Abstract
Aims
To document the prevalence and 9-month incidence of elevated diabetes distress (DD) and the stability of DD over time using both single threshold and minimal clinically important differences (MCID) approaches.
Methods
Adults with type 1 diabetes (T1D) (N=224) completed the 28-item T1-Diabetes Distress Scale (T1-DDS) at baseline and 9 months. A T1-DDS threshold was identified with spline analysis and MCID was calculated from the standard error of measurement.
Results
Analyses supported a cut-point of ≥2.0 for elevated DD. The prevalence and 9-month incidence of elevated DD was 42.1% and 54.4%, respectively. MCID was +/−0.19 but varied by subscale (.26 to .50). Elevated DD was stable: only 20% crossed 2.0 over 9 months. MCID analyses showed that change also occurred among those who remained either below or above 2.0 over time. Change varied by source of distress, with Powerlessness the most prevalent and stable. Using MCID, only participant age, gender and number of complications predicted change.
Conclusions
The prevalence, 9-month incidence and stability of elevated DD is high among adults with T1D, with change based on source of DD. We propose a combined cut-point/MCID framework for measuring change in DD, since each approach reflects unique characteristics of change over time.
Keywords: diabetes distress, prevalence, incidence, change over time
1.0 INTRODUCTION
Diabetes distress (DD) refers to the emotional distress associated with the ongoing worries, burdens and concerns that occur when managing a demanding chronic disease like diabetes over time [1]. Although often confused with depression, unlike depression DD is directly linked with poor glycemic control and problematic self-care behaviors [2–6]. Recent studies have indicated that among type 2 adults (T2D) elevated DD is highly stable over time and that the point prevalence of elevated DD is approximately 46%, suggesting a widespread clinical problem in this population [7]. To date, however, no systematic analysis of the prevalence, incidence and stability of DD over time among adults with type 1 diabetes (T1D) has occurred.
Given the importance of DD with respect to quality of life and disease management, one important but often neglected question is: How much change in DD among adults with T1D over time should be considered meaningful? For clinicians and individuals with T1D, this highlights the need to identify how much DD must decrease before an intervention might be considered successful or how much DD must increase before an intervention should be initiated; for clinical researchers, this issue is critical in order to demonstrate meaningful change in DD as a result of a new device or treatment [8]. Standardizing the cut-points and parameters of stability and change in DD over time also permits studies of correlates of DD and predictors of change so that individuals at risk can be identified. The goals of the present study were to identify criteria for elevated DD, clock patterns of stability and change over time and document how much change in DD among adults with T1D needs to occur to be meaningful.
The Type 1 Diabetes Distress Scale (T1-DDS) is a reliable and valid 28-item self-report instrument developed to identify the specific sources of DD for adults with T1D and to provide an index of overall diabetes-related emotional distress for use in clinical and research settings [9]. It contains a total score plus seven reliable, factor-derived, diabetes-specific subscales: powerlessness, management distress, hypoglycemia distress, negative social perceptions, physician distress, hypoglycemia distress, eating distress, family/friends distress.
There are two primary strategies for documenting meaningful change over time on measures like the T1-DDS. First are those based on a rational or empirically-based scale cut-point that divides a sample into those who have a particular health condition vs. those who do not – a diagnostic threshold. “Significant” change is then indicated when a respondent’s score rises above or drops below the threshold or scale cut-point, thus reaching or no longer reaching the criterion for a “health condition” or diagnosis. For example, a common marker of “poor” glycemic control is when an A1C level rises above 7.0% [53 mmol/mol] [10], or when a depression score on the Patient Health Questionnaire-9 (PHQ9) increases to ≥ 10 [11].
The diagnostic approach, however, does not document meaningful change across the entire range of participant experience. This is true when considering A1C levels, scores on depression scales, or scores on the T1-DDS. A simple diagnostic cut-point cannot identify a significant increase or decrease between scores over time that are either both below or both above the scale cut-point. For example, a diagnostic cut-point approach does not indicate whether a reduction in A1C from 10.0% [86 mmol/mol] to 9.5% [80 mmol/mol] is significant because both the original and subsequent A1C levels are well above the diagnostic level of 7.0% [53 mmol/mol]. Although a reduction of 5% may reflect a very meaningful change in glycemic control, its significance would not be captured by the diagnostic approach. The same is true of scales such as the PHQ9 and T1-DDS.
A second approach addresses this omission and is generally called minimal clinically important differences (MCID). MCID is defined as a numerical score indicating the smallest meaningful value of change anywhere along the entire range of a continuous measure, such as A1C, PHQ9, or T1-DDS [8]. A host of statistical methods have been proposed as a basis for defining MCID, e.g., the standard error of measurement (SEM) or a half-standard deviation unit [12, 13]. In overview, both diagnostic and MCID approaches are important and helpful; they simply address the documentation of change in different ways.
Given the importance of DD and the need to create reliable criteria to document meaningful change among T1D adults over time, we sought to document the benchmarks for meaningful change on the T1-DDS over time. Toward this end, we asked the following questions. First, using both exploratory and confirmatory approaches, what T1-DDS total scale score cut-point should be used to indicate significantly elevated DD? Second, using this score cut-point, what is the prevalence and incidence of elevated DD over 9 months and how much change occurs during this interval? Third, what is a reliable MCID for the T1-DDS, and how much change occurs over time using an MCID approach? In supplementary analyses, we also asked which respondent baseline characteristics predict change in DD over time, using either a cut-point or MCID approach?
2.0 MATERIALS AND METHODS
2.1 Subjects
Adults with type 1 diabetes were recruited from four community diabetes clinics in California to assure a diverse sample. Inclusion criteria were type 1 diabetes ≥ 12 months, age ≥ 19 years, and no severe substance abuse, cognitive deficit, or psychosis. Clinic staff identified all eligible individuals during regular visits or sent letters to all eligible individuals in their T1D registry informing them that they would receive a telephone call from a project representative if they did not opt out by either calling a toll-free number or returning an enclosed postcard. All participants were screened for eligibility by telephone, and, if interested, were emailed a HIPAA-protected personal link to the online survey, which included an informed consent form. Participants also provided permission for their health care provider to release any HbA1C results within the past three months. Participants received a $15 electronic gift card for participation. Nine months after initial assessment, a new survey was sent to individuals who agreed to allow us to contact them to assess stability and change in DD over time. The study received approval from the UCSF Committee on Human Research and data were collected in 2015–2016.
2.2 Measures
Demographic measures included age, gender, ethnicity (White/non-White), education (years), and duration of diabetes. Diabetes status included clinic-recorded A1C within 3 months, body mass index (BMI based on self-reported weight and height), current form of insulin delivery (pump vs. multiple daily injections), number of diabetes complications from a list of eight, and number of times in the past week that they had a blood glucose (BG) reading < 70 mg/dl. In addition, respondents estimated the average number of times per day in the past 2 weeks that they checked their BG, along with how many times per day they probably should have checked but did not. Similarly, they estimated the average number of times per day in the last two weeks that they had taken an insulin bolus, along with how many times per day in the last 2 weeks they probably should have bolused but did not. A percent of total minus missed tests or boluses provided an index of adherence for each. At both baseline and 9-month follow-up, participants completed the T1-DDS (28 items; alpha = .91) [9]. Items are scored on a 6-point response scale from 1 (”not a problem”) to 6 (“a very serious problem”).
2.3 Data Analyses
Descriptive statistics were computed to review item and scale distributions. Spline regression models were specified using nonlinear regression procedures [14]. These analyses estimated where a knot or cut-point occurred along the distribution of T1-DDS scores that reflected a change in the linear relationship between T1-DDS and A1C [15, 16]. Then, piecewise regression models were performed to test for significant differences both between the two slopes and intercepts of the curves defined by that cut-point and by the change in slope as the distribution moved across the cut-point. Prevalence/incidence rates for DD based on this cut-point were then calculated, and chi-square tests were used to evaluate the percentage of four groups of individuals: those who became distressed, were no longer distressed, or remained below or above the threshold.
Following Hilliard, et al. [8], we employed SEM as an indicator of MCID for the T1-DDS. We used the SEM small effect formula [1*(SD * √(1−α)], and utilized the standard deviation and Cronbach’s alpha from the baseline DD scales. The prevalence of participants who scored ≥ +1 MCID, ≥ −1 MCID, or remained within 1 MCID over nine months was then calculated. To evaluate participant correlates of change in DD, separate analyses of variance (ANOVA) or chi-square models were specified for the four threshold groups and the three MCID groups on each participant characteristic. A priori specific contrasts were examined with least significant difference (LSD) follow-up tests.
3.0 RESULTS
3.1 Sample Characteristics
Of 348 eligible individuals identified from the four clinics, 305 completed the baseline survey (87.0%) and 224 completed the follow-up assessment 9 months later (73%). There were no significant differences between those who completed the 9-month assessment and those who did not on any demographic or diabetes status variable tested. Average age was 43.0 (15.2) years, 56.0% were female, over 90% received some post-high school education, and mean HbA1C was 7.4% (1.12) [57.2 (12.8) mmol/mol] (Table 1).
Table 1.
Participant Characteristics at Baseline (N=224)
| Mean (SD) or % | |
|---|---|
| Current age (years) | 43.0 (15.2) |
| Age at DM onset (years) | 20.9 (13.3) |
| Gender (% female) | 56% |
| Ethnicity (% white) | 84.4% |
| Education (years) | 16.8 (2.1) |
| Duration of diabetes (years) | 22.2 (14.3) |
| HbA1c | |
| % | 7.4 (1.2) |
| mmol/mol | 57.2 (12.8) |
| Body mass index (kg/m2) | 25.4 (4.1) |
| Pump use (%) | 68.8% |
| No. complications | 2.0 (2.3) |
| % of prescribed GM done in past 2 weeks | 78.5 (18.8) |
| % of prescribed boluses taken in past 2 weeks | 87.5 (15.1) |
| # times past week BG <70 | 2.9 (2.5) |
3.2 T1-DDS Scale Cut-Point
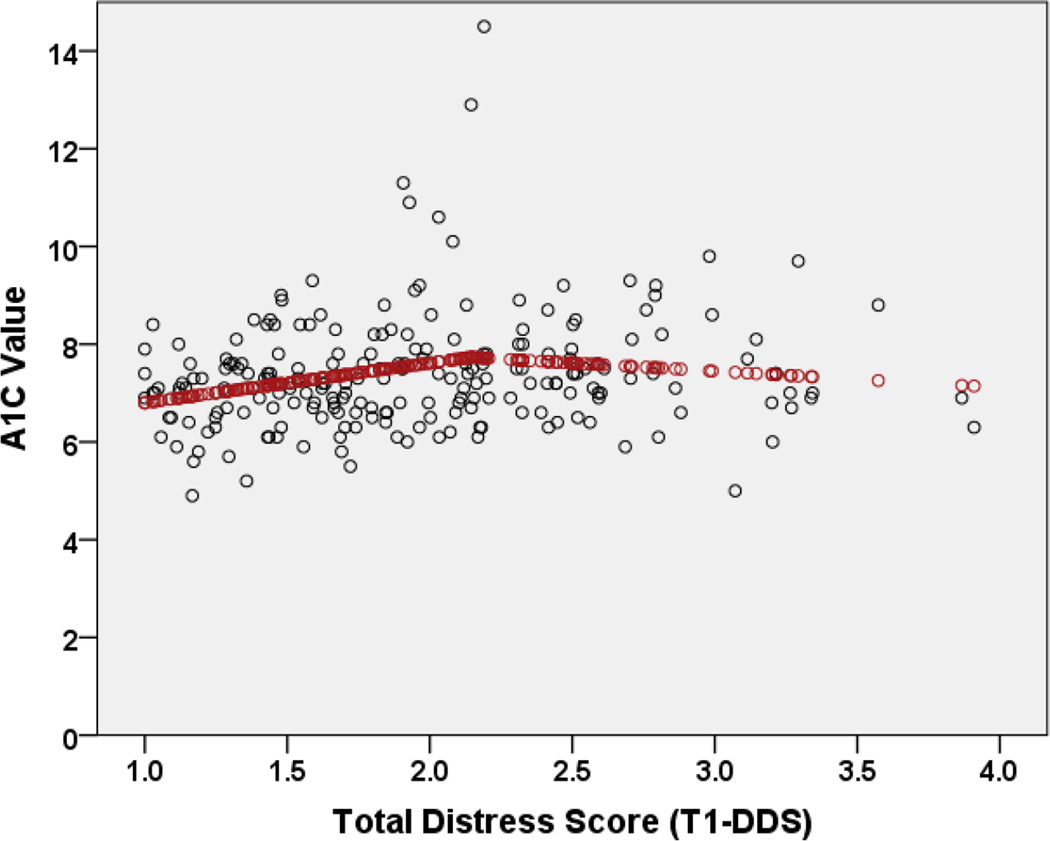
In exploratory analyses with baseline data, we sought to identify a data-based diagnostic cut-point for the total T1-DDS scale score, using patient A1C as the dependent variable. Results of spline regression procedures, specifying a linear relationship between T1-DDS and A1C along the full T1-DDS range, indicated a knot at the T1-DDS score of 2.081 (R2 = .053), with a positive linear coefficient for the slope in the first phase and a negative adjustment for the second phase (Figure 1). Specifying a linear relationship below the knot and a quadratic relationship above the knot moved the cut-point to 2.145, but did not change the R2; thus, the more parsimonious linear/linear model was preferred.
Figure 1.
Bivariate relationship of total distress (T1-DDS) with actual A1C values (dots) and predicted A1C values (line), suggesting a T1-DDS cut-point of 2.0.
The cut-point estimated in spline analyses, rounded to 2.0, was used to establish two patient groups: those with T1-DDS < 2 and those with T1-DDS ≥ 2. In piecewise regression models using this cut-point, a significant linear relationship was found between T1-DDS and A1C below the cut-point (B = .83 [SE = .37], Beta = .05, t = 2.27, p = .02) but not above the cut-point (B = −.31 [SE = .28], Beta = −.02, t = −1.14, p = .26). Specifically, below the cut-point, predicted A1C levels increased 0.83% for each 1-point increase in T1-DDS. There was no significant increase in mean A1C levels as T1-DDS scores exceeded the 2.0 cut-point. There was also a statistically significant result for a change in slope from T1-DDS < 2.0 and T1-DDS ≥ 2.0 (B = −1.14 [SE = .46], Beta = −.38, t = −2.50, p = .01.). These analyses suggested the viability of a T1-DDS total scale cut-point of 2.0 to identify elevated DD, since the relationship between T1-DDS and A1C was significantly linear until 2.0, after which no significant relationship occurred.
3.3 Prevalence, Incidence and Change in DD Over Time
The prevalence and 9-month incidence of DD, using the ≥ 2 threshold on the T1-DDS, was 42.1% and 54.4%, respectively. Individuals who were distressed at baseline tended to remain distressed at 9 months (71%), and those who were not distressed at baseline tended to remain so at 9 months (81%) (Table 2). Thus, elevated DD, when it occurred, was persistent over time, and between 20% and 30% of participants became distressed or were no longer distressed over 9 months. However, the proportion of participants who were distressed at both at baseline and 9-months differed across the seven T1-DDS subscales (Cochran’s Q: χ2 = 320.79, p < .0001). For example, 90% of participants who scored ≥ 2.0 on the Powerlessness subscale at baseline scored similarly at 9 months, whereas only 60% who scored at ≥ 2.0 at baseline on Physician Distress did so at 9 months. Thus, stability and change over time varied significantly as a function of the source of DD.
Table 2.
Prevalence, Incidence, and Change/Stability Over Time in Diabetes Distress (N=224)
| Below Cut-Point at T1 |
Above Cut-Point at T1 |
||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) |
Baseline Prevalence |
9-Month Incidence |
Above at T2 (Worse) |
Below at T2 (Same- Low) |
Above at T2 (Same- High) |
Below at T2 (Better) |
|
| Total T1-DDS | 1.96 (.64) |
42.1% | 54.4% | 19% | 81% | 71% | 29% |
| Powerlessness | 2.84 (1.21) |
72.0% | 85.3% | 39% | 61% | 90% | 10% |
| Management Distress |
1.99 (.91) |
42.7% | 54.9% | 20% | 80% | 77% | 23% |
| Hypoglycemia Distress |
1.98 (.97) |
42.9% | 57.1% | 25% | 75% | 73% | 27% |
| Negative Social Perceptions |
1.82 (1.01) |
32.3% | 39.3% | 10% | 90% | 77% | 23% |
| Eating Distress |
2.25 (1.08) |
54.3% | 73.7% | 34% | 66% | 78% | 22% |
| Physician Distress |
1.33 (.62) |
11.4% | 16.1% | 6% | 94% | 60% | 40% |
| Family/Friend Distress |
1.49 (.69) |
23.6% | 31.7% | 11% | 89% | 65% | 35% |
Notes. “Worse” indicates the percentage of individuals < 2.0 at baseline who scored ≥ 2.0 at 9 months, “same” indicates the percentage of individuals ≥ 2.0 at baseline who remained ≥ 2.0 or ≤ 2.0 at 9 months, and “better” indicates the percentage of individuals ≥ 2.0 at baseline who scored < 2.0 at 9 months. T1= baseline, T2 = 9 months.
3.4 Minimal Clinical Important Difference (MCID)
With a baseline T1-DDS total score mean of 1.96 (0.64), an alpha of 0.91 and a small effect size of 1.0, the MCID for the total T1-DDS score was 0.19 (Table 3). MCIDs for the subscales varied from 0.26 to 0.50: smaller values than for the total scale were expected, given that MCID was based in part on the number of scale items and alpha.
Table 3.
T1-DDS MCID Values and Percent Change Over Time (N = 224)
| Number of items |
Alpha | MCID | Increased ≥ 1 MCID (Worsened) |
Remained within 1 MCID (No Change) |
Decreased ≥ 1 MCID (Improved) |
|
|---|---|---|---|---|---|---|
| Total T1-DDS | 28 | .91 | .19 | 27.7% | 44.2% | 28.1% |
| Powerlessness | 5 | .87 | .44 | 18.3% | 53.6% | 28.1% |
| Management distress |
4 | .76 | .44 | 30.8% | 54.0% | 15.2% |
| Hypoglycemia distress |
4 | .79 | .44 | 23.7% | 50.0% | 26.3% |
| Negative social perceptions |
4 | .84 | .40 | 14.3% | 61.2% | 24.6% |
| Eating distress | 3 | .78 | .50 | 23.7% | 53.6% | 22.8% |
| Physician distress |
4 | .82 | .26 | 10.3% | 79.0% | 10.7% |
| Friend/family distress |
4 | .80 | .31 | 20.1% | 66.1% | 13.8% |
Because MCID is based on change along the entire scale distribution and not just on a single score cut-point, more than twice as many individuals demonstrated a change of at least one MCID compared to a change across the single T1-DDS threshold of ≥ 2.0: 125 vs. 52, respectively (Cochran’s Q: χ2(1) = 65.79, p < .0001). There was considerable variability in stability and change in MCID over time across the T1-DDS subscales (Cochran’s Q: χ2(7) = 90.64, p < .0001). For example, 79.0% of individuals remained within one MCID over time on Physician Distress, whereas only 50.0% did so on Hypoglycemia Distress. Again, based on MCID, the source of DD affected stability and change over time.
3.5 Supplementary Analyses: Predictors Of Change
We undertook two sets of analyses of predictors of T1-DDS total score change across the ≥ 2.0 threshold over time. First, we compared those who scored below the threshold at baseline but who scored above it at 9 months (became distressed), compared to those who remained below it at both time points. Second, we compared those who scored above the threshold at baseline but who scored below it at 9 months (were no longer distressed), compared to those who remained above the threshold at both time points. In the first set of analyses no variable significantly predicted change from low to elevated DD over time. In the second set of analyses, only those with good self-monitored BG adherence at baseline had T1-DDS scores below the cut-point at 9 months (82.0% vs. 69.9%, p < .001). Overall, very few participant baseline characteristics predicted a change across the DD threshold over time.
We next computed how baseline variables predicted change over time across three participant groups using MCID: those whose T1-DDS score increased by at least one MCID (became worse), those who remained within one MCID (no change), and those whose T1-DDS score decreased by at least one MCID (improved) (Table 4). Age, gender and number of complications either approached or reached statistical significance: females (p < .03), younger adults (p < .07) and those with more complications (p < .03) displayed a higher probability of reduced DD ≥ 1 MCID over time than males, older adults, and those with fewer complications.
Table 4.
Predictors of Change of One MCID or More (N = 224)
| Increased ≥1 MCID (Worsened) n = 62 |
No Change in MCID (Same) n = 99 |
Decreased ≥1 MCID (Improved) n = 63 |
p Value of Omnibus Test |
|
|---|---|---|---|---|
| Age | 42.5 (14.4) | 45.4 (15.4)x | 39.8 (14.3)y | .07 |
| Gender (% female) | 54.5%x | 48.5%x | 69.8%y | .03 |
| Ethnicity (% white) | 85.5% | 83.8% | 84.1% | .96 |
| Education (years) | 17.2 (1.8) | 16.7 (2.1) | 16.6 (2.3) | .25 |
| Percent using insulin pump |
67.7% | 65.7% | 74.6% | .48 |
| Diabetes duration | 19.8 (13.9) | 23.4 (14.7)) | 22.7 (13.9) | .30 |
| BMI | 25.5 (4.0) | 24.9 (4.1) | 26.1 (4.1) | .19 |
| Hypo episodes requiring assistance (% yes) |
16.9% | 22.6% | 26.7% | .44 |
| Number of complications |
1.7 (2.2)x | 1.7 (2.3)x | 2.6 (2.5)y | .03 |
| HbA1C -% mmol/mol |
7.4 (1.3) 59.6 (13.9) |
7.3 (1.0) 56.1 (10.7) |
7.6 (1.3) 59.1 (14.7) |
.36 |
| BG monitoring adherence (%) |
75.6 (19.2) | 78.9 (19.3) | 80.5 (17.6) | .33 |
| Bolus adherence (%) |
87.5 (15.6) | 88.5 (13.9) | 85.8 (16.3) | .55 |
Note. Mean (SD) or percent; ANOVA or chi square analyses, respectively.
Significant contrasts (p < .05) between x and y made with least significant difference follow-up tests.
4.0 DISCUSSION
The first aim of this research was to identify a T1-DDS score cut-point to define elevated DD. Data-driven exploratory analyses highlight a single knot at 2.0, marking a significant shift in the bivariate relationship between T1-DDS and A1C. Confirmatory analyses show a significant linear relationship between T1-DDS and A1C below but not above 2.0; and, secondly, a significant change occurs in the slope as the distribution moves beyond the 2.0 cut-point. The scale response options indicate that significant DD occurs when respondents report even a “slight problem” on average across all 28 items. DD, therefore, is a potent experience—even at low levels it displays a significant relationship with glycemic control. Interestingly, the same 2.0 cut-point was demonstrated with A1C and diabetes management variables when a different scale to assess DD was administered to adults with T2D [17]. These findings suggest that across the diabetes spectrum, even at relatively low levels of intensity, DD has significant implications for disease management and glycemic control. It also suggests that interventions not target only patients who report high DD; even modest levels of DD warrant clinical exploration and targeted intervention.
Our second aim was to identify the prevalence and 9-month incidence of elevated DD and to document change in DD over time. The point-prevalence and 9-month incidence of elevated DD among adults with T1D, based on the ≥ 2.0 cut-point, are strikingly high -- 42.1% and 54.4%, respectively. These figures are similar to the 46.2% prevalence and 9-month 54.3% incidence of DD among adults with T2D, re-calculated from an earlier study (N = 502) [7]. They are also substantially higher than the reported prevalence/incidence of clinical depression or elevated depression symptoms in this population [18, 19].
Without intervention, elevated DD is relatively stable over time when using the ≥ 2.0 threshold. Among those with elevated DD at baseline, 71% report similarly high levels at 9 months; and of those who report low DD at baseline, 81% report similarly at 9 months. Overall, 76.8% of the total sample evidenced no change in DD across the 2.0 threshold. Thus, unlike depression [7], elevated DD does not appear to be episodic, ebbing and flowing over time; instead, when it occurs, without intervention it is generally persistent and continuous. Given this stability and the relationships among DD, behavioral management and glycemic control, DD is worthy of clinical attention and intervention.
The prevalence, incidence and stability of DD over time vary significantly by the source of DD. Feelings of powerlessness are the most prevalent and persistent source of DD among those assessed, pointing to the discouraging impact of varying and sometimes chaotic BG numbers over time, despite an individual’s best efforts. These findings suggest that, although the overall level of DD is important as a generic indicator of DD, it is also helpful to identify specific sources of distress as targets for intervention.
As found in other studies [8], without intervention few baseline demographic and diabetes-related variables predict significant change in DD over time. Since DD reflects diabetes-related affective status, variables like initial levels of quality of life and related psychosocial variables may better predict change over time than participant demographics or diabetes status, and may be an area of future research.
Our third goal was to explore the use MCID as an indicator of change. SEM was selected to calculate MCID because it is a property of the scale and not a property of a particular sample’s T1-DDS score distribution. Thus, its use can be better applied to diverse T1D populations. Although not presented here, we also calculated MCID using half-standard deviation units and found very similar results. Furthermore, we calculated MCID with SEM using a large, diverse, national sample of 279 T1Ds and again found results similar to those reported here. These findings, therefore, support the relative stability of MCID values across methods of calculation and samples of T1D adults.
MCID analyses indicate that a far greater amount of meaningful change in DD occurs over time than is reflected by the use of a single score threshold. For example, whereas 10.7% of individuals display increases and 12.5% display decreases in DD across the 2.0 threshold, in MCID analyses 27.7% of the sample increased by one or more MCIDs and 28.1% decreased. Across the entire T1DDS score range, over twice as many individuals display a significant change using MCID than using the diagnostic criterion. Thus, focusing exclusively on the presence or absence of elevated DD based on a score of ≥ 2.0 will miss the documentation of much meaningful change in DD over time.
It is important to note, however, that an increase or decrease of at least 1 MCID in DD over time does not necessarily mean that the level of an individual’s DD is no longer of concern. Individuals with initially low levels of DD (< 2.0) may increase one or more MCIDs over time but still fail to cross the threshold for elevated DD; and individuals with initially high levels of DD (≥ 2.0) may demonstrate a reduction of at least one MCID and still not cross the threshold. This latter patient group also may demonstrate a significant increase from their initially high level, thus raising additional clinical concern. MCID, therefore, provides an yardstick for observing change even among patients who remain above or below the threshold over time.
Given the differences in the amount of change noted when using the diagnostic and MCID approaches, how might meaningful change in DD over time best be documented? We suggest a two-stage approach that includes both methods, since each makes a unique contribution to the documentation of change. First, given that elevated DD is significantly related to disease management, glycemic control and quality of life, it is important to identify those individuals who score above and below the score threshold. Having an agreed-upon standard around which a definition of elevated DD can be operationalized increases consistency across clinical settings and studies. Second, the MCID findings indicate that a full explication of change requires additional, more fine-grained analyses; otherwise, important aspects of change among those who score consistently high or consistently low over time will be missed and an increase in the risk of type 1 error will occur - not documenting a significant change when in fact one occurred. For example, of the patients who scored above the 2.0 threshold at both time points (31% of the total sample), 32.9% demonstrated a reduction in DD of at least one MCID but stayed within the elevated range (≥ 2.0); and 25.7% demonstrated an increase in DD of at least one MCID. A similar pattern of change occurred for patients who scored below 2.0 at both time points: some displayed significant reductions in DD, whereas others displayed significant increases. Thus, including MCID into the analysis of change permits the detection of significant change even among those who score consistently high or low in DD over time, with implications for clinical care.
This study has several strengths: it included a diverse, community sample of adults with T1D, permitted a careful comparison of different approaches to change, included assessment of several potential predictors of change, and allowed for a sufficiently long time interval to detect meaningful change. Several cautions are noteworthy. First, larger and more diverse samples should be recruited that include a broader range of patient age and glycemic control than in the present sample. Furthermore, our sample was not sufficiently large to enable subgroup analyses. Second, the study included a 9-month time frame with only two assessments. Patterns of stability and change may vary based on the time intervals and multiple assessments. Third, the study was observational and no intervention was included to stimulate change, thus reducing the opportunity to identify important predictors of change. Fourth, the variability in MCID among the T1-DDS subscales might in part be accounted for by the underlying statistical robustness of the sub scale itself. Analyses using other psychometric methods, like Rasch analysis, might be helpful.
In summary, we find that a score of 2.0 serves as a useful cut-point to indicate elevated DD among adults with T1D. The prevalence and 9-month incidence of elevated DD are 42.1% and 54.4%, respectively, and elevated DD is highly stable over time. Patterns of stability and change however, vary significantly by the source of DD, emphasizing the need to consider the source of distress clinically. MCID analyses indicate that significant change in DD also occurs among patients who display either consistently low or elevated DD over time. Last, few variables predict change over time, with only age, gender, number of complications and frequency of BG testing predicting change beyond one MCID. A 2-stage framework for measuring change in DD is suggested to maximize detection of meaningful change over time.
Acknowledgments
Appreciation is expressed to the following for their assistance with patient recruitment: Anna Chang, M.D., Paul FitzGerald, M.D., Martha Nolte Kennedy, M.D., Grace S. Kim, M.D., Sara Kim, M.D., Pooja Sherchan, M.D., Peter M Sklarin, M.D., Amber Wheeler, M.D., Douglas W. Zlock, M.D.
Funding source: DK094863 from the National Institute of Diabetes, Digestive and Kidney Disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
No author reported a conflict of interest. Lawrence Fisher: consultant to or advisory board member for Roche Diagnostics, Elli Lilly, Novo Nordisk, Sanofi, Abbott Diabetes Care; William Polonsky: consultant or advisory board with Sanofi, Novo Nordisk, Elli Lilly, Dexcom, Abbott, J&J, Boehringer Ingelheim, Takeda, Roche; Anne Peters: consultant or advisory board with Amgen, Abbott Diabetes Care, Becton Dickinson, Biodel, Bristol Myers Squibb/Astra-Zeneca, Janssen, Lexicon, Lilly, Medtronic Minimed, Novo Nordisk, OptumRx, sanofi, Takeda, Thermalin. Speakers bureau - Bristol Myers Squibb/Astra-Zeneca, Novo Nordisk, Janssen.
References
- 1.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes a call for greater precision and clarity. Diabetic Med. 2014;31:764–772. doi: 10.1111/dme.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher L, Hessler DH, Glasgow RE, Arean PA, Masharani U, Naranjo D, et al. REDEEM: a practical trial to reduce diabetes distress. Diabetes Care. 2013;36:2551–2558. doi: 10.2337/dc12-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hessler DH, Fisher L, Glasgow RE, Stryker LA, Dickinson LM, Arean PA, et al. Reductions in diabetes distress are associated with improved management and glycemic control over time. Diabetes Care. 2014;37:617–624. doi: 10.2337/dc13-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd CE, Smith JKW. Stress and diabetes: a review of the literature. Diabetes Spectrum. 2005;18:121–127. [Google Scholar]
- 5.Delahanty LM, Grant RW, Wittenberg E, Bosch JL, Wexler DJ, Cagliero E, et al. Association of diabetes-related emotional distress with diabetes treatment in primary care patients with type 2 diabetes. Diabetic Med. 2007;24:48–54. doi: 10.1111/j.1464-5491.2007.02028.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogbera A, Adeyemi-Doro A. Emotional distress is associated with poor self-care in type 2 diabetes mellitus. J Diabetes. 2011;3:348–352. doi: 10.1111/j.1753-0407.2011.00156.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress among adults with type 2 diabetes. Diabetic Med. 2008;25:1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilliard ME, Lawrence JM, Anderson A, Crume T, Dolan LM, Mechant AT, et al. Identification of minimal clinically important difference scores of the PedsQL in children, adolescents, and young adults with type 1 and type 2 diabetes. Diabetes Care. 2013;36:1891–1897. doi: 10.2337/dc12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher L, Polonsky WH, Hessler DH, Masharani U, Blumer I, Peters AL, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Compl. 2015;29:572–577. doi: 10.1016/j.jdiacomp.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ADA. Clinical practice recommendations: glycemic targets. Diabetes Care. 2015;38S:S33–S40. [Google Scholar]
- 11.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: The validity of a brief depression severity measure. J Gen Int Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai SK, Yazdany J, Fortin PR, Avina-Zubieta JA. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res Therapy. 2015;17:143–155. doi: 10.1186/s13075-015-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum cliically important difference: a review of concepts and methods. Spine. 2007;7:541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 14.IBM Corp. SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.; 2010. [Google Scholar]
- 15.Marsh LC. Spline Regression Models. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 16.Motulsky HJ, Ransnas LA. Fitting curved to data using nonlinear regression: a practical and nonmathamatical review. The Fed Amer Soc Exp Biol J. 1987;1:365–374. [PubMed] [Google Scholar]
- 17.Fisher L, Hessler DM, Polonsky WH, Mullan JT. When is diabetes distress clinically meaningful? Estabishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259–264. doi: 10.2337/dc11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trief PM, Xing D, Foster NC, Maahs DM, Kittelsrud JM, Olson BA, et al. Depression in adults in the T1D Exchange Clinic Registry. Diabetes Care. 2014;37:1563–1572. doi: 10.2337/dc13-1867. [DOI] [PubMed] [Google Scholar]
- 19.Fisher L, Hessler DH, Polonsky WH, Masharani U, Peters AL, Blumer I, et al. The prevalence of depression in type 1 diabetes and the problem of over-diagnosis. Diabetic Med. 2015 Oct 3; doi: 10.1111/dme.12973. [DOI] [PubMed] [Google Scholar]