Abstract
Episodic memory involves remembering the details that characterize a prior experience. Successful memory recovery has been associated with the reinstatement of brain activity patterns in a number of sensory regions across the cortex. However, how the hippocampus and surrounding medial temporal lobe (MTL) cortex contribute to this process is less clear. Models of episodic memory posit that hippocampal pattern reinstatement, also referred to as pattern completion, may mediate cortical reinstatement during retrieval. Empirical evidence of this process, however, remains elusive. Here, we use high-resolution fMRI and encoding-retrieval multi-voxel pattern similarity analyses to demonstrate for the first time that the hippocampus, particularly right hippocampal subfield CA1, shows evidence of reinstating individual episodic memories. Furthermore, reinstatement in perirhinal cortex (PrC) is also evident. Critically, we identify distinct factors that may mediate the cortical reinstatement in PrC. First, we find that encoding activation in PrC is related to later reinstatement in this region, consistent with the theory that encoding strength in the regions that process the memoranda is important for later reinstatement. Conversely, retrieval activation in right CA1 was correlated with reinstatement in PrC, consistent with models of pattern completion. This dissociation is discussed in the context of the flow of information into and out of the hippocampus during encoding and retrieval, respectively.
Keywords: CA1, medial temporal lobes, high-resolution fMRI, encoding-retrieval similarity
Introduction
When we think about the past, we are able to bring to mind rich images of our previous experiences, which is a defining feature of our episodic memories (Tulving, 1972). Indeed, successful remembering of a past experience has been associated with the reactivation of the brain regions activated during that experience. Initial attempts to measure the reactivation of encoding experiences during retrieval found that cortical regions active during the encoding of particular information, for example, pictures versus sounds, are reactivated later when they are remembered even in the absence of the picture or sound (Wheeler et al., 2000; Polyn et al., 2005). Critically, according to some theories, cortical reinstatement may be mediated by the hippocampus (McClelland et al., 1995; Norman and O’Reilly, 2003). Specifically, it has been proposed that reactivation of hippocampal patterns, or pattern completion, can result in or mediate cortical reinstatement during remembering (McClelland et al., 1995; MAR model: Davachi and Danker, 2013). Much current work has focused, however, on reactivation and its relationship to successful remembering, leaving the predictors that may mediate reinstatement underexplored. Here, we use high-resolution fMRI to identify signatures of pattern completion in the hippocampus and to explore the factors that mediate cortical reinstatement within the medial temporal lobe (MTL) cortex.
Recent advances in multivariate fMRI analyses, which measure the similarity of patterns of activation elicited during encoding and retrieval, have provided highly sensitive tools that can bee used to characterize the reinstatement of memories in the human brain. One approach measures the reinstatement of particular classes of content, e.g. faces or objects, by comparing ‘average’ encoding and retrieval trials that involved the same class of content. Prior work using this approach has shown that successful remembering is associated with the similarity between average encoding and retrieval patterns of activation in a variety of cortical regions including ventral temporal cortex (Polyn et al., 2005; Gordon et al., 2014; Davachi and Danker, 2013), prefrontal and parietal cortex (Kuhl et al., 2012; Kuhl and Chun, 2014) and even early visual regions (Bosch et al., 2014). An alternative approach used here employs encoding-retrieval similarity analyses (ERS). These analyses assess evidence for the reinstatement of individual memories or episodes by directly correlating encoding and retrieval patterns for specific events (Staresina et al., 2012; Ritchey et al., 2013; Wing et al., 2015). However, because this phenomenon has mainly been studied in sensory regions, it is less clear if cortical regions in the medial temporal lobe (MTL) similarly reinstate patterns based on the content or category of encoded information (cf: Staresina et al., 2012), despite their known role in both encoding (Davachi et al., 2003; Ranganath et al., 2003; Staresina et al., 2011; Uncapher and Rugg, 2005; for a review, see: Davachi, 2006) and retrieval (Kirwan and Stark, 2004; Eldridge et al., 2005) operations.
Thus, despite an abundance of research investigating the encoding and retrieval operations of the hippocampus and surrounding MTL cortex, much less work has focused on how the MTL system as a whole coordinates the neural reinstatement of specific episodic memories. Theoretical models of hippocampal function in memory posit that hippocampal pattern completion may be necessary to reactivate, or bring back to mind, the details associated with a past experience and that hippocampal pattern completion may, thus, mediate cortical reinstatement (McClelland et al., 1995; MAR model: Davachi and Danker, 2013). While there is some indication that hippocampal activation patterns can disambiguate individual memories (Chadwick et al., 2010), and that hippocampal univariate activation at retrieval correlates with cortical reinstatement (Ritchey et al., 2013; Staresina et al., 2012; Gordon et al., 2014; Bosch et al., 2014; Wing et al., 2015), and associative memory recovery more generally (Eldridge et al., 2000; Cabeza et al., 2001), to our knowledge there is no direct evidence for hippocampal pattern completion revealed through the reinstatement of event-specific patterns of neural activity that were present at encoding. Furthermore, the ‘memory as reinstatement’ or MAR model (Davachi and Danker, 2013) predicts that subsequent reinstatement, mediated by pattern completion, should be mediated by encoding strength, indexed by activation in the cortical regions that process memoranda. Past studies have shown that univariate activation in the hippocampus at the time of encoding is related to later memory (Davachi et al., 2003; Ranganath et al., 2003) However, a remaining question is whether encoding strength in the hippocampus or in MTL cortex mediates later neural reinstatement.
To address these questions, in the current study, participants underwent high-resolution fMRI during both associative memory encoding and retrieval. To measure the reinstatement of individual episodic memories, we used ERS to compute the match between encoding and retrieval multi-voxel patterns in hippocampal subfields and surrounding MTL cortex. We chose an associative memory task that involved forming a novel association between two objects, a function that has been shown to both depend on an intact hippocampus (Ryan et al., 2000; Holdstock, 2005; Hannula et al., 2006) as well as evoke strong perirhinal cortical activation because of its selectivity for object stimuli (Brown and Aggleton, 2001; Davachi, 2006; Awipi and Davachi, 2008; Miyashita, 1988). First, we predicted that the hippocampus would show greater encoding-retrieval similarity for successful memories, in line with its predicted function of reinstating encoding experiences during retrieval. An abundance of prior studies employing ERS have, thus far, failed to find evidence of reinstatement in the hippocampus, (Staresina et al., 2012; Ritchey et al., 2013; Wing et al., 2015). This is puzzling given evidence for hippocampal reinstatement in electrophysiological recordings (Frankland and Bontempi, 2005; Tayler et al., 2013), and may be explained by the possibility that patterns of activation in the hippocampus are more fine-grained than those in cortical areas. Using high-resolution fMRI would help to provide finer-grained resolution to examine reinstatement in different hippocampal subfields. Second, given its role in object encoding, we hypothesized that perirhinal cortex (PrC) would also exhibit changes in ERS as a function of object memory. Critically, we then asked how univariate activation during encoding and retrieval in these regions correlate with ERS in perirhinal cortex to gain leverage on factors during encoding and retrieval that contribute to cortical reinstatement.
Methods
Results from this dataset investigating the contribution of functional connectivity to later memory have been previously published (Duncan et al., 2014; Tompary et al., 2015). Seventeen students from New York University (8 female, mean age: 27.1, range: 22-35) participated. One participant was excluded due to experimenter error. All participants were right-handed with normal or corrected to normal vision. All experimental protocols were approved by the Institutional Review Board at New York University.
Experiment design
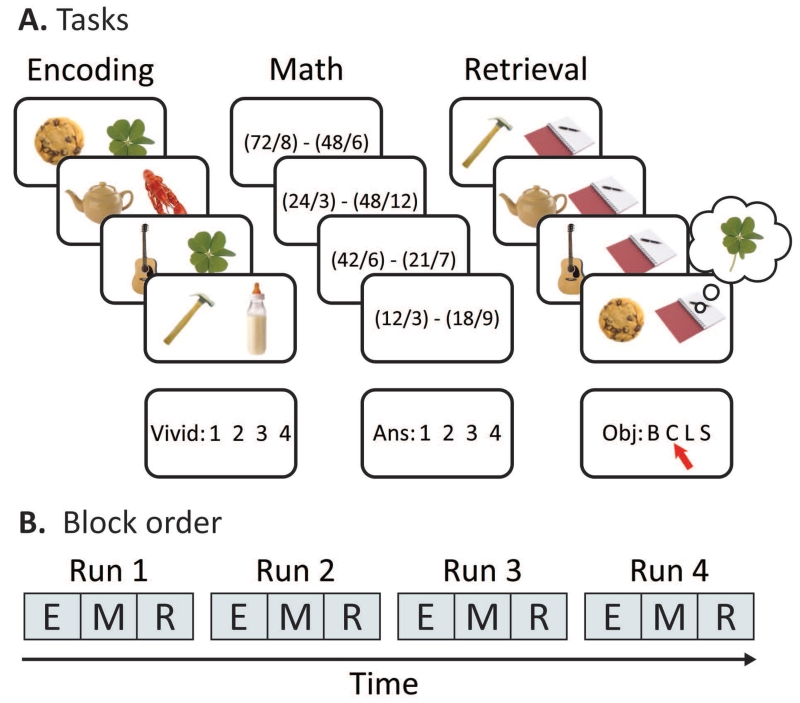
Participants were scanned while they completed four interleaved blocks each of encoding, math and retrieval tasks (Figure 1). In total, the experiment comprised one 5.5-min baseline math scan, followed by four 17-min scans that included one block each of encoding, post-encoding math, and retrieval. Each block lasted for 5.5 min, and the stimulus onset and response window of each task was identical: the stimulus displayed for 3 sec, then the response display for 2 sec, followed by 4 sec of fixation. The next day, participants returned to complete a long-term memory task that included a recognition test and a cued recollection test. We also collected confidence ratings for both long-term memory tests. For the purposes of this study, we only report the encoding and retrieval data from the first session.
Figure 1. Experiment design.
A. An overview of the experiment design. Participants completed blocks of encoding, math, and retrieval, preceded by one baseline block of math. During encoding, participants imagined two objects interacting, and later performed cued recall by choosing the associate (baby bottle, clover, lobster, or scissors) originally presented with each cue. Between each encoding and retrieval block, participants solved math problems. The math blocks were used as a filler task to incorporate a delay between encoding and retrieval.
Encoding
Over four blocks lasting 5.5 min, we presented participants with 144 object-object pairs and instructed them to associate each pair by vividly imagining the two objects interacting. Each object pair was presented for 3 sec, allowing participants to create and elaborate on a scenario in which the objects interacted. Critically, one object in each pair was unique to that trial (‘cue’), and the other object was one of four repeating objects: a baby bottle, clover, lobster or scissors (‘associate’). These four objects always appeared on the right side of the screen and repeated throughout the entire experiment, pseudo-randomized such that each associate was paired with 36 different cues. In the following 2-sec response period, participants rated the vividness of the imagined scenario on a scale of 1 to 4 (most to least vivid). The response mappings to the button box for the vividness ratings were counterbalanced across participants. If participants could not successfully imagine the objects interacting, they were able to opt out of the trial using the thumb button.
Math
In the scanner, participants completed a series of math problems between encoding and retrieving blocks of object pairs as well as at the beginning of the session. Each problem required the subtraction of two dividends. All possible solutions to each problem corresponded with the four buttons participants used to answer the problems. Each problem was presented on screen for 3 seconds, followed a 2-sec response window with the response options displayed on screen, followed by 4 seconds of fixation. Participants completed 36 problems during each 5.5-min block immediately following each encoding block. If participants could not solve the problem, they were encouraged not to guess and indicate that they did not know by pressing the thumb button.
Retrieval
After each math block, participants performed a cued recall task for all cues that they saw in the immediately preceding encoding block. On each trial, participants were instructed to match the presented trial-unique cue with the correct associate. As in the encoding task, the object was presented on screen for 3 seconds, followed by a 2-sec response window with the response options displayed (B, C, L and S), corresponding to the four possible associates (baby bottle, clover, lobster and scissors). An image of a notepad appeared next to each object, in order to equate the number of objects on screen across retrieval and encoding trials. Critically, this meant that the visual display seen on corresponding encoding and retrieval trials were only partially overlapping because the to-be-recalled associate was never presented on retrieval trials. Participants were encouraged not to guess and were instructed to use the thumb button to indicate that they did not remember the correct associate. The retention interval for each trial was approximately 11 minutes on average.
Long-term memory test
Data from the next day’s memory tests are not relevant to the aims of this experiment and therefore are not reported. In brief, participants were brought back to the lab approximately 24 hours after their scan sessions for a surprise memory test, Participants were tested for recognition of each cue, as well as memory for the associate studied with each cue (Duncan et al., 2014; Tompary et al., 2015).
fMRI
All scanning was performed using a 3T Siemens Allegra MRI system with a whole-head coil. Visual stimuli were projected onto a screen that was viewed through a mirror attached to the participant’s head coil. Functional data was collected using a zoomed high-resolution echo-planar pulse (EPI), with oblique coronal slices aligned perpendicular to the long axis of the hippocampus (1500-ms TR, 22-ms echo time (TE), FOV=192 × 96, 21 slices, 1.5 × 1.5 × 3-mm voxels, 77° flip angle). The field of view was decreased in the phase encode direction to reduce the total read out time and, thus, minimize distortions and artifacts (Olman et al., 2009). Saturation bands were used to suppress signal for tissue superior and inferior to the coverage of the scan. For whole brain coverage, we collected a T1-weighted high-resolution magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence (1 × 1 × 1 mm voxels, 176 sagittal slices). We also collected a T2-weighted 2D image (5100-ms TR, 88-ms echo time (TE), .898 × .898 × 1.5 mm voxels) in the same plane as the functional volumes. Finally, a field map sequence was collected to obtain estimates of the magnetic field and an in-plane spin-density image.
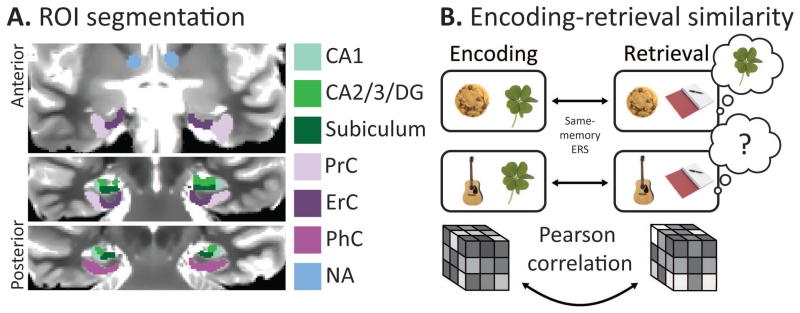
ROI Segmentation
Regions of interest (ROIs) were manually drawn using the coronal slices of each participant’s MPRAGE as an anatomical reference, with a focus on the hippocampus and surrounding MTL cortex. Within the hippocampus, right and left CA1, CA2/3/dentate gyrus and subiculum were isolated by referencing both an atlas and a previous high-resolution fMRI study (Duvernoy, 2005; Kirwan et al., 2007). A bilateral hippocampus ROI was also created comprising the three subfields. ROIs of the surrounding MTL cortex were also created, including perirhinal cortex (PrC), parahippocampal cortex (PhC) and entorhinal cortex (ErC) (Insausti et al., 1998). Due to the limited coverage outside of the MTL afforded by high-resolution scans, control ROIs outside the MTL included the ventral tegmental area (VTA), nucleus accumbens and cerebral spinal fluid (CSF). We chose these three control regions because they were within the coverage of the functional scans in every participant, and because unlike ventral temporal areas (Ritchey et al., 2013; Gordon et al., 2014), there is no prior evidence that these regions are involved in memory reinstatement. Therefore we predicted that ERS within the control regions would not be modulated by memory. All regions were originally segmented for use in prior work (Duncan et al., 2014; Tompary et al., 2015), and the same segmentations were used here. All ROIs were then resampled and aligned with the functional volumes. Finally, the ROIs were masked to remove voxels that had substantial dropout or distortions in the EPI images, first by excluding all voxels outside of a brain mask created by AFNI’s 3dAutomask function (available online at http://afni.nimh.nih.gov/afni). Each ROI was then verified by visual comparison to an undistorted functional scan, generated by each participant’s field map image. Due to disproportionate dropout in ErC, with an average of 28% voxels excluded per participant (SD: 17%), we did not use this ROI in any analyses.
Preprocessing
The data were preprocessed using custom scripts combining FSL command line tools (http://www.fmrib.ox.ac.uk/fsl) and AFNI (http://afni.nimh.nih.gov/afni). The first 11 volumes of all functional runs were discarded to allow for scanner stabilization. Then all functional volumes were slice time corrected, corrected for motion within each run, and finally aligned to the field map sequence. In order to retain high spatial resolution critical for pattern analyses, and to reduce the risk of blurring the boundaries of our small ROIs, the data were not spatially smoothed (Zeineh, 2003; Carr et al., 2010).
Encoding-retrieval similarity
Representational similarity analysis (Kriegeskorte et al., 2008) was used to assess encoding-retrieval similarity (ERS) as a measure of trial-specific episodic reinstatement (Xue et al., 2010; Ritchey et al., 2013; Staresina et al., 2012; Wing et al., 2015). In each of the four runs, every encoding and retrieval trial was modeled as 3-second boxcar function in a voxel-wise general linear model (GLM) using AFNI’s 3dDeconvolve function. This resulted in four GLMs per subject, each with 72 regressors (one for each encoding and retrieval trial). Again, data entering the GLM were not smoothed or normalized. Each model also included a single regressor for all math trials and block regressors to account for any differences between encoding, math, and retrieval blocks. Two regressors were included to account for scanner drift, as well as six motion regressors derived from the motion correction process. The resulting beta values for each encoding and retrieval trial were transformed into t values (Kriegeskorte et al., 2008), and then z-scored across voxels in each ROI such that the pattern of activity in each trial and run was centered around zero, ensuring that any results we found could not be driven by trial-to-trial fluctuations in mean activation across the ROI. Then, in each ROI, the pattern for each encoding trial was transformed into a vector and correlated with its corresponding retrieval vector. ERS for each trial was calculated as the correlation coefficient (Pearson’s correlation) of these two vectors. The coefficients were Fisher transformed before being entered in statistical tests.
In our main analysis of interest, the correlation coefficients for each participant were sorted according to memory for that trial, with the critical comparison between ERS for correctly remembered trials relative to those that were forgotten. In addition to standard statistical tests, permutation tests were employed to account for differences in accuracy across participants, which influenced the number of correct and incorrect trials that were entered into the comparison for each participant. To do this, accuracy of the trials in a given participant’s run was shuffled across the run, thus maintaining the true distribution of remembered and forgotten trials per run. The original correlation coefficients were sorted by the shuffled accuracy. This computation was repeated 1,000 times, generating a null distribution of average correlation coefficients for remembered and forgotten trials for each subject. The true difference in ERS as a function of memory performance was compared to this null distribution.
ERS and behavior
To further investigate the relationship between ERS and memory performance, correlations between ERS and overall memory across participants were computed. A memory score was created for all participants based on their performance at the immediate memory test: [Source Correct - (Source Incorrect)/4]. The number of incorrect trials was divided by four to account for the higher number of incorrect options presented on each trial (Duncan et al., 2014). An ERS score was computed for every participant: [Remembered ERS – Forgotten ERS] and then ERS score and memory score were correlated across participants. To ensure that our results were robust and would generalize outside of our sample, our results were confirmed with robust regression and permutation tests. Specifically, memory performance was shuffled across participants 10,000 times and shuffled scores were correlated with ERS in each ROI to create a null distribution. The true correlations were then compared to the shuffled distribution.
Univariate activation
BOLD activation for each encoding and retrieval trial was also extracted. BOLD activation was calculated by averaging each trial’s beta estimate over all voxels in an ROI. We then z-scored all trials across each run to account for differences in activation between scans. Correlations between ERS and BOLD activation were Fisher transformed before being submitted to statistical tests.
Results
Behavior
Associative memory for the repeated object that matched each cue was reliably above chance (mean = 0.79, SD = .15, t(15) = 14.47, p < 10 −11). Three participants reported falling asleep at during the experiment (one participant during one run, two participants during two runs), and performance on these runs were > 2 SD relative to the group mean. Thus, those runs were excluded from all subsequent analyses. In addition, we required all participants to have seven or more trials in each accuracy bin, and as a result we fully excluded one participant with an insufficient number of forgotten trials.
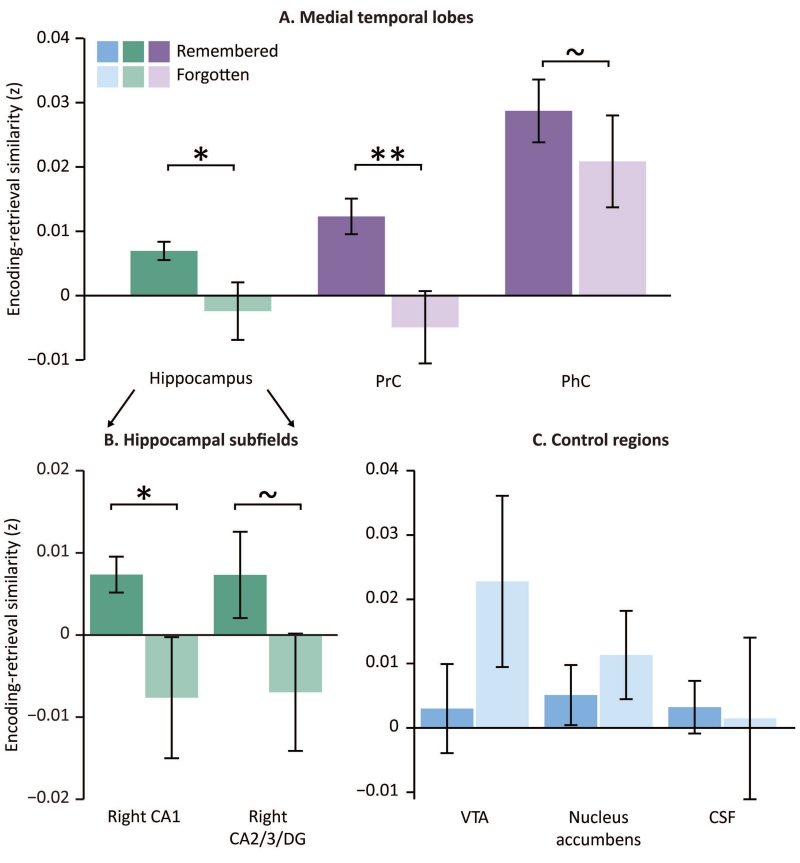
Memory reinstatement
We first tested for differences in encoding-retrieval similarity, or ERS, for remembered versus forgotten trials. If ERS is related to memory success, there should be significantly greater ERS for remembered relative to forgotten trials. When considering the entire hippocampal regions bilaterally, we found that ERS is indeed greater for remembered compared to forgotten trials (t(14)=2.26, p < 0.05). Focusing in on hippocampal subfields revealed that ERS in right CA1, but not other subregions, shows significant modulation by memory (t(14)=2.21, p < 0.05), with a trend in right CA3/DG in the same direction (t(14)=2.14, p = 0.05). We confirmed these results with a permutation test (CA1: p < 0.002; CA3/DG: p = 0.06). While we only found significant differences in the right subfields, separate 2 (hemisphere: right, left) by 2 (memory: remembered, forgotten) ANOVAs for each subfield revealed no significant difference between right and left CA1 (F(1,14)=0.44, p=0.52) or in right and left CA3 (F(1,14)=0.37, p=0.55). Furthermore, to compare ERS across the different hippocampal subfields, we ran a 3 (CA1, CA2/3/DG, subiculum) by 2 (remembered, forgotten) repeated measures ANOVA and found no interaction between memory and subfield (F(2,28)=1.66, p=0.21) and a trending main effect of memory accuracy (F(1,28)=4.21, p=0.06). Although ERS modulation by memory was not significantly greater in right CA1 than in other subfields, we focus on this region for subsequent analyses to maximize the sensitivity of the subsequent analyses.
Finally, ERS in perirhinal cortex (PrC) was also significantly higher for remembered compared to forgotten trials (PrC: t(14)=3.64, p < 0.005, Figure 2). Of note, ERS in parahippocampal cortex (PHC) revealed a trend in the same direction t(14)=1.87, p < 0.08). Critically, however, ERS was not significantly modulated by memory in the three control regions (all p’s > 0.28).
Figure 2. fMRI methods.
A. An example of the ROI boundaries over a T2 weighted scan. All ROIs were hand-drawn while referencing coronal slices over T1 and T2 weighted images. B. A schematic of the ERS analysis. We computed same-memory ERS for each trial by correlating its pattern of activation at encoding and retrieval.
ERS and region size
The BOLD signal in the medial temporal lobe, particularly in cortex, is particularly susceptible to dropout and distortion due to its proximity to the ear canals. All of our ROIs were masked to exclude voxels with insufficient signal intensity (see Methods). To ensure that this procedure did not bias the results, we measured whether the number of voxels included for each participant influenced ERS values. In right CA1, no voxels were removed from each participant’s ROI as a result of this process, with exception of 1 voxel removed from one participant’s ROI. The number of voxels in each participant’s rCA1 ROI did not relate to memory across participants (r(11)=0.10, p = 0.76). Furthermore, when using a partial correlation to account for the number of voxels in rCA1, there was still a trend for ERS correlating with memory across participants (r(11)=0.56, p = 0.09). In PrC, neither the size of the ROIs nor the proportion of voxels included correlated with memory across participants (size: r(11)=0.20, p = 0.53; proportion: r(11)=0.17, p = 0.58). When accounting for either of these variables in partial correlations, ERS still strongly related to memory (size: r(11)=0.83, p < 0.01; proportion: r(11)=0.79, p < 0.01). These results suggest that the ERS values represent the successful reinstatement of the initially encoded information, and are not confounded by variations in signal quality that are known to be especially problematic in anterior MTL cortex.
Across participant ERS correlations with behavioral memory performance
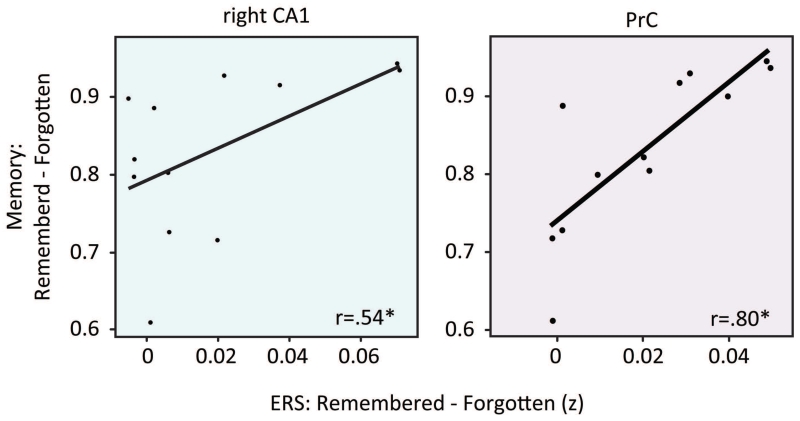
To test if the extent to which participants exhibit ERS on individual trials relates to their overall memory performance, we asked to what extent ERS predicted memory performance across participants. Here the three participants who reported falling asleep during the study were entirely excluded, to avoid any qualitative differences in the relationship between memory and ERS that could be related to sleeping. To this end, an ERS ‘difference score’ was computed for each participant and ROI by subtracting the average ERS of forgotten trials from the average ERS of remembered trials. We then correlated this difference score with overall memory performance for each participant. Interestingly, we found significant positive correlations between participants’ ERS difference scores and overall memory performance in right CA1 and PrC (CA1: r(10)=.54, p=.07; PrC: r(10)=.80, p= .002; all other ROIs, p>.23; Figure 3). This suggests that participants who show stronger mnemonic reinstatement in CA1 and PrC are those who also have better associative memory performance. To ensure that these results are generalizable outside of our sample size, we replicated these results using a permutation test where memory performance was shuffled across participants (CA1: p<0.05, PrC: p < 0.005). Robust regression further confirmed this relationship in PRC but not right CA1 (CA1: ß=0.13, p=0.13, PrC: ß=0.15, p < 0.005).
Figure 3. Influence of ERS on memory.
A. ERS for the hippocampus and MTL cortex, specifically PrC and PhC. ErC was not analyzed due to excessive dropout. B. ERS for hippocampal subfields: right CA1 and right CA2/3/DG. C. ERS for three control regions: VTA, nucleus accumbens, and CSF. Error bars denote standard error of the mean. ~ indicates p < 0.10 ,* indicates p <0.05, ** indicates p <0.005.
Predictors of ERS during encoding and retrieval
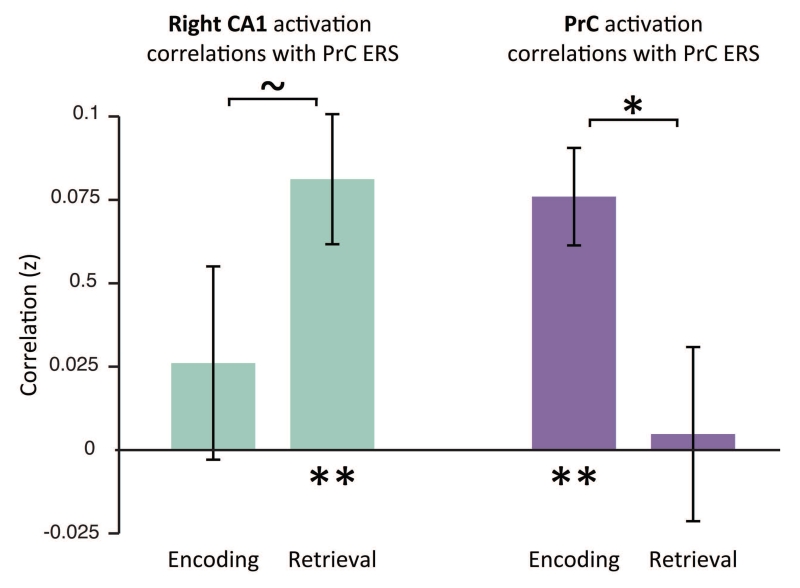
We next wanted to ask how univariate activation in MTL regions during encoding and retrieval are related to reinstatement, as measured by ERS. Specifically, we hypothesized that both encoding strength, measured by univariate encoding activation, and hippocampal signals related to retrieval, perhaps a proxy for pattern completion, may both be related to cortical reinstatement. For this analysis, we used PrC as our cortical region because it is known to be important for encoding and retrieval of object memories (Brown and Aggleton, 2001; Staresina and Davachi, 2008; Awipi and Davachi, 2008) and in our own dataset, shows significant memory modulated ERS. Thus, to test these predictions, we assessed whether our measure of ERS in PrC related to trial-by-trial variability in mean activation in the MTL ROIs (CA1 and PrC) within each participant. To this end, we correlated the trial-by-trial encoding and retrieval univariate activation in right CA1 and PrC with reinstatement measured in PrC for each participant. We found a dissociation between region and task, such that encoding activation in PrC correlated with PrC ERS (t(14)=5.20,p<0.001), while right CA1 retrieval activation correlated with PrC ERS (t(14)=4.16, p<0.005) (Figure 4). Furthermore, when we restricted the trials in this analysis to only include trials that were successfully remembered, we replicated our effects (PrC encoding activation related to PRC ERS: t(14)=4.24, p < 0.001; CA1 retrieval activation related to PrC ERS: t(14)=3.90, p < 0.01). Critically, to assess specificity, we found that PrC encoding activation correlated more with PrC ERS than PrC retrieval activation (t(14)=2.34, p < 0.05). On the other hand, right CA1 retrieval activation correlated marginally more with ERS in PrC relative to right CA1 encoding activation (t(14)=1.76, p = 0.10). Conversely, in keeping with the idea that specific cortical regions involved in encoding and retrieval are often sensitive to the content of the memory, neither encoding nor retrieval activation in parahippocampal cortex (PhC) correlated with PrC ERS (both p’s < 0.17). While we can make no assumptions about the directionality of the correlations, these results are in line with the hypothesis that cortical memory reinstatement is related to encoding strength, as measured by PrC univariate activation during encoding and hippocampal retrieval-related activation, perhaps related to pattern completion (McClelland et al., 1995; Norman and O’Reilly, 2003; Davachi and Danker, 2013).
Figure 4. Relationship between ERS and memory across participants.
The correlation between same-memory ERS (remembered – forgotten) and mean memory performance across participants. Pearson correlation r values for right CA1 and PrC are displayed. * indicates p <0.05.
Comparing the contribution of ERS and univariate activation to memory
While we predicted a relationship between ERS and univariate activation that reflected the flow information during encoding and retrieval periods, we also expected that our measure of reinstatement captured patterns of activity in the BOLD signal that are more informative than mean BOLD activation in these regions (Staresina et al., 2012; LaRocque et al., 2013; Ritchey et al., 2013). To ensure that ERS predicted memory accuracy when controlling for fluctuations in BOLD activation, we ran separate binomial logistic regressions for right CA1 and PrC to predict the memory success of each trial with three regressors: 1) trial-by-trial ERS values, 2) trial-by-trial activation during encoding, and 3) trial-by-trial activation during retrieval. We included random intercept and slope terms for each participant, and all other variables were modeled as fixed effects. When entered into the regression with encoding and retrieval activation, ERS in PrC remained significant when accounting for univariate encoding and retrieval activity (ß=2.61, p < 0.01) and predicted memory significantly better than univariate encoding and retrieval activation in PrC (encoding: wald χ2 = 6.77, p < 0.01; retrieval: wald χ2 = 6.85, p < 0.01) (Table 1). ERS from right CA1 remained a significant predictor of memory accuracy (ß=1.53, p<0.05) and was also significantly more correlated with memory compared both to encoding and retrieval activation (encoding: wald χ2=4.34, p<0.05; retrieval: wald χ2=4.87, p<0.05). Critically, these results indicate that ERS relates to memory accuracy more than both encoding and retrieval activation, and continues to relate to memory accuracy even when accounting for mean BOLD activation at encoding and retrieval.
Table 1. Logistic regression of ERS, ROI activation and memory.
We computed separate binary logistic regressions for CA1 and PrC to predict memory success for each trial using ERS, encoding activation, and retrieval activation as predictors for each ROI. Bold statistic indicates significant effects.
| Perirhinal cortex | β | StdErr | z | p |
|---|---|---|---|---|
| Intercept | 1.61 | 0.24 | 6.78 | <0.001 |
| ERS | 2.60 | 1.00 | 2.61 | <0.01 |
| Encoding activity | 0.03 | 0.05 | 0.69 | 0.49 |
| Retrieval activity | 0.01 | 0.06 | −0.26 | 0.80 |
| Right CA1 | β | StdErr | z | p |
|---|---|---|---|---|
| Intercept | 1.59 | 0.22 | 7.12 | <0.001 |
| ERS | 1.53 | 0.68 | 2.24 | 0.02 |
| Encoding activity | 0.11 | 0.09 | 1.24 | 0.21 |
| Retrieval activity | 0.02 | 0.07 | 0.29 | 0.77 |
Specificity of reinstatement
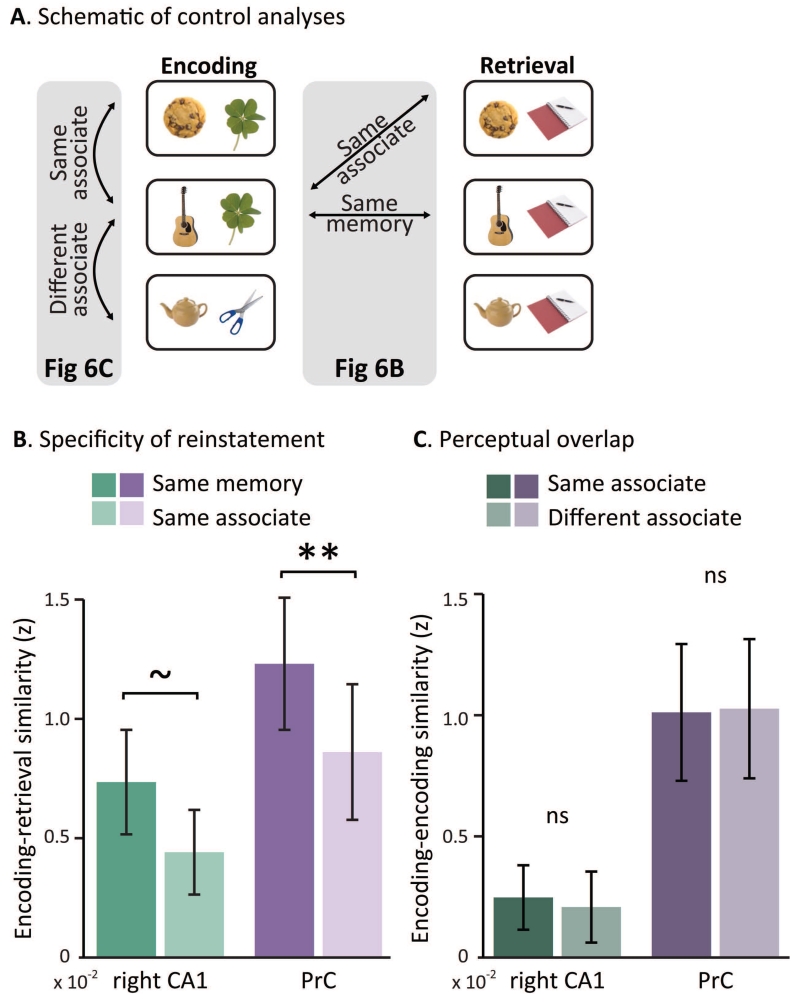
While previous work has identified item-level reinstatement in cortical regions (Staresina et al., 2012; Ritchey et al., 2013; Wing et al., 2015), evidence for item-level reinstatement in the hippocampus remains elusive. We next assessed whether the ERS seen in right CA1 and PrC represented reinstatement of specific episodes – specifically, trial-unique pairs of objects – and were not, for example, more driven by the act of retrieving one of the four associates which trials had in common. To test this, we conducted two control analyses: the first probed ERS for trials that shared an associate and the second focused on comparisons across encoding trials to asses how perceptual similarity contributes to measures of pattern similarity (Figure 6A).
Figure 6. Specificity of reinstatement.
A. Schematic of control analyses used to assess item-level reinstatement in the MTL. B. Group mean of same-memory and same-associate ERS in CA1 and PrC for all correctly remembered trials. Same-associate ERS was computed by correlating each encoding trial with all retrieval trials that shared an associate, and correlation each retrieval trial with all encoding trials that shared an associate. ** indicates p < 0.01, ~ indicates p < 0.1 using permutation tests. Error bars denote standard error of the mean. C. Group mean of encoding-encoding similarity in CA1 and PrC for all correctly remembered trials, sorted by similarity across trials with either the same or different associate. Error bars denote standard error of the mean.
For the first analysis, following an approach from Staresina and colleagues (2012), we created same-associate ERS scores by correlating: 1) the pattern of each encoding trial with all the retrieval trials studied with the same associate, and 2) the pattern of each retrieval trial with all encoding trials that shared its associate. The two scores were averaged to create one same-associate ERS score for each trial, which was then compared to the original ERS values (same-memory ERS). Because same-associate ERS is a measure of similarity across all episodes that shared an associate, while same-memory ERS is a measure of the reinstatement of a specific episode, we predicted that same-memory ERS would be greater than same-associate ERS for remembered items (Figure 6B). In addition to traditional statistical comparisons, we also employed a permutation test that took into account the different number of comparisons in each condition (1 measure for same-memory ERS and up to 8 for same-associate ERS). To do this, we shuffled each participant’s accuracy and trial type across each run and re-computed the difference between correlations for same-memory ERS and same-associate ERS 1,000 times. We then compared the true difference against this null distribution. First, in a repeated measures ANOVA with PrC ERS (same-memory, same-associate) and memory performance (remembered, forgotten) as factors, we found a significant interaction (F(1,14)= 10.45, p<0.005) such that same-memory ERS was greater than same-associate ERS for remembered trials (t(14)=3.37, p <0.005). We confirmed this difference with the permutation test (p<0.005). We found a trend for the same interaction in right CA1 using a separate ANOVA (F(1,14)= 4.34, p=0.06), driven by lower same-memory ERS than same-associate ERS for forgotten trials (t(14)=−1.71, p=0.11), and no difference between same-memory ERS than same-associate ERS for remembered trials (t(14)=1.41, p=0.18). However, when employing a permutation test, we found that same-memory ERS was marginally greater than same-associate ERS in right CA1 (p=0.07). Furthermore, neither region exhibited changes in same-associate ERS as a function of memory success (CA1: t(14)=1.61, p=0.13, PrC: t(14)=−1.52, p = 0.15). The fact that same-memory ERS was consistently greater than same-associate ERS for remembered trials reinforces the claim that same-memory ERS is sensitive to the unique combination of objects in each trial. That same-associate ERS did not reflect memory success lends support for our claim that specifically same-memory ERS is sensitive to reinstatement of unique, trial-specific memories.
Our second control analysis addressed the possibility that the difference between same-memory ERS and same-associate ERS is not due to the unique reinstatement of a cue with its associate, but instead is driven by differences in perceptual overlap. Same-memory ERS may be greater than same-associate ERS because the cue is on screen both during encoding and retrieval, while trials with a different cue at encoding and retrieval were used to compute same-associate ERS and therefore had less perceptual overlap across encoding and retrieval relative to same-memory ERS trials. Because cues never repeated within the encoding or retrieval tasks, we instead used the repeating associates to see if differences in perceptual similarity could explain any differences in pattern similarity. Specifically, we correlated the pattern similarity of each encoding trial with all other encoding trials with the same associate (‘same associate’), and also correlated this pattern with all other encoding trials with a different associate (‘different associate’). We limited this analysis to all correctly remembered trials to equate for successful encoding. To minimize any potential differences in temporal distance, we correlated each encoding trial only with encoding trials in different runs. In both regions, pattern similarity for same associate and different associate encoding trials were not significantly different (CA1: t(14)=−0.61, p<1; PrC: t(14)=−1.19, p<1, Figure 6C). Unfortunately the field of view used in this study did not include coverage of regions where we might expect that perceptual similarity would result in increased pattern similarity, for instance in ventral temporal or occipital cortex. We cannot completely rule out the influence of perceptual similarity with null results, but this does suggest that any effects of perceptual similarity are negligible in this analysis.
Discussion
The reinstatement of patterns of encoding-related activation is quickly becoming recognized as a hallmark of successful memory retrieval. Prior work has shown that cortical regions reactivate and that patterns of activity in cortical regions of interest also re-emerge when associations from a prior episode are brought back to mind. The current experiment was designed to query the role of hippocampal subregions and MTL cortical regions in memory reinstatement. Using encoding-retrieval similarity to measure the reinstatement of episodic memories, our results provide evidence for the reinstatement of unique episodic memories, both in the hippocampus, specifically right CA1, and in the perirhinal cortex. In addition, we find that participants who show more pronounced modulation of ERS during successful remembering are those whose memories are better, on average, which strengthens the proposed link between neural reinstatement and memory success. Critically, we show that cortical reinstatement in PrC differentially relates to univariate activation in CA1 and PrC, such that PrC ERS is related to trial-by-trial fluctuations in activation in PrC during encoding, but related to right CA1 activation during retrieval. Finally, we employ several control analyses to ensure that the match between encoding and retrieval patterns reflects the reinstatement of individual associative memories.
Past studies have found correlations between hippocampal activation at retrieval and ERS in a wide variety of cortical regions (Staresina et al., 2012; Ritchey et al., 2013) as well as cortical reinstatement more generally (Kuhl et al., 2010; Wimmer and Shohamy, 2012), but none to date have found this effect in the hippocampus. We see evidence that pattern reinstatement in the hippocampus is not only greater for correctly remembered object pairs relative to forgotten pairs, but also relative to the reinstatement seen during successfully remembered trials that share the same associate. This pattern suggests that the match between encoding and retrieval patterns in the hippocampus may represent individual episodes. However, the stimuli in our experiment are not entirely unique, due to the repeating associates that overlap amongst many pairs. This brings up the question of whether our ERS measure is indeed sensitive to the reinstatement of individual memories or simply sensitive to the reinstatement of the repeating associate. If ERS is only driven by the retrieved associate that is shared by many of the individual memories, then we would expect the ERS computation to reveal equivalent values when grouping all trials that share an associate and comparing them to individual memory trials, which also share an associate. However, we find that the match between patterns elicited by the encoding and retrieval of a specific object-object pair is significantly greater than the match between encoding and retrieval patterns of all pairs that share an associate (Fig 6B). Furthermore, we also asked whether ERS is related to perceptual overlap that is evident when comparing the encoding and retrieval of specific object-object associations by comparing trials across encoding that share perceptual overlap. Here, again, we do not see any evidence that patterns elicited in PrC or right CA1 are sensitive to the perceptual overlap of the presented paired associates during encoding (Fig 6C).
We attribute our novel finding to two factors. First, retrieval performance in our studies was quite high relative to prior studies, and it is possible that memories in our study were more vivid, which raises the possibility that ERS may be specifically related to the recollection of detailed associations. Indeed, in a prior study that decoded memories in the hippocampus, participants were repeatedly shown the encoding memoranda and were trained to vividly recall details of each episode (Chadwick et al., 2012) . In addition, our study differed from past work through use of high-resolution fMRI, which may be more sensitive to patterns of activation in small regions (Kirwan et al., 2007). Using high-resolution fMRI also enabled us to investigate the independent contribution of hippocampal subfield CA1 and generate specific predictions about its role in neural reinstatement based on past research. For instance, past work has implicated CA1 in both memory encoding and retrieval processes (Eldridge et al., 2005; Duncan et al., 2014) and suggests that CA1 may act as a comparator between past memories and incoming sensory information in the environment (Chen et al., 2011; Duncan et al., 2012). While we found that ERS in all subfields was numerically higher for trials that were successfully remembered, the effect was only statistically significant in right CA1.
We also find a higher match between encoding and retrieval for successfully remembered object pairs in the perirhinal cortex, which is consistent with previous demonstrations of reinstatement within the category-specific ventral and medial temporal cortical regions that are involved in encoding those memories (Polyn et al., 2005; Staresina et al., 2013). Furthermore, we find that encoding activation in PrC is correlated with ERS in this region. While we cannot make any claims about the causal relationship between activation and ERS, this finding is consistent with a large body of work that has linked stimulus-specific cortical encoding activation to later successful memory (for example, Davachi et al., 2003; Uncapher et al., 2006) in addition to cortical reinstatement (Wheeler et al., 2000; Polyn et al., 2005; Kuhl et al., 2012). It is well known that greater univariate encoding activation relates to subsequent memory, with activation being positively correlated with encoding success. Thus, greater activation in PrC related to ERS may reflect stronger memory encoding, which likely is related to both information processing and effort.
Past studies using ERS found evidence for reinstatement of scenes in the parahippocampal cortex, a region previously implicated in scene processing (Epstein and Kanwisher, 1998) and involved in memories involving scenes or contextual information more broadly (Awipi and Davachi, 2008; Davachi, 2006; Weis et al., 2004; Diana et al., 2007), while using word-scene stimuli exclusively (Staresina et al., 2012; Ritchey et al., 2013). This is consistent with work linking parahippocampal activation to the encoding and retrieval of scenes (Staresina et al., 2011, 2013). By contrast, prior work has shown that the perirhinal cortex is differentially important for the encoding and retrieval of objects, or items (Awipi and Davachi, 2008; Staresina and Davachi, 2008; Staresina et al., 2011) and our finding that the perirhinal cortex reinstates objects associations is consistent with these results. Our study does not directly test the difference in cortical reinstatement by using different categories of stimuli, nor did the coverage of our scans allow us to use other regions in the cortex, such as the lateral occipital complex (Grill-Spector et al., 2001), to further investigate the specificity of cortical reinstatement. However, the fact that ERS in the perirhinal cortex is modulated by memory success when using objects as stimuli lends support for the idea that reinstatement is specific to relevant cortical areas within the medial temporal lobes.
CA1 may be uniquely equipped to initiate the reinstatement of a memory in cortical regions, as it is the only subfield with direct reciprocal connections to the MTL cortex (Amaral and Witter, 1989) and it receives input from CA3 (Amaral and Witter, 1989; Hasselmo et al., 1995). Models of hippocampal function in memory predict that encoding and retrieval may depend on switches in communication with CA1. During encoding, CA1 may bias the input it receives from cortical regions that carry sensory information about the current environment via the MTL cortex, while during retrieval, CA1 may selectively prioritize input from CA3 that then propagates out through the MTL cortex to relevant cortical regions in order to coordinate cortical reinstatement, (O’Reilly and McClelland, 1994; Norman and O’Reilly, 2003). Consistent with these models, we found that those trials with greater univariate encoding activation in the perirhinal cortex resulted in higher reinstatement in the perirhinal cortex, while right CA1 activation during retrieval correlated with reinstatement in the perirhinal cortex. Hippocampal involvement in cortical reinstatement at retrieval is consistent with previous work (Kuhl et al., 2010; Staresina et al., 2012; Wimmer and Shohamy, 2012; Ritchey et al., 2013) and is in line with the theoretical role of CA1 in reinstating a memory and then transferring that information to cortex, thus driving cortical reinstatement. Furthermore, a prior analysis of this dataset has found elevated CA1-CA3/DG connectivity during retrieval relative to encoding (Duncan et al., 2014). While we cannot make any claims about the directionality of these correlations, the relationships between activation and ERS provide some leverage for models of encoding strength and pattern completion that detail the flow of input during encoding from cortex to hippocampus and, during retrieval, the reinstatement from hippocampus back to cortex.
It is possible that ERS is only sensitive to processes related to general memory success instead of representations of specific memories. To adjudicate between these two possibilities, we compared pattern similarity between encoding and retrieval trials for unique memories (same-memory ERS) with pattern similarity between encoding and retrieval trials for object pairs that shared the same associate but did not share the same cue (same-associate ERS). We found greater same-memory ERS for successfully retrieved memories relative to same-associate ERS, both in right CA1 and in PrC, which replicates a similar effect in nearby parahippocampal cortex when learning scene-word pairs (Staresina et al., 2012). This result builds confidence that ERS is not just picking up on general successful memory retrieval in CA1 and the perirhinal cortex. Further control analyses suggested that this difference was also not driven by increased perceptual overlap in the same-memory condition. In fact, only same-memory ERS exhibited reliable differences that related to memory success, which suggests that the signal used in this analysis is sensitive to patterns of activity that are unique to different memories, rather than reflecting a more general process that underlies memory formation. Nor could it be that our measure of reinstatement merely reflected differences in mean activation in these regions, since we computed a binary logistic regression (Ritchey et al., 2013; LaRocque et al., 2013) to confirm that ERS both in right CA1 and perirhinal cortex reliably predicted memory performance when controlling for encoding and retrieval activation.
In conclusion, our findings suggest that successful memory for unique episodic events is reflected by neural reinstatement of patterns of activity in the hippocampus, particularly in right CA1 and in the perirhinal cortex. To our knowledge, this is the first demonstration that the hippocampus reinstates patterns of individual episodic memories. Furthermore, we find that distinct MTL interactions at encoding and retrieval may independently contribute to successful cortical reinstatement in the MTL, giving us further insight into the mechanisms underlying widespread memory reinstatement throughout cortex.
Figure 5. Predictors of ERS during encoding and retrieval.
Group mean of correlations between PrC ERS and univariate activation at retrieval and encoding. For each participant, we correlated PrC ERS and univariate activation in right CA1 and PrC across all trials. Green bars indicate right CA1 activation and dark purple bars indicate PrC activation. ~ indicates p < 0.10; * indicates p < 0.05; ** indicates p < 0.01. Error bars denote standard error of the mean.
Acknowledgments
Grant Sponsor: National Institute of Mental Health; Grant number: MH074692
Grant Sponsor: National Science Foundation GRFP
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. J Exp Psychol Learn Mem Cogn. 2008;34:769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch SE, Jehee JFM, Fernández G, Doeller CF. Reinstatement of associative memories in early visual cortex Is signaled by the hippocampus. J Neurosci. 2014;34:7493–7500. doi: 10.1523/JNEUROSCI.0805-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the Human Medial Temporal Lobe with High-Resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Bonnici HM, Maguire EA. Decoding information in the human hippocampus: a user’s guide. Neuropsychologia. 2012;50:3107–3121. doi: 10.1016/j.neuropsychologia.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, Maguire EA. Decoding Individual Episodic Memory Traces in the Human Hippocampus. Curr Biol. 2010;20:544–547. doi: 10.1016/j.cub.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: Hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18:523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Danker JF. The cognitive neuroscience of episodic memory. In: Ochsner KN, Kosslyn SM, editors. The Handbook of Cognitive Neuroscience. Vol. 1. Oxford University Press; New York: 2013. [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: A high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22:389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Tompary A, Davachi L. Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area CA1 pathways. J Neurosci. 2014;34:11188–11198. doi: 10.1523/JNEUROSCI.0521-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: functional anatomy, vascularization, and serial sections with MRI. 3rd ed. Springer; Berlin: 2005. [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex. 2014;24:3350–3364. doi: 10.1093/cercor/bht194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS. The role of the human medial temporal lobe in object recognition and object discrimination. Q J Exp Psychol B. 2005;58:326–339. doi: 10.1080/02724990444000177. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Ruff DA, Kiani R, Bodurka J, Esteky H, Tanaka K, Bandettini PA. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J Neurosci. 2014;34:8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Wagner AD. Multi-voxel patterns of visual category representation during episodic encoding are predictive of subsequent memory. Neuropsychologia. 2012;50:458–469. doi: 10.1016/j.neuropsychologia.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque KF, Smith ME, Carr VA, Witthoft N, Grill-Spector K, Wagner AD. Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. J Neurosci. 2013;33:5466–5474. doi: 10.1523/JNEUROSCI.4293-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature. 1988;335:817–820. doi: 10.1038/335817a0. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Olman CA, Davachi L, Inati S. Distortion and signal loss in medial temporal lobe. PloS One. 2009;4:e8160. doi: 10.1371/journal.pone.0008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex N Y N. 2013;1991;23:2818–2828. doi: 10.1093/cercor/bhs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Cooper E, Henson RN. Reversible information flow across the medial temporal lobe: The hippocampus links cortical modules during memory retrieval. J Neurosci. 2013;33:14184–14192. doi: 10.1523/JNEUROSCI.1987-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci Off J Soc Neurosci. 2011;31:8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RNA, Kriegeskorte N, Alink A. Episodic reinstatement in the medial temporal lobe. J Neurosci. 2012;32:18150–18156. doi: 10.1523/JNEUROSCI.4156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. Consolidation of associative and item memory is related to post-encoding functional connectivity between the ventral tegmental area and different medial temporal lobe subregions during an unrelated task. J Neurosci Off J Soc Neurosci. 2015;35:7326–7331. doi: 10.1523/JNEUROSCI.4816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. In: Episodic and semantic memory. Organization of memory. Tulving E, Donaldson W, editors. Academic Press; New York: 1972. [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: A functional magnetic resonance imaging study. J Neurosci. 2005;25:7260–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, Elger CE, Fernández G. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004;15:2729–2733. [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: How memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci. 2015;27:679–691. doi: 10.1162/jocn_a_00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen C, Lu Z, Mumford JA, Poldrack RA. Greater neural pattern similarity across repetitions is associated with better memory. Science. 2010;330:97–101. doi: 10.1126/science.1193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]