Abstract
Objective
We tested the hypothesis that osmotherapy with hypertonic saline (HS) attenuates cerebral edema following experimental cardiac arrest (CA) and cardiopulmonary resuscitation (CPR) by exerting its effect via the perivascular pool of aquaporin-4 (AQP4). We used mice with targeted disruption of the gene encoding α-syntrophin (α-Syn-/-) that demonstrate diminished perivascular AQP4 pool but retain the minor endothelial pool.
Design
Laboratory animal study.
Setting
University animal research laboratory.
Interventions
Isoflurane-anesthetized adult male wild type (WT) C57B/6 or α-Syn-/- mice were subjected to CA/CPR and treated with either a continuous intravenous (IV) infusion of 0.9% saline (NS) or various concentrations of hypertonic saline (HS). Serum osmolality, regional brain water content, blood-brain barrier (BBB) disruption, and AQP4 protein expression were determined at 24 hr after CA/CPR.
Measurements and Main Results
7.5% HS treatment significantly attenuated water content in the caudoputamen (CP) complex and cortex as compared with NS-treatment in WT mice subjected to CA/CPR. In contrast in α-syn-/- mice subjected to CA/CPR, 7.5% HS treatment did not attenuate water content. Treatment with 7.5% HS attenuated BBB disruption at 24 hr following CA/CPR in WT mice but not in α-Syn-/- mice. Total AQP4 protein expression was not different between NS and HS-treated WT mice.
Conclusions
Following experimental CA/CPR: 1) continuous HS therapy maintained to achieve serum osmolality of ∼350 mOsm/L is beneficial for the treatment of cerebral edema; 2) perivascular pool of AQP4 plays a critical role in water egress from brain; 3) HS attenuates BBB disruption via perivascular AQP4 pool.
Keywords: hypertonic saline, osmotherapy, global cerebral ischemia, cardiac arrest, cerebral edema, aquaporins
Cardiac arrest (CA) is a leading cause of mortality and poor neurologic outcome (1-4). The pathophysiological mechanisms of brain injury following CA are complex and multifactorial, including the primary ischemic neuronal injury and secondary injury from consequent cerebral edema (5-8).
Aquaporin-4 (AQP4), the predominant water channel in brain, has been implicated in the pathogenesis of ischemia-evoked cerebral edema (9-14). There are three AQP4 pools in the brain: a major perivascular pool localized to the astrocytic endfeet, a minor endothelial pool, localized to the endothelial non-endfeet pool localized in astrocytic processes in the neuropil (14) and an ependymal pool (15). In addition, AQP4 is expressed in subpial astrocytic processes. The astrocytic endfoot pool of AQP4 is anchored by the dystrophin associated protein complex (16) and is deleted in mice with targeted disruption of the gene encoding α-syntrophin (α-Syn-/-). α-Syn-/- mice show a reduced severity of cerebral edema after ischemic stroke (12) and thus it has been suggested that the perivascular pool of AQP4 is rate limiting for water influx during ischemia-evoked edema formation.
Thus far limited data are available on treatment for CA-evoked cerebral edema. Hyperosmolar therapy with hypertonic saline (HS) solutions is a commonly used treatment for cerebral edema from diverse etiologies (17-19). We have demonstrated in well-characterized animal models of focal ischemia that continuous intravenous (IV) HS infusion to target serum osmolality levels of ∼350 mOsm/L attenuates cerebral edema, ameliorates blood-brain barrier (BBB) disruption, and improves mortality and these actions are dependent on the presence of perivascular AQP4 (20, 21).
Using α-syn-/- mice and their WT counterparts in a well-characterized murine model of brain injury following experimental CA,we tested the hypotheses that: 1) HS attenuates cerebral edema; 2) the perivascular pool of AQP4 is selectively involved in the elimination of water from the brain with HS; and 3) HS protects the integrity of the BBB.
Material and Methods
General Preparation and Animal Surgery
The experimental protocol was approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines. WT C57B/6 (Charles River, Hollister, CA, USA) mice were used as controls. α-syntrophin (α-Syn-/-) mice were bred on a C57B/6 background more than 10 generations to avoid effects of differing genetic strains (22).
Cardiac Arrest Model
The experimental procedures in WT and α-Syn-/- (20-28 g) were performed randomly by a single investigator blinded to treatment, as previously described (23-25). Briefly, all surgical procedures were performed in mice anesthetized (induction with 5% isoflurane and maintenance with 2% isoflurane in 20% oxygen/80% air) via face mask under controlled rectal temperature of 37 °C. An internal jugular venous catheter was placed for intravenous access. Mice were endotracheally intubated with a 22G IV catheter, and mechanically ventilated (Minivent, Hugo Sacs Elektronik, March-Hugstetten, Germany) and CA was induced by IV injection of 50μL cold 0.5 mol/l KCL. This well-characterized and unique animal model utilizes selective heating of the animal's head to a target temporalis muscle temperature of 39 °C while cooling the body temperature to 29 °C during CA as described previously (23). Cardiopulmonary resuscitation (CPR) was initiated 8 min after induction of CA by slow injection of 0.5 mL of epinephrine (8μg), chest compressions (approximately 300/min), and ventilation with 100% oxygen. Thirty minutes after return of spontaneous circulation (ROSC) and confirmation of sufficient spontaneous breathing (> 30/min), the endotracheal tube was removed.
Assessment of Regional Brain Edema
Brains were removed at desired endpoints and dissected into 4 regions (cortex, caudoputamen (CP) complex, hippocampus and cerebellum). Brain edema was assessed by comparing wet-to-dry ratios (WDR) as described previously (20, 21, 26).
Assessment of BBB Integrity
BBB permeability was assessed by the Evans blue (EB) extravasation method (27) with modifications (20) and quantified as nanograms per whole brain.
Assessment of Serum Osmolality
At the end of the experiment, serum osmolality (mOsm/L) was determined with an automated freezing point depression micro-osmometer (Advanced Instruments, Inc., Norwood, MA) (20, 21).
Neurobehavioral Assessment
Neuro-behavioral testing and scoring was performed after completion of treatment by an investigator (EM) blinded to the experimental group and scores summed for consciousness, interaction, eye appearance, breathing, food/water intake and overall activity (0 = no impairment and 20 = maximum impairment) (24, 28).
Immunoblotting for AQP4
AQP4 protein expression was determined with a Western blot protocol for AQP4 as previously described with modifications (29) following sub-dissection of the CP complex, hippocampus, cerebral cortex, and cerebellum from WT mice treated with normal saline or 7.5% HS or sham operation.
Experimental Groups
In the first series of experiments, WT mice (n=90) subjected to CA were randomized to receive either continuous IV infusion via a programmable infusion pump KDS 250 (KD scientific Inc., Holliston, PA, USA) of 0.9% saline (NS; 308 mOsm/L), 3% HS (1023 mOsm/L), 5% HS (1900 mOsm/L), or 7.5% HS (2310 mOsm/L). α-Syn-/- mice (n=38) subjected to CA were randomized to receive either continuous IV infusion of NS or 7.5% HS. Sham-operated mice subjected to all surgical procedures (intubation and vascular catheterization) except for CA in both strains served as controls. HS was instituted as a mixture of acetate:chloride (50:50; pH=6.5–7.0) to avoid hyperchloremic acidosis. All infusions were given at rate of 1 ml/kg/hr. Treatments were started 30 min after ROSC and continued for 24 hr. Serum osmolality and regional brain water content by WDR were determined at the end of the experiment.
In the second series of experiments, WT (n=25) and α-Syn-/- (n= 28) mice subjected to CA were randomized to receive continuous IV infusion of NS or 7.5% HS for 24 hr. BBB integrity was assessed by EB extravasation method at the end of the experiment. Sham-operated WT and α-Syn-/- mice served as controls (n=7 each).
In the third series of experiments (n=12), WT mice subjected to CA and treated with IV infusion of NS or 7.5% HS for 24 hr were used to determine AQP4 protein expression. Sham-operated WT mice and naïve WT mice served as controls (n=4 each). AQP4 expression in α-Syn-/- mice was not analyzed because the total level of AQP4 is normal in brain of α-Syn-/- mice (16).
In the fourth series of experiments, physiological parameters (blood pressure, arterial blood gases) during CA/CPR were measured at baseline and 30 min after ROSC in WT and α-Syn-/- mice (n=5 each).
Statistical Analysis
All values are expressed as mean ± SEM. Physiologic parameters, CA-related parameters, plasma osmolality, differences in regional brain water content, and EB extravasation among treatment groups were determined by one-way analysis of variance (ANOVA) with post hoc Student-Newman-Keuls test. Survival rates among groups were analyzed by Chi-square Test. Neuroscore is presented as median (with 25% and 75% quartiles) and analyzed by the non-parametric Mann-Whitney U test. Densitometric analysis of AQP4 expression in different treatment groups was analyzed by Student's t test. P < 0.05 was considered statistically significant.
Results
Assessment of Serum Osmolality and Regional Brain Water Content
In the first series of experiments conducted to determine if HS attenuates regional cerebral edema associated with CA/CPR, 5 mice were excluded from the final analysis because of dislocation of the venous catheter. Serum osmolality was elevated in WT mice treated with HS in a dose-dependent manner (NS: 314±1; 3% HS: 318±2; 5% HS: 326±2; 7.5% HS: 345±5 mOsm/L; Table 1). Similarly, serum osmolality was significantly elevated in α-Syn-/- mice treated with 7.5% HS compared with NS treatment (7.5% HS: 351±7, NS: 316±4; Table 2). Serum sodium concentration was significantly elevated in mice treated with 5% HS and 7.5% HS compared with NS and 3% HS treatment (Table 1). In WT mice treated with NS, 3% HS, and 5% HS, CA led to an increase in brain water content in the CP complex and cortex as compared with those in sham-operated mice. While 3% HS and 5% HS treatment did not attenuate water content in WT mice subjected to CA/CPR, 7.5% HS treatment significantly attenuated water content as compared with NS treatment (cortex:78.6±0.1% vs 79.2±0.2%; CP complex: 77.9± 0.3% vs 79±0.4%, P < 0.05; Figure 1). Water content increased in both NS- and 7.5% HS-treated α-Syn-/- mice subjected to CA/CPR in the CP complex but not in the cortex, hippocampus, or cerebellum as compared with those in sham-operated mice. In contrast to WT mice, 7.5% HS treatment in α-Syn-/- mice did not attenuate regional brain water content compared to NS treatment (Figure 2). No differences were observed in mortality rates in both strains treated with HS as compared with NS.
Table 1. Summary of physiologic variables in WT mice used for experiments on brain water content at 24 hr following CA/CPR.
| NS Surgical Shams | NS CA/CPR | 3% HS Surgical Shams | 3% HS CA/CPR | 5% HS Surgical Shams | 5% HS CA/CPR | 7.5% HS Surgical Shams | 7.5% HS CA/CPR | |
|---|---|---|---|---|---|---|---|---|
| N | 8 | 15 | 8 | 13 | 7 | 12 | 9 | 13 |
| Body weight (g) | 24.5±0.4 | 24.1±0.6 | 24.2±0.5 | 24.9±0.4 | 24.5±0.5 | 24.7±0.4 | 24.7±0.5 | 24.3±0.4 |
| CPR duration (sec) | 61±6 | 60±5 | 60±7 | 61±8 | ||||
| Epinephrine (μg) | 8.8±0.2 | 8.7±0.2 | 8.8±0.2 | 8.9±0.2 | ||||
| Survival rate (%) | 100% | 80% | 100% | 85% | 100% | 83% | 100% | 77% |
| Sodium (mmol/L) at end of experiment | 145±1 | 146±1 | 147±1 | 147±1 | 152±1* | 152±1* | 155±2* | 156±3* |
| Osmolality (mOsm/L) at end of experiment | 317±2 | 314±1 | 317±1 | 318±2 | 325±2¶ | 326±2¶ | 347±9¶ | 345±5¶ |
| Neuroscore | 7 (4.5–8) | 7 (4–9) | 6 (3–9) | 4 (3–4.5)* |
Neuroscore is presented as median with 25 and 75% quartiles. All other data indicates mean ± standard error of the mean (SEM); CA/CPR, cardiac arrest and cardiopulmonary resuscitation; NS, 0.9% saline; HS, hypertonic saline
P < 0.05 vs. NS and 3% treatment groups;
P < 0.05 vs. all other treatment groups
Table 2. Physiologic variables in α-Syn-/- mice used for experiments on brain water content at 24 hr following CA/CPR.
| NS Surgical Shams | NS CA/CPR | 7.5% HS Surgical Shams | 7.5% HS CA/CPR | |
|---|---|---|---|---|
| N | 6 | 12 | 6 | 14 |
| Body weight (g) | 24.5±0.4 | 24.1±0.6 | 24.7±0.5 | 24.3±0.4 |
| CPR duration (sec) | 65±5 | 64±6 | ||
| Epinephrine (μg) | 8.9±0.2 | 9.0±0.2 | ||
| Survival rate (%) | 100% | 75% | 100% | 71% |
| Sodium (mmol/L) at end of experiment | 145±1 | 146±1 | 152±2* | 157±4* |
| Osmolality (mOsm/L) at end of experiment | 316±3 | 316±4 | 340±5* | 351±7* |
| Neuroscore | 7 (7–9) | 8 (5.5–8.5) |
Neuroscore is presented as median with 25 and 75% quartiles.
All other data indicates mean ± standard error of the mean (SEM).
CA/CPR, cardiac arrest and cardiopulmonary resuscitation; NS, 0.9% saline;
HS, hypertonic saline.
P < 0.05 vs. NS treatment groups
Figure 1.
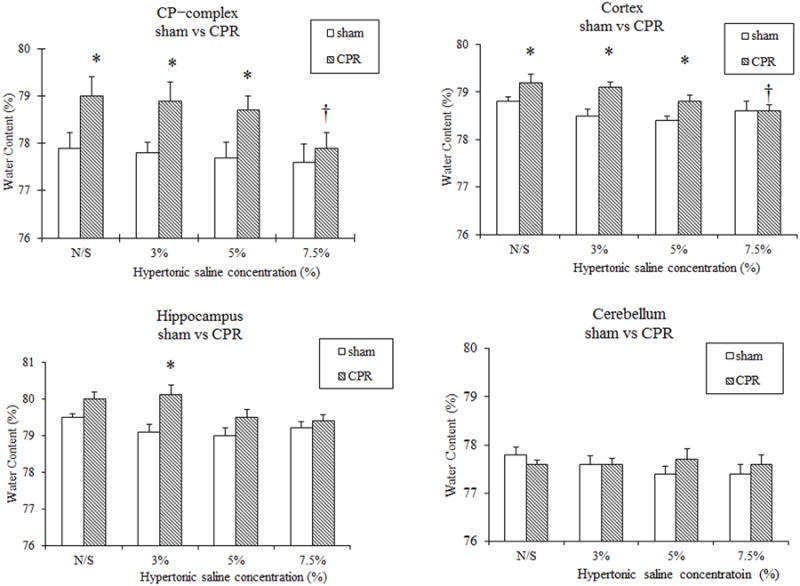
% Regional brain water contents in the caudoputamen (CP) complex, cortex, hippocampus, and cerebellum of WT mice treated with NS, 3% HS, 5% HS, or 7.5% HS for 24 hr following 8 min CA/CPR. * P < 0.05 versus surgical shams. †P < 0.05 versus CPR with NS treatment.
Figure 2.
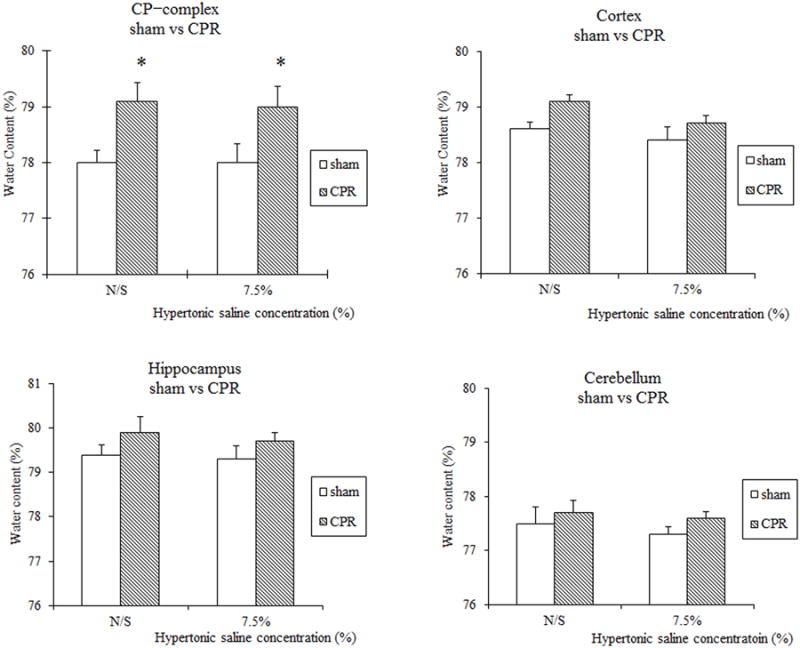
% Regional brain water contents in the caudoputamen (CP) complex, cortex, hippocampus, and cerebellum of α-Syn-/- mice treated with NS or 7.5% HS for 24 hr following 8 min CA/CPR. * P < 0.05 versus surgical shams.
Assessment of BBB integrity
In the second series of experiments conducted to determine if HS attenuates BBB disruption following CA, in the absence and presence of perivascular AQP4 pool. Following sham operations, the fluorescent quantification of Evans Blue showed that BBB disruption was not different between WT and α-Syn-/- mice. The severity of BBB disruption following CA was similar in WT and α-Syn-/- treated with NS. Treatment with 7.5% HS attenuated BBB disruption in WT mice but not in α-Syn-/- mice (Figure 3).
Figure 3.
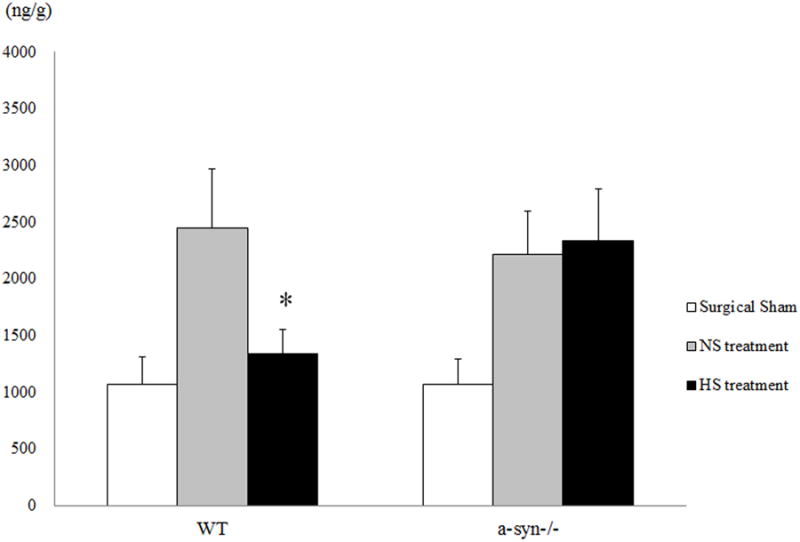
Blood brain barrier disruption as estimated by EB extravasation in various treatment groups at 24 hr following CA/CPR. * P < 0.05 versus NS treatment in WT mice.
AQP4 expression with Western blotting
In the third series of experiments conducted to determine if HS modulates AQP4 expression 24 hr after CA/CPR. There were no significant difference in AQP4 expression in all brain regions between NS and 7.5% HS-treated WT mice (Figure 4).
Figure 4.
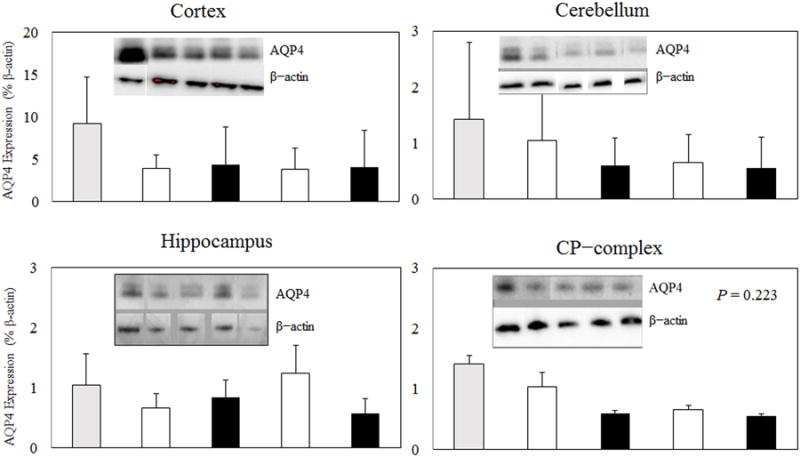
Immunoblot of membrane protein isolated from the surgical shams or 8min CA mice treated with either NS or 7.5% HS for 24 hr. Naïve WT mice were used as controls. AQP4 was identified as a major band at ∼ 30 kDa. Densitometric analysis (intensity ratio) for the AQP4 protein was corrected to a standard protein (β–actin).
Cardiac Arrest and Cardiopulmonary Resuscitation Related Parameters
Body weight, CPR duration, epinephrine dose, and survival rate were not different (Table 1 and 2). In the fourth series of experiments conducted to determine if α-Syn deletion alters physiologic parameters during CA/CPR experiment, temporal profiles of mean arterial pressure during CA/CPR were similar between WT and α-Syn-/- mice groups (Figure 5). Blood analyses including blood gases, pH, base excess, blood glucose, sodium and potassium levels at baseline and at 30 min after ROSC were similar between WT and α-Syn-/- mice (Table 3).
Figure 5.
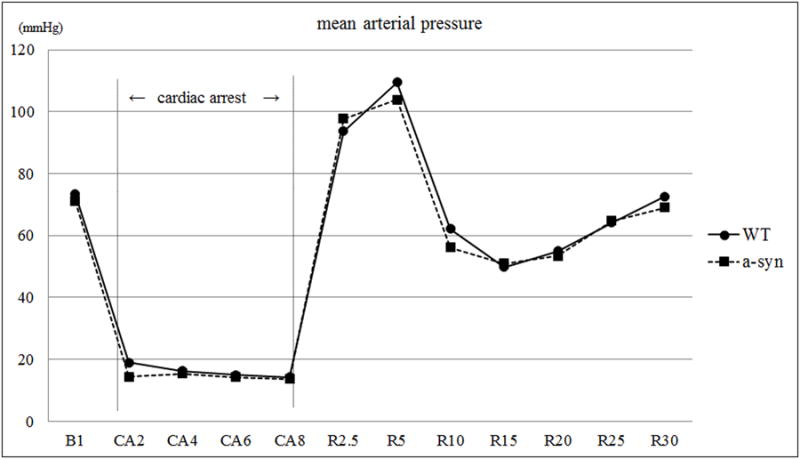
Mean arterial pressure in WT and α-Syn-/- mice at baseline (B1), 8 min of CA (CA2 to CA8) and CPR (R 2.5 to R 30 in min).
Table 3. Blood gas analysis, serum sodium, and Glucose data at baseline 10 min before induction of CA and at recovery (30 min after ROSC).
| WT (n=5) | α-Syn-/- (n=5) | |
|---|---|---|
| pH | ||
| Baseline | 7.35 ± 0.02 | 7.35 ± 0.02 |
| Recovery | 7.12 ± 0.02 | 7.02 ± 0.03 |
| PaCO2, mm Hg | ||
| Baseline | 37.9 ± 3.2 | 37.4 ± 2.3 |
| Recovery | 44.3 ± 3.9 | 42.8 ± 2.6 |
| PaO2, mm Hg | ||
| Baseline | 170.6 ± 6.1 | 163.8 ± 5.6 |
| Recovery | 287.4 ± 8.4 | 269.0 ± 6.7 |
| Base Excess, mEq/L | ||
| Baseline | -5.1 ± 1.2 | -4.6 ± 0.5 |
| Recovery | -14.3 ± 1.8 | -15.9 ± 0.6 |
| Sodium, mmol/L | ||
| Baseline | 146.0 ± 1.4 | 147.6 ± 1.3 |
| Recovery | 146.4 ± 2.3 | 147.6 ± 1.3 |
| Potassium, mmol/L | ||
| Baseline | 4.1 ± 0.1 | 4.1 ± 0.1 |
| Recovery | 4.6 ± 0.2 | 4.6 ± 0.2 |
| Glucose, mg/dL | ||
| Baseline | 176 ± 11 | 174 ± 4 |
| Recovery | 204 ± 11 | 208 ± 10 |
No significant difference between experimental groups
CA= Cardiac Arrest; ROSC=Return of Spontaneous Circulation
Discussion
This study demonstrates several important findings. First, in WT mice, brain water content in the cortex and the CP complex following CA/CPR is attenuated with continuous HS infusion when serum osmolality is maintained at level of ∼350 mOsm/L. In contrast, in mice that lack perivascular AQP4 (α-Syn-/-), brain water content is not altered with HS treatment. Second, HS attenuates BBB disruption depending on the presence of the perivascular pool of AQP4. These findings provide evidence that the perivascular pool of AQP4 mediates the effect of osmotherapy with HS on cerebral edema following CA/CPR and implicate that this pool constitutes a therapeutic target to reduce cerebral edema and to protect the integrity of BBB.
Cerebral edema is an important modulator of outcome following brain injury from CA/CPR; pathophysiological mechanisms include elevations in intracranial pressure from cerebral edema formation leading to diminution in cerebral blood flow with consequent loss of brain function (30, 31). Thus far, limited treatment strategies aimed at CA-evoked cerebral edema are available including osmotic agents, and mechanisms of beneficial effects of osmotherapy are not fully elucidated. For example, a previous study demonstrated no improvement in functional and histopathological outcome at 7 days following a single bolus injection of hypertonic saline hydroxyethyl starch 1.5 min following ROSC in a rat model of CA despite accentuating local cerebral blood flow (32). In our previous studies, osmotherapy with continuous infusion of HS ameliorated stroke-evoked cerebral edema following experimental focal ischemia (20, 26, 29). The beneficial effect of HS was not observed in mice genetically engineered to lack perivascular pool of AQP4 expression (21). In keeping with these previous studies, results from the present study demonstrate that HS attenuates regional cerebral edema following CA/CPR in WT mice but not in α-Syn-/- mice. While the pathophysiological mechanisms of edema formation may differ between the 2 injury paradigms (focal and global ischemia), HS was equally efficacious in attenuating brain water content via the perivascular pool of AQP4.
AQP4, the most abundant water channel in the brain (33), is known to contribute to water homeostasis in brain injury from diverse etiologies (34). In murine models of focal ischemia, AQP4 is up-regulated around the peri-infarct border early after stroke (35, 36). The degree of increase in AQP4 is correlated with the magnitude of cytotoxic cerebral edema (33). Conversely, different strategies to reduce the AQP4 pool, perivascularly (11, 12) or in the brain at large (9) prevent edema formation, indicating that the presence of AQP4 facilitates the entry of water in astrocytes. At later time points, AQP4 plays beneficial role in the clearance of water from the brain into blood vessels. In this edema resolution phase, AQP4 expression in the peri-infarct zone is increased after 48 hr in focal ischemia models, which can facilitate resorption of excess brain water (35, 37). In a traumatic brain injury animal model that replicates vasogenic edema, AQP4 knockout mice develop more cerebral edema than WT mice because efflux of water from brain to blood vessels is limited in the transgenic animals (38). These results implicate that AQP4 plays dual role of water movement in a variety of brain injury paradigms. Although no specific inhibitor to block AQP4 has been available, the evidence from AQP4 deletion mice (9, 12) and knockdown of AQP4 expression using siRNA technique (39) suggests that AQP4 contributes to the regulation of edema formation and resolution of both cytotoxic and vasogenic cerebral edema. However, from a practical standpoint, different patterns of AQP4 expression and their alterations in a variety of pathological conditions make the treatment for cerebral edema complex and difficult.
Serum osmolality is a critical determinant of cerebral edema because hyperosmolar state causes water efflux from brain to blood vessels. HS solutions reappeared in clinical use in the 1980s and are increasingly being used for the treatment of cerebral edema resulting in various brain injury paradigms (17, 40). In keeping with our previous studies with focal cerebral ischemia (26, 41), our study demonstrates that maintenance of serum osmolality ≥ 350 mOsm/L with 7.5% HS attenuated both regional cerebral edema and BBB disruption following CA/CPR. Lower HS concentrations (3% or 5%), however, had no effect on reducing regional brain water content. These results suggest that maintenance of a constant osmotic gradient by maintaining a “euvolemic hyperosmolar” state to eliminate water from the injured and non-injured brain is important in ameliorating CA-evoked cerebral edema.
Another important finding of our study is that the osmotic action of HS depends on the presence of the perivascular pool of AQP4. Indeed, we observed that HS treatment failed to attenuate both cerebral edema and BBB disruption in mice that lack perivascular AQP4 (α-Syn-/-). The lack of edema resolution observed in α-Syn-/- mice indicates that the water efflux from the brain is mainly mediated by the perivascular AQP4. Furthermore, the present findings are consistent with the notion that AQP4 mediates bidirectional water flux (12),where perivascular AQP4 helps to regulate not only the initial cerebral edema formation, but also subsequent edema resolution.
In addition to the water elimination with HS via the perivascular AQP4, severity of BBB disruption may have a significant impact on the magnitude of cerebral edema. We have shown that HS attenuates BBB disruption in the ischemic hemisphere after focal brain ischemia depending on the integrity of perivascular AQP4 pool (21). Likewise, we found that BBB disruption at 24 hr following CA/CPR was attenuated with HS treatment in WT mice but not in α-Syn-/- mice while BBB disruption was similar in both WT and α-Syn-/- mice with NS treatment. HS has been shown to have anti-inflammatory properties, which may lead to attenuation of BBB disruption (42-44). Taken together, it can be presumed that HS has its anti-inflammatory action, attenuating BBB disruption when the perivascular AQP4 exists after ischemic injury. This may further explain the beneficial effect of HS on eliminating water from brain to blood vessels across the intact BBB region.
We have demonstrated that cerebral edema is maximal at 24 hr and the BBB disruption is maximal at 48 hr in our model of CA in WT mice (unpublished data). We determined the effect of HS on regional cerebral edema at 24 hr following CA/CPR because there is selective vulnerability of certain brain regions following global cerebral ischemia; these include the medium sized neurons of the striatum (CP complex), CA1 neurons of the hippocampus, cerebral cortex, and the Purkinje cells of the cerebellum (45). At this time point where BBB remains relatively intact, cytotoxic edema component plays a dominant role. During this cytotoxic edema formation phase, up-regulation of AQP4 could facilitate water transport into the brain. However, we found no difference in AQP4 expression between mice subjected to sham surgery and CA. It is difficult to clearly differentiate cytotoxic and vasogenic edema components at the time point we observed. Nevertheless, the fact that the presence of perivascular AQP4 with osmotherapy ameliorated cerebral edema following CA/CPR implies that modulators of AQP4, together with the osmotic agents would offer a new therapeutic option to reduce post-CA cerebral edema and to protect the integrity of the BBB. Further studies are needed to elucidate the spatial and temporal patterns of AQP4 expression following global cerebral ischemia. The translational significance of the present study are significant, in that, regional brain water content was attenuated by 1–2% in mice treated with HS which translates to > 90 ml reduction in human brain volume and can be life-saving for patients following CA (46, 47).
Our study has several limitations. First, the water flux across the BBB is not mediated exclusively by AQP4. There is a possibility that other water transporting molecules are being disrupted by the α-syn gene deletion. We have shown that α-syn gene deletion does not alter the expression of these known water transporting molecules (14). Second, the α-syn gene deletion may have affected the integrity of BBB; however, previous studies using AQP4-/- mice demonstrated no alteration of the BBB integrity (48). Our results also showed that BBB remains intact in α-Syn-/- mice, which concurs with our previous study in experimental focal cerebral ischemia (21). Moreover, BBB disruption was not induced following inhibition of AQP4 gene using RNA interference (39), and BBB remained intact in glial conditional AQP4-/- mice (49). Together, these data indicate that AQP4 deletion does not alter BBB integrity in the normal brain. Third, we did not explore long-term outcomes because we sought to determine the effect of HS on cerebral edema that was maximal at 24 hr after CA/CPR in our preliminary experiments. Fourth, both HS treatment and CA insult could have altered ischemia-evoked changes in AQP4 expression in a time-dependent manner. The changes in AQP4 expression has never been examined in global ischemia models. It is suggested that severely damaged brain is unable to express sufficient AQP4 proteins. For instance, in a model of more severe stroke following permanent vessel occlusion, an early up-regulation of AQP4 expression was not observed (50). Finally, our study cannot address if bolus administration of HS is more efficacious than continuous infusion (or combination) in this injury paradigm. Our study was not designed to study other effects of HS on physiological parameters such as augmentation of regional cerebral blood flow that may confer neuroprotection.
Conclusions
In summary, this is the first study to demonstrate in a well-characterized murine model of CA/CPR that HS infusion maintained to achieve serum osmolality ∼350 mOSm/L attenuates cerebral edema and BBB disruption via the perivascular pool of AQP4. These results suggest that treatment with HS targeted to the perivascular pool of AQP4 is a promising approach for ameliorating CA/CPR-evoked cerebral edema.
Acknowledgments
We thank Marvin Adams, MD, PhD, and Stanley C. Froehner, PhD for providing the α-syntrophin knockout mice and Stephanie J. Murphy, DVM, PhD, and Sarah Mader for maintaining the animal colony.
Drs. Bhardwaj and Nakayama received support for article research from the National Institutes of Health (NIH). Their institutions received funding (This work was supported by Public Health Service NIH grant NS046379).
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to declare.
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 3.Vaillancourt C, Stiell IG Canadian Cardiovascular Outcomes Research T. Cardiac arrest care and emergency medical services in Canada. Can J Cardiol. 2004;20(11):1081–1090. [PubMed] [Google Scholar]
- 4.Richmond TS. Cerebral resuscitation after global brain ischemia: linking research to practice. AACN Clin Issues. 1997;8(2):171–181. doi: 10.1097/00044067-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 5.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(S 1-2):1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 6.Bulkley GB. Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function. Lancet. 1994;344(8927):934–936. doi: 10.1016/s0140-6736(94)92276-4. [DOI] [PubMed] [Google Scholar]
- 7.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Naples R, Ellison E, Brady WJ. Cranial computed tomography in the resuscitated patient with cardiac arrest. Am J Emerg Med. 2009;27(1):63–67. doi: 10.1016/j.ajem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 10.Badaut J, Lasbennes F, Magistretti PJ, et al. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22(4):367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Vajda Z, Pedersen M, Fuchtbauer EM, et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A. 2002;99(20):13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiry-Moghaddam M, Otsuka T, Hurn PD, et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100(4):2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amiry-Moghaddam M, Williamson A, Palomba M, et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100(23):13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiry-Moghaddam M, Xue R, Haug FM, et al. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18(3):542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 15.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neely JD, Amiry-Moghaddam M, Ottersen OP, et al. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98(24):14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004;10(2):126–131. doi: 10.1097/00075198-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ziai WC, Toung TJ, Bhardwaj A. Hypertonic saline: first-line therapy for cerebral edema? J Neurol Sci. 2007;261(1-2):157–166. doi: 10.1016/j.jns.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Hays AN, Lazaridis C, Neyens R, et al. Osmotherapy: use among neurointensivists. Neurocrit Care. 2011;14(2):222–228. doi: 10.1007/s12028-010-9477-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Toung TJ, Sapirstein A, et al. Effect of duration of osmotherapy on blood-brain barrier disruption and regional cerebral edema after experimental stroke. J Cereb Blood Flow Metab. 2006;26(7):951–958. doi: 10.1038/sj.jcbfm.9600248. [DOI] [PubMed] [Google Scholar]
- 21.Zeynalov E, Chen CH, Froehner SC, et al. The perivascular pool of aquaporin-4 mediates the effect of osmotherapy in postischemic cerebral edema. Crit Care Med. 2008;36(9):2634–2640. doi: 10.1097/CCM.0b013e3181847853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams ME, Kramarcy N, Krall SP, et al. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150(6):1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kofler J, Hattori K, Sawada M, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. Neurosci Methods. 2004;136(1):33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Allen D, Nakayama S, Kuroiwa M, et al. SK2 channels are neuroprotective for ischemia-induced neuronal cell death. J Cereb Blood Flow Metab. 2011;31(12):2302–2312. doi: 10.1038/jcbfm.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguchi N, Nakayama S, Tanaka M. Fluoxetine has neuroprotective effects after cardiac arrest and cardiopulmonary resuscitation in mouse. Resuscitation. 2012;83(5):652–656. doi: 10.1016/j.resuscitation.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Toung TJ, Chen CH, Lin C, et al. Osmotherapy with hypertonic saline attenuates water content in brain and extracerebral organs. Crit Care Med. 2007;35(2):526–531. doi: 10.1097/01.CCM.0000253309.44567.A6. [DOI] [PubMed] [Google Scholar]
- 27.Uyama O, Okamura N, Yanase M, et al. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8(2):282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama S, Vest R, Traystman RJ, et al. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J Mol Neurosci. 2013;51(1):92–98. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CH, Xue R, Zhang J, et al. Effect of osmotherapy with hypertonic saline on regional cerebral edema following experimental stroke: a study utilizing magnetic resonance imaging. Neurocrit Care. 2007;7(1):92–100. doi: 10.1007/s12028-007-0033-9. [DOI] [PubMed] [Google Scholar]
- 30.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22(5):E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto Y, Kemmotsu O, Kitami K, et al. Acute brain swelling after out-of-hospital cardiac arrest: pathogenesis and outcome. Crit Care Med. 1993;21(1):104–110. doi: 10.1097/00003246-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Noppens RR, Kelm RF, Lindermann R, et al. Effects of a single-dose hypertonic saline hydroxyethyl starch on cerebral blood flow, long-term outcome, neurogenesis, and neuronal survival after cardiac arrest and cardiopulmonary resuscitation in rats. Crit Care Med. 2012;40:2149–2156. doi: 10.1097/CCM.0b013e31824e6750. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14(4):265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro Mde C, Hirt L, Bogousslavsky J, et al. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83(7):1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- 36.Hirt L, Ternon B, Price M, et al. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab. 2009;29(2):423–433. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- 37.Frydenlund DS, Bhardwaj A, Otsuka T, et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci U S A. 2006;103(36):13532–13536. doi: 10.1073/pnas.0605796103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadopoulos MC, Manley GT, Krishna S, et al. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. Faseb Journal. 2004;18(9):1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 39.Badaut J, Ashwal S, Adami A, et al. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab. 2011;31(3):819–831. doi: 10.1038/jcbfm.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28(9):3301–3313. doi: 10.1097/00003246-200009000-00032. [DOI] [PubMed] [Google Scholar]
- 41.Chang Y, Chen TY, Chen CH, et al. Plasma arginine-vasopressin following experimental stroke: effect of osmotherapy. J Appl Physiol. 2006;100(5):1445–1451. doi: 10.1152/japplphysiol.00763.2005. [DOI] [PubMed] [Google Scholar]
- 42.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocha-e-Silva M, Poli de Figueiredo LF. Small volume hypertonic resuscitation of circulatory shock. Clinics (Sao Paulo) 2005;60(2):159–172. doi: 10.1590/s1807-59322005000200013. [DOI] [PubMed] [Google Scholar]
- 44.Cao C, Yu X, Liao Z, et al. Hypertonic saline reduces lipopolysaccharide-induced mouse brain edema through inhibiting aquaporin 4 expression. Crit Care. 2012;16(5):R186. doi: 10.1186/cc11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 46.Paczynski RP. Osmotherapy. Basic concepts and controversies. Crit Care Clin. 1997;13(1):105–129. doi: 10.1016/s0749-0704(05)70298-0. [DOI] [PubMed] [Google Scholar]
- 47.Adrie C, Haouache H, Saleh M, et al. An underrecognized source of organ donors: patients with brain death after successfully resuscitated cardiac arrest. Intensive Care Med. 2008;34(1):132–137. doi: 10.1007/s00134-007-0885-7. [DOI] [PubMed] [Google Scholar]
- 48.Saadoun S, Tait MJ, Reza A, et al. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161(3):764–772. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 49.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A. 2011;108(43):17815–17820. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman B, Schachtrup C, Tsai PS, et al. Acute vascular disruption and aquaporin 4 loss after stroke. Stroke. 2009;40(6):2182–2190. doi: 10.1161/STROKEAHA.108.523720. [DOI] [PMC free article] [PubMed] [Google Scholar]


