Abstract
Objective
The aim of this study is to identify the prognostic significance of X-ray cross-complementing gene 1 (XRCC1) in patients with gastric cancer undergoing surgery and platinum-based adjuvant chemotherapy.
Methods
Immunohistochemistry (IHC) was used to evaluate XRCC1 protein expression profiles on surgical specimens of 612 gastric cancer patients. The relationship between XRCC1 expression and existing prognostic factors, platinum-based adjuvant chemotherapy, disease-free survival (DFS) and overall survival (OS) were analyzed.
Results
Among 612 patients staged Ⅱ/Ⅲ in our study, 182 (29.74%) were evaluated as XRCC1 IHC positive. XRCC1 expression was not significantly related to OS (P = 0.347) or DFS (P = 0.297). Compared with surgery only, platinum-based adjuvant chemotherapy significantly improved the OS (P = 0.031). And the patients with negative XRCC1 expression benefited more from platinum-based adjuvant chemotherapy (P = 0.049). Multivariate analysis demonstrated that tumor size, T category, N category, vascular or nerve invasion and platinum-based chemotherapy were good prognostic factors for OS (P < 0.05). Though XRCC1 plays an important role in DNA repair pathways, no significant relationship is found in XRCC1 expression and OS among gastric cancer in our study.
Conclusions
XRCC1 might be an alternative prognostic marker for the patients of gastric cancer after radical resection. The patients with negative XRCC1 expression can benefit more from platinum-based adjuvant chemotherapy.
Keywords: Gastric cancer, X-ray cross-complementing gene 1 (XRCC1), platinum drugs prognosis
Introduction
Gastric cancer in spite of its decreasing incidence remains one of the most common causes of cancer-related deaths worldwide (1). Surgery is the preferred method for the treatment of gastric cancer. Meanwhile, platinum-based adjuvant chemotherapy after surgery has been widely accepted as a standard treatment for several decades. However, chemotherapy has limited efficacy in both resectable and unresectable gastric cancer cases (2, 3). New molecular markers pivotal to tumor biology, to prediction of the prognosis and adjuvant treatment regimens are urgently needed.
Since the antineoplastic mechanism of platinum agents is to cause DNA damage by forming intrastrand and interstrand platinum-DNA cross-links, DNA repair systems have been increasingly implicated in chemotherapyresistance (4). Thus, proteins involved in the DNA repair pathways, such as the excision repair cross-complementing 1 (ERCC1), the breast and ovarian cancer susceptibility gene 1 (BRCA1) and X-ray cross-complementing gene 1 (XRCC1) are probably related to platinum-based chemotherapy responsiveness and prognosis.
XRCC1 is located on chromosome 19q13.2-13.3 with a length of 33 kilobase. It plays an important role in DNA repair-pathways, acting as a scaffolding protein for the base excision repair (BER) and single-strand break repair (SSBR). XRCC1 is the first gene to be isolated which is sensitive to ionizing radiation. It is one of the most important DNA repair genes, and interacts with at least three other proteins (poly-ADP-ribose polymerase, DNA ligase 3, and DNA polymerase β) to repair single-strand breaks in DNA (5). The current studies of XRCC1 mainly focused on the relationship between gene polymorphism and cancer susceptibility. There are three polymorphisms in the XRCC1 have been extensively investigated: codon 194 (Arg194Trp), codon 280 (Arg280His) and codon 399 (Arg399Gln) (6). However, few studies have investigated the relationship between the expression of XRCC1 and the prognostic significance in tumors (7). Therefore, we evaluated the expression of XRCC1 in the surgically resected gastric cancer tissues and tried to determine if XRCC1 can provide any role in predicting the prognosis.
Materials and methods
Patients
A total of 612 patients with gastric cancer were included in our study, provided by the First Affiliated Hospital of Nanjing Medical University between January 2006 and December 2009. Eligibility criteria used for the patients selection were as follows: (Ⅰ) diagnosis of gastric cancer; (Ⅱ) staged Ⅱ or Ⅲ; (Ⅲ) D2 surgical resection; (Ⅳ) received adjuvant chemotherapy after surgery based on cisplatin or oxaliplatin combined with 5-fluorouracil (5-FU); and (Ⅴ) follow-up data was available, and the end of follow-up was May 2013. The study protocol was approved by The Ethical Committee of this hospital which is equivalent to IRB. Each subject had signed an informed consent before entry into the study. The details were shown in Table 1.
1.
Patient characteristics and relationship between XRCC1 expression and existing prognostic factors
| Patient characteristics | No. of patients (%) | XRCC1 expression (%) | P | |
| Negative | Positive | |||
| XRCC1, X-ray cross-complementing gene 1. | ||||
| Age | 0.328 | |||
| <60 | 268 (43.8) | 194 (31.7) | 74 (12.1) | |
| 60 | 344 (56.2) | 236 (38.6) | 108 (17.6) | |
| Gender | 0.488 | |||
| Male | 444 (72.5) | 308 (50.3) | 136 (22.2) | |
| Female | 168 (27.5) | 122 (20.0) | 46 (7.5) | |
| Tumor size (cm) | 0.426 | |||
| <5 | 316 (51.6) | 217 (35.5) | 99 (16.1) | |
| 5 | 296 (48.4) | 213 (34.8) | 83 (13.6) | |
| Differentiation | 0.269 | |||
| Well moderate | 122 (19.9) | 91 (14.9) | 31 (5.0) | |
| Poorly | 490 (80.1) | 339 (55.4) | 151 (24.7) | |
| T category | 0.886 | |||
| T1-T2 | 65 (10.6) | 45 (7.4) | 20 (3.2) | |
| T3-T4 | 547 (89.4) | 385 (62.9) | 162 (26.5) | |
| N category | 0.291 | |||
| N0 | 106 (17.3) | 68 (11.1) | 38 (6.2) | |
| N1 | 129 (21.1) | 87 (14.2) | 42 (6.9) | |
| N2 | 174 (28.4) | 128 (20.9) | 46 (7.5) | |
| N3 | 203 (33.2) | 147 (24.0) | 56 (9.2) | |
| Vascular or nerves invasion | 0.130 | |||
| Negative | 269 (44.0) | 198 (32.4) | 71 (11.6) | |
| Positive | 343 (56.0) | 232 (37.9) | 111 (18.1) | |
| Stage | 0.129 | |||
| Ⅱ | 158 (25.8) | 103 (16.8) | 55 (9.0) | |
| Ⅲ | 454 (74.2) | 327 (53.4) | 127 (20.8) | |
| Adjuvant chemotherapy | 0.922 | |||
| No | 176 (28.8) | 123 (20.1) | 53 (8.7) | |
| Yes | 436 (71.2) | 307 (50.1) | 129 (21.1) | |
Immunohistochemistry (IHC)
Tissue samples were formalin-fixed and paraffin-embedded; 4-μm thick sections were cut and stained by using the avidin-biotin complex method. After that, the slides were pretreated with microwaves for antigen retrieval in 10 mM citrate buffer (pH 6.0) and incubated in the primary antibody at 4 ℃ overnight. The antibody used for the detection of XRCC1 was monoclonal mouse anti-XRCC1 antibody (1:300 dilution; Abcam). In addition, each case included a negative and a positive control. If the staining was uncertain, it was repeated to confirm it.
Scoring of XRCC1
The staining intensity of XRCC1 expression was scored on a scale of 1-3 as follows: 0 score for no staining; 1 for weak staining; 2 for moderate staining; and 3 for strong staining. The percentage of positive cancer cells was scored as follows: 0 score for 0%; 0.1 for 1-9%; 0.5 for 10-49%; 1.0 for 50% or more. We multiplied the staining intensity by the proportion score of the percentage of positive cancer cells. Thus, we divided the patients into two groups: positive ones (the product >1) and negative ones (the product ≤1) (8).
Statistical analysis
χ2 test was performed to evaluate the associations between XRCC1 expression and the existing prognostic factors. Survival analysis was done by using the log-rank test and Kaplan-Meier curve. Cox proportional hazard model was applied to find predictors for the overall survivals (OS) and disease-free survivals (DFS). For these analyses, P < 0.05 was considered to be statistically significant. All statistical analyses were performed using the SPSS 17.0 software package.
Results
Study population
In total 612 gastric cancer patients were enrolled into our study with median age of 61 years old (61±11.1, range, 24-93 years old). There are more male patients than female (male: 72.5%, female: 27.5%). The mean tumor size was 4.954 cm. Patients with stage Ⅲ and poorly differentiated gastric cancer were more prevalent (put the 74.0% here); 436 (71.2%) patients received platinum-based adjuvant chemotherapy. The clinical characteristics of 612 patients were presented in Table 1.
XRCC1 expression
In gastric cancer, XRCC1 protein was mostly located in the cell nucleus (Figure 1A). The intensity of staining was varied from absent to strong (Figure 1B-E). Of 612 patients, 182 samples (29.7%) showed an IHC score of more than 1 point, and they were evaluated as XRCC1 IHC positive. The other 430 samples were evaluated as XRCC1 IHC negative.
1.
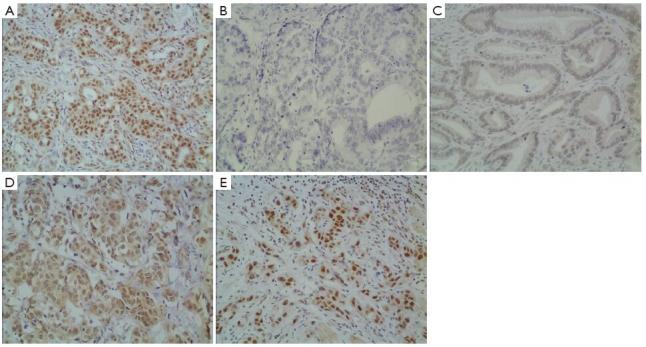
Immunohistochemical detection of XRCC1 protein in gastric cancer. (A) In gastric cancer, XRCC1 expression was located in the nucleus, XRCC1 expression located in the nucleus was varied; (B) no staining; (C) weak staining; (D) moderate staining; (E) strong staining (×200).
Survival and XRCC1 expression
The time-to-event was defined as the time from the surgery to death caused by gastric cancer (for event) and from surgery to last follow-up (for censoring). At the end of follow-up, in May 2013,250 (40.8%) patients were still alive. There is gastric cancer recurrence rate of 49.8% (305) from our study. The median OS and DFS were 37.357 months with 95% CI: 31.049-43.218 months and 18.072 months with 95% CI: 16.807-21.059 months, respectively.
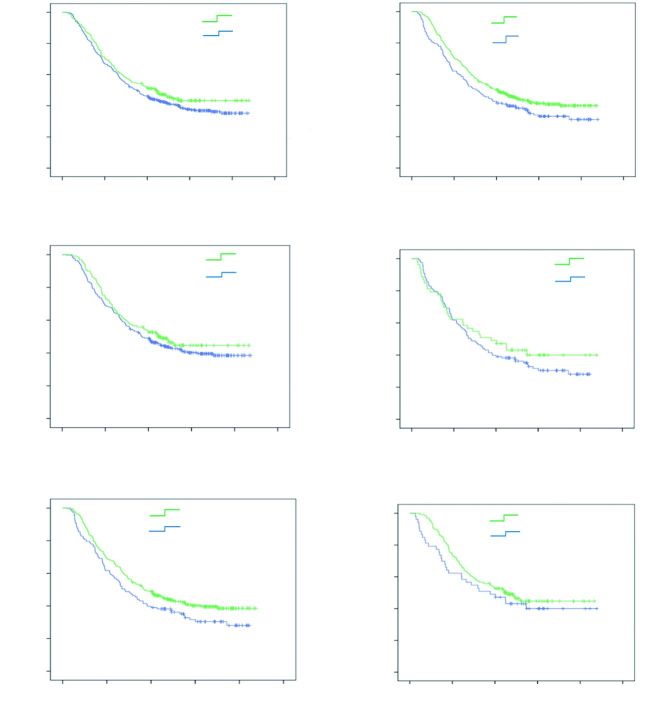
Neither OS (P = 0.206, Figure 2A) nor DFS (P = 0.973) was significantly prolonged among the patients with positive XRCC1 expression when compared with patients with negative XRCC1 expression. The patients received combination of surgery and platinum-based adjuvant chemotherapy were associated with better OS compared with patients received surgery alone (P = 0.031, Figure 2B). There was no statistically significant difference of OS between tumors with and without XRCC1 expression in the patients received platinum-based adjuvant chemotherapy (P = 0.326, Figure 2C), as well as the patients that only received surgery (P = 0.414, Figure 2D). However, after stratification by XRCC1 expression, this survival benefit was only found among the patients without XRCC1 expression (P = 0.049, Figure 2E), and it was not found among the patients with positive XRCC1 expression (P = 0.327, Figure 2F).
2.
Overall cumulative survival rates of patients according to XRCC1 expression. (A) OS between tumors with and without XRCC1 expression (P = 0.206); (B) OS between patients with and without platinum-based adjuvant chemotherapy (P = 0.031); (C) OS between tumors with and without XRCC1 expression in the patients received platinum-based adjuvant chemotherapy (P = 0.326); (D) OS between tumors with and without XRCC1 expression in the patients that did not received platinum-based adjuvant chemotherapy (P = 0.414); (E) OS of patients with negative XRCC1 expression received platinum-based adjuvant chemotherapy or not (P = 0.049); (F) OS of patients with positive XRCC1 expression received platinum-based adjuvant chemotherapy or not (P = 0.327).
There was no significant association between the expression of XRCC1 and age, gender, tumor size, differentiation, T category, N category, Vascular or nerves invasion, stage and adjuvant chemotherapy. Tumor size, T category, N category, vascular or nerves invasion, and adjuvant chemotherapy were significant predictors for OS according to multivariate analysis (Table 2). Age, gender, tumor differentiation and XRCC1 expression were not significant prognostic predictors in this study.
2.
Predictors from Cox proportional hazard model
| Factors | RR | 95% CI | P |
| XRCC1, X-ray cross-complementing gene 1. | |||
| Age | 1.059 | 0.859–1.307 | 0.590 |
| Gender | 0.946 | 0.748–1.196 | 0.641 |
| Tumor size | 1.381 | 1.116–1.709 | 0.003 |
| Differentiation | 0.909 | 0.679–1.218 | 0.523 |
| T category | 2.847 | 1.755–4.620 | 0.000 |
| N category | 1.602 | 1.447–1.773 | 0.000 |
| Vascular or nerve invasion | 1.366 | 1.087–1.718 | 0.008 |
| Adjuvant chemotherapy | 0.620 | 0.492–0.781 | 0.000 |
| XRCC1 | 0.862 | 0.683–1.088 | 0.347 |
Discussion
One of the most challenging problems in oncology is how to select the right candidates for treatment with good response. Even in patients with similar clinical or pathological features, their survival outcomes were quite different. Thus discovery of new biomarkers predicting better response and avoiding unnecessary toxicity in adjuvant chemotherapy is urgently needed. Two previous studies have suggested that gastric cancer patients with low expression of either BRCA1 (9) or ERCC1 (10) could benefit more from platinum-based chemotherapy, whereas the expression of ERCC1 or BRCA1 contributed to a significantly prolonged OS, respectively. Since XRCC1, BRCA1 and ERCC1 are all DNA repair genes, we considered that the expression of XRCC1 could reflect the cell’s internal ability to repair DNA damage to some extent and XRCC1 might have similar prognostic significance in gastric cancer (11). In a recent study, Wang et al. (12) showed that XRCC1 protein levels were significantly down-regulated in gastric cancer lesions compared with normal tissues, and low expression of XRCC1 was significantly associated with unfavorable clinical and pathological parameters and decreased OS. Similar phenomenon was also found in pancreatic cancer (13). However, in our study, there was no significant difference between patients with XRCC1-positive expression and patients with XRCC1-negative expression in OS (P = 0.206). And XRCC1 might not be a good prognostic predictor according to the Cox Proportional hazard model. In our view, this consequence may partly attribute to the polymorphism of XRCC1, since an altered DNA repair activity has been suggested to be associated with the XRCC1 polymorphism. On the other hand, human cells have evolved a set of complex DNA repair systems and the multiple effects may cause the function of XRCC1 to be less obvious. Besides, the prognostic power of XRCC1 might be hampered by the sample size and retrospective nature of this study.
Molecular epidemiologic studies indicate that single nucleotide polymorphisms (SNP) of XRCC1 were associated with the risk of various cancers including gastric cancer as well as being predictive for chemotherapy outcomes (14-16). The current studies of XRCC1 mainly focused on the relationship between gene polymorphisms and cancer susceptibility. Several studies have reported the association of XRCC1-399 with the risk in non-small-cell lung cancer (NSCLC) (17), colorectal cancer (18), gastric cancer (19) and prostate cancer (20). Some previous studies also showed that the polymorphism of XRCC1 could influence the effect of the platinum agents by altering the DNA repair capacity. However, the relationship between the expression of XRCC1 and the sensitivity to platinum-based chemotherapy in gastric cancer was rarely reported. In our analysis, patients in XRCC1 IHC-negative could benefit from platinum-based adjuvant chemotherapy in a certain degree compared with which in XRCC1 IHC-positive subgroup (P = 0.049). This outcome supports the notion that XRCC1 negative expression sensitized cancer to platinum-based adjuvant chemotherapy. Some recent reports also showed that XRCC1 plays an important part in repairing cisplatin adducts through DNA BER pathway (21), and XRCC1 negative expression would sensitize cancer to cisplatin or oxaliplatin as a result of the reduced BER capacity (22).
In conclusion, our study suggested that the patients with XRCC1-negative expression benefited more from platinumbased adjuvant chemotherapy. Detecting the expression of XRCC1 in gastric cancer tissues may provide clinical guidance in choosing the right candidate for adjuvant chemotherapy. However, further large-scale studies are called to find out the exact mechanisms.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81100274,81001428) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
The authors have no conflicts of interest to declare.
Contributor Information
Ping Liu, Email: liu-ping@csco.org.cn.
Xiqiao Zhou, Email: Zhouxiqiao@njmu.edu.cn.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin, 2011,61:69-90.] [DOI] [PubMed] [Google Scholar]
- 2.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010,303:1729-37.] [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase Ⅲ study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase Ⅲ study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol, 2006,24:4991-7.] [DOI] [PubMed] [Google Scholar]
- 4.Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle, 2008,7:1353-9.] [DOI] [PubMed] [Google Scholar]
- 5.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111:1843–50. doi: 10.1289/ehp.6632. [Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect, 2003,111:1843-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehouse CJ, Taylor RM, Thistlethwaite A, et al. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104:107–17. doi: 10.1016/s0092-8674(01)00195-7. [Whitehouse CJ, Taylor RM, Thistlethwaite A, et al. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell, 2001,104:107-17.] [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Ma J, Zong HT, et al. Pharmacogenetic role of XRCC1 polymorphisms on the clinical outcome of gastric cancer patients with platinum-based chemotherapy: a systematic review and meta-analysis. Genet Mol Res. 2014;13:1438–46. doi: 10.4238/2014.March.6.2. [Xu J, Ma J, Zong HT, et al. Pharmacogenetic role of XRCC1 polymorphisms on the clinical outcome of gastric cancer patients with platinum-based chemotherapy: a systematic review and meta-analysis. Genet Mol Res, 2014,13:1438-46.] [DOI] [PubMed] [Google Scholar]
- 8.Vilmar A, Garcia-Foncillas J, Huarriz M, et al. RTPCR versus immunohistochemistry for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1 in NSCLC. Lung Cancer. 2012;75:306–12. doi: 10.1016/j.lungcan.2011.08.016. [Vilmar A, Garcia-Foncillas J, Huarriz M, et al. RTPCR versus immunohistochemistry for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1 in NSCLC. Lung Cancer, 2012,75:306-12.] [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Wang J, Li X, et al. Prognostic significance of BRCA1 expression in gastric cancer. Med Oncol. 2013;30:423. doi: 10.1007/s12032-012-0423-5. [Chen W, Wang J, Li X, et al. Prognostic significance of BRCA1 expression in gastric cancer. Med Oncol, 2013,30:423.] [DOI] [PubMed] [Google Scholar]
- 10.Ozkan M, Akbudak IH, Deniz K, et al. Prognostic value of excision repair cross-complementing gene 1 expression for cisplatin-based chemotherapy in advanced gastric cancer. Asian Pac J Cancer Prev. 2010;11:181–5. [Ozkan M, Akbudak IH, Deniz K, et al. Prognostic value of excision repair cross-complementing gene 1 expression for cisplatin-based chemotherapy in advanced gastric cancer. Asian Pac J Cancer Prev, 2010,11:181-5.] [PubMed] [Google Scholar]
- 11.Lu Z, Luo T, Nie M, et al. Genetic polymorphisms of XRCC1 gene and susceptibility to gastric cancer in Chinese Han population. Biomarkers. 2013;18:542–6. doi: 10.3109/1354750X.2013.822562. [Lu Z, Luo T, Nie M, et al. Genetic polymorphisms of XRCC1 gene and susceptibility to gastric cancer in Chinese Han population. Biomarkers, 2013,18:542-6.] [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Wu X, Chen Y, et al. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res. 2012;18:2987–96. doi: 10.1158/1078-0432.CCR-11-2863. [Wang S, Wu X, Chen Y, et al. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res, 2012,18:2987-96.] [DOI] [PubMed] [Google Scholar]
- 13.Crnogorac-Jurcevic T, Efthimiou E, Nielsen T, et al. Expression profiling of microdissected pancreatic adenocarcinomas. Oncogene. 2002;21:4587–94. doi: 10.1038/sj.onc.1205570. [Crnogorac-Jurcevic T, Efthimiou E, Nielsen T, et al. Expression profiling of microdissected pancreatic adenocarcinomas. Oncogene, 2002,21:4587-94.] [DOI] [PubMed] [Google Scholar]
- 14.Ju H, Lim B, Kim M, et al. A regulatory polymorphism at position -309 in PTPRCAP is associated with susceptibility to diffuse-type gastric cancer and gene expression. Neoplasia. 2009;11:1340–7. doi: 10.1593/neo.91132. [Ju H, Lim B, Kim M, et al. A regulatory polymorphism at position -309 in PTPRCAP is associated with susceptibility to diffuse-type gastric cancer and gene expression. Neoplasia, 2009,11:1340-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding WJ, Fang JY, Chen XY, et al. The expression and clinical significance of DNA methyltransferase proteins in human gastric cancer. Dig Dis Sci. 2008;53:2083–9. doi: 10.1007/s10620-007-0145-2. [Ding WJ, Fang JY, Chen XY, et al. The expression and clinical significance of DNA methyltransferase proteins in human gastric cancer. Dig Dis Sci, 2008,53:2083-9.] [DOI] [PubMed] [Google Scholar]
- 16.Motoya S, Kitamura K, Matsuda A, et al. Interaction between CD45-AP and protein-tyrosine kinases involved in T cell receptor signaling. J Biol Chem. 1999;274:1407–14. doi: 10.1074/jbc.274.3.1407. [Motoya S, Kitamura K, Matsuda A, et al. Interaction between CD45-AP and protein-tyrosine kinases involved in T cell receptor signaling. J Biol Chem, 1999,274:1407-14.] [DOI] [PubMed] [Google Scholar]
- 17.Kiyohara C, Takayama K, Nakanishi Y. Association of genetic polymorphisms in the base excision repair pathway with lung cancer risk: a meta-analysis. Lung Cancer. 2006;54:267–83. doi: 10.1016/j.lungcan.2006.08.009. [Kiyohara C, Takayama K, Nakanishi Y. Association of genetic polymorphisms in the base excision repair pathway with lung cancer risk: a meta-analysis. Lung Cancer, 2006,54:267-83.] [DOI] [PubMed] [Google Scholar]
- 18.Stern MC, Siegmund KD, Conti DV, et al. XRCC1, XRCC3, and XPD polymorphisms as modifiers of the effect of smoking and alcohol on colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2384–90. doi: 10.1158/1055-9965.EPI-06-0381. [Stern MC, Siegmund KD, Conti DV, et al. XRCC1, XRCC3, and XPD polymorphisms as modifiers of the effect of smoking and alcohol on colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev, 2006,15:2384-90.] [DOI] [PubMed] [Google Scholar]
- 19.Huang WY, Chow WH, Rothman N, et al. Selected DNA repair polymorphisms and gastric cancer in Poland. Carcinogenesis. 2005;26:1354–9. doi: 10.1093/carcin/bgi084. [Huang WY, Chow WH, Rothman N, et al. Selected DNA repair polymorphisms and gastric cancer in Poland. Carcinogenesis, 2005,26:1354-9.] [DOI] [PubMed] [Google Scholar]
- 20.Hirata H, Hinoda Y, Tanaka Y, et al. Polymorphisms of DNA repair genes are risk factors for prostate cancer. Eur J Cancer. 2007;43:231–7. doi: 10.1016/j.ejca.2006.11.005. [Hirata H, Hinoda Y, Tanaka Y, et al. Polymorphisms of DNA repair genes are risk factors for prostate cancer. Eur J Cancer, 2007,43:231-7.] [DOI] [PubMed] [Google Scholar]
- 21.Weaver DA, Crawford EL, Warner KA, et al. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005;4:18. doi: 10.1186/1476-4598-4-18. [Weaver DA, Crawford EL, Warner KA, et al. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer, 2005,4:18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Niu Y, Zhou Y. Increase the cisplatin cytotoxicity and cisplatin-induced DNA damage in HepG2 cells by XRCC1 abrogation related mechanisms. Toxicol Lett. 2010;192:108–14. doi: 10.1016/j.toxlet.2009.10.012. [Zhang R, Niu Y, Zhou Y. Increase the cisplatin cytotoxicity and cisplatin-induced DNA damage in HepG2 cells by XRCC1 abrogation related mechanisms. Toxicol Lett, 2010,192:108-14.] [DOI] [PubMed] [Google Scholar]