapy for at least 6 months) before being considered for local therapy. The preferred local therapy was chemoradiation because of the morbidity associated with surgery. Chemotherapy included a fluoropyrimidine (intravenous or oral) and either a platinum compound or a taxane. The dose of radiation ranged from 45 – 65 Gy being delivered in 25 – 28 fractions. Surgery, when agreed upon, was performed 6–8 weeks after the end of chemoradiation. The chemotherapy regimen, radiation dose, and surgical techniques were at the discretion of the treating physician or surgeon.
After local therapy was completed, patients were followed at 3–6 month intervals in the first 3 years and then less frequently.
Survival
Date of death was identified based on electronic health records, tumor registry, or the Social Security Database.
Statistical Methods
Patient characteristics were summarized using descriptive statistics. OS was defined as the number of years between date of chemoradiation start and death from any cause, and was censored at last follow-up for living patients. Survival curves were estimated using the Kaplan-Meier methods [10] and median time was reported with a 95% confidence interval (CI). Univariate and multivariate Cox proportional hazards regression models [11] were used to assess the association between patient characteristics and OS. Surgery was treated as a time-varying covariate changing from no to yes on the date of surgery. Duration of chemotherapy and any patient characteristics that were significant in the univariate model at the 0.10 level were included in a multivariate model (full model). Duration of Chemotherapy was required to remain in the multivariate model regardless of significance level. Then, backward elimination was implemented until all remaining predictors had a p-value less than 0.05 (reduced model). A nomogram [12] was built to predict OS based on univariate Cox analyses for information available at the start of chemoradiation, including patient characteristics that were significant at the 0.10 level. Discrimination was evaluated using the concordance index (C-index), with 200 bootstrap samples. Statistical analyses were performed in SAS 9.3 [The SAS Institute, Cary, NC], figures were created in Stata 13.1 [Stata Corp, College Station, TX], and the nomogram was created in S-plus [TIBCO Corporation, Palo Alto, CA].
Results
Patient Characteristics
A total of 64 patients with MGEAC who received local consolidation after systemic chemotherapy between February 2003 and January 2014 were identified. Table 1 shows that patients were primarily men (81%), with poorly differentiated MGEAC (63%), good ECOG PS scores (≤1; 90%), distant lymph node metastasis (52%), received chemoradiation with dose range 50 – 55 Gy (61%), and did not undergo surgery (78%).
Table 1.
Patient Characteristics
| Characteristics | N (%) |
|---|---|
| All | 64 (100%) |
|
| |
| At Diagnosis | |
|
| |
| Age at Presentation at MDACC - median (min,max) | |
| N=64 | 58 (32,75) |
| Chemotherapy Duration (months) - median (min,max) | |
| N=64 | 3.7 (0.9,14.1) |
| SUV Uptake of Primary At Baseline | |
| N=46 | 13.6 (0,50) |
| SUV Uptake of Primary After Chemotherapy | |
| N=38 | 5.7 (0,42) |
| Gender | |
| Male | 52 (81%) |
| Female | 12 (19%) |
| Histology Grade | |
| G2 Moderately differentiated | 24 (38%) |
| G3 Poorly differentiated | 40 (63%) |
| ECOG PS | |
| 0 | 20 (31%) |
| 1 | 38 (59%) |
| 2 | 4 (6%) |
| Missing | 2 (3%) |
| Location of Tumor | |
| Esophagus | 6 (9%) |
| AEG 1 | 16 (25%) |
| AEG 2 | 15 (23%) |
| AEG 3 | 13 (20%) |
| Gastric | 14 (22%) |
| Distribution of distant metastasis | |
| Cytology | 13 (20%) |
| Peritoneum | 9 (14%) |
| Distant lymph nodes | 33 (52%) |
| Peritoneum and distant lymph nodes | 1 (2%) |
| Visceral | 8 (13%) |
| HER2 | |
| Positive | 3 (5%) |
| Negative | 27 (42%) |
| N/S | 34 (53%) |
| Adenocarcinoma Subtype | |
| SRC- Signet Ring Carcinoma | 10 (16%) |
| M & SRC | 2 (3%) |
| NE- Neuro Endocrine | 2 (3%) |
| NOS- Not otherwise specified | 50 (78%) |
|
| |
|
Treatment
| |
| Dose of Consolidation (Gy) | |
| 40–50 | 21 (33%) |
| 50–55 | 39 (61%) |
| >55 | 3 (5%) |
| Unknown | 1 (2%) |
| Surgery | |
| Yes | 14 (22%) |
| No | 50 (78%) |
ECOG PS= Eastern Cooperative Oncology Group Performance Score; AEG=Adenocarcinoma of Esophagogastric Junction; HER2=Human Epidermal Growth Factor Receptor 2; M= Mucinous
Overall Survival
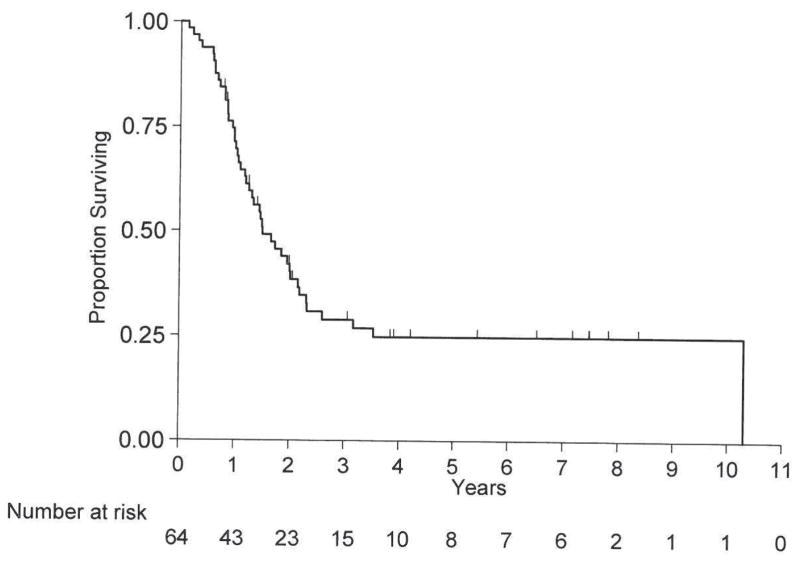
The median follow-up time for survivors was 3.9 years (range, 0.8 to 8.4 years). Forty-five (70%) of 64 patients died and 19 (30%) were alive at the last follow-up. The median OS was 1.5 years (95% CI, 1.2 – 2.2 years). The 1-year OS rate was 71% (SE=6%). The 5-year OS rate was 25% (SE=6%; Figure 1). Table 2 shows additional OS information. Only surgery (p=0.03) was significantly associated with OS. Ninety-two percent of patients who had surgery and 67% of patients who did not have surgery were alive at 1 year.
Figure 1.
Overall Survival
Table 2.
Survival Outcomes by Patient Characteristics
| Characteristic | Level | OS
|
||
|---|---|---|---|---|
| Deaths / Total | 1-yr (SE) | P-value | ||
| All Patients | 45 / 64 | 71%(6%) | ||
|
| ||||
| Univariate | ||||
|
| ||||
| Age* | 0.45 | |||
| <60 | 23 / 37 | 75%(7%) | ||
| >=60 | 22 / 27 | 65%(9%) | ||
|
| ||||
| Chemotherapy Duration* | 0.15 | |||
| <4 | 29 / 38 | 68%(8%) | ||
| ≥4 | 16 / 26 | 74%(9%) | ||
|
| ||||
| Log(SUV Uptake of Primary At Baseline)* | 0.76 | |||
| <2.61 | 17 / 22 | 72%(10%) | ||
| ≥2.61 | 19 / 24 | 62%(10%) | ||
|
| ||||
| Log(SUV Uptake of Primary After Chemotherapy)* | 0.30 | |||
| <1.74 | 10 / 19 | 78%(10%) | ||
| ≥1.74 | 16 / 19 | 62%(11%) | ||
|
| ||||
| Gender | 0.20 | |||
| Male | 38 / 52 | 66%(7%) | ||
| Female | 7 / 12 | 92%(8%) | ||
|
| ||||
| Histology Grade | 0.76 | |||
| G2 Moderately differentiated | 17 / 24 | 62%(10%) | ||
| G3 Poorly differentiated | 28 / 40 | 77%(7%) | ||
|
| ||||
| ECOG PS | 0.83 | |||
| 0 | 13 / 20 | 70%(10%) | ||
| ≥1 | 31 / 42 | 70%(7%) | ||
|
| ||||
| Location of Tumor | 0.11 | |||
| Esophagus/AEG 1/AEG 2 | 28 / 37 | 62%(8%) | ||
| Gastric/AEG 3 | 17 / 27 | 85%(7%) | ||
|
| ||||
| Distribution of Distant Metastases | 0.86 | |||
| Cytology | 9 / 13 | 68%(13%) | ||
| Distant lymph node | 24 / 33 | 72%(8%) | ||
| Peritoneum/Peritoneum+ Distant lymph node | 7 / 10 | 70%(14%) | ||
| Visceral | 5 / 8 | 71%(17%) | ||
|
| ||||
| Adenocarcinoma Subgroup | 0.40 | |||
| SRC/M &SRC | 9 / 12 | 64%(14%) | ||
| NE/NOS | 36 / 52 | 73%(6%) | ||
|
| ||||
| Type of Consolidation | 0.08 | |||
| 40–50 | 11 / 21 | 90%(7%) | ||
| >50 | 33 / 42 | 62%(7%) | ||
|
| ||||
| Surgery** | 0.03 | |||
| Yes | 8 / 14 | 92%(7%) | ||
| No | 37 / 64 | 67%(7%) | ||
OS=Overall Survival; SE=Standard Error; SUV=Standardized Uptake Value; ECOG PS= Eastern Cooperative Oncology Group Performance Score; AEG=Adenocarcinoma of Esophagogastric Junction; SRC=Signet Ring Carcinoma; M= Mucinous; NE= Neuro Endocrine; NOS=Not Otherwise Specified.
P-values were calculated based on continuous values for age at presentation at MDACC, duration of all chemo, and logarithm of SUV uptake of primary at baseline and after chemotherapy, and excluded patients with missing values for that characteristic.
Time varying covariate. All patients begin in the “No” group. Patients who receive surgery are censored in the “No” group on the date of surgery. Then they start in the “Yes” group on that date. 1-year OS for “Yes” patients is the time since surgery.
Table 3 shows the multivariate analysis for OS. Duration of chemotherapy before chemoradiation, dose of consolidation chemoradiation and surgery were included in the full model. Duration of chemotherapy was required to remain in the model and only surgery remained significant in the reduced model. Patients with surgery were 63% less likely to die than those without surgery (HR=0.37; p=0.02) after accounting for chemotherapy duration, and including surgery as a time-varying covariate.
Table 3.
Multivariate Cox Proportional Hazards Models for OS
| Characteristic | Full Model (Death/Total=44/63) | Reduced Model (Death/Total=45/64) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Chemotherapy Duration (years)* | 0.91 | (0.81,1.02) | 0.10 | 0.90 | (0.80,1.01) | 0.08 | |
| Dose of Consolidation (Gy) | >50 vs. 40–50 | 1.58 | (0.77,3.25) | 0.21 | |||
| Surgery** | Yes vs. No | 0.41 | (0.18,0.95) | 0.04 | 0.37 | (0.16,0.85) | 0.02 |
HR=Hazard Ratio; CI=Confidence Interval.
Duration of Chemotherapy was required to remain in the model regardless of significance level.
Time varying covariates. All patients begin in the “No” group. Patients who receive surgery are censored in the “No” group on the date of surgery. Then they start in the “Yes” group on that date.
Nomogram
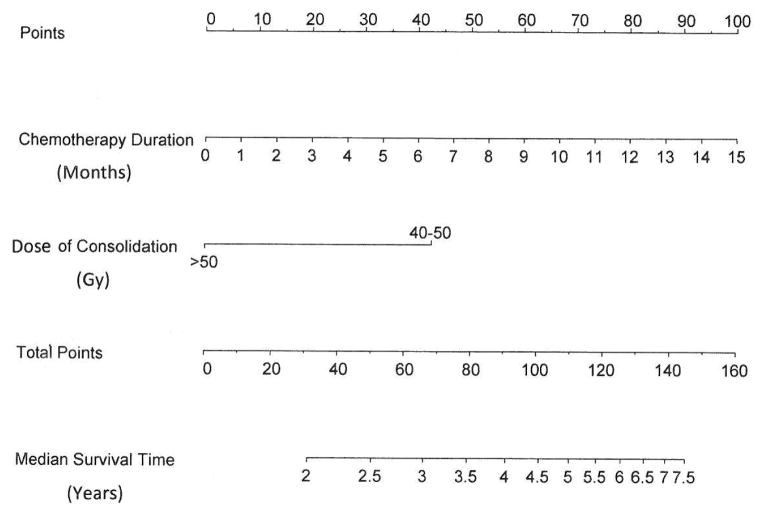
A nomogram (Figure 2) was developed to predict OS. The predictors included duration of chemotherapy and dose of consolidation since they had p-values less than 0.10 and are readily available before chemoradiaton. The ‘total points’ correspond to the median OS time in years. Internal validation showed that the C-index for the model was 0.61.
Figure 2.
Nomogram Predicting Overall Survival at the start of CRT
Discussion
Prognosis of MGEAC is poor with a median OS between 8.6 and 13.8 months [5, 13]. However, some patients with MGEAC do achieve favorable outcomes. In this analysis, 8 patients survived longer than 5 years but had no specific clinical variable that could prospectively identify them. Therefore, our multidisciplinary team cannot identify them at their initial presentation but they can be selected based on their clinical course and the decision to administer local consolidation. Currently, there is no algorithm to help with this selection process.
We present one of the largest cohorts of patients who first received systemic therapy and then received consolidative local therapy. A major characteristic of these patients is that they have excellent tumor regression from systemic therapy and the duration of response is unusually long (>6 months). These patients also do not develop new metastases. However, since systemic therapy is not curative and the primary tumor is rarely eradicated by systemic therapy it is rationale to selectively use local consolidation. Our preference is also to use chemoradiation rather than surgery. The nomogram was developed to help construct selection of these patients. However, it should be emphasized that this nomogram is prognostic only, and it is also not yet validated.
It should be emphasized that our report has shortcomings given that it is a retrospective review of data from a single institution. However, since literature lacks guidance on this issue, we believe the results may be useful for other groups. We encourage more reports on this subject and improved awareness of the fact that even patients with stage IV disease can have long-term OS (>5 years) with this type of approach.
Acknowledgments
This study is supported by generous grants from the Caporella, Dallas, Sultan, Park, Smith, Frazier, Oaks, Vanstekelenberg, McNeil, Planjery, and Cantu Families, as well as from the Schecter Private Foundation, Rivercreek Foundation, Kevin Fund, Myer Fund, Dio Fund, Milrod Fund, and multidisciplinary grants from the University of Texas M. D. Anderson Cancer Center, Houston, USA. It is also supported in part by the National Cancer Institute awards CA138671, CA172741, CA129926 (JAA) and P30CA016672 and used the Biostatistics Resource Group.
Footnotes
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Cancer Facts and Figures. 2015 [ http://www.cancer.org/cancer/esophaguscancer/detailedguide/esophagus-cancer-key-statistics]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.SEER Stat Fact Sheets. Esophagus. [ http://seer.cancer.gov/statfacts/html/esoph.html]
- 4.Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:194–227. doi: 10.6004/jnccn.2015.0028. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, Pauligk C, Luley K, Bichev D, Schumacher G, et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:2068–2073. doi: 10.1093/annonc/mdt141. [DOI] [PubMed] [Google Scholar]
- 8.Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14:4400–4407. doi: 10.1158/1078-0432.CCR-07-4713. [DOI] [PubMed] [Google Scholar]
- 9.AJCC. AJCC cancer staging manual. 6. New York: 2002. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 11.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 12.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; 2001. [Google Scholar]
- 13.Elimova E, Shiozaki H, Wadhwa R, Sudo K, Chen Q, Estrella JS, Blum MA, Badgwell B, Das P, Song S, et al. Medical management of gastric cancer: a 2014 update. World J Gastroenterol. 2014;20:13637–13647. doi: 10.3748/wjg.v20.i38.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]