Abstract
Background
Racial variation in the relationship between blood glucose and hemoglobin A1c (HbA1c) complicates diabetes diagnosis and management in racially mixed populations. Understanding why HbA1c is persistently higher in blacks than whites could help reduce racial disparity in diabetes outcomes.
Objective
Test the hypothesis that neighborhood disadvantage is associated with inflammation and poor metabolic control in a racially mixed population of pediatric type 1 diabetes patients.
Methods
Patients (n = 86, 53 white, 33 black) were recruited from diabetes clinics. Self-monitored mean blood glucose (MBG) was downloaded from patient glucose meters. Blood was collected for analysis of HbA1c and C-reactive protein (CRP). Patient addresses and census data were used to calculate a concentrated disadvantage index (CDI). High CDI reflects characteristics of disadvantaged neighborhoods.
Results
HbA1c and MBG were higher (p < 0.0001) in blacks [10.4% (90.3 mmol/mol), 255 mg/dL] than whites [8.9% (73.9 mmol/mol), 198 mg/dL). CDI was higher in blacks (p < 0.0001) and positively correlated with HbA1c (r = 0.40, p = 0.0002) and MBG (r = 0.35, p = 0.0011) unless controlled for race. CDI was positively associated with CRP by linear regression within racial groups. CRP was not different between racial groups, and was not correlated with MBG, but was positively correlated with HbA1c when controlled for race (p = 0.04).
Conclusions
Neighborhood disadvantage was associated with inflammation and poor metabolic control in pediatric type 1 diabetes patients. Marked racial differences in potential confounding factors precluded differentiation between genetic and environmental effects. Future studies should recruit patients matched for neighborhood characteristics and treatment regimen to more comprehensively assess racial variation in HbA1c.
Keywords: hemoglobin A1c, inflammation, racial bias, socioeconomic factors, type 1 diabetes mellitus
Metrics of metabolic control used to monitor and manage diabetes include hemoglobin A1c (HbA1c) and direct measurement of blood glucose concentration. Several studies have shown, however, that HbA1c and blood glucose are not interchangeable estimates of metabolic control (1–3). Moreover, both diabetic and non-diabetic individuals of African heritage tend to have higher HbA1c levels than individuals of European heritage even when blood glucose levels are similar between racial groups (4–7). Racial variation in the quantitative relationship between blood glucose and HbA1c complicates the diagnosis and management of diabetes in racially mixed populations. Understanding why HbA1c levels are persistently higher in blacks than whites could help reduce racial disparity in diabetes outcomes.
Although genetic factors may contribute to racial disparities in HbA1c, public health research suggests that community-level social and environmental factors also play a role (8, 9). For example, psychosocial variables like depression can lead to poor adherence to physician-recommended treatment and worse diabetes outcomes (10, 11). Obesity (12) and exposure to social or environmental stressors have been associated with inflammation (11, 13) which in turn can induce insulin resistance (14) and contribute to racial disparity in diabetes outcomes. Gaskin et al. (15) reported that diabetes prevalence was associated with individual poverty for both blacks and whites but neighborhood poverty was associated with diabetes prevalence among blacks and only poor whites. Black children were also reported to have poorer metabolic control and higher mortality rates than non-Hispanic white children even after controlling for individual-level factors like behavior, treatment regimen, and socioeconomic status (16). To address the possibility that segregation may account for variation in neighborhood risk exposure, LaVeist (17) found no difference in diabetes outcomes among whites and blacks living in the same low-income neighborhoods. The complex interplay between environmental stress, inflammation, and racial disparities in diabetes outcomes is the focus of this study.
Addressing the impact of social and environmental factors on outcomes in pediatric diabetes necessitates combining public health and biochemical perspectives. The concentrated disadvantage index (CDI) is a community-level measure of neighborhood disadvantage derived from factor analysis using six census measures (18). The use of CDI as a measure of ‘concentrated disadvantage’ is derived from the work of Sampson et al. (19) on the Project on Human Development in Chicago Neighborhoods. Since that time, CDI has become a well-established, robust, and highly cited measure across the social sciences and public health. In addition, CDI was adopted by the Social Environments Working Group for incorporation in the PhenX Toolkit (20) a widely used compendium of valid, consensus measures of environmental exposures for use in biomedical research.
C-reactive protein (CRP) is a commonly used biomarker of inflammation associated with poor metabolic control as assessed by both higher HbA1c levels and insulin resistance (14, 21, 22). This project was conducted to test the hypothesis that neighborhood disadvantage is associated with inflammation and poor metabolic control in a racially mixed population of pediatric type 1 diabetes patients.
Methods
Subjects and samples
Type 1 diabetes patients (n = 86) were recruited between March and October 2014 with informed consent from pediatric diabetes clinics at Children’s Hospital, New Orleans, LA as approved by the Institutional Review Board at the Louisiana State University Health Sciences Center. Patients included in this convenience sample were aged 5–21 yr with duration of type 1 diabetes of at least 1 yr. Patients excluded from the study included those less than 18 yr without a parent or legal guardian present to give consent, who did not have their HbA1c analysis performed by Children’s Hospital laboratory, and who did not have their blood glucose meters with them at the enrollment clinic visit. Four participants were enrolled at one recruitment clinic per week. This slow recruitment rate was due to analytical constraints imposed by a concurrent biochemical study performed on the same study participants. We attempted to recruit two white and two black participants at each enrollment clinic in order of their appointment times but this was not always possible. After a nurse coordinator or physician provided potential participants with a brief overview of the study, those who agreed to participate met with a clinical trial coordinator who executed informed consent and completed a demographic form using data from patient records and information provided by the participant. Addresses, age, gender, self-identified race, duration of diabetes, height, weight, systolic and diastolic blood pressure, and treatment regimen (two shots a day, multiple daily injections, or pump therapy) were collected at each clinic visit. BMI-z scores were calculated as described by Wang and Chen (23). Blood samples were collected in EDTA (ethylenediaminetetraacetic acid) tubes for analysis of HbA1c and CRP.
Mean blood glucose
Blood glucose data were downloaded from patient glucose meters using each manufacturer’s dedicated software. The meter model used by each subject was determined by patient preference or the patient’s health care provider. Self-monitored mean blood glucose (MBG) was calculated for the 30 days prior to the clinic visit and recorded on each patient’s chart by a nurse or physician. It should be noted that self-monitored blood glucose measurements typically include a disproportionate number of pre-prandial events.
Hemoglobin A1c
HbA1c was measured by immunoassay at each clinic visit in the Children’s Hospital laboratory using the VITROS 5, 1 FS Chemistry System (Ortho-Clinical Diagnostics, Rochester, NY, USA) with an upper reporting limit of 16%. Results are reported in National Glycohemoglobin Standardization Program equivalents.
C-reactive protein
CRP was measured using a fluorescent bead-based immunoassay system from EMD Millipore (Billerica, MA, USA). Plasma samples were stored at −80°C and underwent two freeze–thaw cycles before being analyzed. CRP was measured using human cardiovascular disease panel 3 in 96-well plates according to the manufacturer’s instructions. Assays were performed in duplicate on a Luminex MAGPIX imaging system using 25 µL of diluted (1:40 000) plasma from each subject. Standard curves were generated using the premixed lyophilized standards supplied by the manufacturer. Median fluorescence intensities were collected using milliplex analyst 5.1 software.
Concentrated disadvantage index
Neighborhood disadvantage was assessed using CDIs generated by factor analysis (18). A total of 86 patient’s home addresses were geocoded to the census tracts of their residence using ArcGIS v10.2 (Environmental Systems Research Institute, Redlands, CA, USA). The index reflects a linear combination of six US census tract characteristics, including (i) percentage of individuals on public assistance; (ii) percentage of individuals below poverty; (iii) percentage unemployed; (iv) percentage female-headed households, (v) percentage African-American, and (vi) percentage less than 18 yr old.
Data analysis
Analyses were carried out using pc-sas version 9.3 (SAS Institute, Cary, NC, USA) and GraphPad Prism v. 5 (GraphPad Software, San Diego, CA, USA). Differences between group means for categorical and continuous variables were assessed by chi-squared and Wilcoxon–Mann–Whitney tests, respectively. Associations between CDI, CRP, and metrics of metabolic control (HbA1c and MBG) were assessed by Somers’ D statistic and by Spearman’s correlation with or without adjustment for potential confounders (age, gender, race, and BMI-z). Log-transformed CRP was used to account for right skew in CRP values. Simple linear regression was used to graphically assess relationships between HbA1c, CRP, and CDI. Results were considered significant at p < 0.05.
Results
Demographic and clinic data are presented in Table 1. CDI could not be determined for two white participants with rural addresses. CRP was too low to measure in plasma from two white and one black participant. Five white participants and three black participants had CRP levels indicative of acute infection and inflammation (CRP > 10 mg/L). One participant was omitted from analyses involving BMI-z because of age limits to BMI-z calculation. The proportion of males and females, duration of diabetes, BMI-z, mean systolic and diastolic blood pressures, and CRP were similar between racial groups. Mean HbA1c, MBG, and CDI were higher in black patients. Somers’ D analysis suggested that a randomly selected black patient was 87% more likely to have a higher CDI than a randomly selected white patient.
Table 1.
Population demographics and clinic data*
| Variable | Total | Black | White | p-value† |
|---|---|---|---|---|
| n | 86 | 33 | 53 | |
| Male/female | 44/42 | 14/19 | 30/23 | 0.20 |
| Age (yr) | 14.0 ± 0.4 | 13.1 ± 0.6 | 14.5 ± 0.5 | 0.08 |
| Duration of diabetes (yr) | 8.3 ± 0.4 | 8.3 ± 0.7 | 8.3 ± 0.5 | 0.95 |
| HbA1c (%) | 9.5 ± 0.2 | 10.4 ± 0.4 | 8.9 ± 0.2 | <0.0001 |
| HbA1c (mmol/mol) | 80.2 ± 2.1 | 90.3 ± 3.9 | 73.9 ± 1.9 | <0.0001 |
| MBG (mg/dL)‡ | 220 ± 8 | 255 ± 16 | 198 ± 6 | 0.0002 |
| BMI-z | 0.47 ± 0.11 | 0.67 ± 0.16 | 0.35 ± 0.16 | 0.22 |
| SBP (mmHg) | 119 ± 1 | 118 ± 2 | 119 ± 1 | 0.54 |
| DBP (mmHg) | 70 ± 1 | 70 ± 1 | 70 ± 1 | 0.92 |
| CDI | 0.000 ± 0.114 | 0.955 ± 0.156 | −0.617 ± 0.078 | <0.0001 |
| CRP (mg/L) | 5.00 ± 1.42 | 5.27 ± 2.91 | 4.83 ± 1.45 | 0.88 |
BMI, body mass index; CDI, concentrated disadvantage index; CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; MBG, mean blood glucose; SBP, systolic blood pressure; SEM, standard error of the mean.
Values are means ± SEM.
Differences between groups were determined by chi-squared or Wilcoxin–Mann–Whitney tests.
MBG was calculated from an average of 111 ± 9 glucose meter observations during the 30-day collection period.
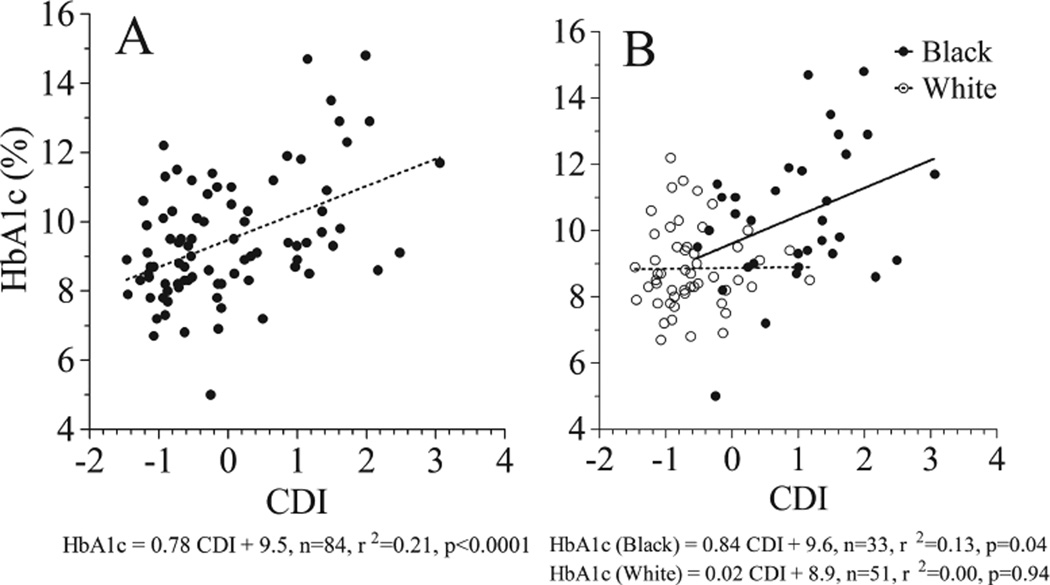
Unadjusted Spearman’s correlation analysis (Table 2) showed that CDI was positively correlated with both HbA1c and MBG but not CRP. The positive correlation observed between CDI and metrics of metabolic control remained significant when adjusted for age, gender, or BMI-z but was no longer observed after adjusting for race. Linear regression analysis (Fig. 1A) confirmed that CDI was strongly positively associated with HbA1c in the study sample. However, regression analysis within racial groups (Fig. 1B) indicated that CDI was only positively associated with HbA1c in black patients.
Table 2.
Relationships between CDI, metrics of metabolic control and CRP
| CDI vs. HbA1c | CDI vs. MBG | CDI vs. CRP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Controlling for | n | r | p-value* | n | r | p-value* | n | r | p-value* |
| Unadjusted | 84 | 0.40 | 0.0002 | 84 | 0.35 | 0.0011 | 81 | 0.16 | 0.1454 |
| Age | 84 | 0.41 | 0.0001 | 84 | 0.34 | 0.0012 | 81 | 0.18 | 0.1043 |
| Gender | 84 | 0.40 | 0.0001 | 84 | 0.36 | 0.0007 | 81 | 0.14 | 0.1871 |
| Race | 84 | 0.11 | 0.3016 | 84 | 0.15 | 0.1488 | 81 | 0.33 | 0.0021 |
| BMI-z | 83 | 0.38 | 0.0004 | 83 | 0.34 | 0.0014 | 80 | 0.15 | 0.1730 |
BMI, body mass index; CDI, concentrated disadvantage index; CRP, C-reactive protein; HbA1c, hemoglobin A1c; MBG, mean blood glucose.
Statistical associations were assessed by Spearman’s correlation
Fig. 1.
Linear regression analysis of concentrated disadvantage index (CDI) vs. HbA1c in the mixed race study sample (A) and separately by racial subgroup (B).
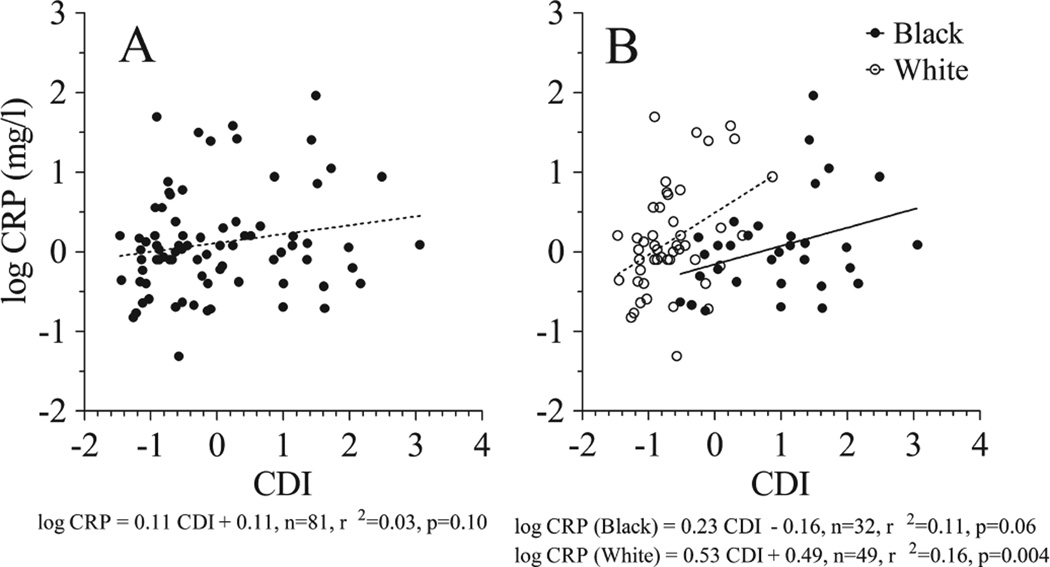
The lack of association between CDI and CRP (Table 2) remained unchanged when adjusted for age, gender, or BMI-z. In contrast, CDI was significantly positively correlated with CRP after adjusting for race. This observation was confirmed by linear regression analysis which showed that CDI was not associated with log CRP in the study sample (Fig. 2A) but was positively associated with log CRP (Fig. 2B) when assessed separately in white patients (p = 0.004) and black patients (p = 0.06).
Fig. 2.
Linear regression analysis of concentrated disadvantage index (CDI) vs. C-reactive protein (CRP) in the mixed race study sample (A) and separately by racial subgroup (B).
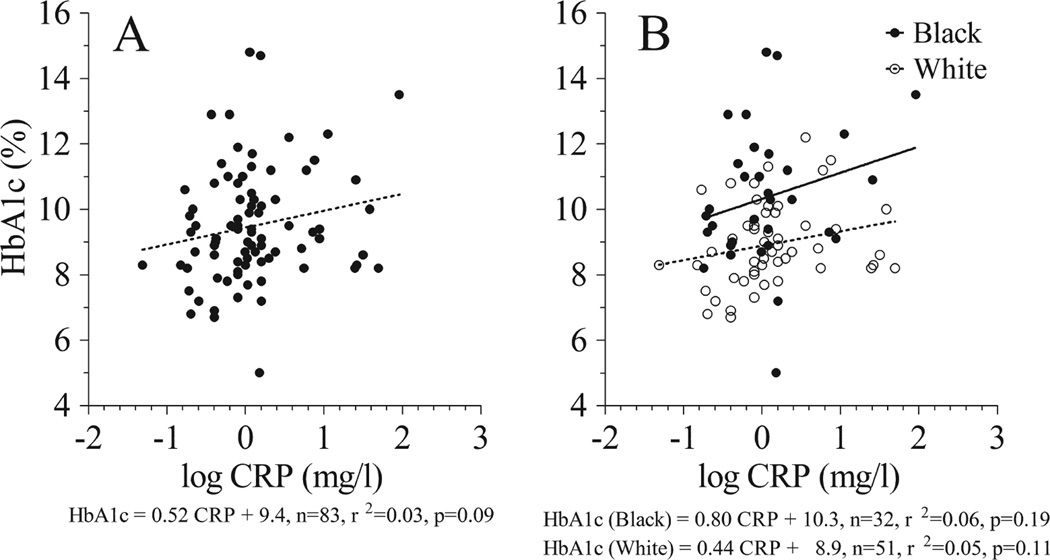
Unadjusted Spearman’s correlation analysis (Table 3) showed that CRP was not correlated with HbA1c or MBG. Associations between CRP and metrics of metabolic control remained unchanged when adjusted for age, gender, or BMI-z. A significant positive correlation was observed between CRP and HbA1c, but not MBG, after adjusting for race. Linear regression analysis (Fig. 3A) indicated that log CRP was not significantly associated with HbA1c in the study sample. Analysis within racial groups (Fig. 3B) produced similar results. The separate regression lines for black and white patients had similar slopes but different intercepts, indicating fixed bias in the relationship between CRP and HbA1c across the range of CRP. Linear regression analysis within racial groups, excluding subjects with acute inflammation (CRP >10 mg/L), showed that log CRP was positively associated with HbA1c in white patients (HbA1c = 1.12 CRP + 9.0, n = 46, r2 = 0.17, p = 0.005) but not in black patients (HbA1c = 0.06 CRP + 10.2, n = 29, r2 = 0.0, p = 0.95).
Table 3.
Relationships between CRP and metrics of metabolic control
| CRP vs. HbA1c | CRP vs. MBG | |||||
|---|---|---|---|---|---|---|
| Controlling for | n | r | p-value* | n | r | p-value* |
| Unadjusted | 83 | 0.18 | 0.10 | 83 | 0.12 | 0.28 |
| Age | 83 | 0.17 | 0.13 | 83 | 0.14 | 0.22 |
| Gender | 83 | 0.18 | 0.10 | 83 | 0.13 | 0.26 |
| Race | 83 | 0.23 | 0.04 | 83 | 0.15 | 0.19 |
| BMI-z | 82 | 0.15 | 0.18 | 82 | 0.09 | 0.41 |
BMI, body mass index; CRP, C-reactive protein; HbA1c, hemoglobin A1c; MBG, mean blood glucose.
Statistical associations were assessed by Spearman’s correlation.
Fig. 3.
Linear regression analysis of C-reactive protein (CRP) vs. HbA1c in the mixed race study sample (A) and separately by racial subgroup (B).
Discussion
Racial differences in the relationship between CDI, CRP, and HbA1c complicate the interpretation of the results observed in our mixed race population of pediatric type 1 diabetes patients. For example, the strong positive correlation between CDI and HbA1c or MBG observed in the study sample disappeared when race was included as a cofactor in the analysis. And although regression analysis confirmed that CDI was strongly positively associated with HbA1c in the study sample as a whole, subgroup analysis by race showed that CDI was associated with HbA1c in black participants but not in white participants. Similarly, regression analysis and unadjusted Spearman’s correlation showed that CDI was not associated with CRP in the study sample. However, subgroup analysis by race, and Spearman’s correlation controlled for race, both indicated that CDI was positively associated with CRP in both black and white participants.
Although previous studies have routinely reported positive associations between HbA1c and biomarkers of inflammation (21, 22, 24), an association between CRP and HbA1c was not consistently observed in this study. For example, Spearman’s correlation analysis detected a weak but significant positive correlation between CRP and HbA1c but only when race was included as a cofactor. This observation was not supported by regression analysis which failed to detect an association between CRP and HbA1c in the mixed race study sample as a whole or in black or white participants analyzed separately. Although mean HbA1c was significantly different between racial groups, mean CRP was not. Graphical evidence of fixed bias (different intercepts) in the relationship between CRP and HbA1c in black vs. white participants indicates that HbA1c tended to be higher in black diabetes patients at all observed levels of inflammation. Obesity is a potential source of inflammation (12) but BMI-z was not significantly different between blacks and whites in this study. Moreover, the lack of correlation between CRP and HbA1c in the study sample remained non-significant after statistically controlling for variation in BMI-z.
The apparent lack of association between CDI and HbA1c in white participants is qualified by the fact that fewer white participants lived in disadvantaged neighborhoods as indicated by their lower mean CDI and narrower observed CDI range. The lack of CDI overlap between racial groups makes it impossible to separate neighborhood characteristics from racial characteristics as causes of variability in HbA1c in the study sample as a whole. This issue is partly addressed in this study by evaluating some associations within each racial group separately. Failure to adequately consider group characteristics in drawing inferences about causes of variability across individuals can result in what has been termed as psychologistic or individualistic fallacy, i.e., assuming that an individual-level outcome like HbA1c can be explained exclusively in terms of individual-level characteristics like race (25, 26). Such an approach fails to consider neighborhood limitations like access to healthy food, health care, and safe physical activity environments.
Uncontrolled behavioral or environmental factors limited our ability to differentiate between genetic and environmental effects in this study. For example, the SEARCH for Diabetes in Youth study showed that patients using insulin pumps had lower HbA1c levels (27). When we analyzed our data, we unexpectedly found clear racial disparity in treatment regimen in the study sample, with 64% of white patients using insulin pumps but only 9% of black patients. Statistically controlling for racial differences in treatment regimen post hoc was not feasible in this study due to the low proportion of black insulin pump users. A recent study by Willi et al. (28) found similar racial disparity in insulin pump use that could not be explained by socioeconomic status alone. Poor glycemic control in pediatric diabetes patients has also been associated with Helicobacter pylori infection (29, 30) and with depression (31) which can in turn lead to poor adherence to physician-recommended treatment and worse diabetes outcomes (10, 11). Controlling for these and other variables can alter the interpretation of study results, as evidenced by the different picture that emerged in the relationship between CRP and HbA1c in white subjects when individuals with acute inflammation were excluded from regression analyses.
Collectively, the results of this study show that neighborhood disadvantage (higher CDI) was associated with poor metabolic control (higher HbA1c) in blacks and with inflammation (higher CRP) in both blacks and whites. Differentiating between genetic and environmental effects was not possible, however, due to racial differences in treatment regimen and the lack of significant racial overlap in CDI. This study provides an important lesson for the design of future studies that seek to assess the relative contributions of genetic and environmental factors on racial disparity in HbA1c in diabetes patients. In addition to controlling for characteristics like age, gender, and BMI-z, we strongly suggest that patients should be recruited under protocols that match participants from different racial groups based on neighborhood characteristics and diabetes treatment regimen. Statistically controlling for other behavioral factors (e.g., depression and treatment adherence) and other environmental factors (e.g., ongoing infection or access to health care) may also be warranted.
Acknowledgments
Research reported in this paper was supported in part by the Mid-South Transdisciplinary Collaborative Center for Health Disparities Research through the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health under Award Number U54MD008176. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided by Children’s Hospital of New Orleans, the Louisiana State University Health Sciences Center, and the P and C Carroll Foundation, New Orleans, LA.
Footnotes
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author contribution
J. M. H. and S. A. C. were responsible for the conception and design of the study. S. J. C., P. C., R. G., A. V., S. S., and J. Z. collected data and/or analyzed samples. C. V., J.M. H., and S. J. C. analyzed the data. All authors contributed to interpreting the data and drafting the manuscript and are responsible for the intellectual content and final approval of the published version.
References
- 1.Hempe JM, Soros AA, Chalew SA. Estimated average glucose and self-monitored mean blood glucose are discordant estimates of glycemic control. Diabetes Care. 2010;33:1449–1451. doi: 10.2337/dc09-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care. 2006;29:352–355. doi: 10.2337/diacare.29.02.06.dc05-1594. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DM, Xing D, Beck RW, et al. Hemoglobin A1c and mean glucose in patients with type 1 diabetes: analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care. 2011;34:540–544. doi: 10.2337/dc10-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamps JL, Hempe JM, Chalew SA. Racial disparity in hemoglobin A1c independent of mean blood glucose in children with type 1 diabetes. Diabetes Care. 2010;33:1025–1027. doi: 10.2337/dc09-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067–1072. doi: 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract. 2010;87:415–421. doi: 10.1016/j.diabres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Dagogo-Jack S. Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol. 2010;6:589–593. doi: 10.1038/nrendo.2010.126. [DOI] [PubMed] [Google Scholar]
- 8.Delamater AM, Shaw KH, Applegate EB, et al. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care. 1999;22:700–705. doi: 10.2337/diacare.22.5.700. [DOI] [PubMed] [Google Scholar]
- 9.Auchincloss AH, Diez Roux AV, Brown DG, Erdmann CA, Bertoni AG. Neighborhood resources for physical activity and healthy foods and their association with insulin resistance. Epidemiology. 2008;19:146–157. doi: 10.1097/EDE.0b013e31815c480. [DOI] [PubMed] [Google Scholar]
- 10.Hood KK, Rausch JR, Dolan LM. Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes: rates, magnitude, and moderators of change. Pediatr Diabetes. 2011;8:718–723. doi: 10.1111/j.1399-5448.2011.00771.x. [DOI] [PubMed] [Google Scholar]
- 11.Holt RIG, de Groot M, Lucki I, Hunter CM, Sartorius N, Golden SH. NIDDK international conference report on diabetes and depression: current understanding and future directions. Diabetes Care. 2014;37:2067–2077. doi: 10.2337/dc13-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007;378:48–52. doi: 10.1016/j.cca.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 14.Mauvais-Jarvis F. Novel link between inflammation, endothelial dysfunction, and muscle insulin resistance. Diabetes. 2013;62:688–690. doi: 10.2337/db12-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaskin DJ, Thorpe RJ, Jr, McGinty EE, et al. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104:2147–2155. doi: 10.2105/AJPH.2013.301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton R, Good G, Mikhailov T, Freels S, Donoghue E. Ethnic differences in mortality from insulin-dependent diabetes mellitus among people less than 25 years of age. Pediatrics. 1999;103:952–956. doi: 10.1542/peds.103.5.952. [DOI] [PubMed] [Google Scholar]
- 17.La Veist T, Pollack K, Thorpe R, Jr, Fesahazion R, Gaskin D. Place, not race: disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Aff (Millwood) 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson RJ, Sharkey P, Raudenbush SW. Durable effects of concentrated disadvantage on verbal ability among African-American children. Proc Natl Acad Sci USA. 2008;105:845–852. doi: 10.1073/pnas.0710189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Hempe JM, McCarter RJ, Li S, Fonseca VA. Association between inflammation and biological variation in hemoglobin A1c in U.S. non-diabetic adults. J Clin Endocrinol Metab. 2015;100:2364–2371. doi: 10.1210/jc.2014-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T, Dorn JP, Donahue RP, Sempos CT, Trevisan M. Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002;155:65–71. doi: 10.1093/aje/155.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Chen H-J. Use of percentiles and Z-scores in anthropometry. In: Preedy VR, editor. Handbook of Anthropometry. New York: Springer; 2012. pp. 29–48. [Google Scholar]
- 24.King DE, Mainous AG, III, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26:1535–1539. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- 25.Riley MW. Sociological Research I: A Case Approach. New York: Harcourt, Brace and World; 1963. Special problems of sociological analysis; pp. 700–725. [Google Scholar]
- 26.Diez Roux AV. A glossary for multilevel analysis. J Epidemiol Community Health. 2002;56:588–594. doi: 10.1136/jech.56.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155:183–189. e1. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 28.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424–434. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begue RE, Mirza A, Compton T, Gomez R, Vargas A. Helicobacter pylori infection and insulin requirement among children with type 1 diabetes mellitus. Pediatrics. 1993;103:e83. doi: 10.1542/peds.103.6.e83. [DOI] [PubMed] [Google Scholar]
- 30.Begue RE, Gomez R, Compton T, Vargas A. Effect of Helicobacter pylori eradication in the glycemia of children with type 1 diabetes: a preliminary study. South Med J. 2002;95:842–845. [PubMed] [Google Scholar]
- 31.Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149:526–531. doi: 10.1016/j.jpeds.2006.05.039. [DOI] [PubMed] [Google Scholar]