Abstract
Prenatal alcohol exposure (PAE) results in fetal alcohol spectrum disorder (FASD), which is characterized by a wide range of cognitive and behavioral deficits that may be linked to impaired hippocampal function and adult neurogenesis. Preclinical studies in mouse models of FASD indicate that PAE markedly attenuates enrichment-mediated increases in the number of adult-generated hippocampal dentate granule cells (aDGCs), but whether synaptic activity is also affected has not been studied. Here, we utilized retroviral birth-dating coupled with whole cell patch electrophysiological recordings to assess the effects of PAE on enrichment-mediated changes in excitatory and inhibitory synaptic activity as a function of DGC age. We found that exposure to an enriched environment (EE) had no effect on baseline synaptic activity of 4 week or 8 week old aDGCs from control mice, but significantly enhanced the excitatory/inhibitory ratio of synaptic activity in 8 week old aDGCs from PAE mice. In contrast, exposure to EE significantly enhanced the excitatory/inhibitory ratio of synaptic activity in older pre-existing DGCs situated in the outer dentate granule cell layer (i.e., those generated during embryonic development; dDGCs) in control mice, an effect that was blunted in PAE mice. These findings indicate distinct electrophysiological responses of hippocampal DGCs to behavioral challenge based on cellular ontogenetic age, and suggest that PAE disrupts EE-mediated changes in overall hippocampal network activity. These findings may have implications for future therapeutic targeting of hippocampal dentate circuitry in clinical FASD.
Keywords: Neurogenesis, Electrophysiology, Fetal alcohol spectrum disorder, Environmental Enrichment
Introduction
Fetal alcohol spectrum disorder (FASD) is a prevalent condition (2–5% in U.S.) that encompasses a range of clinical outcomes, from severe mental retardation as a consequence of high dose alcohol exposure (fetal alcohol syndrome), to more subtle behavioral abnormalities as a consequence of moderate gestational alcohol exposure (Riley et al., 2011) (May et al., 2009). Few empirically tested therapeutic interventions exist for mitigating the effects of FASD (Kodituwakku and Kodituwakku, 2011). Common behavioral problems in clinical FASD include those associated with hippocampal dysfunction that impact cognition and mood (Mattson et al., 2011). These behavioral disorders are thought to be due, in part, to long-lasting neurochemical and electrophysiological alterations in hippocampal circuitry (Berman and Hannigan, 2000).
The hippocampal dentate is a unique brain structure comprised of a heterogeneous population of DGCs of various cellular ages which exhibit ontogenetic age-dependent differences in network function (Kim et al., 2012). The adult hippocampal subgranular zone continuously generates cohorts of immature DGCs, which display unique connectivity, hyper-excitability and plasticity during a critical period between 4–8 weeks of cellular age. As their maturation progresses, aDGCs eventually resemble older, less active granule cells that are generated during embryonic and early postnatal development (dDGCs), in terms of both connectivity and electrophysiological properties (Laplagne et al., 2006). Although immature aDGCs only constitute an estimated 5–6% of the total DGC population, they exert unique and profound effects on hippocampal network activity, and are critical for the encoding of certain forms of episodic memory (Gu et al., 2012; Nakashiba et al., 2012; Piatti et al., 2013). Optogenetic silencing of immature aDGCs impairs spatial memory (Gu et al., 2012), whereas silencing of older, more mature DGCs enhances spatial learning performance (Nakashiba et al., 2012), further underscoring cellular age-dependent differences in network function. Recent work has also elucidated unique presynaptic inputs onto immature aDGCs, which are temporally regulated during cellular maturation and dynamically regulated by experience (Bergami et al., 2015; Vivar et al., 2015).
A number of studies using preclinical rodent models of FASD have demonstrated that prenatal alcohol exposure (PAE) exerts long-lasting impairments in the production of newborn DGCs in adulthood (Boehme et al., 2011; Choi et al., 2005; Gil-Mohapel et al., 2010; Helfer et al., 2009; Ieraci and Herrera, 2007; Kajimoto et al., 2013; Klintsova et al., 2007; Redila et al., 2006). We have previously demonstrated that moderate PAE had no effect on the production of aDGCs in mice exposed to standard laboratory housing conditions, but severely impaired the neurogenic response to social and physical enrichment (Choi et al., 2005; Kajimoto et al., 2013). Although prior studies have demonstrated that PAE can exert long-lasting effects on DGC function (Berman and Hannigan, 2000; Valenzuela et al., 2012), those studies did not take into account functional differences based on ontogenetic cellular age. Here, we utilized retroviral birth-dating coupled with whole cell patch electrophysiological recordings to assess the effects of PAE on enrichment-mediated changes in excitatory and inhibitory synaptic activity of DGCs in adult hippocampus. Our findings demonstrate that PAE alters baseline synaptic activity under conditions of enrichment in a manner that is dependent upon DGC maturational age.
Materials and Methods
Animals and PAE
All animal experiments were performed in accordance with protocols approved by the University of New Mexico Animal Care and Use Committee. C57/BL6J male mice (Jackson Laboratory, Bar Habor, ME) housed under reverse 12-hour dark / 12-hour light cycle (lights off at 08:00 hours) were used for all experiments. PAE was performed using a previously described limited access paradigm of maternal drinking (Brady et al., 2012; Kajimoto et al., 2013). Briefly, 60 day old female mice were subjected to a ramp-up period with 0.066% saccharin containing 0% ethanol (2 days), 5% ethanol (2 days) and finally 10% ethanol for 4 hrs per day from 10:00–14:00 daily beginning 2 weeks prior to pregnancy, and continued throughout gestation. Average daily alcohol consumption was 6.98 ± 0.76 gm/kg/d (n=24 dams). This paradigm has previously been shown to result in an average daily blood ethanol concentration (BEC) of ~88 mg/dl after 4 hours of drinking, with ethanol consumption rate predictive of BEC (Brady et al., 2012; Kajimoto et al., 2013). Female mice offered 0.066% saccharin without ethanol during the same period of time throughout pregnancy served as controls. There was no effect of alcohol consumption on the number of pups per litter (Control litter size; 7.09 ± 1.59 pups and PAE litter size; 6.50 ± 1.55 pups) or the percentage of male vs. female offspring. On the day of birth, ethanol and saccharin concentrations were gradually decreased, with return to water only by postnatal day 5. All offspring were gender segregated at weaning and placed into standard housing or enriched environment, as described below. Given the technical challenges for patch clamp recordings across multiple treatment groups, only male pups were used for further study to avoid variability due to gender-specific effects of PAE.
Retrovirus injection
Between postnatal days 40–50, all PAE and control offspring received stereotaxic injection of replication-deficient murine retrovirus to confer expression of green fluorescent protein (GFP) to hippocampal DGC progenitor cells, targeting the dorsal and ventral hilar regions of the hippocampal dentate, as previously described (Ge et al., 2006). Briefly, 0.5 µl of retrovirus was stereotaxically delivered to 4 sites targeting the dorsal and ventral hippocampal subgranular zone bilaterally, using a 1 µl Hamilton syringe. Stereotaxic coordinates were measured from bregma as follows: −2 mm AP, ± 2.0 mm L, and −2 mm DV for dorsal hippocampus and −3 mm AP, ± 2.6 mm L and −2.5 mm DV for sites in the ventral hippocampus.
Enriched Environment (EE)
Standard housing included a standard mouse cage (28 cm × 18 cm × 13 cm) without running wheels or toys (3 mice/cage). EE included a larger cage (48 cm × 27 cm × 20 cm) with 2 running wheels and multiple objects (ladder, tunnel and hanging toys that were changed out daily), and 6 mice/cage (Supplementary Figure 1). Mice were housed under EE for 4 or 8 weeks until sacrifice. Mice were weighed weekly. There was a significant main effect of housing on weight (p<0.001), but no effects of alcohol treatment (Supplementary Figure 2). Previous studies using EthoVision™ Motion Tracking demonstrated no difference between PAE and control mice in the duration of time spent in the various EE cage zones (Kajimoto et al., 2013).
Electrophysiological recordings
Mice were anesthetized with ketamine (250 mg/kg, i.p.), transcardially perfused with pre-oxygenated (95% O2/5% CO2) cold cutting solution containing (in mM): 220 sucrose, 2 KCL, 1.25 NaH2PO4, 26 NaHCO3, 12 MgSO4, 10 glucose, 0.2 CaCl2. Brains were removed, incubated for 2 minutes in pre-oxygenated (95% O2/5% CO2) ice-cold cutting solution, and bisected in the midsagittal plane, with the right hemisphere used for electrophysiological recordings. Transverse hippocampal slices (250 µm thick) were prepared using a vibratome and allowed to recover in artificial cerebrospinal fluid (ACSF) as described previously (Diaz et al., 2014). GFP+ aDGCs were detected using an Olympus BX51WI microscope equipped with epifluorescence illumination, whereas GFP− dDGCs were identified based on their position in the outermost dentate granule cell layer (Mathews et al., 2010; Muramatsu et al., 2007). All recordings were performed with an Axopatch 200B amplifier and Clampex 9.2 software (Molecular Devices, Sunnyvale, CA,) as previously described (Diaz et al., 2014). Miniature spontaneous excitatory postsynaptic currents (mEPSCs) were detected using an internal pipet solution containing (in mM) 120 K-gluconate, 15 KCL, 4 MgCl2, 0.1 EGTA, 10 HEPES, 4 MgATP, 0.3 NaGTP, 7 phosphocreatine, 5 QX-314 Br (pH 7.4, 290–300 mOsm), with 100 µM of picrotoxin and 0.5 µM tetrodotoxin (TTX) added to the recording ACSF. Miniature spontaneous inhibitory postsynaptic currents (mIPSCs) were detected using the following internal pipet solution (in mM): 19 K-gluconate, 121 KCl, 5 NaCl, 4 MgCl2, 0.1 EGTA, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 phosphocreatine, 5 QX-314 Cl (pH 7.4, 300–310 mOsm), in the presence of 3 mM of kynurenic acid and 0.5 µM TTX added to the recording ACSF. Event data were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA). 3–5 total DGCs were recorded for each mouse (for both mEPSC and iPSC, which were performed in separate recording sessions), with at least one of these cells from the GFP+ aDGC population and one from GFP− dDGC population in the outermost region of the dentate granule cell layer. Recordings were averaged for each mouse, and n=number of mice (see statistics below).
Intracellular biocytin cell filling and staining
Where indicated, dDGCs recorded from the outermost region of the dentate granule cell layer were backfilled with biocytin for morphological analysis using 0.5% of biocytin (Sigma) added to the internal pipet solution. The slices were then incubated in the recording chamber for 15–20 minutes to allow biocytin to fill distal processes, followed by fixation in 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS) overnight. Slices were washed with PBS for 30 minutes, permeabilized with 0.4% Triton-X100 in PBS for 30 minutes and incubated with Cy-3-conjugated Streptavidin (0.125 µg/ml in PBS containing 1% BSA) for 90 minutes. Slices were mounted on glass slides and coverslipped with Fluoromount-G™ (Electron Microscopy Sciences, Hatfield, PA).
Morphological analysis
All images were taken using a Zeiss 510 META confocal microscope. Dendritic branching complexity was analyzed using Sholl analysis of biocytin-filled dDGCs. Briefly, an image stack was acquired using a 20× objective with 1 µm optical intervals, and compressed into a single image plane by applying the maximum intensity projection function. All dendrites from a single biocytin-filled neuron were traced using Neurolucida software (MBF Bioscience, Inc. Williston, VT). Dendritic intersections crossing each concentric ring, (beginning 10 um from the center of the cell soma), were counted manually, with concentric rings spaced at 10 µm intervals. For spine density measurements, the left hemisphere of each brain was immediately fixed with 4% PFA overnight, followed by overnight cryoprotection with 30% sucrose in PBS. Transverse hippocampal sections (60 µm thickness) were obtained using a freezing sliding knife microtome. Sections were mounted on glass slides and coverslipped with Fluoromount-GTM. Confocal image stacks were taken using a 100× oil objective with 0.2 µm optical section intervals. For each mouse, >50 µm of distal dendrite segments were sampled.
Statistics
Data were analyzed by two-way ANOVA with post-hoc multiple comparison (Sidak’s or Tukey tests) using Prism (Graph Pad, San Diego, CA). N's represent the number of mice (n=6 mice per group; with whole cell patch electrophysiological recordings from 3–4 neurons per mouse, (which included at least 1 GFP− and 1 GFP+ cell in each recording session). To avoid litter effects, each mouse within any given treatment/housing group was taken from a different litter. Data are expressed as means ± S.E.M., with p<0.05 considered statistically significant.
RESULTS
PAE facilitates excitatory synaptic activity in aDGCs following EE
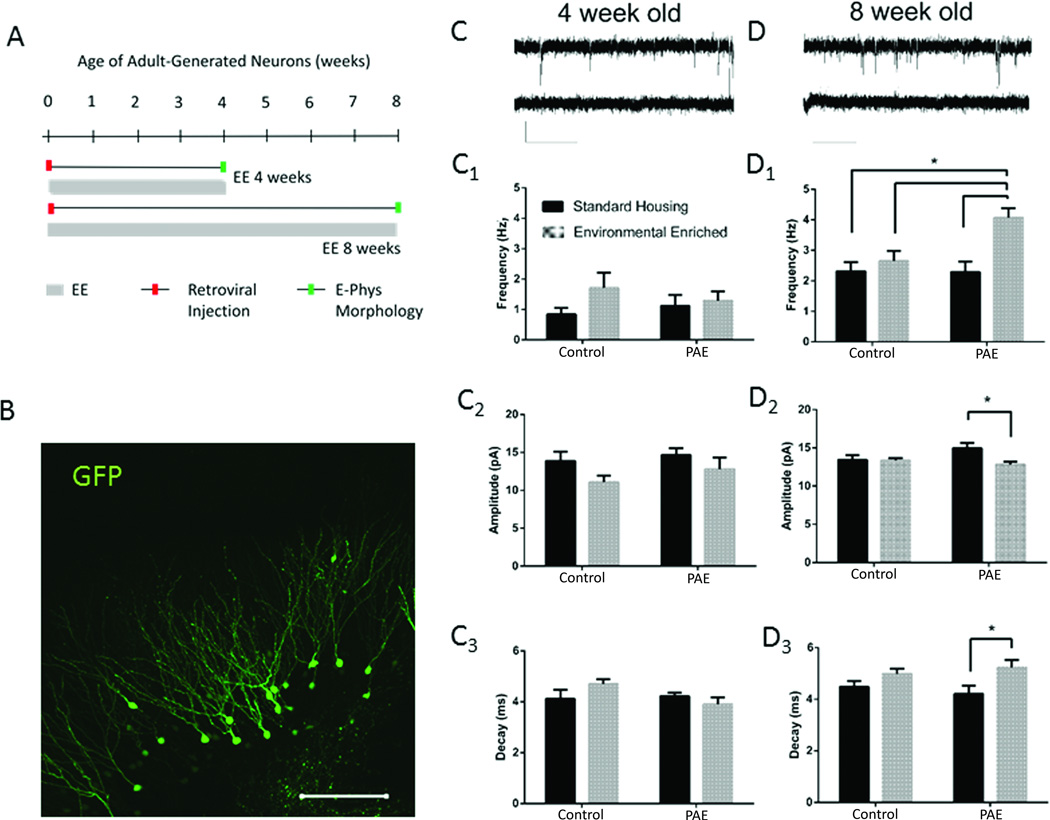
Utilizing the limited access PAE model, we previously demonstrated that exposure to moderate levels of alcohol throughout gestation significantly impaired the numerical neurogenic response to enriched environment (EE) (Choi et al., 2005; Kajimoto et al., 2013). To evaluate the impact of prenatal alcohol on functional synaptic activity in newly generated aDGCs in this PAE/EE paradigm, we applied a retroviral labeling and birth-dating approach coupled with whole cell patch clamp recordings. Following stereotaxic delivery of GFP-retrovirus, which targets dividing hippocampal progenitors, PAE and control mice were exposed to standard housing (SH) or EE conditions. Whole cell patch electrophysiological recordings of GFP+ aDGCs were performed at 4 and 8 weeks post retroviral injection, a period that spans a critical window of cellular maturation in which aDGCs are thought to uniquely influence hippocampal network activity (Piatti et al., 2013). Experimental design and GFP+ aDGC labeling is shown in Figure 1A and 1B, respectively.
Figure 1. mEPSC Recordings from GFP+ Adult-Generated DGCs.
(A) Experimental design for retroviral labeling of newborn DGCs and EE. Hippocampal progenitors of control and PAE mice were labeled with GFP retrovirus by stereotaxic delivery and placed into EE or standard cages for 4 or 8 weeks prior to electrophysiological and morphological analysis. (B) GFP immunofluorescence of 4 week old aDGCs. Scale bar=100 um. (C and D) Whole cell patch trace recordings from individual GFP+ aDGCs at 4 wpi (C) or 8 wpi (D) in the absence (top trace) or presence (bottom trace) of 3 mM kynurenate. Scale bar = 20 pA and 1 s. Frequency, amplitude and decay values for mEPSCs recorded from GFP+ aDGCs at 4 (C1–C3) and 8 (D1–D3) wpi. Graphed data represent means ± SEM, *p<0.05 using Tukey’s (D1) or Sidak’s post-hoc multiple comparisons, n=6 mice/group.
Glutamatergic excitatory synaptic transmission
As shown in Figure 1C and 1D, spontaneous miniature excitatory postsynaptic currents (mEPSCs) were readily detectable in both 4 and 8 week-old GFP+ aDGCs from all mice, as indicated by whole cell patch electrophysiological recordings in ACSF containing picrotoxin (100 µM; representative traces are depicted in Figs. 1C,1D). The mEPSCs were abolished by 3 mM kynurenate (representative lower trace in Figs. 1C, 1D), indicating that these events were mediated by ionotropic glutamate receptors.
As anticipated, the frequency of spontaneous mEPSCs increased ~2-fold between 4 and 8 weeks in GFP+ aDGCs from both control-SH and PAE-SH groups (p<0.05; Figs. C1,D1), reflecting the maturation and synaptic integration of aDGCs that occurs during this period (Ge et al., 2006). At 4 weeks, there were no significant effects of EE on excitatory synaptic transmission in any group (Fig. C1–C3). However, at 8 weeks, aDGCs from PAE-EE mice displayed an approximate 2-fold increase in mEPSC frequency that was not observed in aDGCs from control-EE mice (Fig. 1D1; treatment × housing interaction (F (1, 20) = 5.056, p= 0.04). PAE-EE aDGCs also displayed a small but significant decrease in mEPSC amplitude (Fig. 1D2) and an increase in mEPSC decay time (Fig. 1D3) at 8 weeks, neither of which were observed in control-EE mice (post hoc analysis, p<0.03 for both amplitude and decay, PAE-EE vs. PAE-SH). These observations indicate that PAE significantly enhances action potential-independent glutamatergic synaptic transmission in aDGCs under conditions of EE in an ontogenetic age-dependent manner. Although the net effect of increased frequency and decay time of mEPSCs may be partially offset by reduced amplitude, all changes in glutamatergic neurotransmission in response to EE were only observed in PAE mice, indicating a potential impact of PAE on network activity.
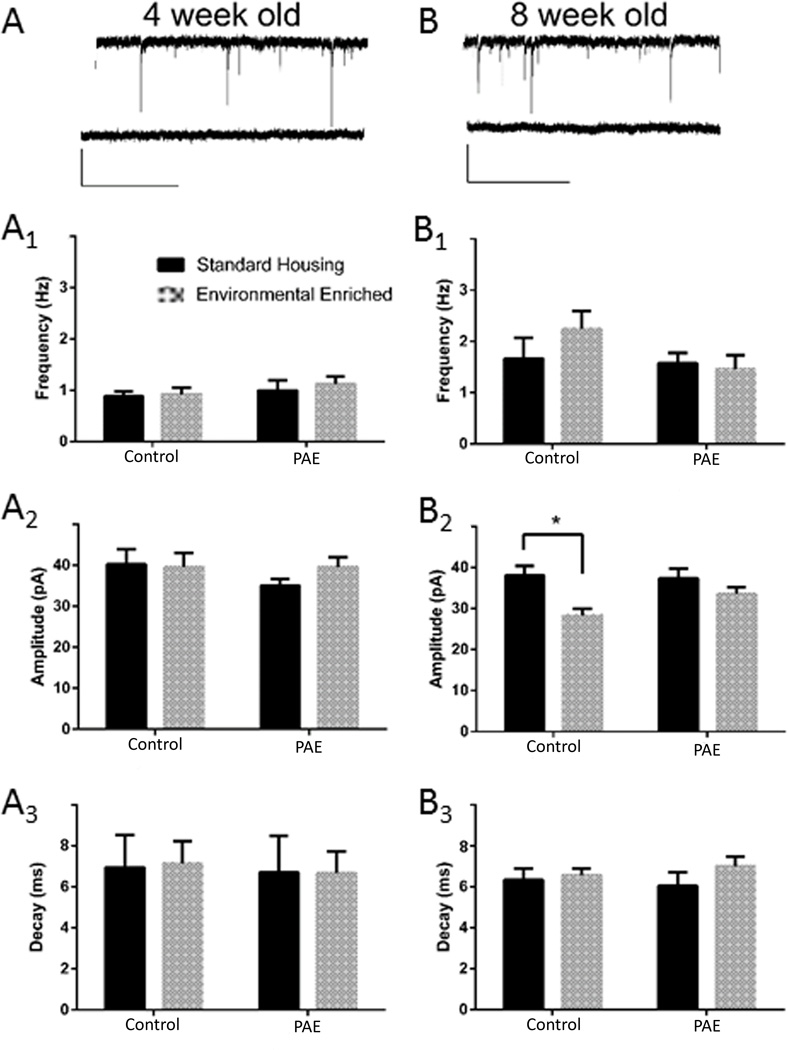
GABAergic inhibitory synaptic transmission
As shown in Figure 2, spontaneous miniature inhibitory postsynaptic currents (mIPSCs) were also readily detectable in GFP+ aDGCs in all mice, with a slight increase in frequency between 4 and 8 weeks as assessed by whole cell patch electrophysiological recordings in ACSF containing 3 mM kynurenate (Fig 2). Miniature IPSCs were blocked in the presence of 100 µM bicuculline (Figs. 2A,B, bottom traces), indicating that these events were mediated by GABAA receptors. In contrast to glutamatergic excitatory transmission described above, EE had no significant influence on mIPSC frequency (Figs. 2A1,B1) or decay (Figs. 2A3,B3) in 4 or 8 week old GFP+ aDGCs in any group. However, EE induced a small but significant decrease in the amplitude of mIPSCs in 8 week-old aDGCs recorded from control mice (F (1, 20) = 11.39; p= 0.003), which was not observed in PAE mice (p= 0.005).
Figure 2. mIPSC Recordings from GFP+ Adult-Generated DGCs.
(A and B) Whole cell patch traces recordings from individual GFP+ aDGCs at 4 wpi (A) or 8 wpi (B) in the absence (top trace) or presence (bottom trace) of 100 µM bicuculline. Scale bar = 40 pA and 2 s. Frequency, amplitude and decay values for mIPSCs recorded from GFP+ aDGCs at 4 (A1–A3) and 8 (B1–B3) wpi. Graphed data represent means ± SEM, *p<0.05 using Sidak’s post-hoc multiple comparison, n=6 mice/group.
Dendritic spine density in adult-generated DGCs
Environmental enrichment had no significant impact on distal spine density of GFP+ aDGCs in PAE mice (PAE-SH, 1.72 ± 0.055 vs. PAE-EE, 1.69 ± 0.22 spines/µm) or control mice (control-SH, 1.68 ± 0.15 vs. control-EE, 1.87 ± 0.21 spines/µm; means ± SEMs, n=6 mice/group; 8 weeks).
PAE attenuates excitatory/inhibitory ratio of synaptic currents in dDGCs following EE
To compare synaptic activity of newly generated DGCs (aDGCs) with that of older, pre-existing DGCs generated during embryonic and early postnatal development (dDGCs), we recorded from GFP− neurons located in the outermost region of the dentate granule cell layer during each electrophysiological recording session. Previous studies have demonstrated that a cell's birthdate correlates with its subsequent location within the granule cell layer; DGCs born during embryonic and early postnatal development comprise the outermost dentate granule layer compared to adult-generated cells that layer within the inner and middle regions (Mathews et al., 2010; Muramatsu et al., 2007).
Glutamatergic and GABAergic synaptic transmission
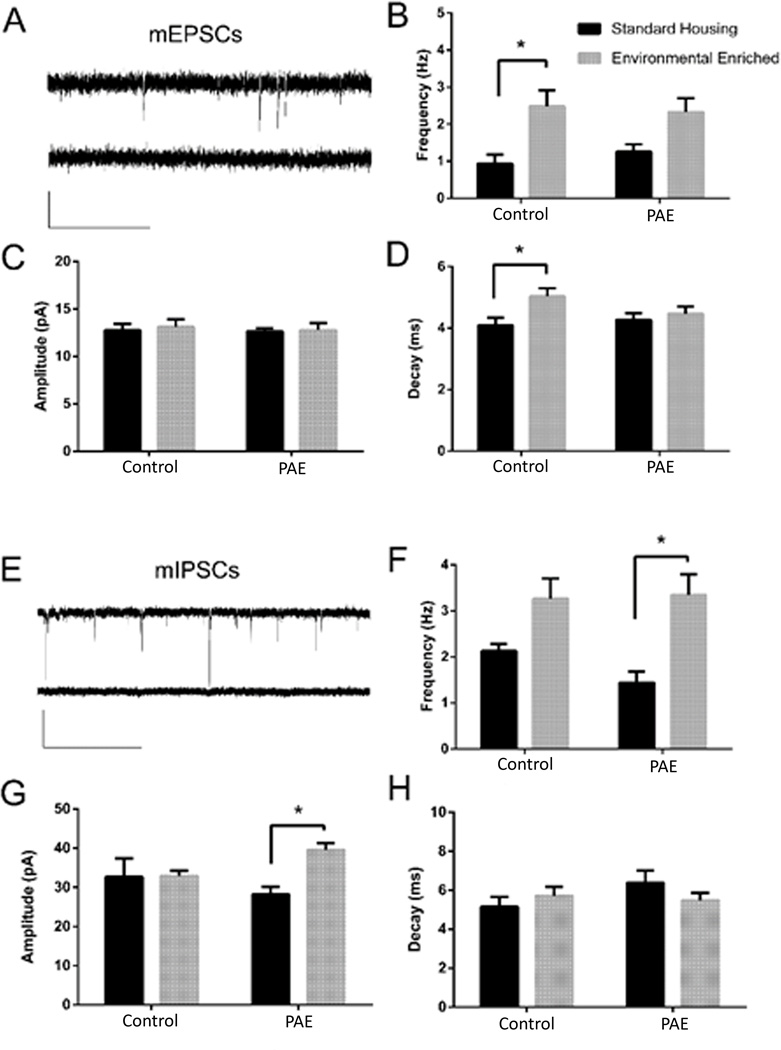
Spontaneous mEPSCs were readily detectable in GFP− dDGCs recorded in the outermost dentate granule cell layer from all mice, and these were blocked by 3 mM kynurenate (Fig. 3A). As shown in Figure 3B, dDGCs displayed an approximate 2-fold increase in glutamatergic synaptic current frequency (mEPSC) after EE in control mice. Although there was a trend toward increased frequency of mEPSCs in PAE-EE mice compared to PAE-SH mice, this did not reach statistical significance (p=0.062). Two-way ANOVA revealed that both the frequency (F (1, 20) = 16.07; p<0.001) and decay of mEPSCs (F (1, 20) = 5.39; p=0.031) were significantly affected by the housing conditions in control mice, with no significant effect on amplitude (Figure 3B–D). Thus, EE stimulated enhanced action potential-independent glutamatergic neurotransmission in dDGCs from control mice, which was attenuated in PAE mice.
Figure 3. mEPSC and mIPSC Recordings from dDGCs.
(A) mEPSC trace recordings from an individual DGC in the absence (top) or presence (bottom) of 3 mM kynurenate. Scale bar = 20 pA and 1 s. (B–D) Frequency, amplitude and decay values for mEPSCs (E) sample traces of mIPSC recordings from an individual DGC at in the absence (top) or presence (bottom) of 100 µM bicuculline. Scale bar = 40 pA and 2 s. (F–H) Frequency, amplitude and decay values for mIPSCs. *p<0.05, Sidak’s multiple comparison, n=6 mice/group.
Bicuculline-sensitive mIPSCs were readily detectable in dDGCs from all mice (Fig. 3E). As shown in Figure 3F, EE stimulated a significant (~2-fold) increase in mIPSC frequency in dDGCs recorded from PAE-EE mice (p=0.002). Although there was a trend toward increased frequency of mIPSC in control-EE mice, this did not reach statistical significance (p=0.058). Two-way ANOVA revealed that both the frequency (F (1, 20) = 19.55, p<0.001) and amplitude (F (1, 20) = 4.367, p=0.049), but not the decay, of mIPSCs were significantly influenced by housing in PAE mice (Fig. 3 F–H). The amplitude of mIPSC was significantly increased by EE only in PAE mice (p= 0.018). These data suggest that dDGCs display a trend toward increased GABAA receptor-mediated synaptic input in both control and PAE mice following EE, which only reached statistical significance in the PAE group.
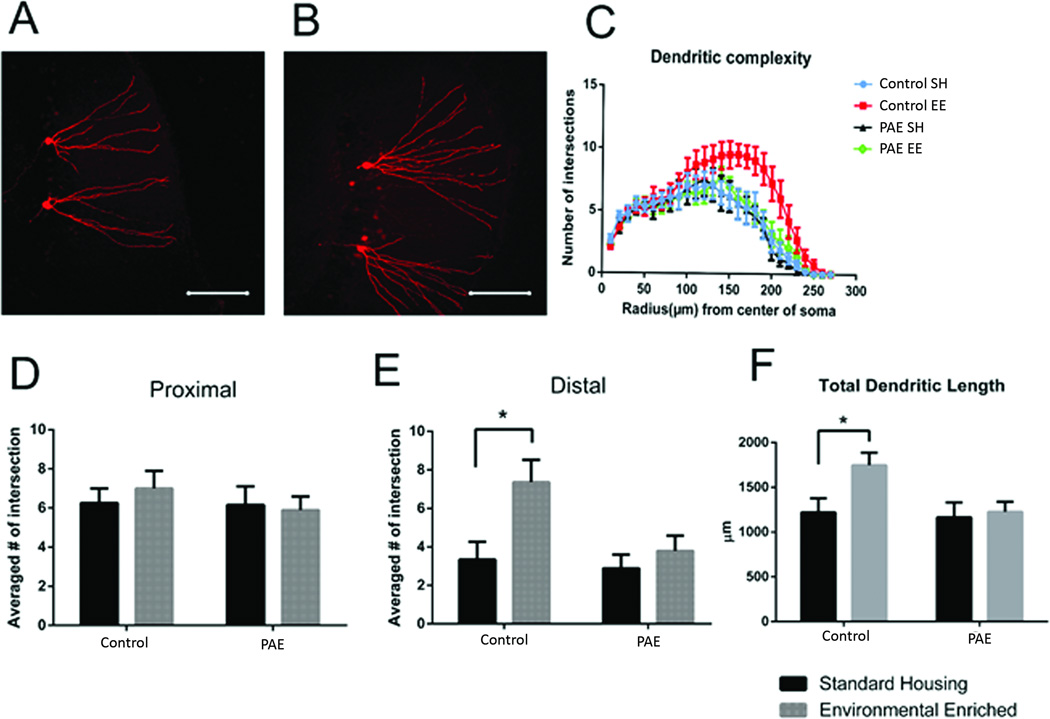
Dendritic branching
EE increased branching complexity in distal dendrites of dDGCs in control mice (control-EE), and this effect was abolished by PAE (PAE-EE) (Figure 4). As shown in Figure 4D–E, two-way ANOVA revealed significant housing (F (1, 20) = 7.3; p= 0.014) and treatment (F (1, 20) = 4.86; p= 0.039) effects on distal dendritic segments >170 µm from soma (Fig 4D–E). Post-hoc analysis confirmed that the effect of EE on dendritic branching was only significant in control mice (Fig. 4E, p<0.01). In addition, total dendritic length was increased by EE treatment only in control mice (Fig. 4F, p<0.05). Therefore, distal dendritic complexity is enhanced by environmental stimulation in control mice, an effect that is significantly attenuated by PAE.
Figure 4. Dendritic Complexity in dDGCs.
(A, B) Representative images of dendritic morphology from control mice housed under standard (A) vs. EE (B) conditions. (C) Sholl anlaysis of dendritic branching (D) Branching complexity of proximal dendrites (60–110 µm). (E) Branching complexity of distal dendrites (170–220 µm). (F) Total dendritic length. *p<0.05, Sidak’s multiple comparison, n=6 mice/group.
Discussion
In this study, we asked whether PAE results in long-lasting changes in synaptic activity in adult hippocampal dentate granule cells following exposure to enriched environment. We used a GFP-retroviral labeling/birth-dating approach coupled with whole cell patch electrophysiological recordings to assess synaptic activity in adult-generated DGCs (aDGCs) at 4 and 8 weeks of age following exposure to standard or enriched housing conditions (SH vs. EE). To assess synaptic activity in older, pre-existing DGCs generated during embryonic and early postnatal development (dDGCs), we performed whole cell patch electrophysiological recordings from GFP− cells in the outermost dentate granule cell layer where these cells make up the vast majority of DGCs (Mathews et al., 2010). Our results demonstrate distinct electrophysiological responses to EE based on cellular ontogenetic age, and provide evidence that PAE disrupts EE-mediated changes in hippocampal network activity.
Adult-Generated DGCs
We previously demonstrated in the limited access PAE paradigm that exposure to moderate levels of alcohol throughout gestation has no effect on adult neurogenesis under SH conditions, but abolishes the numerical neurogenic response to EE (Kajimoto et al., 2013). Here, we extend those findings to demonstrate altered synaptic activity in aDGCs from PAE-EE mice that is characterized, in part, by heightened excitatory synaptic transmission. Importantly, this effect was dependent upon aDGC maturational state, observed in 8 week but not 4 week old aDGCs from PAE-EE mice. Using rabies virus-based retrograde transynaptic tracing methods, Bergami et al., recently demonstrated that EE exposure between 2–6 weeks following the birth of aDGCs profoundly affects the pattern of monosynaptic input by both local interneurons and long-distance cortical neurons (Bergami et al., 2015). These changes were not observed in 13 week old aDGCs, indicating important changes in network connectivity that occur with a specific time window of aDGC maturation, corresponding to the well-known critical period of enhanced synaptic plasticity of aDGCs previously described (Ge et al., 2007; Kheirbek et al., 2012). Our observation of heightened excitatory neurotransmission in response to EE in aDGCs from PAE, but not from control mice, may represent a compensatory hyper-responsiveness to EE either due to a lower number of aDGCs in PAE-EE compared to their control-EE counterparts, or a disruption in afferent connectivity during a critical period of cellular maturation.
Interestingly, the heightened excitatory response in PAE-EE mice was not associated with increased spine density. Previous studies have shown that neither EE, nor exercise, has a significant impact on total spine density in aDGCs, although both promote increased density of mushroom spines, which are thought to represent sites of enhanced excitatory synaptic transmission (Piatti et al., 2011; Zhao et al., 2015; Zhao et al., 2006). On the other hand, training on discrete spatial tasks stimulates enhanced dendritic complexity and spine density in aDGCs in proportion to the intensity of cognitive demand (Lemaire et al., 2012; Tronel et al., 2010). Although few studies have assessed the electrophysiological correlates of these behavioral paradigms and anatomical changes, a recent study demonstrated a profound effect of EE on the pattern of monosynaptic input to aDGCs that was paradoxically associated with a reduction in frequency of spontaneous excitatory currents, suggesting a dissociation of spine density and excitatory input (Bergami et al., 2015). It is currently unknown whether the increased mEPSC frequency in aDGCs from PAE-EE mice reflects a persistent increase of presynaptic glutamate release, activation of existing silent synapses, and/or increased number of synapses. Further investigation will be required to parse out these mechanisms.
In addition to increased frequency of mEPSCs in aDGCs from PAE-EE mice, we also observed small but significant changes in the glutamatergic and GABAergic amplitude and decay, indicating qualitative alterations in synaptic transmission. Several mechanisms could underlie alteration of current amplitude and decay, including changes in receptor expression or subunit composition, resulting in altered gating kinetics (Koike-Tani et al., 2005; Rouach et al., 2005; Sommer et al., 1990). Indeed, PAE is known to influence the expression and function of NMDA and GABA receptors within the adult hippocampus (Allan et al., 1998; Samudio-Ruiz et al., 2010; Savage et al., 1992), but these have not been studied in aDGCs or within the context of combined PAE and EE.
Regardless of the underlying mechanisms, the enhancement of basal excitatory synaptic transmission following EE in aDGCs from PAE mice raises the question of whether this response is physiologically relevant for hippocampal function. In previous studies, we demonstrated that the impaired neurogenic response to EE in PAE mice is associated with delayed learning in an A-B contextual discrimination learning task, a behavior that is thought to be reliant on neurogenic function (Kajimoto et al., 2013). However, in those studies, PAE-EE mice eventually learned the task to perform at the same level as control-EE mice. This suggests that the surviving subpopulation of aDGCs in PAE-EE mice are functionally competent to mediate this behavioral improvement, albeit over a slower time course compared to control-EE mice.
Mature DGCs in outer region of dentate granule cell layer (dDGCs)
Previous extracellular slice electrophysiological studies have yielded conflicting results regarding the effect of EE on synaptic transmission in dDGCs (Eckert and Abraham, 2013). We are aware of only a few studies that have used patch-clamp techniques to study EE effects on spontaneous excitatory or inhibitory currents in hippocampal DGCs generated during embryonic or early postnatal development, and these focused on the CA1 region. Parsley et al (2007) reported that EE had no significant effect on either the amplitude or the frequency of mEPSCs in CA1 pyramidal neurons (Parsley et al., 2007). In contrast, Malik and Chattarji (2012) found that EE increases the frequency but not the amplitude of mEPSCs in CA1 pyramidal neurons (Malik and Chattarji, 2012). Here, we demonstrate that EE produces a similar effect on mEPSC frequency in dDGCs and also increases the decay time of these events. However, in contrast to our findings of enhanced excitatory synaptic transmission in aDGCs from PAE-EE mice, we found electrophysiological evidence for the suppression of this EE-mediated effect on mEPSCs in dDGCs from PAE mice. PAE not only attenuated the EE-mediated increase in basal excitatory synaptic transmission in dDGCs, but also exacerbated inhibitory neurotransmission as indicated by an increase in mIPSC frequency that reached significance only in PAE-EE mice. Several studies have demonstrated impaired LTP within DGCs from rodent models of PAE, as measured by field excitatory postsynaptic potential potentiation (Brady et al., 2013; Sutherland et al., 1997; Varaschin et al., 2010). In addition, PAE has been shown to attenuate EE-mediated enhancement of dendritic spine density in CA1 pyramidal neurons (Berman and Hannigan, 2000). Our studies add to these findings, to demonstrate an imbalance between excitatory and inhibitory synaptic transmission in favor of the latter in PAE-EE mice, which is correlated with impaired morphological plasticity in dDGCs.
Conclusion
The overall findings from this study indicate that PAE impairs the responsiveness of the dentate gyrus to EE-stimulated inputs. In newly generated aDGCs, the increased excitatory transmission in 8 week old cells perhaps reflects increased response to EE in the context of significantly fewer numbers of aDGCs in the PAE group. By contrast, our data suggest a blunted responsiveness to EE in older DGCs that are generated earlier in development (dDGCs), with a concomitant increase in GABAergic transmission. Together, both of these effects suggests that PAE significantly impairs the dentate responsiveness to EE, which may have therapeutic implications in FASD.
Supplementary Material
Acknowledgments
Grant Information:
Sponsor: National Institutes of Health (NIH)
Grant numbers: 5R01-AA017449, P50-AA022534, R37-AA015614, P30-CA118100.
References
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. The Journal of pharmacology and experimental therapeutics. 1998;284(1):250–257. [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Gobel J, Yang SM, Conzelmann KK, Schinder AF, Gotz M, et al. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron. 2015;85(4):710–717. doi: 10.1016/j.neuron.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Boehme F, Gil-Mohapel J, Cox A, Patten A, Giles E, Brocardo PS, Christie BR. Voluntary exercise induces adult hippocampal neurogenesis and BDNF expression in a rodent model of fetal alcohol spectrum disorders. The European journal of neuroscience. 2011;33(10):1799–1811. doi: 10.1111/j.1460-9568.2011.07676.x. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcoholism, clinical and experimental research. 2012;36(3):457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(3):1062–1067. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29(11):2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Jotty K, Locke JL, Jones SR, Valenzuela CF. Moderate Alcohol Exposure during the Rat Equivalent to the Third Trimester of Human Pregnancy Alters Regulation of GABAA Receptor-Mediated Synaptic Transmission by Dopamine in the Basolateral Amygdala. Frontiers in pediatrics. 2014;2:46. doi: 10.3389/fped.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MJ, Abraham WC. Effects of environmental enrichment exposure on synaptic transmission and plasticity in the hippocampus. Current topics in behavioral neurosciences. 2013;15:165–187. doi: 10.1007/7854_2012_215. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain research reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nature neuroscience. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT, Klintsova AY. Binge-like postnatal alcohol exposure triggers cortical gliogenesis in adolescent rats. The Journal of comparative neurology. 2009;514(3):259–271. doi: 10.1002/cne.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiology of disease. 2007;26(3):597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Kajimoto K, Allan A, Cunningham LA. Fate analysis of adult hippocampal progenitors in a murine model of fetal alcohol spectrum disorder (FASD) PLoS ONE. 2013;8(9):e73788. doi: 10.1371/journal.pone.0073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(25):8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR, Christian K, Ming GL, Song H. Time-dependent involvement of adult-born dentate granule cells in behavior. Behavioural brain research. 2012;227(2):470–479. doi: 10.1016/j.bbr.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcoholism, clinical and experimental research. 2007;31(12):2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kodituwakku EL. From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychology review. 2011;21(2):204–223. doi: 10.1007/s11065-011-9170-1. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Saitoh N, Takahashi T. Mechanisms underlying developmental speeding in AMPA-EPSC decay time at the calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(1):199–207. doi: 10.1523/JNEUROSCI.3861-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Tronel S, Montaron MF, Fabre A, Dugast E, Abrous DN. Long-lasting plasticity of hippocampal adult-born neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(9):3101–3108. doi: 10.1523/JNEUROSCI.4731-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Chattarji S. Enhanced intrinsic excitability and EPSP-spike coupling accompany enriched environment-induced facilitation of LTP in hippocampal CA1 pyramidal neurons. Journal of neurophysiology. 2012;107(5):1366–1378. doi: 10.1152/jn.01009.2011. [DOI] [PubMed] [Google Scholar]
- Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. The Journal of comparative neurology. 2010;518(22):4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental disabilities research reviews. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Muramatsu R, Ikegaya Y, Matsuki N, Koyama R. Neonatally born granule cells numerically dominate adult mice dentate gyrus. Neuroscience. 2007;148(3):593–598. doi: 10.1016/j.neuroscience.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsley SL, Pilgram SM, Soto F, Giese KP, Edwards FA. Enriching the environment of alphaCaMKIIT286A mutant mice reveals that LTD occurs in memory processing but must be subsequently reversed by LTP. Learning & memory. 2007;14(1–2):75–83. doi: 10.1101/lm.356607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(21):7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Ewell LA, Leutgeb JK. Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Frontiers in neuroscience. 2013;7:50. doi: 10.3389/fnins.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychology review. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nature neuroscience. 2005;8(11):1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Sheema S, Caldwell KK. Hippocampal N-methyl-D-aspartate receptor subunit expression profiles in a mouse model of prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2010;34(2):342–353. doi: 10.1111/j.1530-0277.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Queen SA, Sanchez CF, Paxton LL, Mahoney JC, Goodlett CR, West JR. Prenatal ethanol exposure during the last third of gestation in rat reduces hippocampal NMDA agonist binding site density in 45-day-old offspring. Alcohol. 1992;9(1):37–41. doi: 10.1016/0741-8329(92)90007-w. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7(2):232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(17):7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in neurosciences. 2012;35(5):284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, Savage DD. Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. The Journal of pharmacology and experimental therapeutics. 2010;334(1):191–198. doi: 10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Jou J, Wolff LJ, Sun H, Gage FH. Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. The Journal of comparative neurology. 2015;523(10):1588. doi: 10.1002/cne.23800. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.