Abstract
The use of aggressive crystalloid resuscitation to treat hypoxemia, hypovolemia and nutrient deprivation promoted by massive blood loss may lead to the development of the blood vicious cycle of acidosis, hypothermia, and coagulopathy and, utterly, death. Metabolic acidosis is one of the many metabolic derangements triggered by severe trauma/hemorrhagic shock, also including enhanced proteolysis, lipid mobilization, as well as traumatic diabetes. Appreciation of the metabolic benefit of plasma first resuscitation is an important concept. Plasma resuscitation has been shown to correct hyperfibrinolysis secondary to severe hemorrhage better than normal saline. Here we hypothesize that plasma first resuscitation corrects metabolic derangements promoted by severe hemorrhage better than resuscitation with normal saline. Ultra-high-performance liquid chromatography-mass spectrometry-based metabolomics analyses were performed to screen plasma metabolic profiles upon shock and resuscitation with either platelet-free plasma or normal saline in a rat model of severe hemorrhage. Of the 251 metabolites that were monitored, 101 were significantly different in plasma vs normal saline resuscitated rats. Plasma resuscitation corrected lactate acidosis by promoting glutamine/amino acid catabolism and purine salvage reactions. Plasma first resuscitation may benefit critically injured trauma patients by relieving the lactate burden and promoting other non-clinically measured metabolic changes. In the light of our results, we propose that plasma resuscitation may promote fueling of mitochondrial metabolism, through the enhancement of glutaminolysis/amino acid catabolism and purine salvage reactions. The treatment of trauma patients in hemorrhagic shock with plasma first resuscitation is likely not only to improve coagulation, but also to promote substrate-specific metabolic corrections.
Keywords: Metabolomics, Shock, Plasma first, normal saline
Introduction
Hemorrhage is the most common cause of preventable death after injury (1). Improving survival in patients who are actively bleeding requires prompt resuscitation and the mechanical control of active exsanguination. Impaired tissue perfusion from massive blood loss coupled with aggressive crystalloid resuscitation leads to the development of the blood vicious cycle of acidosis, hypothermia, and coagulopathy, which in turn result in inexorable shock and death (2). Preemptive resuscitation strategies to prevent progression to lethal systemic derangement of metabolism and coagulation are a logical strategy for patients at risk of continued bleeding and death. Plasma resuscitation is therefore a logical resuscitation fluid, as it has abundant proteins which buffer metabolic changes and regulate coagulation (3).
The proposed concept of preemptive plasma in trauma was described over 30 years ago (2), and was largely ignored for the following two decades until retrospective review of cases in the military (4) and civilian (5) centers identified a survival benefit in patients receiving plasma early during resuscitation. More recently, the survival advantage of early plasma resuscitation has been prospectively validated in a large multicenter observational study (6). While there is an associated risk of developing organ failure with plasma transfusions (7), the survival benefit outweighs this risk in critically injured patients. The next generation of clinical studies are underway to evaluate the therapeutic potential of pre hospital plasma first resuscitation in hypotensive trauma patients (8, 9).
While most research on hemorrhagic shock and trauma focuses on coagulation changes, there are few studies addressing the metabolic effect of plasma during resuscitation beyond lactate and pH. It has recently been identified that multiple changes related to carbohydrate, protein, and nucleic acids metabolism occur within minutes of hemorrhage and progressively build up with ongoing shock (10). The metabolic pathways that change in this model of profound lethal shock are consistent with those that change in human trauma patients’ blood that is withdrawn upon admission in the emergency department (11).
A partial resuscitation experimental design was created in the interest of replicating the pre hospital setting, as to test the metabolic effect of plasma first versus crystalloid resuscitation without overwhelming dilution of whole blood. This model has been recently exploited to demonstrate that rats that develop systemic fibrinolysis from severe hemorrhagic shock have an exacerbation of fibrinolytic activity with crystalloid resuscitation, a phenomenon that is attenuated with plasma resuscitation (12). As a result, plasma resuscitation resulted in a marked survival advantage in comparison to resuscitation with crystalloid. With the hundreds of proteins that buffer the plasma from metabolic derangement (3), it was suspected that the survival advantage was not completely attributable to the improvements in coagulation. Analogously, the rich composition in terms of small molecule compounds prompts us to hypothesize that plasma resuscitation in rodents subjected to near lethal shock would correct metabolic derangements better than resuscitation with saline.
Methods
Subjects
The University of Colorado Institutional Animal Care and Use Committee approved this animal study protocol #90814. Twelve male Sprague Dawley rats (age 14-16 weeks) were used with weights between 300 and 400 grams.
Animal Induction and Cannulation
Anesthesia was induced with pentobarbital (5mg/kg, intraperitoneal) followed by tracheostomy and femoral artery cannulation. The level of anesthesia was monitored and maintained as described as follows. The rats had a cannula (PE-50 tubing) from the right femoral artery and it was connected to a Propag (Well Allyn model 224) monitor for continuous physiological parameters: heart rate, blood pressure, respiratory rate, base deficit, etc. The temperature was measured continuously with a rectal thermometer. Based on this constantly monitoring, we maintained the proper level of anesthesia and we insured that the rat was comfortable without any discomfort or pain. The animals were allowed to recover from these initial procedures for airway and vascular access and then randomized into resuscitation groups as described below. Recovery was defined as animal blood obtaining normothermia (>36C) via heat lamp and waiting for the mean arterial blood pressure to increase to greater than 80 mmHg. In all animals this was obtained by a rest period with no fluid resuscitation.
Hemorrhagic shock
Hemorrhagic shock was initiated in both experimental arms (twelve rats) through the femoral artery catheter over five minutes to achieve a mean arterial pressure (MAP) of 25 mmHg. A MAP of 25 mmHg has been selected as previous experiments with a MAP >30 mmHg were insufficient in producing coagulation changes (13) and rodents with a MAP<20 mmHg did not survive a second blood draw. Animals’ blood pressure was maintained at a MAP of 25 mmHg ± 2 mmHg for 30 minutes with additional blood draws as needed. The thirty minutes of shock was based on a previous model in which 30 minutes was adequate to increase systemic tPA levels (14).
Replicating Pre Hospital Partial Resuscitation
Resuscitation prior to arrival to the hospital has limitations on the amount of fluid that can be delivered to critically injured patients. It is not uncommon for patients who manifest coagulation changes to be incompletely resuscitated before they arrive to the hospital. Through the femoral cannulation an estimated 10% total blood volume of the rat (0.06ml/gram*rat weight grams*0.10) was infused over 2 minutes (plasma vs normal saline – n = 6 per group), with an additional 3 minutes of resuscitation (saline only) to obtain a MAP>30 mmHg. This initial 10% volume resuscitation was based on the estimate of what a trauma patient would receive prior to arrival to the hospital in our ongoing pre hospital plasma trial (9). It was anticipated that plasma resuscitation would increase blood pressure more effectively than crystalloid due to oncotic forces of proteins. An additional 3 minutes of resuscitation of NS allowed this arm of the experiment to increase blood pressure to a MAP >30 mmHg. Following 5 minutes of resuscitation both arms of the experiment were observed for 15 minutes. Additional resuscitation was not performed during this time frame to reduce confounding of ongoing whole blood dilution.
Blood samples
Blood sampling was taken at the same time intervals for both experimental arms (n = 6 for each group, either normal saline - NS or platelet-free plasma - PFP). A baseline blood sample was obtained after cannulation and tracheostomy. A second blood draw was done following 30 minutes of shock. A third blood sample was obtained after 15 minutes of observation following resuscitation. To keep blood sampling volume constant between different animal weights 8% of estimated total blood volume was drawn at each time point. Blood samples after shock reduced blood pressure dramatically to a point at which animals would not survive without immediate resuscitation, which was performed at this point. Animals that did not survive resuscitation or died during the final blood draw had an immediate laparotomy and sternotomy with blood draw through the supra-hepatic vena cava using a 20 gauge needle.
Whole blood was collected in 3.2 % sodium citrate tubes at a 1:10 ratio, based on a standardized model for assessment of coagulation in rodents (12). Individual microcentrifuge tubes were prefilled with citrate and marked to an appropriate fill level to ensure reproducible ratios of whole blood to citrate. Whole blood was centrifuged at 6,000 g for 10 minutes at 4 °C. Plasma was removed and then spun at 12,500 g for 10 minutes at the same temperature to remove contaminating platelets and acellular debris. The remaining plasma was flash frozen in liquid nitrogen and stored at −80°C until analyzed. Thromboelastography (TEG) assays were performed as extensively described (12).
Blood Component Banking
Blood banking technique in rodents has been described by Gilson et al (15). A modified protocol was developed to minimize the number of animals required by eliminating the need to sacrifice animals to obtain blood products. During hemorrhage the first 3 ml of blood were collected in citrate phosphate dextrose adenine (CPDA-1) with a final concentration of 14%. These blood samples underwent serial centrifuge spins to remove red cells and platelets to reduce the contamination of cellular lysate, which has previously been shown to alter fibrinolysis (8). The remaining platelet free plasma (PFP) was flash frozen in liquid nitrogen and stored at −80 degrees Celsius. Prior to transfusion PFP from 3 animals was warmed in a 37°C incubator for 15 minutes and pooled. Pooling was performed to reduce variability in animal plasma.
Blood Samples collection for metabolomics analysis
Whole blood was centrifuged at 1,500 g for 10 minutes at 4°C. Plasma supernatants were collected and then spun at 12,500 g for 10 minutes at the same temperature to remove contaminating platelets and acellular debris. The final plasma supernatant was flash frozen in liquid nitrogen and stored at −80°C until analyzed.
Metabolomics analyses
Plasma samples (10 μl) were immediately extracted in −20°C lysis/extraction buffer (methanol:acetonitrile:water 5:3:2) at 1:25 dilutions. Samples were then agitated at 4°C for 30 min and then centrifuged at 10,000g for 15min at 4°C. Protein and non-methanol soluble lipid pellets were discarded, while supernatants were stored at −80°C prior to metabolomics analyses.
Metabolomics analyses were performed as previously reported (10). Ten μl of sample extracts were injected into an UHPLC system (Ultimate 3000, Thermo, San Jose, CA, USA) and run on a linear 9 min gradient through a Kinetex XB-C18 column (150×2.1 mm, 1.7 μm particle size – Phenomenex, Torrance, CA, USA) at 250 μl/min from 5-95% B (mobile phase: A: 18 mΩ H2O, 0.1% formic acid; B: acetonitrile, 0.1% formic acid). The UHPLC system was coupled online with a QExactive system (Thermo, San Jose, CA, USA), scanning in Full MS mode (2 μscans) at 70,000 resolution in the 60-900 m/z range, 4kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated in negative and then positive ion mode (separate runs). Calibration was performed before each analysis against positive or negative ion mode calibration mixes (Piercenet – Thermo Fisher, Rockford, IL, USA) to ensure sub ppm error of the intact mass. Metabolite assignments were performed using the software Maven (Princeton, NJ, USA), upon conversion of .raw files into .mzXML format through MassMatrix (Cleveland, OH, USA). The software allows for peak picking, feature detection and metabolite assignment against the KEGG pathway database. Assignments were further confirmed against chemical formula determination (as gleaned from isotopic patterns and accurate intact mass), and retention times against a library of >620 standard compounds (SIGMA Aldrich, St. Louis, MO, USA; MLSMS, IROA Tech, Bolton, MA, USA).
Data analysis
Relative quantitation was performed by exporting integrated peak area values into Excel (Microsoft, Redmond, CA, USA) for statistical analysis (repeated measures ANOVA with Tukey multiple column comparison test, significance threshold for p-values < 0.05 in the figures, T test of NS vs PFP resuscitation measurements in supporting table 1) and partial least square discriminant analysis (PLS-DA), calculated through the macro MultiBase (freely available at www.NumericalDynamics.com).
Hierarchical clustering analysis (HCA) was performed through the software GENE-E (Broad Institute, Cambridge, MA, USA – freely available at http://www.broadinstitute.org/cancer/software/GENE-E/). Box and whisker plots were graphed through GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA) and figure panels were assembled through Photoshop CS6 (Adobe, Mountain View, CA, USA).
Results
Plasma metabolomics analyses were performed on 12 Sprague Dawley rats exposed to hemorrhagic shock and resuscitation either with NS or PFP. This model of severe hemorrhagic shock is known to induce systemic hyperfibrinolysis [12], as confirmed here via TEG (LY30 > 3%) (Supporting Figure 1). Overall, 251 metabolites were monitored throughout each stage, and results are reported in Figure 1 (HCA map – vectorial version in Supporting Figure 2) and Supporting Table 1, together with the metabolite name, KEGG pathway ID, super-pathway assignment, the polarity in which each metabolite has been detected, the experimentally observed mass to charge ratios, line plots showing the trends throughout each sample on the basis of the median values across each biological replicate, statistical analyses comparing resuscitation values in both arms of the study – NS vs PFP). Metabolites covered by the present analyses are highlighted as dark grey nodes in the KEGG pathway database map 01100 (Supporting Figure 3).
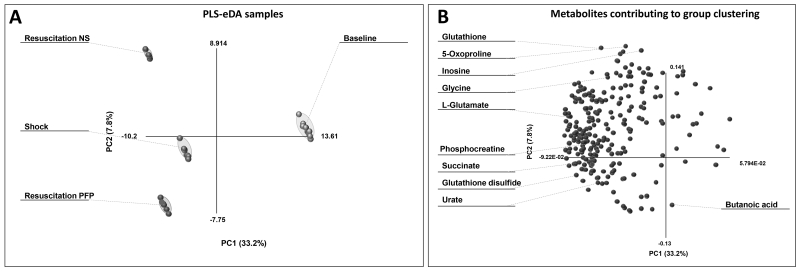
Figure 1.
Heat map of clustering analyses (1-Pearson correlation) based upon metabolic changes in rat plasma upon hemorrhagic shock (Shock) and resuscitation with either normal saline (NS) or platelet-free plasma (PFP) in comparison to the baseline. Each square is representative of the levels of that metabolite (names and hierarchical clustering are provided in Supporting Figure 1). Row values are Z-score normalized for each metabolite across all biological replicates per each time point, while quantitative changes are color-coded from black (low) to white (high). Pathways assignment is provided in the right y axis of the map.
The partial least square discriminant analysis (PLS-DA) of the results showed that PC1+PC2 accounted for the 41% of the total covariance. The top ten metabolites showing the highest loading values across the principal components are labeled in the loading plot shown in Figure 2. Top metabolites distributed across PC2 (mostly reflecting the differential effect of resuscitation strategies) were mainly involved in glutathione homeostasis and redox poise (reduced glutathione – GSH, oxidized glutathione – GSSG, glycine, glutamate and 5-oxoproline). Other key compounds include metabolites involved in the tricarboxylic acid (TCA) cycle, such as succinate, metabolites involved in lipid metabolism (butanoic acid), purine/nitrogen catabolism substrates and byproducts (inosine and urate). A total of 101 metabolites were differentially affected by the resuscitation strategy (p<0.05 T-test - Supporting Table 1). The strong anions urate and succinate increased upon shock, but only the former was differentially affected by resuscitation (higher in PFP-resuscitated rats).
Figure 2.
Partial least square analysis of metabolic changes in rat plasma (baseline: control) upon exposure to hemorrhagic shock and resuscitation with either normal saline (NS) or platelet-free plasma (PFP). In A, clusters are indicative of each sample group (from baseline to Shock and resuscitation). In B, metabolites (variables) are graphed contributing to maximize the covariance (PC1 = 33.2%; PC2 = 7.8%) throughout all the samples (observations) in A. The top ten metabolites contributing the most to covariance are highlighted in B.
Energy metabolism
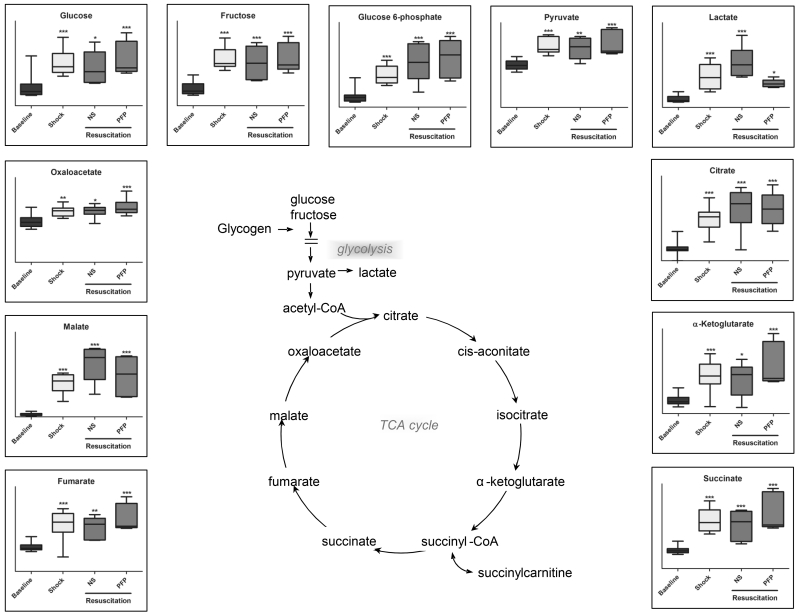
Plasma accumulation of glucose and fructose was observed upon hemorrhagic shock, together with the increase of late glycolytic end-products, pyruvate and lactate. Anionic TCA cycle intermediates followed a similar trend (Figure 3). Resuscitation with NS further increased glyceraldehyde 3-phosphate and lactate levels, while PFP resulted in glyceraldehyde 3-phosphate and lactate decrease in comparison to shock plasma and NS resuscitated rats (p<0.05 T-test - Figure 3; Supporting Table 1).
Figure 3.
An overview of glycolysis and Krebs cycle metabolites detected in plasma samples from rats undergoing hemorrhagic shock (probably due to tissue injury and cell lysis) and resuscitation with either normal saline (NS or platelet-free plasma – PFP). Results are graphed as box-plots indicating median values (line), mean values (+) and upper/lower quartile distributions for each group. Asterisks indicate significance upon repeated measures ANOVA test (p-values: * ≤ 0.1; ** ≤ 0.01; *** ≤ 0.001).
Shock promoted lipolysis and beta-oxidation of lipids, as suggested by the shock-dependent accumulation of glycerophospholipid breakdown products (N-methylethanolamine phosphate, glycerol-3-phosphoethanolamine, ethanoloamine phosphate – Supporting Table 1), fatty acid-mobilizing acyl-carnitines (propanoyl-carnitine, butanoylcarnitine – Figure 5), and ketone bodies (acetoacetate and hydroxyisobutyrate and butanoate – Supporting Table 1). Butanoate further increased in the PFP group in comparison to the NS arm (p=0.008 T-Test – Supporting Table 1). On the other hand, resuscitation with PFP prevented the accumulation of acetoacetate and propanoyl-carnitine observed in NS resuscitated rats (p=0.008 T-Test – Supporting Table 1), suggestive of controversial effect of resuscitative strategies on lipolysis at different stages. Plasma levels of the carboxylic acid citramalate increased after hemorrhagic shock, and they decreased upon resuscitation with NS but not PFP (Supporting Table 1).
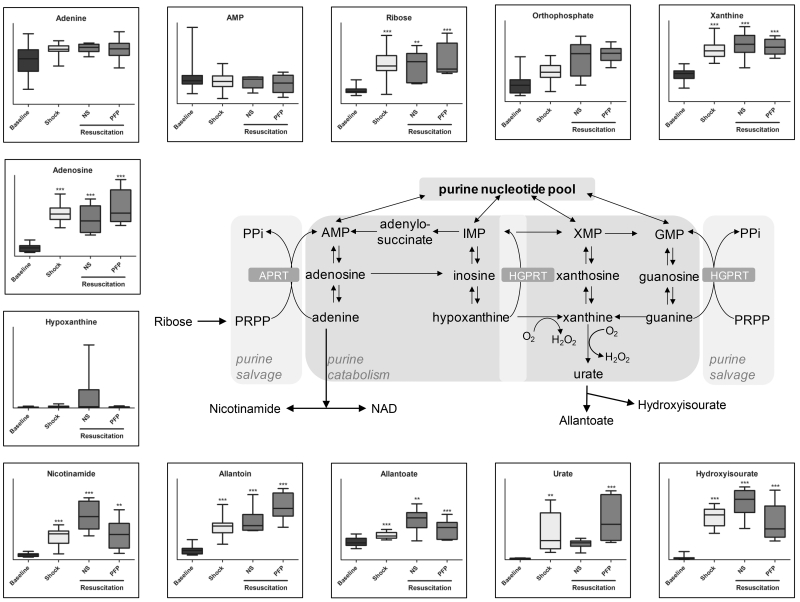
Figure 5.
An overview of purine catabolites detected in plasma samples from rats undergoing hemorrhagic shock and resuscitation with either normal saline (NS or platelet-free plasma – PFP). Results are graphed as box-plots indicating median values (line), mean values (+) and upper/lower quartile distributions for each group. Asterisks indicate significance upon repeated measures ANOVA test (p-values: * ≤ 0.1; ** ≤ 0.01; *** ≤ 0.001).
Amino acids: proteolysis, transamination and glutathione homeostasis
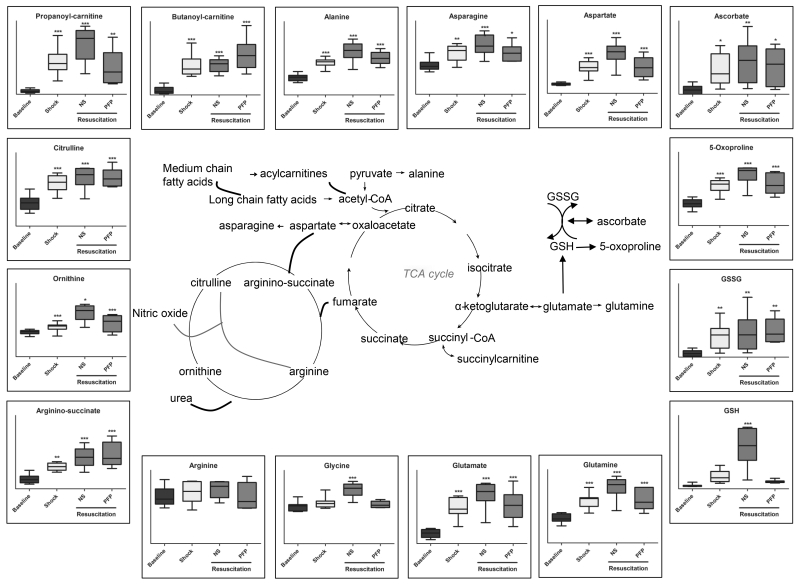
Shock promoted the accumulation of plasma amino acid levels, except for arginine and glycine (Figure 4, Supporting Table 1). Transamination byproducts and substrates, alanine, asparagine, aspartate and glutamate remained stable after resuscitation with PFP, while they kept increasing when resuscitating with NS. This holds true especially for asparagine, aspartate, glutamine, glycine, lysine, histidine, methionine, phenylalanine, and threonine significantly (p<0.05 T-Test) increasing in the NS group in comparison to PFP resuscitated rats (Supporting Table 1). Similar results were observed for GSH (p=0.003 – T-Test – Supporting Table 1) and its precursors (5-oxoproline, glycine, glutamate, cysteine), significantly increasing after shock and corrected by PFP, but not NS resuscitation.
Figure 4.
An overview of arginine metabolism, glutathione and anti-oxidant homeostasis and transamination pathway-related metabolites detected in plasma samples from rats undergoing hemorrhagic shock and resuscitation with either normal saline (NS or platelet-free plasma – PFP). Results are graphed as box-plots indicating median values (line), mean values (+) and upper/lower quartile distributions for each group. Asterisks indicate significance upon repeated measures ANOVA test (p-values: * ≤ 0.1; ** ≤ 0.01; *** ≤ 0.001).
Urea cycle and purine catabolism
Arginine did not increase in response to hemorrhagic shock (Figure 4). Increases in the levels of all amino acids but arginine are suggestive of the post-shock consumption of this metabolite. Catabolism products of arginine through the urea cycle (ornithine, citrulline and arginine-succinate) increased significantly upon late hemorrhagic shock (Figure 4). However, resuscitation with NS but not PFP further promoted ornithine accumulation (p=0.005 T-test - Figure 4, Supporting Table 1). Shock-induced accumulation of polyamines (e.g. putrescine, atherospermidine, spermidine and spermine) was significantly (p<0.05 T-test) exacerbated in NS-resuscitated rats in comparison to PFP (Supporting Table 1), suggesting that in the former group nitrogen metabolism might preferentially follow this pathway rather than nitric oxide generation.
Nitrogen unbalance following shock might be further affected by purine catabolism (Figure 5). Purine catabolites urate and allantoin further increased (p<0.05 T-test) upon resuscitation with PFP in comparison to NS (Figure 5). Nicotinamide, hypoxanthine, allanoate and hydroxyisourate (increasing after shock) were further accumulating in response to NS resuscitation (Figure 5). The accumulation of the aforementioned metabolites is affected by the activity of oxygen-dependent oxidative-stress sensitive enzymes (e.g. xanthine dehydrogenase), which is consistent with the hypothesis of PFP performing better than NS in restoring redox homeostasis after hemorrhagic shock.
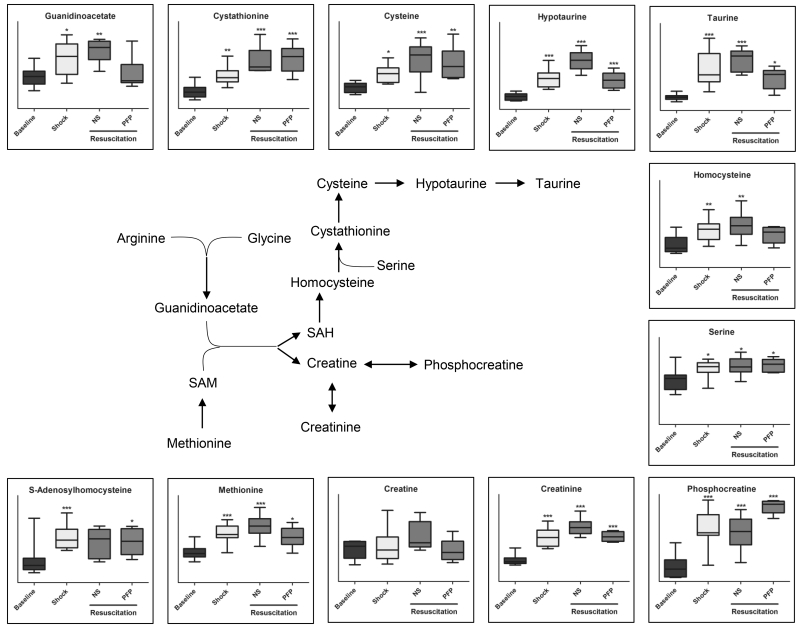
Creatine and sulfur metabolism
As anticipated above, arginine and glycine were the only two amino acids not increasing after shock. Both metabolites can fuel guanidinoacetate biosynthesis and contribute to creatine metabolism in response to shock (Figure 6). Creatine and, more significantly, phosphocreatine and creatinine, increased after shock and did not decrease back to baseline level upon resuscitation with either NS or PFP (Figure 6). Conversely, creatinine further increased in the NS group in comparison the PFP rats, while phosphocreatine increased after PFP but not NS resuscitation (p<0.05 T-Test - Figure 6).
Figure 6.
An overview of metabolites involved in arginine and sulphur metabolism detected in plasma samples from rats undergoing hemorrhagic shock and resuscitation with either normal saline (NS or platelet-free plasma – PFP). Results are graphed as box-plots indicating median values (line), mean values (+) and upper/lower quartile distributions for each group. Asterisks indicate significance upon repeated measures ANOVA test (p-values: * ≤ 0.1; ** ≤ 0.01; *** ≤ 0.001).
Guanidinoacetate and S-Adenosylmethionine (SAM) levels influence the accumulation of creatine and S-Adenosylhomocysteine (SAH), a precursor to cysteine and taurine in one-carbon and sulphur metabolism, respectively (Figure 6). Shock induced the increase of all metabolic intermediates of these pathways, a phenomenon that was corrected by resuscitation with PFP but not NS, especially with respect to the accumulation of hypotaurine and taurine (p<0.05 T-Test - Figure 6, Supporting Table 1).
Discussion
Plasma resuscitation improves coagulation and survival in a rodent model of hemorrhagic shock induced hyperfibrinolysis (12), as we hereby confirmed. We thus show that hemorrhagic shock triggers systemic metabolic derangement in rats that is differentially corrected by NS and PFP resuscitation in a significant fashion for almost half of the metabolites monitored in this study. These data suggest that plasma resuscitation may not just contribute to improvements in coagulopathy (12), but also concomitantly affect systemic metabolism by altering substrate specific metabolic derangements. Specifically, the present in depth analysis of the metabolic effect of plasma resuscitation suggested that plasma resuscitation corrects hyperlactatemia, while it sustains glutamine/amino acid catabolism, purine catabolism and salvage reactions.
Shock resulted in the progressive accumulation of glucose, a previously documented phenomenon, referred to as traumatic diabetes or insulin resistance (16, 17). This phenomenon was not reverted by either resuscitation treatment, though the underlying mechanism might be differential. Indeed, shock-induced lactate accumulation, a decade long established marker of trauma-dependent metabolic acidosis (18), was worsened by NS resuscitation and ameliorated by plasma resuscitation. Together with significant decreases in glyceraldehyde 3-phosphate level upon PFP in comparison to NS resuscitation, these results are suggestive of decreased glycolysis/lactate acidosis upon plasma resuscitation.
In the light of this evidence, we hypothesized that alternative energy metabolic pathways might be fueled by resuscitation with plasma. Increased levels of glutamine catabolites and TCA cycle intermediates were present following plasma resuscitation and not NS, suggesting that glutamine was used as an alternative source of energy when recovering from profound shock. This is in line with recent observations about glutaminolysis being one of the main driver of succinate accumulation, a metabolic marker of ischemia/reperfusion injury and mediator of reactive oxygen species generation (ROS) upon reperfusion-triggered reactivation of complex I of the electron transport chain (19). Glutamine supplementation has already been demonstrated to clinically to improve glucose metabolism in severely injured trauma patients (20). In addition, animal work has demonstrated that glutamine deficient rodents subjected to hemorrhagic shock increases post resuscitation organ damage (21). Yang et al. demonstrated the beneficial role of glutamine administration in resuscitating rodents following hemorrhagic shock (22). A tentative mechanism might involve substrate level phosphorylation-dependent generation of purine nucleoside triphosphate in mitochondria at the succinyl co-A synthetase level in an oxygen independent fashion. This hypothesis, that needs to be further tested with flux analyses and pharmacological inhibition of the pathway, suggests that glutaminolysis and succinate generation may represent an extreme measure to generate high energy phosphate compounds in mitochondria in response to systemic hypoxemia following severe hemorrhage.
Arginine plays a protective role on the vascular endothelium (23). Arginine was the only amino acid, together with glycine, not increasing after shock, suggesting increased consumption at this stage. The depletion of arginine corresponded to the generation of citrulline, a phenomenon that was not corrected by either resuscitation. However, ornithine significantly increased in NS resuscitated rats in comparison to the PFP group. Increased citrulline accumulation but decreased ornithine levels suggest that, in PFP resuscitated rats, arginine to citrulline conversion may have fueled the generation of vasodilatory and HIF1α stabilizing nitric oxide. On the other hand, accumulation of both ornithine and citrulline in NS rats suggests that arginine may be fluxed through the urea cycle by arginase in this group. NS resuscitation was also associated with increased levels of polyamines, such as spermidine and spermine. Increased polyamine synthesis has been deemed to play a protective role during ischemia and a pathologic one during reperfusion (25). Therefore, the attenuation of polyamine synthesis with plasma resuscitation represents another potential advantage to plasma first resuscitation. Polyamine synthesis might also represent an adaptive metabolic response to cope with osmotic or oxidative stress following hemorrhagic shock, a well-established physiologic phenomenon in plants, bacterial and mammalian cells challenged by abiotic stresses (25). Polyamines have been implicated in platelet inhibition (25, 26). The metabolic link between polyamine generation and systemic fibrinolysis has not previously been described. However, it has been demonstrated in a large animal model of trauma hemorrhagic shock, that platelets have improved function with plasma resuscitation compared to normal saline (27), which could be through attenuation of polyamine synthesis. The mechanism of polyamines causing platelet inhibition and increasing susceptibility to systemic fibrinolysis is an interesting hypothesis warranting future investigation. In this view, it is relevant to appreciate the potentially protective role of PFP resuscitation in preventing polyamine hyperaccumulation (especially late products of the pathway spermidine and spermine) as observed in NS resuscitated rats.
Numerous amino acids were markedly increased following hemorrhagic shock and NS resuscitation compared to plasma resuscitation, which may be attributable to unregulated proteolysis resulting from the activation of proteases. This is consistent with our recent proteomic analysis of plasma from these animals, in which NS resuscitation was associated with an increase in markers of cellular damage and proteases/protease activators compared to plasma resuscitation (12). For example, this rat model produces elevated levels of tissue plasminogen activation (12). tPA not only activates plasmin, a highly active protease that cleaves fibrin, but it has been demonstrated to increase the activity of matrix metalloproteases (MMP) following an ischemic, cereberal event (28). Plasma contains numerous regulators of proteases that may be reflective of the findings of beneficial plasma resuscitation as it prevents proteolytic activity from spiraling out of control, resulting in the hereby-observed increase in the level of circulating amino acids. In vitro depletion of plasma regulator proteins markedly increases whole blood sensitivity to tPA mediated fibrinolysis (29). This in turn may be reflective of just one of the many proteolytic cascades that become hyperactive with plasma dilution of whole blood, a phenomenon that could be attenuated by plasma resuscitation.
Lipid metabolism increased after shock and was differentially affected by plasma versus saline resuscitation at the level of ketone bodies and acyl-conjugated carnitines.
Resuscitation strategy-specific responses were observed in sulfur metabolism (up-regulated by shock and corrected by plasma resuscitation, but not NS). These observations are relevant in that sulphur and arginine metabolism intermediates guanidinoacetate and taurine indirectly affect energy (creatine) and redox (glutathione) homeostasis. By correcting deranged sulphur metabolism, PFP resuscitation, but not resuscitation with NS, may affect the generation of SO2/SO3 and H2S, a gas that is known to protect against traumatic hemorrhagic shock by attenuating oxidative stress (30). Of note, glutathione homeostasis and turn-over (5-oxoproline) was affected by hemorrhagic shock and worsened by NS resuscitation, while plasma resuscitation corrected it back to baseline values. Altogether, metabolomics results are suggestive of a decreased systemic oxidative stress in PFP resuscitate rats. One additional unexplored mechanism underpinning this statement might imply the generation of urate from purine catabolism, a potential adaptive response to shock to protect against oxidative stress associated with ischemia/reperfusion (31). Urate may scavenge reactive oxygen species and result in the production of allantoin (while lower mammals have a functional uricase, the enzyme to promote the reaction, humans do not) (31, 32). In this view, plasma resuscitation would promote such an adaptive response that is instead partially suppressed by NS. PFP resuscitation further promoted plasma adenosine accumulation (urate precursor), which might also have potential beneficial systemic effects after shock by mediating signaling through specific purinergic receptors, as previously discussed (10). At the same time, it is worth noting that purine catabolism might play a pro-oxidant role through two different pathways. First, xanthine dehydrogenase activity can generate hydrogen peroxide. Second, salvage reactions that convert deaminated IMP back to AMP can generate fumarate at the expenses of aspartate (20). This might in turn result in pro-oxidant cascades mediated by fumarate (such as protein succinylation) (34) a likely mediator of reperfusion-driven oxidative stress. Of note, while hemorrhagic shock triggered fumarate/succinate accumulation, consistently with previous observations (11) NS but not plasma resuscitation partially counteracted accumulation of TCA cycle intermediates. Together with the aforementioned beneficial effects on the correction of lactate accumulation, these results support a role for plasma resuscitation in fueling mitochondrial metabolism rather than anaerobic glycolysis in response to hemorrhagic shock.
This pragmatic animal resuscitation model was intended to represent a pre-hospital setting in which resources are limited and obtaining normal blood pressure in profound hemorrhagic shock is not feasible. However, the model has been designed to mimic the effects of sever hemorrhage in the absence of traumatic injury (rats underwent laparotomy and sternotomy), contrarily to what occurs in real clinical cases. As a result, the study is characterized by several limitations. First of all, this study is limited to contrasting differences in a model of pressure controlled resuscitation with saline versus plasma. The metabolic results of this study are partially attributable to differences in physiologic response between resuscitation fluids. It is not possible to conclude if the protein/metabolic constituents of plasma could contribute to explain the metabolic differences observed between these animals at the end of resuscitation with either fluid. Other long established factors, such as plasma buffering capacity (3), may contribute to mitigate specific metabolic derangements such as acidosis and partially influence the post-shock systemic metabolic phenotype. Animals in the NS group after resuscitation to a MAP of 30 mmHg did not sustain their blood pressure during the observation period. As a result, NS animal showed significantly lower blood pressure than rats in the PFP group, further suggesting that some of the observed metabolic specificities of NS-resuscitated rats may be confounded by animal responses to prolonged hypotension, as we previously noted (12). An additional confounding factor is the dilution effect of NS over plasma. Indeed, NS animal required more than double the resuscitation fluid volume in comparison to plasma rats. However, this suggests that some of the observed changes (e.g. lactate higher in the NS vs PFP group) would be further exacerbated if correcting for volume/volume dilution factors. While an additional arm of the study using a synthetic colloid, albumin, or hypertonic saline to reduce this confounding effect would be helpful, none of these products are used in the pre-hospital setting.
In conclusion, in a rat model replicating the pre-hospital setting, our findings suggest that metabolic derangement triggered by profound hemorrhagic shock and reperfusion is differentially corrected by resuscitation with NS or PFP. The metabolites involved with the pathways of interest, mainly glycolysis, Krebs cycle, redox and sulfur homeostasis, polyamine and nitric oxide metabolism, and purine catabolism/salvage have been previously suggested to promote survival and reduce organ injury in both human and animal models. Overall, despite the limitations of the study pointed out above, our results suggest that plasma resuscitation corrects lactate acidosis, promotes glutamine/amino acid catabolism and purine catabolism/salvage reactions and attenuates oxidative injury better than resuscitation with NS. Plasma first resuscitation in trauma likely has numerous effects on trauma patients beyond beneficial improvements in coagulation. For example, plasma has been demonstrated to reproduce repair the glycocalyx in animals following hemorrhagic shock (35). With the ongoing trial of plasma first resuscitation (9) it will be possible to identify if this animal study is translatable to humans. The appreciation of plasma as multifaceted resuscitation fluid is critical for clinicians, as the indication for utilizing this blood product may be well beyond abnormal coagulation.
Supplementary Material
Supporting Figure 1 LY30 (%) in rats at baseline, shock or resuscitation with normal saline (NS – dashed line) or platelet free plasma (PFP – continuous line).
Supporting Figure 2 Heat map of clustered (1-Pearson correlation) of metabolic changes in rat plasma upon exposure to gradual hemorrhage (H1 to H5), prolonged hemorrhage (S10, S20) and hemorrhagic shock (Shock) in comparison to the baseline. Each square is representative of the levels of that metabolite (names and hierarchical clustering are indicated in the right hand y axis of the map). Row values are Z-score normalized for each metabolite across all biological replicates per each time point, while quantitative changes are color-coded from blue (low) to red (high). Pathways assignment is provided in the right y axis of the map.
Supporting Figure 3 An overview of the KEGG pathway metabolome (map 01100). Each node represents a metabolite. Red nodes are the metabolites identified in this study. Metabolic pathways are color coded.
Acknowledgments
This study was supported in part by National Institute of Health grants: T32-GM008315 (NIGMS), P50-GM0492221(NIGMS), UM-1HL120877(NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support was provided by Haemonetics (inc).
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma--a unified approach. J Trauma. 1982;22(8):672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Traverso LW, Medina F, Bolin RB. The buffering capacity of crystalloid and colloid resuscitation solutions. Resuscitation. 1985;12(4):265–70. doi: 10.1016/0300-9572(85)90007-3. [DOI] [PubMed] [Google Scholar]
- 4.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 5.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 6.Del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S24–30. doi: 10.1097/TA.0b013e31828fa3b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg Chic Ill 1960. 2010;145(10):973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 8.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, et al. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43(1):39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore EE, Chin TL, Chapman MC, Gonzalez E, Moore HB, Silliman CC, et al. Plasma first in the field for postinjury hemorrhagic shock. Shock. 2014;41(Suppl 1):35–8. doi: 10.1097/SHK.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol. 2015;308(12):R1034–44. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltz ED, D’Alessandro A, Moore EE, Chin T, Silliman CC, Sauaia A, et al. Pathologic metabolism: an exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78(4):742–51. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore HB, Moore EE, Morton AP, Gonzalez E, Fragoso M, Chapman MP, et al. Shock-induced systemic hyperfibrinolysis is attenuated by plasma-first resuscitation. J Trauma Acute Care Surg. 2015;79(6):897–904. doi: 10.1097/TA.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohlauer M, Moore EE, Droz N, Harr J, Gonzalez E, Fragoso M, et al. Hemodilution is Not Critical in the Pathogenesis of the Acute Coagulopathy of Trauma. J Surg Res. 2012;173(1):26–30. doi: 10.1016/j.jss.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Lawson PJ, Gonzalez E, Fragoso M, Morton AP, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–92. doi: 10.1016/j.surg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49(8):1546–53. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonizzoli M, Zagli G, Lazzeri C, Degl’Innocenti S, Gensini G, Peris A. Early insulin resistance in severe trauma without head injury as outcome predictor? A prospective, monocentric pilot study. Scand J Trauma Resusc Emerg Med. 2012;20:69. doi: 10.1186/1757-7241-20-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahel PF, Flierl MA, Moore EE. “Metabolic staging” after major trauma - a guide for clinical decision making? Scand J Trauma Resusc Emerg Med. 2010;18:34. doi: 10.1186/1757-7241-18-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127–40. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grintescu IM, Luca Vasiliu I, Cucereanu Badica I, Mirea L, Pavelescu D, Balanescu A, et al. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients--A randomized-controlled clinical study. Clin Nutr Edinb Scotl. 2015;34(3):377–82. doi: 10.1016/j.clnu.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Dhar A, Kujath S, Van Way CW. Glutamine administration during total parenteral nutrition protects liver adenosine nucleotides during and after subsequent hemorrhagic shock. JPEN J Parenter Enteral Nutr. 2003;27(4):246–51. doi: 10.1177/0148607103027004246. [DOI] [PubMed] [Google Scholar]
- 22.Yang R, Martin-Hawver L, Woodall C, Thomas A, Qureshi N, Morrison D, et al. Administration of glutamine after hemorrhagic shock restores cellular energy, reduces cell apoptosis and damage, and increases survival. JPEN J Parenter Enteral Nutr. 2007;31(2):94–100. doi: 10.1177/014860710703100294. [DOI] [PubMed] [Google Scholar]
- 23.Tong BC, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4(8):823–32. doi: 10.2174/1389557043403305. [DOI] [PubMed] [Google Scholar]
- 24.Schlüter K-D, Schulz R, Schreckenberg R. Arginase induction and activation during ischemia and reperfusion and functional consequences for the heart. Front Physiol. 2015;6:65. doi: 10.3389/fphys.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34(1):35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- 26.Corona-de-la-Peña N, Uribe-Carvajal S, Barrientos-Rios R, Matias-Aguilar L, Montiel-Manzano G, Majluf-Cruz A. Polyamines inhibit both platelet aggregation and glycoprotein IIb/IIIa activation. J Cardiovasc Pharmacol. 2005;46(2):216–21. doi: 10.1097/01.fjc.0000171753.43564.7c. [DOI] [PubMed] [Google Scholar]
- 27.Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam A, et al. Fresh frozen plasma resuscitation attenuates platelet dysfunction compared with normal saline in a large animal model of multisystem trauma. J Trauma Acute Care Surg. 2014;76(4):998–1007. doi: 10.1097/TA.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji K, Aoki T, Tejima E, Arai K, Lee S-R, Atochin DN, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke J Cereb Circ. 2005;36(9):1954–9. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 29.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, et al. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. J Am Coll Surg. 2015;220(5):872–9. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai W, Wang Y, Lin J-Y, Sun X-D, Yao L-N, Yang Y-H, et al. Exogenous hydrogen sulfide protects against traumatic hemorrhagic shock via attenuation of oxidative stress. J Surg Res. 2012;176(1):210–9. doi: 10.1016/j.jss.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608–19. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988;263(4):1709–12. [PubMed] [Google Scholar]
- 34.Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. 2015;6:6001. doi: 10.1038/ncomms7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–95. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1 LY30 (%) in rats at baseline, shock or resuscitation with normal saline (NS – dashed line) or platelet free plasma (PFP – continuous line).
Supporting Figure 2 Heat map of clustered (1-Pearson correlation) of metabolic changes in rat plasma upon exposure to gradual hemorrhage (H1 to H5), prolonged hemorrhage (S10, S20) and hemorrhagic shock (Shock) in comparison to the baseline. Each square is representative of the levels of that metabolite (names and hierarchical clustering are indicated in the right hand y axis of the map). Row values are Z-score normalized for each metabolite across all biological replicates per each time point, while quantitative changes are color-coded from blue (low) to red (high). Pathways assignment is provided in the right y axis of the map.
Supporting Figure 3 An overview of the KEGG pathway metabolome (map 01100). Each node represents a metabolite. Red nodes are the metabolites identified in this study. Metabolic pathways are color coded.