Abstract
Objective
Hepatic ischemia-reperfusion (I/R) is a major clinical problem with limited treatment options. The pathophysiology of hepatic I/R is characterized by mitochondrial dysfunction and cellular energy deficits. Sirtuin 1 (Sirt1) is an energy-sensing enzyme known to modulate mitochondrial biogenesis. We hypothesized that pharmacologic activation of Sirt1 is protective after hepatic I/R injury.
Design
Animal study.
Setting
University-based experimental laboratory.
Subjects
Wild-type C57BL/6 mice.
Interventions
C57BL/6 mice were subjected to 60-min partial hepatic I/R and post-treated with Sirt1 activator, SRT1720 (20 mg/kg), or vehicle. Blood and liver were collected at 24 h after I/R for analyses of hepatic injury, ATP levels, mitochondrial mass, autophagy, inflammation, and oxidative stress. H4IIE hepatoma cells and rat primary hepatocytes were incubated with oxyrase to induce hypoxia followed by reoxygenation (H/R) in the presence or absence of SRT1720 for assessment of mitochondrial mass, mitochondrial membrane potential, and autophagy.
Measurements and Main Results
SRT1720 restored the reduction in mitochondrial mass, enhanced autophagy, and preserved ATP levels in the liver after I/R, which was associated with a decrease in I/R-induced hepatic injury, apoptosis, and necrosis. I/R-induced inflammation was also significantly reduced by SRT1720 as measured by systemic and hepatic cytokine and chemokine levels as well as a decrease in neutrophil infiltration to the liver. Further, oxidative stress was markedly attenuated in the SRT1720-treated mice, compared to the vehicle. SRT1720 treatment increased ATP levels and survival of cultured hepatocytes after H/R. SRT1720 not only increased the mitochondrial mass but also increased mitochondrial membrane potential per cell in cultured hepatocytes after H/R. Moreover, SRT1720 prevented the H/R-induced mitochondrial depolarization and resulted in an enhancement of autophagy in cultured hepatocytes after H/R.
Conclusions
Pharmacologic stimulation of Sirt1 attenuates liver injury after hepatic I/R by restoring mitochondrial mass and membrane potential, which is associated with the enhancement of autophagy.
Keywords: Sirtuin 1, SRT1720, hepatic ischemia-reperfusion, mitochondria, mitochondrial depolarization, autophagy
Introduction
Hepatic ischemia-reperfusion (I/R) injury most commonly occurs after oncologic liver resection, transplantation, and circulatory shock (1). Specifically, I/R-injury has been associated with delayed graft function and biliary complications after liver transplantation (2, 3). The pathophysiology of I/R is characterized by energy depletion, sterile inflammation, and oxidative injury, leading to cell death (4). Despite our increased understanding of the mechanisms behind hepatic I/R injury, the clinical management remains limited to preventive and supportive care (5).
Mitochondria are the major organelles responsible for ATP generation in the cell. They work by pumping protons through the respiratory chain into the intermembrane space to form the mitochondrial membrane potential (mtMP) (6). The mtMP drives ATP generation and its disruption, also known as mitochondrial depolarization, halts ATP synthesis (6). Therefore, mtMP can be used as a reliable measure of mitochondrial activity (6, 7). In I/R injury, the ischemic phase is characterized by ATP depletion due to a switch to anaerobic respiration. Reperfusion is usually accompanied by the disruption of mitochondrial function, which in turn hampers the cellular attempt at regaining the energy and metabolic homeostasis for cell survival (8, 9). Given the underlying mitochondrial dysfunction in I/R-induced injury, we sought to investigate a therapeutic strategy aimed at preserving or restoring mitochondrial integrity to protect organs against I/R injury.
The autophagy salvage pathway is an additional means of energy generation in cells and can be activated by various cellular stressors, including hypoxia and nutrient starvation (10). Autophagy is a process of degradation and recycling of large molecules and dysfunctional organelles. In this process, targeted cellular cargo are engulfed by an autophagic vesicle derived from the cell membrane (10). The vesicle is then acidified via lysosomal fusion, which initiates the enzymatic degradation of its cargo (11). In this context, autophagy demonstrates protective effects in various settings of nutrient scarcity and energy depletion (12, 13).
Sirtuins are a family of seven highly conserved mammalian proteins involved in cellular energy homeostasis (14). Among them, sirtuin 1 (Sirt1) is a well-characterized energy-sensing enzyme which responds to stress, starvation, and caloric restriction (15). Sirt1 localizes to both the nucleus and cytoplasm, and its deacetylase activity is dependent on nicotinamide adenine dinucleotide (NAD+) (14). Activation of Sirt1 promotes the transcription of genes in the regulation of mitochondrial biogenesis to maintain energy and metabolic homeostasis (16, 17). Thus, modulation of Sirt1 activity in various disease conditions has been gaining traction and multiple studies have shown its beneficial effects in protecting various organs from I/R induced injury (18-22).
In this study, we hypothesized that pharmacologic activation of Sirt1would restore I/R-induced mitochondrial dysfunction and enhance autophagy, thereby attenuating hepatic I/R injury. To test our hypothesis, we first used an established mouse model of partial hepatic ischemia (23, 24). Animals subjected to ischemia were post-treated with SRT1720, a small molecule allosteric stimulator of Sirt1 (25). The effects of SRT1720 on the liver after I/R were analyzed using various injury, metabolic, and inflammatory parameters. In addition, a cell culture model of hypoxia-reoxygenation (H/R) injury was also employed to validate the effects of SRT1720 on mitochondrial mass, mitochondrial membrane potential, and autophagy at the cellular level in hepatocytes.
Methods
Animal Experiments
Male C57BL/6 mice (10-12 weeks old, n = 8/group) underwent 60 minutes of 70% hepatic ischemia as detailed in Supplementary Methods. SRT1720 (20 mg/kg body weight, AdooQ Bioscience, Irvine, CA) or 10% dimethyl sulfoxide (DMSO) in normal saline (vehicle) was intravenously injected at the start of reperfusion. Control animals did not undergo any surgical procedures. Blood and liver samples were collected at 24 h after reperfusion. Methods for measurement of serum liver enzymes, histologic evaluation of liver tissues, immunohistochemistry, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, measurement of caspase-3 activity, measurement of myeloperoxidase (MPO) activity, measurement of cytokine levels, Western blot technique and RT-PCR analysis are detailed in Supplementary Methods. All experiments were performed in accordance with the National Institutes of Health guidelines for use of experimental animals, and approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Cell Culture Experiments
Rat H4IIE hepatoma cells and primary hepatocytes were plated overnight and subjected to H/R as detailed in Supplementary Methods. Briefly, hypoxia was induced using 5% oxyrase (Oxyrase, Mansfield, OH) in glucose-free, pyruvate-free Dulbecco's Modified Eagle's medium (DMEM). Oxyrase is an enzyme derived from the cytoplasmic membrane of E. coli, which consumes dissolved O2 in the media. Reoxygenation was achieved by refreshing with DMEM medium containing 10% heat-inactivated fetal bovine serum, 1% penicillin-streptomycin and 2 mM glutamine. Cells were treated with 0.1% DMSO or SRT1720 at reoxygenation. Measurements of cell survival, mitochondrial mass, mitochondrial membrane potential, and assessments of autophagic vesicles are detailed in Supplementary Methods.
Statistical Analysis
All data are expressed as mean ± standard error (SE) and compared by one-way analysis of variance and the Student-Newman-Keuls test. Differences in values were considered significant if P < 0.05.
Results
SRT1720 Restores Cellular Energy Status after Hepatic I/R
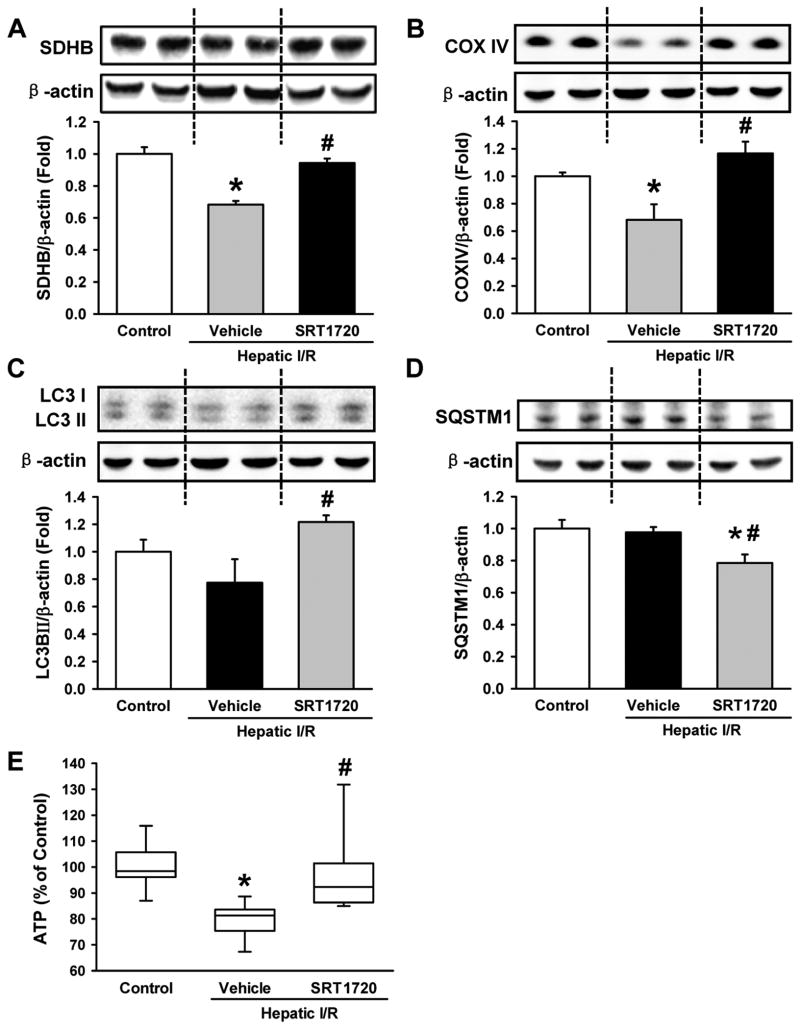
Succinate dehydrogenase subunit B (SDHB) and cytochrome oxidase complex IV (COXIV) are mitochondrial proteins and major components of the respiratory chain, which have been used as markers of mitochondrial mass (26). Both SDHB and COXIV were significantly decreased by 31.8% and 31.9%, respectively, in the livers of the vehicle group compared to the control (Fig. 1A, B). SRT1720-treated mice had near baseline levels of SDHB and COXIV in the liver, which was 1.4- and 1.7-fold higher than the vehicle group, respectively (Fig. 1A, B).
Figure 1.
SRT1720 restores cellular energy status after hepatic ischemia-reperfusion (I/R). Ischemic liver tissues of the vehicle- and SRT1720-treated mice were collected at 24 h after reperfusion. Relative expression of the mitochondrial proteins (A) succinate dehydrogenase subunit B (SDHB) and (B) cytochrome oxidase IV (COXIV) as well as the autophagy markers (C) microtubule associate protein 1 light chain 3 (LC3) and (D) sequesterome 1 (SQSTM1) were measured in the liver by Western blot. (E) Adenosine triphosphate (ATP) levels in the liver lysates were measured. Data are presented as means ± SE (n = 6-8/group). *P < 0.05 versus control; #P < 0.05 versus vehicle.
LC3II protein is a marker of autophagy and its expression level has been correlated to the number of autophagic vesicles (27). We observed a 1.6-fold increase in LC3II expression in the SRT1720-treated mice compared to the vehicle group after I/R (Fig. 1C). Sequesterome 1 (SQSTM1) is an intracellular protein that is degraded by autophagy (27). The level of SQSTM1 was significantly decreased by 19.1% in the SRT1720-treated mice compared to the vehicle. (Fig. 1D). In addition, ATP levels in the liver were decreased by 21% in the vehicle group compared to the control (Fig. 1E). In contrast, ATP levels in the liver of the SRT1720-treated mice were close to control levels (Fig. 1E). However, there were no significant changes in the expression levels of Sirt1 protein among the groups (data not shown). These results suggest that SRT1720 treatment facilitated the recovery of ATP, which was associated with increased mitochondrial mass and autophagy in the liver after I/R.
SRT1720 Attenuates Liver Injury after Hepatic I/R
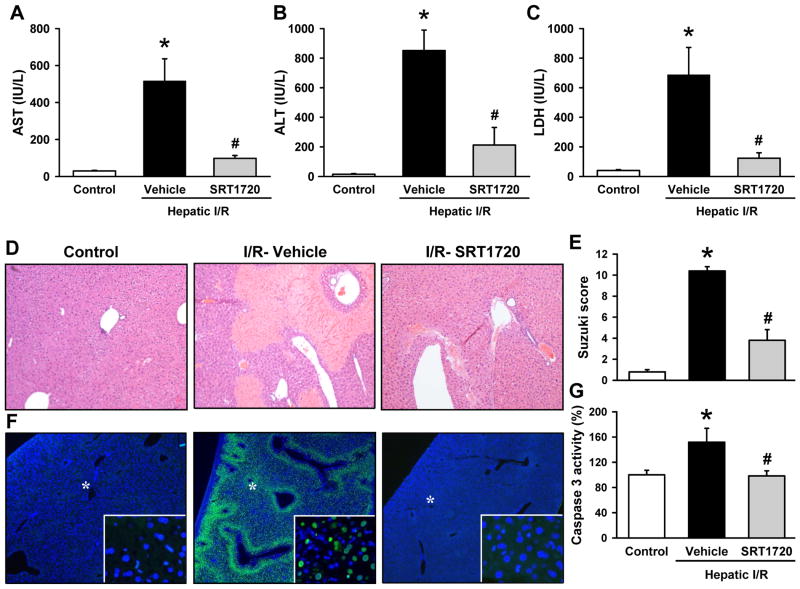
Serum levels of AST, ALT, and LDH were used as indicators of hepatic injury, all of which were dramatically increased in the vehicle group after I/R (Fig. 2A-C). Treatment with SRT1720 decreased the levels of AST by 81.0%, ALT by 75.1%, and LDH by 82.1% when compared to the vehicle group (Fig. 2A-C).
Figure 2.
SRT1720 attenuates hepatic injury after ischemia-reperfusion (I/R). Ischemic liver tissues and blood of the vehicle- and SRT1720-treated mice were collected at 24 h after reperfusion. Serum levels of hepatic injury markers (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), and (C) lactate dehydrogenase (LDH) were measured. (D) Representative photomicrographs of histological staining with hematoxylin & eosin of liver tissues at 100× magnification. (E) Extent of liver injury was graded using the Suzuki score by a blinded investigator as described in the Supplementary Methods. (F) Representative photomicrographs of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (green) and 4′,6-diamidino-2-phenylindole (DAPI) as nuclear counterstained (blue) at 40× magnification. *Indicates the selected areas shown in the insets at 400× magnification. (G) Caspase 3 activity was determined in the liver lysates. Data are presented as means ± SE (n = 6-8/group). *P < 0.05 versus control; #P < 0.05 versus vehicle.
Histologic assessment of the liver on H&E revealed significant areas of necrosis and congestion extending towards the portal triad in the vehicle group (Fig. 2D). This was markedly improved with SRT1720 treatment, where necrosis was limited to the immediate centrilobular area (Fig. 2D). The extent of hepatic injury in the vehicle group and the subsequent protection by the treatment are semi-quantitatively represented using the Suzuki classification (Fig. 2E).
A TUNEL assay was conducted to assess for apoptotic cell death after hepatic I/R (28). The vehicle-treated group had numerous TUNEL-positive nuclei, which were absent in both control and SRT1720-treated mice after I/R (Fig. 2F). This was further supported by a 51.8% increase in caspase 3 activity in the vehicle group as compared to the control, and a decrease to baseline control levels with SRT1720 treatment (Fig. 2G).
SRT1720 Decreases Inflammation after Hepatic I/R
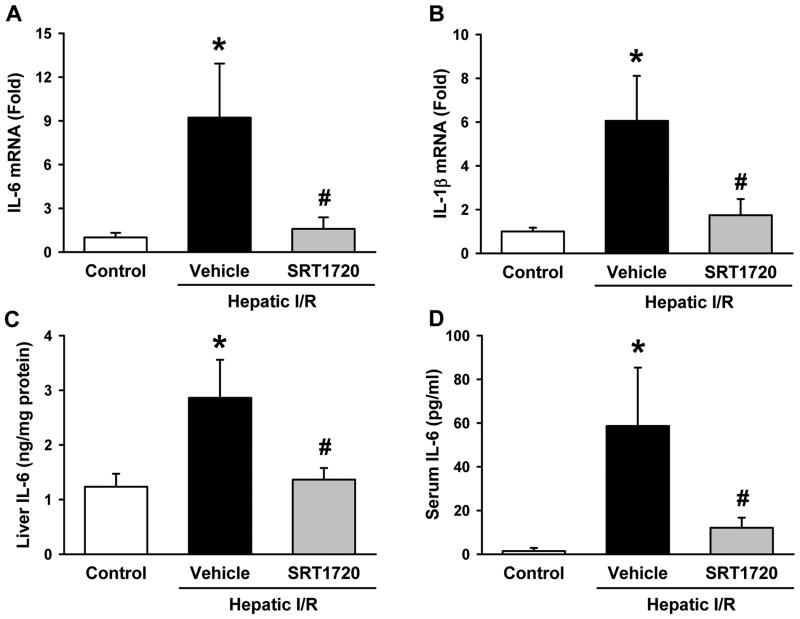
Liver and serum proinflammatory cytokine levels were measured to assess for local and systemic inflammation after hepatic I/R. Liver IL-6 and IL-1β mRNA levels were increased by 9.2- and 6.1-fold compared to the control, respectively (Fig. 3A, B). Their levels were significantly decreased by 82.8% and 71.2%, respectively, with SRT1720 treatment when compared to the vehicle (Fig. 3A, B). Additionally, liver IL-6 protein levels were significantly increased by 1.3-fold in the vehicle group in comparison to the control, while they were markedly reduced by 52.3% in the SRT1720 treatment group (Fig.3C). The change of IL-6 protein levels was also observed in the serum, where there was a 40.7-fold increase in IL-6 levels after I/R in the vehicle group compared to the control (Fig. 3D). Serum IL-6 levels were decreased by 79.5% in the SRT1720-treated group as compared to the vehicle (Fig. 3D).
Figure 3.
SRT1720 decreases inflammation after hepatic ischemia-reperfusion (I/R). Ischemic liver tissue and blood of the vehicle- and SRT1720- treated mice were collected 24 hr after reperfusion. Messenger RNA expression of (A) interleukin-6 (IL-6) and (B) interleukin-1β (IL-1β) as measured by RT-PCR. IL-6 protein levels were measured in the (C) liver tissue lysates and (D) serum by enzyme-linked immunosorbent assay. Data are presented as means ± SE (n = 6-8/group). *P < 0.05 versus control; #P < 0.05 versus vehicle.
SRT1720 Inhibits Neutrophil Infiltration after Hepatic I/R
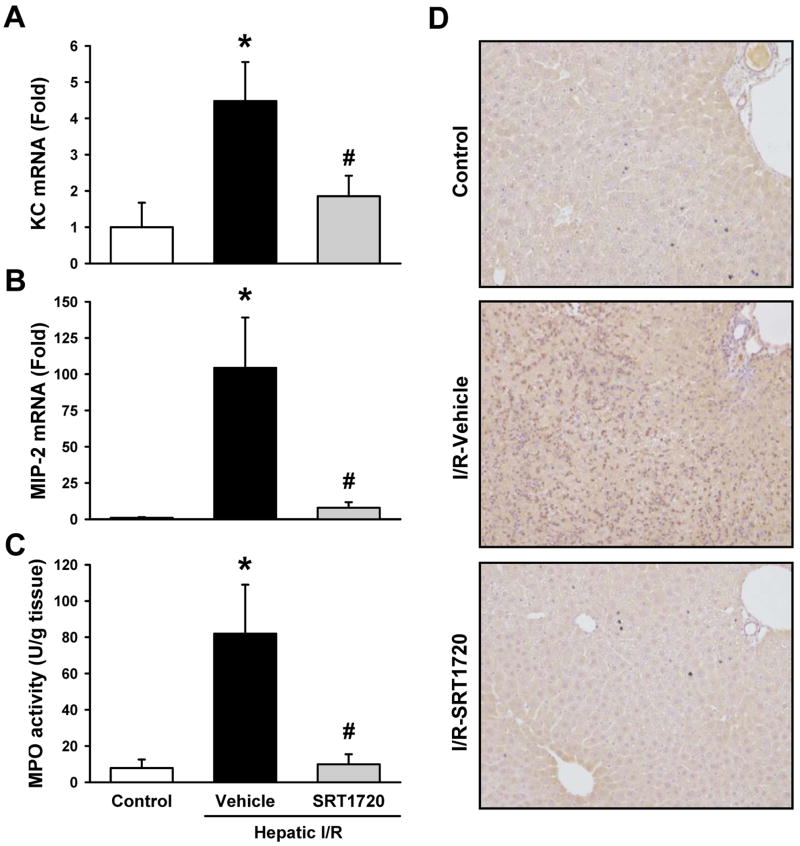
Neutrophil infiltration is a hallmark of hepatic I/R injury (29). As expected, liver mRNA levels of the neutrophil chemoattractants, keratinocyte chemoattractant (KC) and macrophage inhibitory protein-2 (MIP-2), were markedly increased after I/R in the vehicle group compared to the control (Fig. 4A, B). SRT1720 treatment significantly decreased the mRNA levels of KC and MIP-2 by 58.6% and 92.4%, respectively, compared to the vehicle group (Fig. 4A, B). In addition, MPO activity was measured as a marker for neutrophil infiltration and was increased by 10.4-fold in the vehicle group compared to the control, while it was reduced to control levels with SRT1720 treatment (Fig. 4C). The effect of SRT1720 on neutrophil infiltration was further supported by immunohistochemistry against Gr-1, a protein marker of neutrophils (Fig. 4D). The extent of liver neutrophil infiltration in the vehicle-treated mice was in stark contrast to that in the control and SRT1720-treated mice, where neutrophils were rare (Fig. 4D).
Figure 4.
SRT1720 inhibits liver neutrophil infiltration after hepatic ischemia-reperfusion (I/R). Ischemic liver tissues of the vehicle- and SRT1720-treated mice were collected at 24 h after reperfusion. The mRNA levels of (A) keratinocyte-derived chemoattractant (KC) and (B) macrophage inhibitory protein-2 (MIP-2) were measured by RT-PCR. (C) Myeloperoxidase (MPO) activity was determined spectrophotometrically. (D) Immunohistochemistry staining of neutrophils using anti-granulocyte receptor 1 (anti-Gr-1) antibody. Data are presented as means ± SE (n = 6-8/group). *P < 0.05 versus control; #P < 0.05 versus vehicle.
SRT1720 Reduces Oxidative Stress after Hepatic I/R
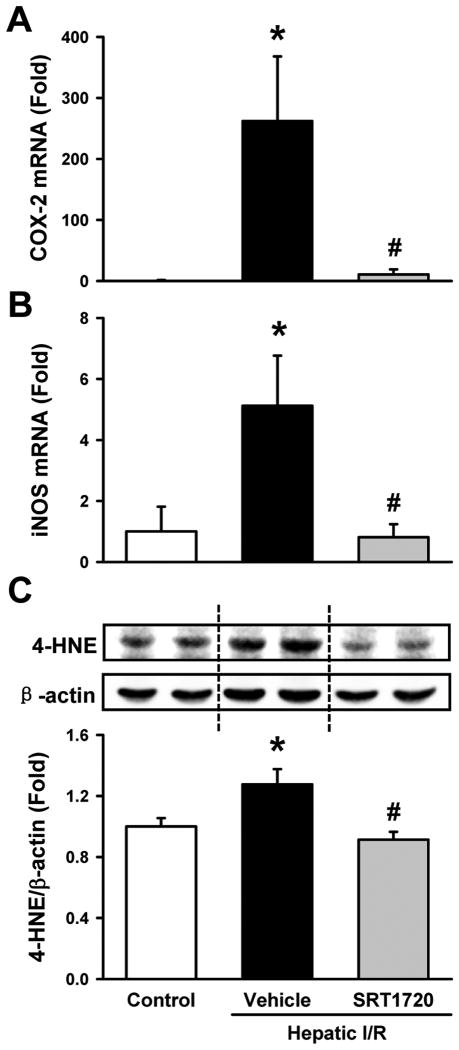
Increased oxidative stress is another characteristic of I/R injury (4). Liver mRNA levels of the oxidative stress mediators, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), were markedly increased after I/R in the vehicle group as compared to the control (Fig. 5A, B). Their levels were decreased by 96.0% and 84.2%, respectively, in the SRT1720-treated mice as compared to the vehicle (Fig. 5A, B). Further, measurement of the levels of 4-hydroxynonenal (4-HNE) adducts, a marker of lipid peroxidation (30), showed a 27.6% increase in the vehicle group as compared to the control, while they were reduced to baseline control levels by SRT1720 treatment (Fig. 5C).
Figure 5.
SRT1720 decreases oxidative stress after hepatic ischemia-reperfusion (I/R). Ischemic liver tissues of the vehicle- and SRT1720-treated mice were collected at 24 h after reperfusion. The mRNA levels of (A) cyclooxygenase 2 (COX-2) and (B) inducible nitric oxide synthase (iNOS) were measured by RT-PCR. (C) 4-hydroxynonenal (4-HNE) protein adducts at 65 kDa was measured by Western blotting. Data are presented as means ± SE (n = 6-8/group). *P < 0.05 versus control; #P < 0.05 versus vehicle.
SRT1720 Increases Survival and ATP Levels of Cultured Hepatocytes after H/R
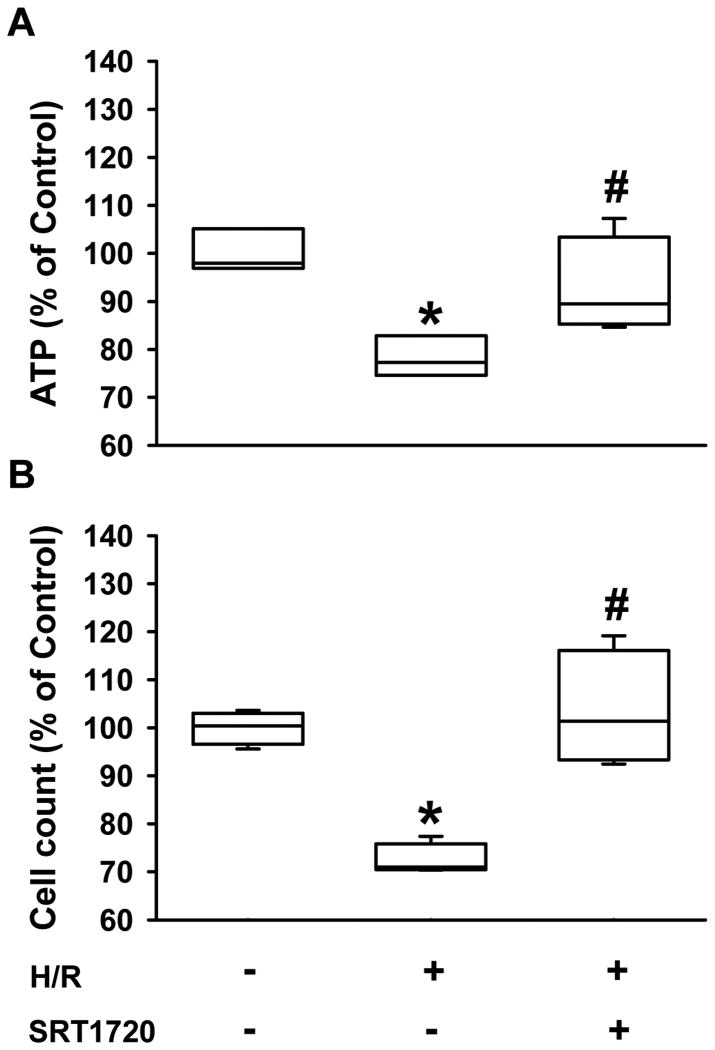
After demonstrating the effect of SRT1720 on mice undergoing hepatic I/R, we sought to investigate the direct role of SRT1720 on cultured hepatocytes after H/R. Rat H4IIE hepatoma cells were incubated with oxyrase to induce hypoxia, followed by reoxygenation with fresh medium for 4 h in the absence or presence of SRT1720. H/R caused a 27.6% and 21.7% decrease in survival rate of H4IIE cells and ATP levels, respectively, compared to the normoxia control (Fig. 6A, B). On the other hand, treatment with SRT1720 improved the survival and ATP levels of H4IIE cells after H/R to 103.6% and 92.7% of the normoxia control, respectively (Fig. 6A, B).
Figure 6.
SRT1720 increases ATP levels and survival of H4IIE hepatocytes after hypoxia-reoxygenation (H/R). H4IIE hepatocytes underwent 6 h of oxyrase-induced hypoxia in glucose-, pyruvate- and serum-free media followed by 4 h of reoxygenation in complete media. (A) ATP levels were measured from total cell lysates. (B) The number of live H4II hepatocytes counted by hemocytometer with a trypan blue exclusion assay. Data are presented as means ± SE (n = 4/group). *P < 0.05 versus control; #P < 0.05 versus H/R.
SRT1720 Enhances Mitochondrial Mass and Membrane Potential of Cultured Hepatocytes after H/R
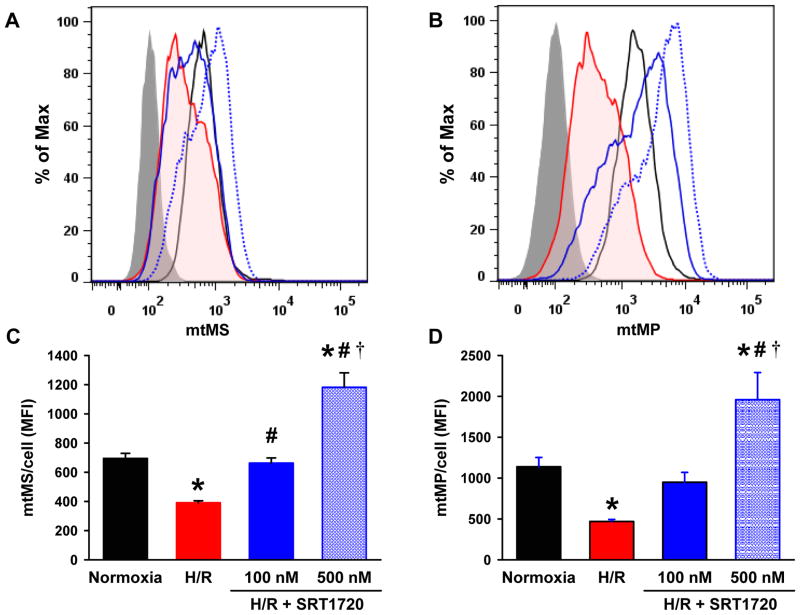
To assess the effects of SRT1720 on the mitochondria after H/R, H4IIE cells were stained with MTG and MTR for measurements of mitochondrial mass (mtMS) and membrane potential (mtMP), respectively, by flow cytometry. At 4 h of reoxygenation, the mean fluorescence intensity (MFI) of both MTG and MTR were significantly decreased by 43.7% and 58.8%, respectively, compared to the normoxia control. In contrast, treatment with 100 and 500 nM of SRT1720 to H/R-injured cells, increased the MFI of MTG by 1.7- and 3.0-fold (Fig. 7A, C) as well as MTR by 2.0- and 4.2-fold, respectively, compared to the untreated H/R-injured cells (Fig. 7B, D).
Figure 7.
SRT1720 increases mitochondrial mass and membrane potential in H4IIE hepatocytes after hypoxia-reoxygenation (H/R). H4IIE hepatocytes underwent 6 h of oxyrase-induced hypoxia in glucose-, pyruvate- and serum-free media followed by 4 h of reoxygenation in complete media. Cells were stained with mitotracker green (A, C) and mitotracker red FM dye (B, D) to measure the mitochondrial mass (mtMS) and mitochondrial membrane potential (mtMP) per cell, respectively, by flow cytometry. (A, B) Representative histograms of flow cytometric anlaysis showing unstained (grey fill), normoxia control (black line), H/R (red shaded line), H/R plus 100 nM (blue line) or 500 nM (blue dotted line) of SRT1720. (C, D) The mean of the fluorescent intensity (MFI) of mitotracker dyes per cell. Data are presented as means ± SE (n = 6/group), representative of three independent experiments. *P < 0.05 versus control; #P < 0.05 versus H/R, †P < 0.05 versus H/R plus 100 nM SRT1720.
SRT1720 Enhances Autophagy in Cultured Hepatocytes after H/R
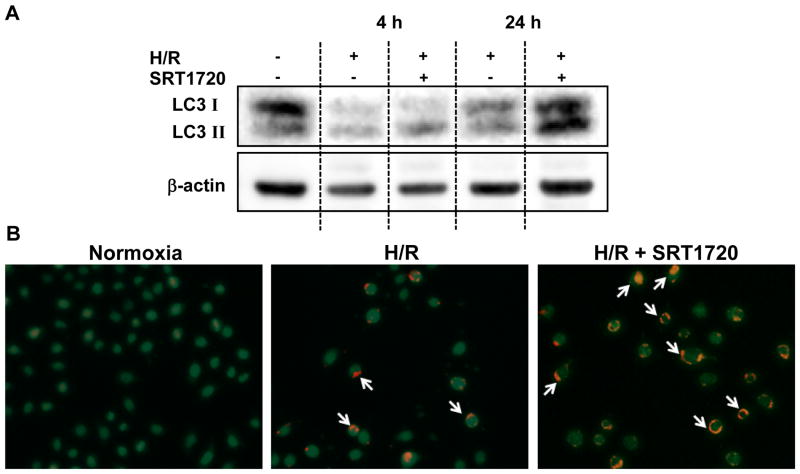
Given the enhancement of autophagy observed in the livers of the SRT1720-treated mice, we next examined whether SRT1720 had a direct effect on autophagy in hepatocytes exposed to H/R. LC3II protein expression in H/R-injured H4IIE cells was slightly decreased compared to the normoxia control; however, with SRT1720 treatment, the LC3II levels were higher than those in both normoxia control and H/R-injured cells, especially at 24 h of reoxygenation (Fig. 8A). Further, cells were stained with acridine orange for the detection of autophagic vesicles under a fluorescence microscope. The autophagic vesicles (shown in red) could be detected in several H4IIE cells exposed to H/R, while they were more abundant and of higher intensity in the SRT1720-treated cells at 24 h of reoxygenation (Fig. 8B).
Figure 8.
SRT1720 enhances autophagy in H4IIE hepatocytes after hypoxia-reoxygenation (H/R). H4IIE hepatocytes underwent 6 h of oxyrase-induced hypoxia in glucose-, pyruvate- and serum-free media followed by 4 h or 24 h of reoxygenation in complete media. At the beginning of reoxygenation, cells were treated with 0.125% DMSO (vehicle) or 500 nM of SRT1720. (A) Western blotting against microtubule associated protein 1 light chain 3 (LC3) in total cell lysate. (B) Representative images of H4IIe cells (green) stained with acridine orange at 24 h of reoxygenation. White arrows point to autophagic vesicles (red). Data representative of three independent experiments.
SRT1720-induced Autophagy is Dependent on the Mitochondrial Membrane Potential after H/R
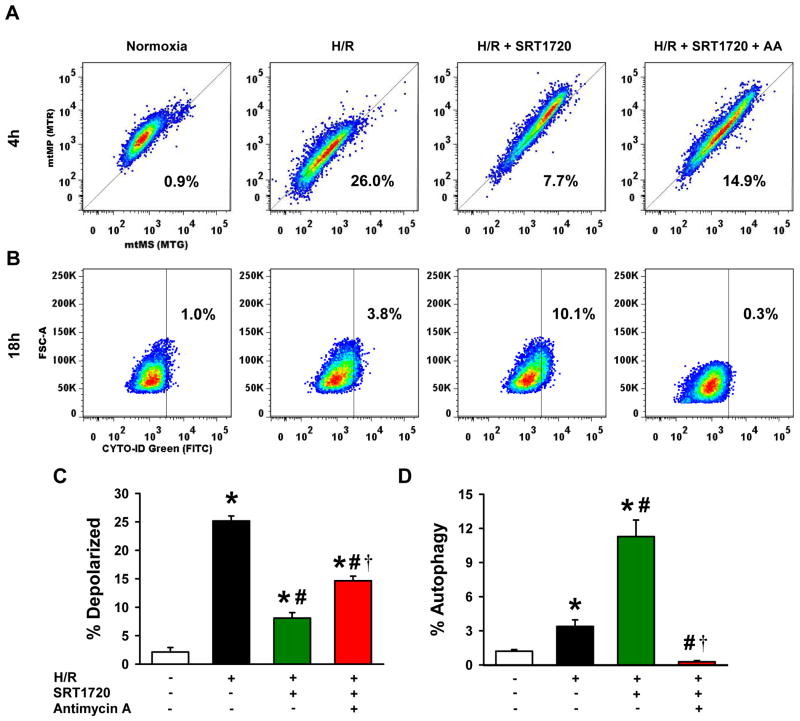
To examine the mitochondrial membrane potential in individual cells, the intensity of MTR staining (indicated mtMP) was re-plotted against MTG staining (indicated mtMS) from the flow cytometric data described in Fig. 9. Under normoxic conditions, healthy H4IIE cells had intact mitochondria, as indicated by 97.9% of the population positioned above the reference line (Fig. 9A). When H4IIE cells were exposed to H/R, at 4 h of reoxygenation, 25.2% of the population lay below the reference line, indicating mitochondrial depolarization (Fig. 9A, C). On the other hand, treatment with SRT1720 decreased the percentage of cells below the reference line to 8.1% (Fig. 9A, C). Co-treatment of SRT1720 with low dose antimycin A, a mitochondrial depolarizing agent (31), increased the percentage of cells with depolarized mitochondria to 14.6% below the reference line (Fig. 9A, C).
Figure 9.
SRT1720-induced autophagy is dependent on mitochondrial membrane potential after hypoxia-reoxygenation (H/R) in H4IIE hepatocytes. H4IIE hepatocytes underwent 6 h of oxyrase-induced hypoxia in glucose-, pyruvate- and serum-free media followed by 4 h or 18 h of reoxygenation in complete media. At the beginning of reoxygenation, cells were treated with 0.125% DMSO (vehicle), 500 nM of SRT1720, or 500nM of SRT1720 plus 500 nM of antimycin A. (A, C) After 4 h of reoxygenation, cells were stained with mitotracker green and mitotracker red FM dye for measurement of mitochondrial mass (mtMS) per cell and mitochondrial membrane potential (mtMP) per cell, respectively. Cells below the reference line (thin diagonal line) were considered as cells with depolarized mitochondria. (B, D) After 18 h of reoxygenation, cells were stained with CYTO-ID green autophagy dye, arbitrary gate drawn at 1% baseline autophagy rate in the normoxia control cells (vertical thin line). Data are presented as means ± SE (n = 6/group), representative of 3 independent experiments. * P < 0.05 versus control; # P < 0.05 versus H/R, † P < 0.05 versus H/R plus 100 nM SRT1720. FSC-A, forward scatter - area.
To establish the relationship between the SRT1720-induced enhancement in autophagy and mitochondrial membrane potential, we further quantified the amount of autophagic vesicles in H4IIE cells by flow cytometry with CYTO-ID staining. H4IIE cells exposed to H/R had an increase in CYTO-ID positive staining to 3.4% of the population in contrast to the normoxia control cells, which were arbitrarily gated at 1% of the population with positive staining for comparison (Fig. 9B, D). Treatment with SRT1720 increased the population of the CYTO-ID-positive cells to 11.3% (Fig. 9B, D). However, addition of antimycin A with SRT1720 reduced the number of the CYTO-ID-positive cells to a level similar to the normoxia control (Fig. 9B, D). Taken together, these results indicate that the increase in the mitochondrial membrane potential stimulated by SRT1720 is needed for the enhancement of autophagy in H/R-injured H4IIE cells.
SRT1720 Increases Mitochondrial Mass, Membrane Potential, and Autophagy in Primary Hepatocytes after H/R
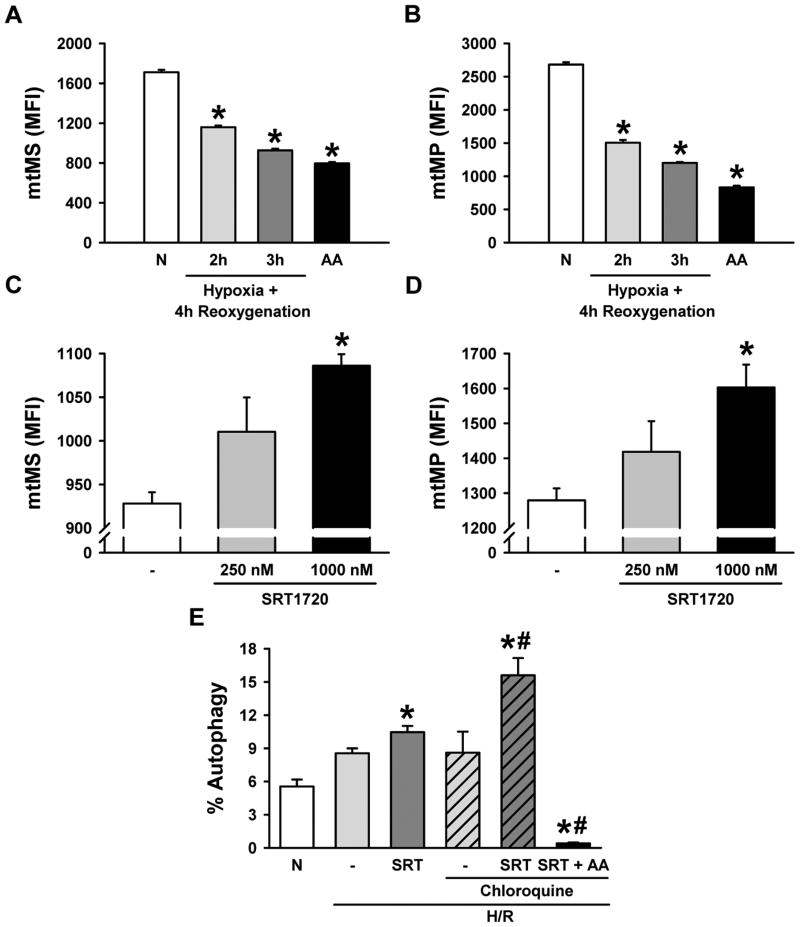
To further verify the conclusion from the study of H4IIE cells, further experiments were performed on primary hepatocytes isolated from rats. The change in mitochondrial mass and membrane potential was determined in primary hepatocytes exposed to different durations of hypoxia. There was a 32.2% and 45.8% decrease in the MFI of MTG as well as a 43.9% and 55.2% decrease in the MFI of MTR after exposure to 2 h and 3 h of hypoxia, respectively, followed by 4 h of reoxygenation, compared to the normoxia control (Fig. 10A, B). Two hours of hypoxia was selected for subsequent experiments. Treatment with 250 nM and 1000 nM of SRT1720 increased the MFI of MTG by 8.9% and 17.0% as well as the MFI of MTR by 10.9% and 25.3%, respectively, after 4 h of reoxygenation (Fig. 10C, D). In addition, 10.5% of H/R-injured hepatocytes treated with SRT1720 were positive for CYTO-ID compared to 5.6% in the normoxia control (Fig. 10E). We then used chloroquine, an inhibitor of the degradation of autophagic vesicles (27), to examine the effect of SRT1720 on autophagic flux. In the presence of chloroquine, the population of the CYTO-ID-positive cells in the H/R-injured hepatocytes with SRT1720 treatment was increased to 15.6% (Fig. 10E). This effect was inhibited by antimycin A. Similar to H4IIE cells, SRT1720 treatment increased the mitochondrial mass and membrane potential which was again associated with the enhancement of autophagy in primary hepatocytes exposed to H/R.
Figure 10.
Effects of hypoxia-reoxygenation (H/R) and pharmacologic Sirt1 activation on mitochondrial mass (mtMS), mitochondrial membrane potential (MtMP), and autophagy in primary hepatocytes. Primary hepatocytes underwent oxyrase-induced hypoxia in glucose- and pyruvate-free media followed by reoxygenation in complete media. Cells were stained with mitotracker green (A, C) and mitotracker red FM dye (B, D) for measurement of mtMS and mtMP per cell, respectively, by flow cytometry. (A, B) Hepatocytes were subjected to 2 h and 3 h of hypoxia followed by 4 h of reoxygenation, or 4 h of treatment with 3 uM of Antimycin A (AA) and compared to the normoxia control (N), * P < 0.05 versus normoxia. (C, D) Hepatocytes were subjected to 2 h of hypoxia followed by 4 h of reoxygenation, and treated with SRT1720 at the time of reperfusion. Bar graphs showing the average mean fluorescent intensity (MFI) of mitotracker dyes per cell, * P < 0.05 versus H/R control. (E) The percent of cells with positive autophagic vesicles was measured using the CYTO-ID cytodetection kit by flow cytometry, * P < 0.05 versus normoxia; # P < 0.05 versus H/R chloroquine control. Data are presented as means ± SE (n = 3-5/group), representative of 3 independent experiments.
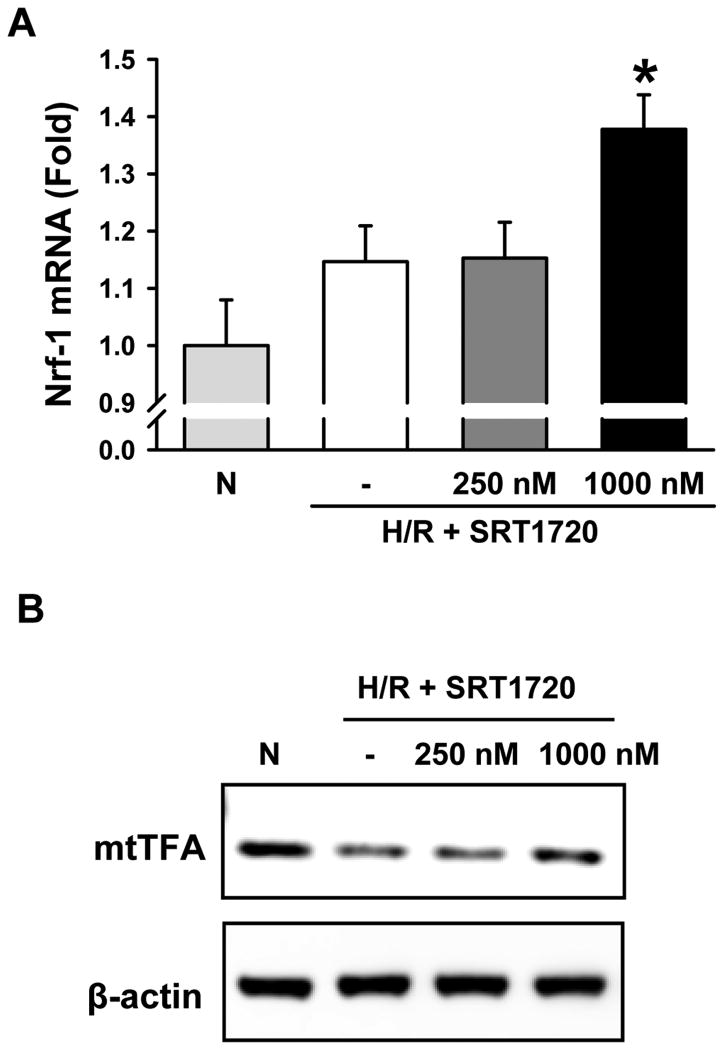
To examine the effects of SRT1720 on stimulating the mitochondrial biogenesis pathway, we measured the expression of nuclear transcription factor-1 (Nrf-1) and mitochondrial transcription factor A (mtTFA), two well know down-stream mediators of the Sirt1 activation pathway (32, 33). There was a 37.8% increase in mRNA levels of Nrf-1 in H/R-injured primary hepatocytes treated with 1000 nM of SRT1720 after 1 h of reoxygenation, compared to normoxia (Fig, 11A). Similarly, there was an increase in the protein expression of mtTFA in the SRT1720-treated primary hepatocytes, compared to H/R alone after 4 h of reoxygenation (Fig. 11B). These results indicate that SRT1720 effectively activated the Sirt1 signaling pathway.
Figure 11.
SRT1720 activates the mitochondrial biogenesis pathway in primary hepatocytes after H/R. Primary hepatocytes underwent oxyrase-induced hypoxia in glucose- and pyruvate-free media followed by reoxygenation in complete media. Cells were lysed and mRNA and protein were used for analysis. Primary hepatocytes were subjected to 2 h of hypoxia followed by 1 h (A) or 4 h of reoxygenation (B). Cells were treated with 250 nM (L) or 1000 nM (H) of SRT1720 at reoxygenation, * P < 0.05 versus normoxia (N). (A) mRNA expression of the mitochondrial biogenesis marker nuclear transcription factor-1 (Nrf-1) and (B) protein expression of mitochondrial transcription factor A (mtTFA) were measured by RT-PCR and western blot, respectively. * P < 0.05 versus Normoxia (N). Data are presented as means ± SE (n = 3/group), representative of 2 independent experiments.
Discussion
Hepatic I/R is primarily a surgical problem with limited clinical interventions available (1). Mitochondrial dysfunction is a central feature of I/R injury due to its importance in energy homeostasis, and its direct involvement in mediating oxidative injury and apoptosis (9). In this study, we show that pharmacologic activation of Sirt1 markedly attenuates hepatic injury, increases mitochondrial mass, and enhances autophagy after I/R. In addition, there is a significant reduction in necrosis, apoptosis, inflammation, neutrophil infiltration, and oxidative stress in the livers of mice post-treated with SRT1720 after hepatic I/R.
SRT1720 is a potent activator of Sirt1 (25). To examine its direct effects on mitochondria, we employ a cell culture model using H4IIE cells and rat primary hepatocytes, which display reductions in mitochondrial mass and membrane potential after exposure to H/R. We demonstrate dose-dependent enhancement of mitochondrial mass and membrane potential per cell with SRT1720 treatment in hepatocytes exposed to H/R. We further demonstrate that SRT1720 treatment increases the expression of Nrf-1 and mtTFA in primary hepatocytes exposed to H/R. These two proteins are important transcription factors involved in the regulation of mitochondrial biogenesis and serve as downstream mediators of Sirt1 (32, 33), indicating that SRT1720 activates the mitochondrial biogenesis pathway in hepatocytes. Consistent with previous studies, we show that stimulation of mitochondrial activity is protective in the setting of I/R (8, 34). In addition, enhancement of mitochondrial biogenesis by Sirt1 protects cultured renal proximal tubule cells from oxidative injury, and protects animals from renal I/R injury (35, 36). In addition, we show an increase in the percentage of H4IIE cells with depolarized mitochondria after H/R, which is partially reversed by treatment with SRT1720. The importance of this finding is highlighted by the fact that mitochondrial depolarization has been identified as a culprit in both necrotic and apoptotic cell death after I/R (37, 38).
In addition to its effect on reversing mitochondrial dysfunction, Sirt1 has been shown to directly inhibit p53-mediated apoptosis after renal I/R (18). In our study, we demonstrate the absence of TUNEL-positive nuclei in the livers of SRT1720-treated mice after hepatic I/R. The contribution of apoptotic cell death to ischemic liver injury has gained attention since it was first reported by Sasaki in 1996 (39). The debate on the predominant mechanism of cell death, whether by necrosis or apoptosis, is complicated by the vast commonalities in the pathways leading to cell death (37). Nevertheless, it has been shown that inhibition of apoptosis through the blockade of caspase activity is protective in the setting of hepatic I/R (40). This is consistent with our results, which show that SRT1720 treatment decreases caspase-3 activity to baseline levels in the liver after I/R.
The sterile inflammatory response after I/R injury can be attributed to the release of damage associated molecular patterns from necrotic and apoptotic cells during the early phase of I/R (29, 41). Herein we demonstrate a significant attenuation of local and systemic inflammation with SRT1720 treatment, which may be directly related to its cellular protective effects against I/R-induced necrosis and apoptosis. In support of this, our cell culture study clearly indicates a direct pro-survival effect of SRT1720 on hepatocytes exposed to H/R. On the other hand, Sirt1 has been shown to inhibit the NF-κB canonical pathway (42), and therefore SRT1720 treatment may also directly attenuate the release of proinflammatory cytokines. Neutrophil infiltration is another characteristic feature of the inflammatory response in hepatic I/R (43). The chemokines KC and MIP-2 have been directly implicated in the process of neutrophil recruitment after hepatic I/R and neutralization of these chemokines is associated with decreased neutrophil infiltration and improved outcomes (43). We show that treatment with SRT1720 markedly decreases the expression of KC and MIP-2, as well as neutrophil infiltration assessed by MPO activity and immunohistochemistry, in the liver after hepatic I/R.
Oxidative stress is another hallmark of I/R injury. Multiple studies have shown partial protection to hepatic I/R using antioxidants (44), with variable clinical outcomes (45, 46). Complicating the picture, oxidative stress and inflammation are highly intertwined. For instance, neutrophils generate reactive oxygen species (ROS) within infiltrated tissue as part of their microbial defense mechanism (47). In addition, ROS can activate NF-κB and increase the transcription of inflammatory mediators such as COX-2 and iNOS (48, 49). Pharmacologic and genetic inhibition of COX-2 has been shown to be protective in the setting of hepatic I/R (50, 51). iNOS is one of several enzymes catalyzing nitric oxide release. Nitric oxide contributes to oxidative injury through the generation of peroxynitrite, which induces cell injury through lipid peroxidation. Several studies have demonstrated that iNOS is in fact deleterious in the setting of hepatic I/R (52, 53). Our results indicate that SRT1720 inhibits COX-2 and iNOS mRNA expression after hepatic I/R, likely contributing to the decrease in oxidative injury as measured by 4-HNE adducts, a marker for lipid peroxidation.
Evidence of the protective effects of autophagy during I/R has been accumulating (54, 55). Bhogal et al. showed that inhibition of autophagy using 3-methyladenine increases apoptosis in primary human hepatocytes under oxidative stress (56). More recently, work by Yun et al. suggests that there is an impaired autophagy response after hepatic I/R, where pharmacologic inhibition of autophagy further exacerbates injury (57). Likewise, our results implicate autophagy as a protective mechanism of SRT1720 after hepatic I/R, as shown by the increase in LC3II expression and decrease in SQSTM-1 protein levels in the liver after I/R. Although we do not observe any change in protein levels of hepatic LC3II after I/R and H4IIE cells after H/R in comparison to their controls, an increase in autophagic vesicles can be detected in the H4IIE cells and primary hepatocytes after H/R by using acridine orange staining and the CYTO-ID autophagy detection kit. In contrast, treatment with SRT1720 increases LC3II expression significantly in the liver and H4IIE cells. These results suggest that Western blotting for LC3II may not be sensitive enough to detect autophagy after ischemia and hypoxia at the time points used in this study. Further kinetic studies are needed to monitor the changes in LC3II expression in this model.
Using the cell culture model, we further identify a correlation between mitochondrial membrane potential and enhancement in autophagy after SRT1720 treatment of hepatocytes exposed to H/R. We detect an increase in mitochondrial mass and membrane potential, as well as an increase in the number of autophagic vesicles, in the H/R-injured hepatocytes after SRT1720 treatment compared to untreated H/R-injured hepatocytes. However, by disrupting the mitochondrial membrane potential with antimycin A, the initial enhancement of autophagy by SRT1720 is significantly diminished. Similar to our observation, the dependence of autophagy on mitochondrial activity has been described in yeast and other mammalian cells (31, 58). On the other hand, the elimination of dysfunctional mitochondria by autophagy has also been reported in hepatocytes exposed to hypoxia (59). Our findings suggest a potential pathway linking Sirt1 and its effect on mitochondria to autophagy regulation, the detailed mechanism of which will be the subject of future investigation.
Conclusions
Post-treatment with SRT1720 is protective in the setting of hepatic I/R. This effect is associated with the restoration of cellular energy status, as well as the recovery of mitochondrial mass and membrane potential, and an enhancement of autophagy in hepatocytes.
Supplementary Material
Acknowledgments
The authors thank Drs. Laura Hansen, Alexandra C. Bolognese, and Cindy Cen for their invaluable feedback and critical review of the manuscript.
Source of Funding: This project was partially supported by grant R01HL076179 (PW) from the National Institute of Health.
Copyright form disclosures: The authors received support for article research from the National Institutes of Health (NIH). This project was partially supported by grant #R01HL076179 (PW). Their institutions received grant support from the NIH.
Footnotes
No reprints will be ordered.
Conflicts of Interest: For the remaining authors, none were declared.
References
- 1.Weigand K, Brost S, Steinebrunner N, et al. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg. 2012;2012:176723. doi: 10.1155/2012/176723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies Transplantation. 1992;53:957–978. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A, Maley W, Li Z, et al. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645–1653. doi: 10.1002/lt.21212. [DOI] [PubMed] [Google Scholar]
- 4.Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–1106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 6.Perry SW, Norman JP, Barbieri J, et al. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woldt E, Sebti Y, Solt LA, et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon KH, Hood BL, Mukhopadhyay P, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135(4):1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 2004;66:514–517. doi: 10.1111/j.1523-1755.2004.761_9.x. [DOI] [PubMed] [Google Scholar]
- 10.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 12.Samokhvalov V, Scott BA, Crowder CM. Autophagy protects against hypoxic injury in C. elegans Autophagy. 2008;4:1034–1041. doi: 10.4161/auto.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang M, Liu K, Luo J, et al. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 17.Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2013;45(1):51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan H, Yang HC, You L, et al. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013;83(3):404–413. doi: 10.1038/ki.2012.394. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CP, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khader A, Yang WL, Kuncewitch M, et al. Sirtuin 1 Activation Stimulates Mitochondrial Biogenesis and Attenuates Renal Injury After Ischemia-Reperfusion. Transplantation. 2014 doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 21.Yan H, Jihong Y, Feng Z, et al. Sirtuin 1-mediated inhibition of p66shc expression alleviates liver ischemia/reperfusion injury. Crit Care Med. 2014;42:e373–381. doi: 10.1097/CCM.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 22.Pantazi E, Zaouali MA, Bejaoui M, et al. Role of sirtuins in ischemia-reperfusion injury. World J Gastroenterol. 2013;19(43):7594–7602. doi: 10.3748/wjg.v19.i43.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda A, Jacob A, Wu R, et al. Milk fat globule--EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J Surg Res. 2013;180:e37–46. doi: 10.1016/j.jss.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe Y, Hines IN, Zibari G, et al. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46:1–7. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo DT. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Methods Mol Biol. 2011;682:3–13. doi: 10.1007/978-1-60327-409-8_1. [DOI] [PubMed] [Google Scholar]
- 29.van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012;23(3):69–84. doi: 10.1016/j.cytogfr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Jin M, Cai Y, et al. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem Biol. 2011;18:1474–1481. doi: 10.1016/j.chembiol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91(4):1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenmoehl J, Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. 2013;13(6):755–761. doi: 10.1016/j.mito.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Saba H, Batinic-Haberle I, Munusamy S, et al. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42(10):1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333(2):593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273(2):345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki H, Matsuno T, Tanaka N, et al. Activation of apoptosis during the reperfusion phase after rat liver ischemia. Transplant Proc. 1996;28:1908–1909. [PubMed] [Google Scholar]
- 40.Cursio R, Gugenheim J, Ricci JE, et al. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. Faseb j. 1999;13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 41.Godwin A, Yang WL, Sharma A, et al. Blocking Cold-Inducible RNA-Binding Protein (CIRP) Protects Liver From Ischemia/Reperfusion Injury. Shock. 2014 doi: 10.1097/SHK.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lentsch AB, Yoshidome H, Cheadle WG, et al. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology. 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 44.Elias-Miro M, Jimenez-Castro MB, Rodes J, et al. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 45.Robinson SM, Saif R, Sen G, et al. N-acetylcysteine administration does not improve patient outcome after liver resection. HPB (Oxford) 2013;15:457–462. doi: 10.1111/hpb.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thies JC, Teklote J, Clauer U, et al. The efficacy of N-acetylcysteine as a hepatoprotective agent in liver transplantation. Transpl Int. 1998;11(Suppl 1):S390–392. doi: 10.1007/s001470050505. [DOI] [PubMed] [Google Scholar]
- 47.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- 49.Hur GM, Ryu YS, Yun HY, et al. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 50.Sunose Y, Takeyoshi I, Ohwada S, et al. The effect of cyclooxygenase-2 inhibitor FK3311 on ischemia-reperfusion injury in a canine total hepatic vascular exclusion model. J Am Coll Surg. 2001;192:54–62. doi: 10.1016/s1072-7515(00)00773-0. [DOI] [PubMed] [Google Scholar]
- 51.Tolba RH, Fet N, Yonezawa K, et al. Role of preferential cyclooxygenase-2 inhibition by meloxicam in ischemia/reperfusion injury of the rat liver. Eur Surg Res. 2014;53:11–24. doi: 10.1159/000362411. [DOI] [PubMed] [Google Scholar]
- 52.Lee VG, Johnson ML, Baust J, et al. The roles of iNOS in liver ischemia-reperfusion injury. Shock. 2001;16:355–360. doi: 10.1097/00024382-200116050-00006. [DOI] [PubMed] [Google Scholar]
- 53.Wang LM, Tian XF, Song QY, et al. Expression and role of inducible nitric oxide synthase in ischemia-reperfusion liver in rats. Hepatobiliary Pancreat Dis Int. 2003;2:252–258. [PubMed] [Google Scholar]
- 54.Ma X, Liu H, Foyil SR, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang JH, Ahn IS, Fischer TD, et al. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology. 2011;141:2188–2199.e2186. doi: 10.1053/j.gastro.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhogal RH, Weston CJ, Curbishley SM, et al. Autophagy: a cyto-protective mechanism which prevents primary human hepatocyte apoptosis during oxidative stress. Autophagy. 2012;8:545–558. doi: 10.4161/auto.19012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yun N, Cho HI, Lee SM. Impaired autophagy contributes to hepatocellular damage during ischemia/reperfusion: heme oxygenase-1 as a possible regulator. Free Radic Biol Med. 2014:168–177. doi: 10.1016/j.freeradbiomed.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signalling pathways. Embo j. 2011;30:2101–2114. doi: 10.1038/emboj.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JS, Nitta T, Mohuczy D, et al. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.