Abstract
We will highlight and put into perspective new lineage tracing data from genetic studies in mice indicating that the genuine progenitors to C cells arise in the endoderm germ layer. This overturns the current concept of a neural crest origin of thyroid C cells referred to in every textbook and dedicated paper to this very day. As will become apparent, except for a single experiment, the neural crest theory has little or no support when the evolution and development of calcitonin-producing cells in the entire chordate family are considered. Instead, a unifying origin of all cells of the ultimobranchial bodies reopens questions on the histogenesis of certain thyroid pathologies previously difficult to explain. On this aspect, medullary thyroid cancer shows a stronger connection to gut neuroendocrine tumours than previously recognized. It is envisaged that novel factors implicated in C cell-derived tumour growth and progression will be discovered as the mechanisms that regulate lineage expansion of embryonic C cell precursors from pharyngeal endoderm are uncovered. We will not discuss why C cells go to the bother of burying themselves in the thyroid - this remains a mystery.
Key Words: Endoderm, Mixed medullary-follicular carcinoma, Neural crest, Neuroendocrine tumours, Thyroid C cells, Ultimobranchial body
Introduction
Thyroid C cells representing the second endocrine cell type of the gland nowadays gain interest mainly as the origin of medullary thyroid cancer (MTC), which despite an often indolent local growth is an invasive and metastatic tumour with until recently few treatment opportunities other than surgery. However, in the past, these calcitonin-secreting cells and their homologous counterparts in lower vertebrates (in which C cells are present outside the thyroid) played a prominent role in the basic characterisation of the neuroendocrine system at large and were in fact prototypic for the proposal that all neuroendocrine cells had a neural crest origin, today regarded as a faulty concept. Functionally, the major C cell hormone calcitonin is essential for calcium homeostasis in water-living animals such as fish and amphibians and of importance also in some mammals (e.g. rodents), whereas, as far as we know, it is not necessary to substitute calcitonin in humans born without a thyroid or subjected to total thyroidectomy. Thyroid C cells were long considered one of few remaining neuroendocrine lineages derived from neural crest cells that in the early embryo migrate ventromedially from the neural tube into the branchial arches of the pharyngeal apparatus. Genetic lineage tracing in mice now overturns this view in favour of an endoderm origin of mammalian C cell progenitors [1]. In this article, we discuss the implications of this discovery in the context of thyroid organogenesis in the vertebrate series and the development of C cell-derived tumours in man. We will set the scene by recapitulating some of the basic features of thyroid C cells, most of which were discovered long before the genetic tools of today were available for developmental and tumour biology studies (for a comprehensive summary of the pre-genetic era on this subject, see Hazard [2]). For other facts and thoughts on C cell development not possible to cover here, we refer to a recent review paper by Kameda [3].
Early History and Histology
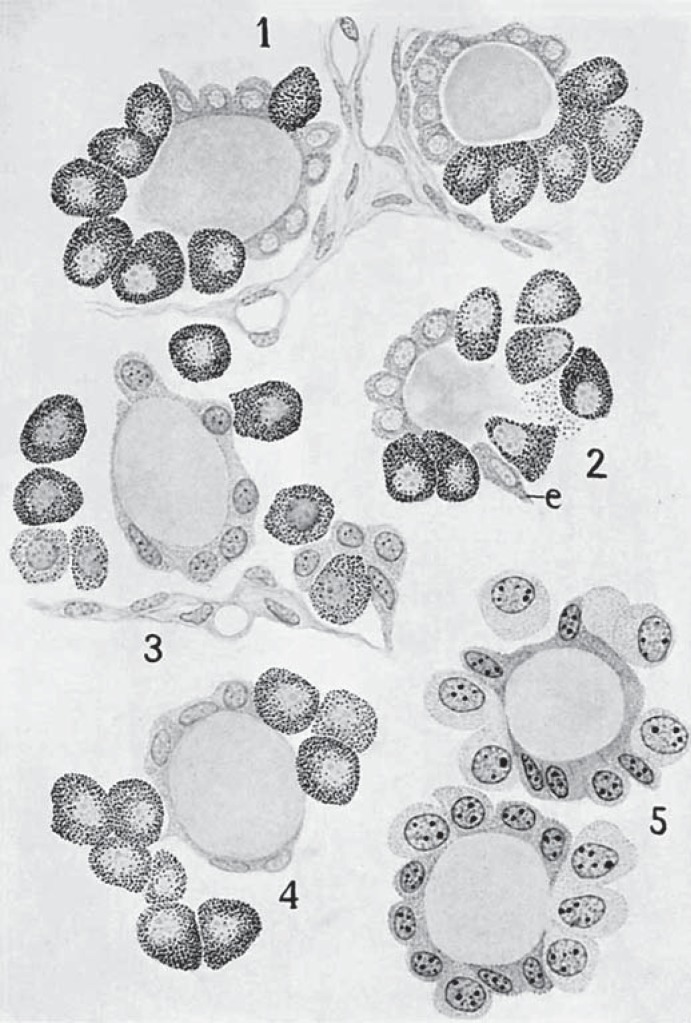
The first indication of a second endocrine cell type dates back to 1876 when E. Cresswell Baber published in the Proceedings of the Royal Society of London[4] the identification in dog thyroid of parenchymatous cells that could be distinguished from the common follicular cells. The name parafollicular cell was introduced in 1932 by José Nonidez [5] and has since been widely used in textbooks although this, strictly speaking, is incorrect considering the fact that thyroid C cells additionally may be located in interfollicular nests and sometimes also integrated with the follicular epithelium i.e. intrafollicularly. In fact, in those early days, the debate concerned whether parafollicular cells developed from thyroid follicles per se and thus moved out or, the reverse process, contributed to thyroid growth by serving as regeneration precursors to the follicular cells thus moving in (fig. 1 reproduced from Nonidez [6]; see online suppl. material for the original text and figures from 1933 summarizing the field to this date; see www.karger.com/doi/10.1159/000447333 for all online suppl. material).
Fig. 1.
Tribute to the first identification of thyroid C cells (long before microscope cameras were invented). Distribution of parafollicular cells in dog thyroid as originally cartooned from observations on tissue sections stained with Cajal's silver nitrate method (reproduced with permission from the paper by Nonidez [6]; see online suppl. material). C cells were distinguished from follicular cells by the presence of argyrophilic granules. The different images (1-5) were thought to represent distinct stages of C cell maturation and integration within the follicular epithelium as observed in puppies (cartoons 1, 2 and 5) and adults (cartoons 3 and 4). Note: the cell shape of this neuroendocrine lineage is consistently epithelial. e = Elongated follicular cell.
It was not until 1966 that Anthony Pearse [7,8] proposed the most appropriate name, C cells, based on the specific expression of calcitonin. Before calcitonin immunostaining on histologic sections was made possible, the scattered distribution of C cells in thyroid tissues and their variable incidence among mammalian species made their identification difficult, especially in humans where they are few in number and generally restricted to a small part of the gland. They are particularly common in both rats and mice, although the animals studied had been maintained on laboratory diets that are rich in both calcium and vitamin D; it is possible that this could have influenced their numbers. C cell tumours have been found to be more common in rats fed high levels of vitamin D [9] and in old bulls maintained on fortified diets [10]. Interestingly, the incidence of medullary carcinomas in humans is higher in those taking vitamin D supplements [11]. Before the era of immunohistochemistry, human C cells were best visualized by the Grimelius silver nitrate method with which the initial discoveries concerning C cells were made [12,13]. Silver techniques were in fact instrumental for the identification of the entire neuroendocrine system and the proposal, also by Pearse, of the now discredited APUD cell concept (to be further commented on below).
In most mammals, C cells are more numerous in the medial centre of the thyroid lobes reflecting the embryonic entry into the gland by fusion with the ultimobranchial bodies that carry the C cell precursors. Thus, C cells are rarely found in the lobe periphery and the isthmus. It is estimated that C cells comprise less than 0.1% of the epithelial mass of the human thyroid [2], and are often found scattered around the so-called solid cell nests which are the remnant of ultimobranchial epithelium. Thyroid C cells vary from polygonal to spindle shape with tapering cell processes underneath the common follicular basement membrane. This may reflect the pro-migratory nature inherited from embryonic time when C cell precursors invaded and disseminated within the prospective thyroid lobes or may be related to a possible paracrine effect: the cytoplasmic processes resemble those found in other neuroendocrine cells such as the G cells in the gastric mucosa. However, simple morphology, as evident to investigators in the late 19th and early 20th centuries, revealed the true epithelial nature of C cells, confirmed today by the expression of E-cadherin [14], consistent with an origin from another source than neural crest-derived mesenchyme.
Calcitonin and Cytodifferentiation
Thyroid C cells are the major and probably only normal source of circulating calcitonin in mammals (ectopic calcitonin secretion has been described in several non-thyroid neuroendocrine tumours but not for normal tissues). The thyroid origin of calcitonin was originally proven by direct measurement in vessels draining the gland in rodents [15]. The lost name thyrocalcitonin was proposed and used for a while to distinguish the thyroid-derived hormone from that mistakenly thought to originate from the parathyroid due to technical difficulties in separating the two in tissue preparations [16]. Of interest, calcitonin expression in C cells is regulated by Nkx2-1 (identical to thyroid transcription factor-1 or TTF-1) [17,18], which is better known for its pivotal roles in thyroid development and differentiation, and in generating functional thyroid tissue from embryonic stem cells [19]. The expression of Nkx2-1, also shared with lung progenitors, does not prove the embryonic origin but strongly suggests a kinship of thyroid follicular and C cells with roots in early development from pharyngeal endoderm.
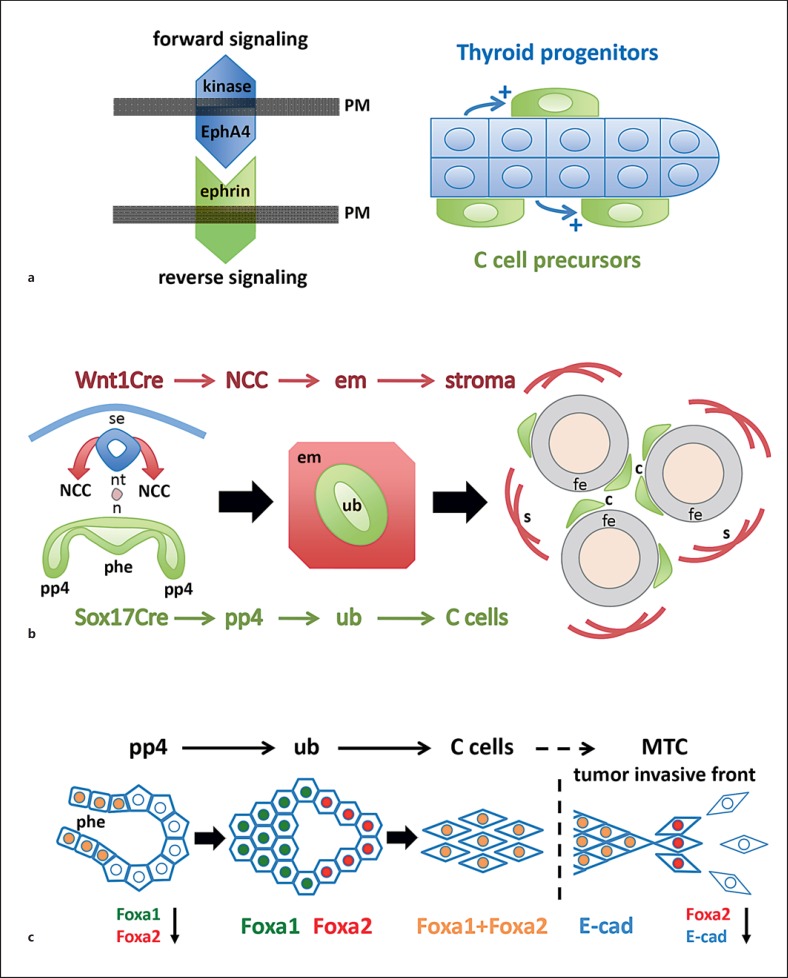
In the embryonic thyroid, the calcitonin gene is switched on in C cells concomitantly with that of thyroglobulin in pre-follicular cells [20], that is terminal differentiation of the two endocrine cell types is synchronous suggestive of a common mechanism. However, although thyroid-stimulating hormone (TSH) has been shown to activate C cells in culture, there is no evidence that TSH directly affects C cells in vivo (ultimobranchial bodies in fish are under pituitary control [21], but this is unrelated to the pituitary-thyroid axis). From studies on mouse thyroid development, it is also known that TSH has no role in the differentiation and histogenesis of embryonic follicular cells [22], indicating that local factors predominate in organogenesis before the pituitary gains control over thyroid function. The final number of C cells appearing in the adult mammalian gland seems to depend on direct interactions with the follicular counterparts that likely start during gland development. Accordingly, before fusion of mouse thyroid primordia and mingling of follicular and C cells, both lineage precursors express EphA4 [23], a tyrosine kinase receptor that guides morphogenesis by bidirectional signalling, forward and reverse, after binding to cognate ligands called ephrins, which are expressed on the surface of interacting adjacent cells that receive the reverse signal (fig. 2a, left). Notably, C cell precursors down-regulate EphA4 before entering the embryonic thyroid whereas follicular progenitors maintain EphA4 expression [23]. Moreover, in EphA4-deficient mice in which forward signalling is lost in the follicular precursors, only a much reduced thyroid C cell number is encountered postnatally, suggesting that propagation of one cell type depends on the other [23]. Since C cells still differentiate in mice lacking EphA4 kinase, this phenotype likely relates to decreased proliferation of C cell precursors and the lack of a growth stimulus generated from nearby follicular cells (fig. 2a, right).
Fig. 2.
C cell development in mouse thyroid. a The interaction of C cell precursors with follicular progenitor cells influences the final number of thyroid C cells (based on data published by Andersson et al. [23]). After integration of the ultimobranchial bodies with the embryonic thyroid only follicular cells express EphA4, a tyrosine kinase receptor that binds promiscuously to ephrin-type ligands on adjacent cells. Genetic deletion or kinase inactivation of EphA4 leads to reduced C cell numbers postnatally. Whether EphA4 on follicular cells affects C cells directly (by altered reverse signalling of ephrins) or indirectly (e.g. by a paracrine growth factor) is unknown. b Lineage tracing proves thyroid C cells originate in endoderm (Johansson et al. [1]). By recombination of a reporter mouse with Cre lines that distinguish endoderm (Sox17Cre) and neural crest (Wnt1Cre) lineages, respectively, it is evident that C cells derived from Sox17+ anterior endoderm, specifically from the pharyngeal pouches from which the paired ultimobranchial bodies develop. In contrast, the neural crest gives rise to ectomesenchymal cells that surround the ultimobranchial body and later, after fusion with embryonic thyroid, converts to the stromal compartment of the gland (see also paper by Kameda et al. [14]). c Differential expression of forkhead box transcription factors in different C cell lineage stages (Johansson et al. [1]). Foxa1 and Foxa2, required in concert for proper endoderm formation foregoing organogenesis, are co-expressed in the pharyngeal endoderm except in the pouch domains from which the ultimobranchial bodies develop. Lineage tracing reveals that all pouch progenitors originate in endoderm proper indicating Foxa1 and Foxa2 are specifically down-regulated as the ultimobranchial bodies form and delaminate. The free ultimobranchial bodies re-express Foxa1 and Foxa2 in a distinct pattern that reveals Foxa1+ (Foxa2-) proliferating and Foxa2+ (Foxa1-) non-proliferating domains of the ultimobranchial epithelium. Eventually, all ultimobranchial cells and embryonic C cells co-express Foxa1 and Foxa2. Invasive cells of human medullary thyroid carcinoma transiently lose Foxa1 and Foxa2 expression. Loss of Foxa2 characterizes epithelial-to-mesenchymal transition accompanied by down-regulation of E-cadherin (E-cad), a key feature of the invasive tumour phenotype. This differs malignant from normal C cells and C cell precursors that express E-cadherin in all developmental stages. a-c PM = Plasma membrane; se = surface ectoderm; nt = neural tube; n = notochord; NCC = neural crest cells; phe = pharyngeal endoderm; pp4 = fourth pharyngeal pouch; em = ectomesenchyme; ub = ultimobranchial body; c = C cells; fe = follicular epithelium; s = stromal cells.
For both cell lineages, the triggering factors responsible for the onset of functional differentiation towards an endocrine and neuroendocrine phenotype, respectively, are not known. In mice, this occurs from embryonic day 15, accompanying lobe formation and tissue reorganization into a follicular thyroid [20]. Nkx2-1 jointly with Pax8 drives thyroid hormone synthesis [19] whereas Nkx2-1 only is required to manufacture C cell differentiation [24]. However, both Nkx2-1 and Pax8 are functional much earlier as thyroid primordial tissues develop from pharyngeal endoderm. This brings us to the core topic of our discussion, namely the developmental origin of progenitors committed to a C cell fate and the site of lineage expansion of C cell precursors. Central players in this process are the ultimobranchial bodies that have been assumed to serve as a vehicle for transportation of neural crest cells to the thyroid in mammals. As a background to this widely accepted but misleading concept, we need to take a closer look on C cell development from the phylogenetic viewpoint.
Ultimobranchial Body and the Neural Crest Hypothesis
Evolution of vertebrates more than 0.5 billion years ago relied upon the invention of a new ectoderm-derived tissue, the neural crest, that after delamination from the neural tube spread into various locations of the embryo to build up new organs including a ‘new head’ adopted for a predatory lifestyle [some authors consider the neural crest the fourth germ layer due to this paramount contribution to evolution; for a recent update, see [25]]. Along with this, also specific for vertebrates, migrating neural crest cells invade the pharyngeal apparatus and contribute to its proper segmentation and formation of endodermal pouches from which the thymus, parathyroid and ultimobranchial bodies develop. In fact, experimental deletion of the neural crest will seriously impair pharyngeal development leading to hypoplasia or agenesis of these glands indicating that neural crest cells migrating there in some way regulate organogenesis [26].
A neural crest origin of thyroid C cells, first proposed by Pearse in a series of papers [8,27,28], was therefore not controversial, although evidence in favour of the hypothesis was at first no more than circumstantial and can be summarized: ultimobranchial cells and neural crest cells share some biomarkers, fusion of the thyroid with the ultimobranchial bodies is required to get C cell precursors there, and adult C cells similar to any other neuroendocrine cell types possess neuronal features. The concept was strengthened by the results of seminal studies on neural crest fate by Nicole Le Douarin and co-workers who, employing an avian chimeric model, showed that quail crest cells transplanted to chick embryos among multiple destinations ended up in the ultimobranchial bodies and that some of these cells were immunoreactive for calcitonin [29,30]. Ever since, major textbooks in developmental biology and medicine refer to mammalian C cells as of neural crest origin, however, ignoring the fact that the ultimobranchial bodies in all vertebrates but mammals constitute a distinct endocrine gland with no connection whatsoever to the thyroid [31]. The demonstration by Le Douarin that the neural crest made a cellular contribution to the developing thyroid gland was not surprising: both connective and neural components would be expected. The proof that the cells were C cells depended on the histochemical localisation of calcitonin in the cytoplasm of cells with the quail nuclear morphology. Current evidence discussed here suggests that this localisation must have been mistaken; the explanation that C cells have a dual origin, with a minor population of neural crest origin, has not been disproved but seems unlikely. The production of calcitonin by cells other than C cells is also an unlikely explanation; the calcitonin gene is indeed active in other cells, for example in neural tissue, but produces calcitonin gene-related peptide, which is proposed to be an ancestral molecule [32], rather than calcitonin that is normally expressed by alternative splicing only in C cells. The Pearse study [30] was carried out with a very early antibody to calcitonin; a cross-reaction to another peptide remains a possibility.
In 1967, Tauber [33] and Copp et al. [34] independently showed in fishes and birds that calcitonin is produced by the ultimobranchial gland and not the thyroid. The ultimobranchial gland of amphibians is simplified in comparison to that of birds, consisting of a single follicular structure. It is evident that in these tiny organs only polarized cells with the apical surface facing the lumen and the basolateral surface towards the extrafollicular space express calcitonin [35,36,37]. Theoretically, it cannot be excluded that neural crest-derived ectomesenchyme that fills out the subpharyngeal space in all vertebrates might enter and be integrated with the ultimobranchial epithelium and differentiate into C cells. However, this would require that mesenchymal cells cross the follicular basement membrane and adopt an epithelial phenotype, i.e. reversal of epithelial-mesenchymal transition which is the opposite process that neural crest cells much earlier undergo as they delaminate from the neural tube and start migrating [25]. To our knowledge, this scenario has never been reported to occur in any studies on ultimobranchial bodies and glands. Follicles in which calcitonin-producing cells border the lumen have been encountered also in the ultimobranchial gland in adult chicken [38] and in the thyroid gland in dogs [39] and humans [40], indicating this is a shared feature among C cells in lower vertebrates and mammals.
Further phylogenetic aspects favour the idea of a sole endoderm origin of the ultimobranchial bodies and glands. Firstly, they are ancient structures that evolved earlier than the parathyroid for control of calcium homeostasis in aquatic species [41], suggesting a developmental trait independently of other pharyngeal derivatives that require a neural crest contribution. Secondly, ancestral calcitonin-producing cells have been identified in the invertebrate series, i.e. before a bona fide neural crest tissue can be recognized [25]. Of particular importance, the endostyle in tunicates harbours not only a thyroid homologue but also a group of argyrophilic cells that express calcitonin and exhibit the typical dense-cored granules conspicuous in C cells [42]. Intriguingly, protochordate C cells occupy a position immediately adjacent to the endostylar zone of iodine-binding cells, suggesting that the close spatial relationship of the two cell types in mammalian thyroids may be traced back to the earliest days of chordate evolution and the advent of endocrine regulation. These observations collectively indicate that the neural crest is not required for C cells to develop and differentiate.
Neuroendocrine Ancestry - The Delusive APUD Concept
The APUD hypothesis coined by Pearse, referring to amine precursor uptake and decarboxylation, embraced all neuroendocrine cells [43]. Sharing these features with neurons, APUD cells and apudomas became for decades the fashionable term for neuroendocrine cells and their tumours, based as we now know on the misleading hypothesis of a developmental origin from neuroectoderm and more specifically the neural crest [44]. Remaining a controversy among scientists and clinicians for many years, the neural crest theory was eventually disproven by modern lineage tracing technology and abandoned in favour of a common stem cell origin in gut endoderm of the entire enteroendocrine series [45,46]. Much of the confusion must be blamed on the fact that neuroendocrine cells are able to handle neurotransmitter precursor amines and generally have a paracrine function, in that sense behaving as neurons. It is now evident that a vesicular amine transporter is required to prevent premature prohormone cleavage [47], which explains why peptide-secreting endocrine cells concentrate and metabolize amines without the necessity of sharing an origin with elements of the nervous system. As pointed out in an editorial [48], this important study suggests that neuronal and endocrine functions are evolutionarily linked by sharing a novel molecular mechanism adapted for divergent functions. The APUD concept is thus in a sense still relevant but with a different meaning than originally assumed.
Endoderm Origin of the Thyroid C Cell Lineage
Further observations in lower vertebrates would not be decisive to finally resolving the embryonic origin of thyroid C cells in mammals. However, significant progress has been obtained by the generation of mouse strains harbouring a chromogenic or fluorescent reporter gene that enables lineage tracing based on directed activation of cre recombinase [49]. So by linking cre to the promoter of a given gene known to be selectively or exclusively expressed in progenitor cells of interest, after recombination with the reporter mouse, those embryonic cells and all offspring will inevitably be labelled and can be tracked directly in the microscope. With this method, targeting cre to the Wnt1+ premigratory neural crest, it was possible to trace streams of migrating crest cells heading for multiple locations, e.g. craniofacial skeleton, branchial arches, adrenal medulla, peripheral nervous system and skin, in the developing embryo [50,51]. This comprised also the endocrine glands derived from pharyngeal endoderm, including the thyroid. However, as already recognized in quail-chick chimeras, ectomesenchymal cells derived from the neural crest build the stromal compartment, not the parenchyma, in mouse thyroid, parathyroid and thymus [14]. And, importantly, thyroid C cells do not belong to the Wnt1+ neural crest lineage [14].
Although it is difficult to see the logic of the one and only exception to the rule, it could always be argued that C cell progenitors diverged before neural crest cells expressed Wnt1 and therefore were excluded from being genetically labelled with Wnt1Cre. Indeed, such tracing data obtained in mouse embryos have turned greater concepts than this on their head [52,53]. If a Wnt1-negative crest progenitor subpopulation holds true this would disunite thyroid C cells and adrenomedullary cells, the only two remaining from the original hypothesis of all neuroendocrine cells being crest derived. This in turn would have implications for the understanding of coincidental tumorigenesis of MTC and phaeochromocytoma in multiple endocrine neoplasia (MEN) syndromes. As we shall see, divergence is actually the case but for another, fundamentally different cause: chromaffin cells are crest derived, thyroid C cells are not. Direct proof of this revision was recently provided by the identification of C cells as a Sox17+ lineage [1]. Sox17, a member of the SRY-related HMG box family of transcription factors, is transiently expressed in undifferentiated endoderm before organ induction and additionally in a subpopulation of genuine mesoderm to become microvasculature. Accordingly, recombination with Sox17Cre uniformly labels parenchyma of all foregut derivatives [54], including ultimobranchial body and thyroid [1], but does not label neural crest tissues. From this, it follows that the ultimobranchial epithelium, which, strictly speaking, is no more than outpocketings of the pharyngeal endoderm, is the bona fide progenitor source for the C cell lineage. Thus, in the mouse thyroid, both follicular cells and C cells descend from anterior endoderm. A graphic summary of these fundamental observations is provided in figure 2b.
Generation of C cell precursors from endoderm most likely takes place gradually as the ultimobranchial body emerges, indicated by the onset of Nkx2-1 expression that eventually encompasses all ultimobranchial cells before fusion with the midline thyroid [1]. Nkx2-1 expression in ultimobranchial cells was first reported by Mansouri et al. [55] investigating the phenotype of Pax8 null mice; in this mutant, no thyroid develops leaving two Nkx2-1+/calcitonin+ structures bilaterally in the neck with the conspicuous shape and position of ultimobranchial bodies. A problem in the past was that Nkx2-1 could not be taken for a genuine marker of C cell precursors since, for example, the ultimobranchial body gives rise to another secretory cell type that forms so-called ultimobranchial follicles embedded among follicles proper in the mammalian thyroid [56,57]. However, plasticity of the ultimobranchial epithelium with signs of transitional states between calcitonin-positive and mucous-secreting cells is evident in amphibians suggesting this may be a residual phenomenon of a phylogenetically ancient mechanism confined to the follicular ultimobranchial gland; switching cell phenotype may serve a distinct purpose in the endocrine control of calcium-dependent functions in aquatic species [31]. In mice, the developing ultimobranchial body consists of subpopulations of cells with different proliferation rate discerned by Ki67 staining that corresponds to the differential expression of forkhead box transcription factors Foxa1 and Foxa2 [1], both known for being crucial to the determination of definitive endoderm and acting in concert in organogenesis from foregut [58]. However, as illustrated in figure 2c, at the time for mergence with the thyroid, all ultimobranchial cells appear uniform and ubiquitously express not only Nkx2-1 but also Foxa1 and Foxa2, and this expression pattern is retained in embryonic C cells as these spread into the gland [1]. This further supports the notion that C cells or ultimobranchial follicular cells have a common embryonic origin, and the precursor cells likely are identical. It also offers a plausible explanation to the variety of epithelial phenotypes comprising both follicular cells and C cells present in cystic ultimobranchial remnants that were analyzed in patients with a lingual thyroid anomaly in which fusion with the ultimobranchial bodies never took place [57].
Developmental Aspects of MTC
Simultaneously with the implementation of the neural crest theory nearly 50 years ago, C cells were discovered to be the tumorigenic origin of MTC in humans [59,60,61]. Since then, MTC has been considered a crest-derived tumour [62], driven in most cases by somatic or germline mutations in the RET proto-oncogene. The neural crest origin was also attractive because phaeochromocytoma, the other main inherited component of MEN type 2, unquestionably is a crest tumour [50]. Ret is naturally expressed in neural crest [63]. However, Ret expression is prominent also in non-crest tissues, for example, the ureteric bud in which Ret-dependent reciprocal signals are critical for kidney organogenesis; abnormal tuning of this process is the likely cause of unilateral renal agenesis incidentally reported for MEN2a patients [64,65]. Of importance, pharyngeal endoderm was included among Ret transcript-positive tissues in the original expression pattern study in mouse embryos [63], providing a plausible explanation for the third manifestation of MEN2a, parathyroid hyperplasia, the cells of which are genuinely endodermal. A previous report showed that mutated RET is indeed expressed in MEN2a parathyroid tumours [66]. It is thus obvious that Ret-mediated growth abnormalities in MEN syndromes are not restricted to cells of neural crest origin.
It is previously inferred that thyroid C cell development relies on Ret expression in a cranial neural crest subpopulation that also, through a vagal contribution, colonizes the gut with presumptive enteric neurons [67]. With our new lineage tracing data at hand [1], this notion needs to be reconsidered. As Ret in endoderm has not been further investigated since first reported [63], we can only speculate on its potential role in glandular development from the pharyngeal apparatus and when and where MTC tumorigenesis may first be initiated. Adult (human) C cells express RET [68] whereas Ret transcripts were not found in the embryonic (mouse) thyroid at the developmental stages tested [69]. Germline activating RET mutations implies that mutant RET receptors are constitutively activated once being naturally expressed, which for C cells probably occur at the progenitor stage in pharyngeal endoderm or in precursor cells present in the ultimobranchial epithelium. In line with this assumption, germline RET 918 mutation may cause metastatic MTC with clinical manifestations already in infancy [70]. For germline mutations associated with a less aggressive MTC than 918 it is conceivable that RET is activated in the same developmental stage. Factors that modify the onset of tumour development and growth in human MTC with different RET point mutations are unknown.
In transgenic mouse models, mutant Ret or Ras under control of the calcitonin/calcitonin gene-related peptide promoter leads to C cell hyperplasia and delayed onset of MTC tumorigenesis at adult age [71,72,73], suggesting a possibility that differentiated C cells might be less responsive to oncogenic activation than C cell precursors. Notably, cell proliferation is abundant in the developing ultimobranchial bodies, but all cells cease to multiply and exit cell cycle as evidenced by loss of Ki67 expression upon entry into the thyroid [1]. Moreover, Ki67+ mouse ultimobranchial cells co-express Foxa1 but not Foxa2, and the same expression pattern is evident in the growth zone of human MTC nodules, indicating that Foxa1 governs proliferation of both embryonic and malignant C cells [1]. In contrast, Foxa2, which is normally expressed in non-proliferating precursors and differentiated C cells, is down-regulated in MTC cells that possess invasive behaviour accompanied by loss of epithelial properties reminiscent of epithelial-mesenchymal transition [1]. The differential expression pattern of Foxa1 and Foxa2 in embryonic C cell development thus seems to be recapitulated in C cell-derived tumours with probable impact on MTC growth and progression (fig. 2c). That embryonic programs of lineage expansion may be recapitulated during oncogenic transformation is currently a bearing concept of relevance for tumour development in many cancers. For thyroid C cells and MTC, this notion alerts for a possibility that different growth capabilities prevailing before and after neuroendocrine differentiation might influence and modify the responsiveness to Ret activation. Since Foxa1 is not expressed in thyroid progenitors of the follicular lineage [1], the Foxa1 promoter may be employed to exclusively target in vivo mutant Ret to embryonic C cell precursors and elucidate whether they grow faster than terminally differentiated cells stimulated by the same oncogene.
Medullary Carcinoma and Mixed C and Follicular Cell Tumours
Mixed medullary-follicular thyroid carcinoma (MMFTC) is a puzzling tumour entity regarding histogenetic origin [74]. Apart from the collision by chance of two cancers with different clonality, a fundamental problem has been to explain how C cells of assumed neuroectodermal origin could attain features typical of differentiated thyroid carcinomas derived from thyroid follicular epithelial cells [75]. Clearly, ultimobranchial cells possess phenotypic pluripotency, elegantly proven in studies on autologous transplants of mouse ultimobranchial bodies [76] and further characterized in human ultimobranchial remnants that never mixed with the thyroid proper [57], but whether this was attributed to contributions from more than one cell type to start with, i.e. of different embryonic origins (endoderm and neural crest), could not be decided. As C cells can no longer be considered of neural crest origin [1], it is conceivable that mixed thyroid carcinomas may arise from the ultimobranchial body epithelium only. In the last part of the paper, we will further elaborate this important aspect focusing on thyroid neoplasias in humans.
Genotype-Phenotype General Considerations
Tumours derived from C cells appear, like tumours derived from neuroendocrine cells in the intestines, always to be malignant. Almost all MTCs show a mutation in the RET proto-oncogene, which in about a quarter of cases is a germline mutation. As already touched upon, the familial syndromes involving MTCs, collectively known as MEN type 2, show an interesting genotype-phenotype correlation. MEN2b patients show medullary carcinoma, commonly also with phaeochromocytomas but rarely if ever parathyroid disease. They also show a variety of neural and skeletal abnormalities. The great majority of these cases have a germline RET 918 mutation. MEN2a patients show medullary carcinoma, with phaeochromocytomas in about half the cases, and parathyroid disease in about a quarter, but no neural or skeletal changes. The commonest germline mutation in MEN2a is RET 634. Families with only occasional members with MTC (FMTC) and with no other associated defects can show one of a variety of germline RET mutations, but not 918 or 634 [77].
C cell hyperplasia is found in both MEN2a and MEN2b patients, it precedes and accompanies tumour development, suggesting that the primary effect of the germline RET mutation is to stimulate C cell growth, and that one or more further mutations in one of the hyperplastic cells leads to tumour development. MEN2b patients, in particular those with a 918 mutation, usually develop marked C cell hyperplasia and MTC at a much earlier age than MEN2a or FMTC patients, and the tumour is more aggressive. Cancer is often diagnosed in the teens or twenties in MEN2b and rarely at this young age in MEN2a. Notably, the youngest reported case in MEN2b was in a 9-week-old infant [70], and a case seen by one author was found at autopsy in a 6-week-old child. These findings suggest that the further mutations needed for carcinogenesis may in some cases occur during foetal life. Some patients belonging to MEN2a families may not develop MTC until very late in life or be obligate carriers and never develop clinical malignancy; obligate carriers are relatively common in FMTC. Total thyroidectomy in early childhood can be curative in MEN2 cases, and examination of the thyroid can find C cell hyperplasia but no cancer. The presumed additional mutations, therefore, must also take place during extra-uterine life, suggesting that growth continues in hyperplastic C cells. Sporadic MTC, often with a somatic RET 918 mutation, is typically found in middle-aged or elderly patients lacking a germline RET mutation. It would be expected to have a longer latency than tumours in patients with a RET germline mutation as the latter require one fewer event to complete the carcinogenic cascade; it is not known when the first mutation occurs in sporadic cases.
Polyclonal Mixed Tumours
The great majority of both germline and sporadic MTCs are cancers composed only of malignant C cells, but there have been many reports of tumours composed of both C and follicular-derived cells in individual patients, with a varying degree of fusion between the different neoplastic tissues. In the past, the accepted origins from the neural crest for C cells and foregut endoderm for follicular cells made it difficult to understand why there should be any association between their tumours. The recognition that C cells are of endodermal rather than neural crest origin [1] justifies a reassessment of the coincidence of the two tumour types. Several reports indicate that papillary thyroid carcinoma (PTC) as a separate tumour occurs more frequently than expected in patients with MTC [78]. Some of these studies are unreliable as allowance has not been made for the greater scrutiny of thyroids resected for MTC than thyroidectomies for other reasons. The search for C cell hyperplasia leads to more thyroid samples being studied, so that small incidental PTCs are more likely to be found.
In a study of spatially separate carcinomas, RET mutations were present in the MTCs and BRAF mutations in the PTCs, supporting the view that the tumours originated in separate cells [79], although the possibility of different second hits in a clone of premalignant cells cannot be excluded. There is a number of individual case reports describing partially fused neoplasms where collision tumours are the likely explanation, and others where the diagnosis of a mixed tumour depends on the identification of immunoreactive thyroglobulin within an MTC; some of these could be explained by inclusion of normal thyroid within an invading MTC. A careful study of a range of cases diagnosed as MMFTC in patients with or without germline RET mutations used several techniques to study clonality in microdissected samples of the different components [80]. In the informative cases, the lesions were not derived from single cells. However, in 8 of the 12 cases, the diagnosis of a mixed medullary follicular tumour was based on the finding of small numbers of thyroid follicles deep in the primary tumour, and the results suggest that such findings should not be used as a basis for this diagnosis. It appears that normal thyroid follicles can survive deep within medullary carcinomas and may even be carried with tumour cells to lymph node metastases. Where the two tumour types were partly or completely separate in the thyroid, differing clonal origins were confirmed. In considering the pathogenesis of the co-occurrence of sporadic medullary and follicular cell tumours that are spatially separate or partly fused but clearly demarcated lesions, it can be assumed that the association is random, with any apparent increase in frequency due to increased ascertainment. For these lesions, in patients with germline RET mutations, increased ascertainment is still relevant, but, as will be discussed further on a low production level of mutant RET protein in follicular cells, could contribute to the frequency with which a coincident carcinoma of follicular cell origin occurs.
Mixed Tumours of Possible Common Origin
There remain tumours where a single well-defined carcinoma contains intimately mixed neoplastic C and neoplastic follicular-derived cells (as an example see fig. 3, from one author's sample collection). The earliest description of mixed tumours as a separate entity was published in 1982 [81]; the primary tumour was a mixture of solid and follicular structures, positive for both calcitonin and thyroglobulin, and all the many metastatic lymph nodes of the tumour showed a similar mixed morphology with the appropriate immunocytochemical findings, and radioiodine uptake was also demonstrated. A second case showed a similar mixture of patterns and staining in both the primary tumour and the lymph node metastases [82]. Since that time, a small number of other cases with similar, solitary, biphasic primary tumours have been described: the medullary component sometimes combined with follicular and sometimes papillary carcinoma, and lymph node metastases showing mixed tumours.
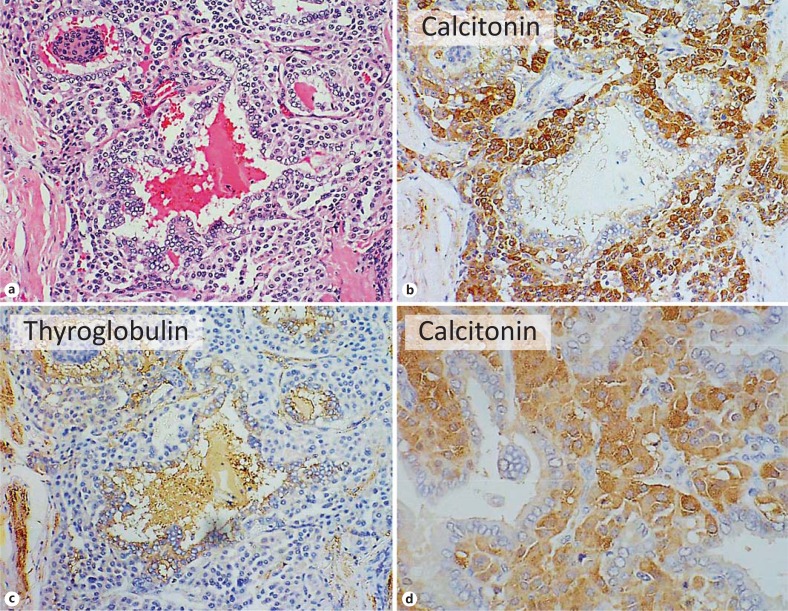
Fig. 3.
Mixed medullary-papillary carcinoma of the thyroid. a-c Near serial sections. a HE. b Immunohistochemistry for calcitonin. c Immunohistochemistry for thyroglobulin. d High-power view of calcitonin-positive cells. Note irregular follicles with papillary projections lined by epithelium and separated by sheets of neoplastic cells. The lining epithelial cells show the nuclear features of papillary carcinoma and both lining cells and luminal colloid are positive for thyroglobulin, while the cells between and spreading around the follicular structures are positive for calcitonin.
Such an admixture of tumours does not prove a common origin, adjacent primary tumours could intermingle, particularly if the two cell types normally show an intimate relationship, and mixed metastases have been described in patients with clearly separate primary tumours [80]. However, there are a number of observations that strongly suggest a common origin for a minority of MMFTC. Bilateral thyroid carcinomas, both mixed medullary-papillary in type, have been reported in a patient with a germline RET 634 mutation [83], and several authors have reported findings of calcitonin and thyroglobulin immunoreactivity in the same tumour cells, including both primary and secondary tumours, with one study using both immunocytochemistry and in situ hybridisation to demonstrate joint expression in a minority of tumour cells [84]. Co-expression of calcitonin and thyroglobulin has also been described in a subset of cells in experimentally induced mouse tumours [73]. The occurrence of mixed tumours in children is also suggestive of a single cell origin from an ultimobranchial stem cell: the first mutation leading to an MTC can almost certainly take place in foetal life, and mixed MMFTC has been found in a child aged 7 [85] and another aged 14 [86].
Mixed endocrine-exocrine differentiation in individual cells and in tumours has been well described in other endodermally derived tissues, with evidence that the two components share a common origin [87,88]. In view of the potential of ultimobranchial cells to give rise to both C and follicular cells, there is no developmental reason why it should not happen in the thyroid. In the other tissues, however, the stem cells that give rise to both exocrine and endocrine cells continue to divide throughout life. In the thyroid, while proof that stem cells capable of C and follicular cell differentiation persist throughout life is lacking, cells in cycle and cells with some stem cell properties have been observed in solid cell nests, the remnant of the ultimobranchial contribution in the human thyroid [89]. Further support for the common origin of some mixed tumours of the thyroid comes from studies of animals, particularly cattle. Here, fusion between the ultimobranchial body and the thyroid is often incomplete, and tumours composed of a mixture of undifferentiated cells, C cells and follicular cells have been described [90]. Several transgenic studies have induced C cell hyperplasia and C cell tumours in mice. One used the long isoform of RET (with a 634 mutation) linked to a calcitonin promoter [91]. Interestingly, abnormal thyroglobulin-containing follicles thought to be of ultimobranchial origin occurred in two founder lines, accompanied in one by tumours, only papillary carcinomas in most mice, but a minority showed both papillary and medullary tumours.
RET in Mixed Tumours
It is thus clear that the potential exists for truly mixed tumours derived from a common origin and showing both C cell and follicular cell differentiation, but their existence in humans has not yet been proven beyond any doubt. A small number of the cases reported are probably monoclonal, but the majority are likely to be tumours derived from different cells, with variable degrees of fusion, and possibly a paracrine trophic interaction between the two different cell types. In this respect, it is relevant to note that although mutations in the proto-oncogene RET are not frequent findings in other tumour types, they are important in the pathogenesis of both MTC and PTC, particularly radiation-induced PTC [92]. The recognition that C and follicular cells share an endodermal origin makes the coincidence that their tumours share involvement of the same oncogene less surprising. Clearly, in both cell types, the pathways through which mutated RET leads to growth are available, whether the mutation is caused by a point mutation (in MTC) or a rearrangement (in PTC). One study where the confounding factor of extra sampling can be assumed not to apply did show that the co-occurrence of MTC and PTC was more common with RET germline mutations involving the intracellular tyrosine kinase domain than with germline mutations involving the extracellular portion [93]. It was suggested that this might be due to a weak oncogenic effect of the mutated RET on follicular cells, even though most authors had failed to find RET expression in normal follicular cells. Observation of a family with a RET 634 mutation, in which all 3 members with MTC also showed one or more PTCs, prompted a study of the effect of RET point mutants on a follicular cell line [94]. A weak mitogenic effect was demonstrated, while wild-type RET had no effect. The authors concluded that RET point mutants can behave as dominant oncogenes for thyroid follicular cells.
The suggestion that a very low basal level of RET activity in normal follicular cells could, when there is a germline RET mutation, lead to increased follicular cell growth and a slightly increased risk of thyroid carcinogenesis is thus plausible, but the fact that these are PTCs rather than FTCs (ʼfollicularʼ in the classifying name MMFTC refers to cell of origin rather than tumour histotype) also raises the possibility that a point mutation in the RET gene leads to instability and a greater chance of a rearrangement. The theory that thyroid C cells were derived from the neural crest was a very persuasive idea, given the association of MTC with neural abnormalities and phaeochromocytoma, itself a tumour of neural crest-derived cells. The reason for these associations must be reconsidered in the light of the evidence that C cells are of endodermal origin. It seems probable that the link lies in the normal level of expression of the RET gene, combined with availability of the downstream signalling mechanisms that interact with the different mutations, leading to different tissue involvement and different clinical syndromes.
Final Comments on Development
The cell of origin of medullary carcinoma is presumed to be the differentiated C cell, and the hyperplastic C cells that precede the development of many familial MTCs would support this. There is little doubt that the great majority of C cells are derived from the ultimobranchial contribution; they are normally found to cluster around the solid cell nests in human thyroid, and are very rare in the periphery and isthmus, regions contributed by the central anlage. However, there is evidence that some human C cells indeed may also be derived directly from progenitor cells of the midline thyroid primordium. In 1993, C cells were found in thyroids from patients with the DiGeorge anomaly [95], a condition where the third and fourth branchial arches and the associated pharyngeal pouches fail to develop. In those with the complete anomaly, in which ultimobranchial bodies likely do not develop, C cells were reduced fourfold in number but not missing. This might not be regarded as proof of a central origin, but in 2008 an MTC was found in an enlarging lingual thyroid in a patient with no normally situated thyroid [96]. It is extremely unlikely that the ultimobranchial body could fuse with an undescended lingual thyroid. In 2012, C cells were directly observed in a lingual thyroid [97]. While the finding of C cells in the central anlage is a further departure from the classical view of thyroid development, it brings the thyroid into line with other organs developed from outgrowths of the primitive endoderm, such as the lung, gall bladder and pancreas, all of which show neuroendocrine cells of endodermal origin.
As previously discussed, bona fide progenitor cells of the central anlage co-express Nkx2-1 and Pax8, both being required for propagation of thyroid organogenesis beyond the bud stage [98]. Ectopic differentiation of C cells would thus require repression of Pax8. Mouse models are of no help to test this since in Pax8 null mutants the thyroid primordium does not survive [55,99]. Conversely, expression of thyroglobulin in the ultimobranchial remnant reported for patients with a lingual thyroid [57] probably requires Pax8 to elicit thyroid differentiation. This possibility could be investigated by targeted expression of Pax8 in C cell precursors with, for example, Foxa2Cre. Notably, observations in human embryos of Pax8 expression in the ultimobranchial anlage (that is the corresponding pouch endoderm) [100], thus differing it from the mouse ultimobranchial, suggest there may be species differences to consider. Nonetheless, these observations in humans further support the close relationship between thyroid tissues that develop from separate locations in foregut endoderm. The very existence of lateral thyroid anlagen does add to the feeling that the ultimobranchial cells must add some function to the thyroid that is not supplied by cells from the central anlage.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgements
This study was supported by grants from the Swedish Research Council (2012-00537), the Swedish Cancer Society (14-0748), and the Sahlgrenska University Hospital (ALFGBG-154401).
References
- 1.Johansson E, Andersson L, Ornros J, Carlsson T, Ingeson-Carlsson C, Liang S, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142:3519–3528. doi: 10.1242/dev.126581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazard JB. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am J Pathol. 1977;88:213–250. [PMC free article] [PubMed] [Google Scholar]
- 3.Kameda Y. Cellular and molecular events on the development of mammalian thyroid C cells. Dev Dyn. 2016;245:323–341. doi: 10.1002/dvdy.24377. [DOI] [PubMed] [Google Scholar]
- 4.Baber EC. Contributions to the minute anatomy of the thyroid gland of the dog. Phil Trans R Soc Lond. 1876;166:557–568. [Google Scholar]
- 5.Nonidez JF. The origin of the ‘parafollicular’ cell, a second epithelial component of the thyroid of the dog. Am J Anat. 1932;49:479–505. [Google Scholar]
- 6.Nonidez JF. The ‘parenchymatous’ cells of Baber, the ‘protoplasmareichen Zellen’ of Huerthle, and the ‘parafollicular’ cells of the mammalian thyroid. Anat Rec. 1933;56:131–141. [Google Scholar]
- 7.Pearse AG. 5-Hydroxytryptophan uptake by dog thyroid ‘C’ cells, and its possible significance in polypeptide hormone production. Nature. 1966;211:598–600. doi: 10.1038/211598a0. [DOI] [PubMed] [Google Scholar]
- 8.Pearse AG. The cytochemistry of the thyroid C cells and their relationship to calcitonin. Proc R Soc Lond B Biol Sci. 1966;164:478–487. doi: 10.1098/rspb.1966.0044. [DOI] [PubMed] [Google Scholar]
- 9.Thurston V, Williams ED. Experimental induction of C cell tumours in thyroid by increased dietary content of vitamin D3. Acta Endocrinol (Copenh) 1982;100:41–45. doi: 10.1530/acta.0.1000041. [DOI] [PubMed] [Google Scholar]
- 10.Krook L, Lutwak L, McEntee K. Dietary calcium, ultimobranchial tumors and osteopetrosis in the bull. Syndrome of calcitonin excess? Am J Clin Nutr. 1969;22:115–118. doi: 10.1093/ajcn/22.2.115. [DOI] [PubMed] [Google Scholar]
- 11.Ron E, Kleinerman RA, Boice JD, Jr, LiVolsi VA, Flannery JT, Fraumeni JF., Jr A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987;79:1–12. [PubMed] [Google Scholar]
- 12.Pearse AG. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci. 1968;170:71–80. doi: 10.1098/rspb.1968.0025. [DOI] [PubMed] [Google Scholar]
- 13.Pearse AG. The calcitonin secreting C cells and their relationship to the APUD cell series. J Endocrinol. 1969;45(suppl):13–14. [PubMed] [Google Scholar]
- 14.Kameda Y, Nishimaki T, Chisaka O, Iseki S, Sucov HM. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J Histochem Cytochem. 2007;55:1075–1088. doi: 10.1369/jhc.7A7179.2007. [DOI] [PubMed] [Google Scholar]
- 15.Foster GV, Baghdiantz A, Kumar MA, Slack E, Soliman HA, Macintyre I. Thyroid origin of calcitonin. Nature. 1964;202:1303–1305. doi: 10.1038/2021303a0. [DOI] [PubMed] [Google Scholar]
- 16.Foster GV. Thyrocalcitonin: failure to demonstrate a parathyroid releasing factor. Nature. 1966;211:1319–1320. doi: 10.1038/2111319a0. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Lavaroni S, Mori A, Okajima F, Kimura S, Katoh R, et al. Thyroid transcription factor 1 is calcium modulated and coordinately regulates genes involved in calcium homeostasis in C cells. Mol Cell Biol. 1998;18:7410–7422. doi: 10.1128/mcb.18.12.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Katagiri N, Ueda M, Tanaka S. Functional analysis of Nkx2.1 and Pax9 for calcitonin gene transcription. Gen Comp Endocrinol. 2007;152:259–266. doi: 10.1016/j.ygcen.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagman H, Andersson L, Nilsson M. The developing mouse thyroid: embryonic vessel contacts and parenchymal growth pattern during specification, budding, migration, and lobulation. Dev Dyn. 2006;235:444–455. doi: 10.1002/dvdy.20653. [DOI] [PubMed] [Google Scholar]
- 21.Pang PK. Calcitonin and ultimobranchial glands in fishes. J Exp Zool. 1971;178:89–99. doi: 10.1002/jez.1401780111. [DOI] [PubMed] [Google Scholar]
- 22.Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA. 2002;99:15462–15467. doi: 10.1073/pnas.242328999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson L, Westerlund J, Liang S, Carlsson T, Amendola E, Fagman H, et al. Role of EphA4 receptor signaling in thyroid development: regulation of folliculogenesis and propagation of the C-cell lineage. Endocrinology. 2011;152:1154–1164. doi: 10.1210/en.2010-0232. [DOI] [PubMed] [Google Scholar]
- 24.Kusakabe T, Hoshi N, Kimura S. Origin of the ultimobranchial body cyst: T/ebp/Nkx2.1 expression is required for development and fusion of the ultimobranchial body to the thyroid. Dev Dyn. 2006;235:1300–1309. doi: 10.1002/dvdy.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green SA, Simoes-Costa M, Bronner ME. Evolution of vertebrates as viewed from the crest. Nature. 2015;520:474–482. doi: 10.1038/nature14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bockman DE, Kirby ML. Dependence of thymus development on derivatives of the neural crest. Science. 1984;223:498–500. doi: 10.1126/science.6606851. [DOI] [PubMed] [Google Scholar]
- 27.Pearse AG, Carvalheira AF. Cytochemical evidence for an ultimobranchial origin of rodent thyroid C cells. Nature. 1967;214:929–930. doi: 10.1038/214929a0. [DOI] [PubMed] [Google Scholar]
- 28.Pearse AG, Polak JM. Cytochemical evidence for the neural crest origin of mammalian ultimobranchial C cells. Histochemie. 1971;27:96–102. doi: 10.1007/BF00284951. [DOI] [PubMed] [Google Scholar]
- 29.Le Douarin N, Le Lievre C. Demonstration of neural origin of calcitonin cells of ultimobranchial body of chick embryo (in French) C R Acad Sci Hebd Séances Acad Sci D. 1970;270:2857–2860. [PubMed] [Google Scholar]
- 30.Polak JM, Pearse AG, Le Lievre C, Fontaine J, Le Douarin NM. Immunocytochemical confirmation of the neural crest origin of avian calcitonin-producing cells. Histochemistry. 1974;40:209–214. doi: 10.1007/BF00501955. [DOI] [PubMed] [Google Scholar]
- 31.Robertson DR. Endocrinology of amphibian ultimobranchial glands. J Exp Zool. 1971;178:101–104. doi: 10.1002/jez.1401780112. [DOI] [PubMed] [Google Scholar]
- 32.Lafont AG, Dufour S, Fouchereau-Peron M. Evolution of the CT/CGRP family: comparative study with new data from models of teleosts, the eel, and cephalopod molluscs, the cuttlefish and the nautilus. Gen Comp Endocrinol. 2007;153:155–169. doi: 10.1016/j.ygcen.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Tauber SD. The ultimobranchial origin of thyrocalcitonin. Proc Natl Acad Sci USA. 1967;58:1684–1687. doi: 10.1073/pnas.58.4.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copp DH, Cockcroft DW, Kueh Y. Calcitonin from ultimobranchial glands of dogfish and chickens. Science. 1967;158:924–925. doi: 10.1126/science.158.3803.924. [DOI] [PubMed] [Google Scholar]
- 35.Robertson DR. Immunohistochemical and morphometric analysis of calcitonin distribution in anuran ultimobranchial glands. Gen Comp Endocrinol. 1988;71:349–358. doi: 10.1016/0016-6480(88)90263-8. [DOI] [PubMed] [Google Scholar]
- 36.Treilhou-Lahille F, Lasmoles F, Taboulet J, Barlet JP, Milhaud G, Moukhtar MS. Ultimobranchial gland of the domestic fowl. Two types of secretory cells involved in calcitonin metabolism. Cell Tissue Res. 1984;235:439–448. doi: 10.1007/BF00217871. [DOI] [PubMed] [Google Scholar]
- 37.Treilhou-Lahille F, Jullienne A, Aziz M, Beaumont A, Moukhtar MS. Ultrastructural localization of immunoreactive calcitonin in the two cell types of the ultimobranchial gland of the common toad (Bufo bufo L.) Gen Comp Endocrinol. 1984;53:241–251. doi: 10.1016/0016-6480(84)90249-1. [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Kameda Y, Tagawa T. An ultrastructural study of the cysts in chicken ultimobranchial glands, with special reference to C-cells. Cell Tissue Res. 1986;246:39–44. doi: 10.1007/BF00218996. [DOI] [PubMed] [Google Scholar]
- 39.Kameda Y. C-cell follicles of canine thyroid glands studied by PAS reaction and electron microscopy. Cell Tissue Res. 1982;225:693–697. doi: 10.1007/BF00214814. [DOI] [PubMed] [Google Scholar]
- 40.Harach HR. Thyroid follicles with acid mucins in man: a second kind of follicles? Cell Tissue Res. 1985;242:211–215. doi: 10.1007/BF00225578. [DOI] [PubMed] [Google Scholar]
- 41.Gorbman A. Evolutionary morphology of endocrine glands. In: Pang PK, Schreibman MP, editors. Vertebrate Endocrinology: Fundamentals and Biochemical Implications. Vol. 1. New York: Academic Press; 1986. pp. 235–259. [Google Scholar]
- 42.Thorndyke MC, Probert L. Calcitonin-like cells in the pharynx of the ascidian Styela clava. Cell Tissue Res. 1979;203:301–309. doi: 10.1007/BF00237244. [DOI] [PubMed] [Google Scholar]
- 43.Pearse AG. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969;17:303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- 44.Pearse AG, Polak JM. Endocrine tumours of neural crest origin: neurolophomas, apudomas and the APUD concept. Med Biol. 1974;52:3–18. [PubMed] [Google Scholar]
- 45.Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells - the last word? J Pathol. 1998;186:117–118. doi: 10.1002/(SICI)1096-9896(1998100)186:2<117::AID-PATH152>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blackmore CG, Varro A, Dimaline R, Bishop L, Gallacher DV, Dockray GJ. Measurement of secretory vesicle pH reveals intravesicular alkalinization by vesicular monoamine transporter type 2 resulting in inhibition of prohormone cleavage. J Physiol. 2001;531(pt 3):605–617. doi: 10.1111/j.1469-7793.2001.0605h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyd CA. Amine uptake and peptide hormone secretion: APUD cells in a new landscape. J Physiol. 2001;531(pt 3):581. doi: 10.1111/j.1469-7793.2001.0581h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckingham ME, Meilhac SM. Tracing cells for tracking cell lineage and clonal behavior. Dev Cell. 2011;21:394–409. doi: 10.1016/j.devcel.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 50.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 51.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 52.Breau MA, Pietri T, Stemmler MP, Thiery JP, Weston JA. A nonneural epithelial domain of embryonic cranial neural folds gives rise to ectomesenchyme. Proc Natl Acad Sci USA. 2008;105:7750–7755. doi: 10.1073/pnas.0711344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weston JA, Thiery JP. Pentimento: neural crest and the origin of mesectoderm. Dev Biol. 2015;401:37–61. doi: 10.1016/j.ydbio.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 54.Engert S, Liao WP, Burtscher I, Lickert H. Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis. 2009;47:603–610. doi: 10.1002/dvg.20540. [DOI] [PubMed] [Google Scholar]
- 55.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 56.Wollman SH, Neve P. Ultimobranchial follicles in the thyroid glands of rats and mice. Recent Prog Horm Res. 1971;27:213–234. doi: 10.1016/b978-0-12-571127-2.50030-4. [DOI] [PubMed] [Google Scholar]
- 57.Williams ED, Toyn CE, Harach HR. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol. 1989;159:135–141. doi: 10.1002/path.1711590208. [DOI] [PubMed] [Google Scholar]
- 58.Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev. 2010;20:527–532. doi: 10.1016/j.gde.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams ED. A review of 17 cases of carcinoma of the thyroid and phaeochromocytoma. J Clin Pathol. 1965;18:288–292. doi: 10.1136/jcp.18.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114–118. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams ED, Brown CL, Doniach I. Pathological and clinical findings in a series of 67 cases of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:103–113. doi: 10.1136/jcp.19.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maguire LH, Thomas AR, Goldstein AM. Tumors of the neural crest: common themes in development and cancer. Dev Dyn. 2015;244:311–322. doi: 10.1002/dvdy.24226. [DOI] [PubMed] [Google Scholar]
- 63.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 64.Lore F, Di Cairano G, Talidis F. Unilateral renal agenesis in a family with medullary thyroid carcinoma. N Engl J Med. 2000;342:1218–1219. doi: 10.1056/NEJM200004203421615. [DOI] [PubMed] [Google Scholar]
- 65.Hibi Y, Ohye T, Ogawa K, Shimizu Y, Shibata M, Kagawa C, et al. A MEN2A family with two asymptomatic carriers affected by unilateral renal agenesis. Endocr J. 2014;61:19–23. doi: 10.1507/endocrj.ej13-0335. [DOI] [PubMed] [Google Scholar]
- 66.Pausova Z, Soliman E, Amizuka N, Janicic N, Konrad EM, Arnold A, et al. Role of the RET proto-oncogene in sporadic hyperparathyroidism and in hyperparathyroidism of multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 1996;81:2711–2718. doi: 10.1210/jcem.81.7.8675600. [DOI] [PubMed] [Google Scholar]
- 67.Manie S, Santoro M, Fusco A, Billaud M. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 2001;17:580–589. doi: 10.1016/s0168-9525(01)02420-9. [DOI] [PubMed] [Google Scholar]
- 68.Fabien N, Paulin C, Santoro M, Berger N, Grieco M, Dubois P, et al. The ret protooncogene is expressed in normal human parafollicular thyroid-cells. Int J Oncol. 1994;4:623–626. doi: 10.3892/ijo.4.3.623. [DOI] [PubMed] [Google Scholar]
- 69.Avantaggiato V, Dathan NA, Grieco M, Fabien N, Lazzaro D, Fusco A, et al. Developmental expression of the RET protooncogene. Cell Growth Differ. 1994;5:305–311. [PubMed] [Google Scholar]
- 70.Shankar RK, Rutter MJ, Chernausek SD, Samuels PJ, Mo JQ, Rutter MM. Medullary thyroid cancer in a 9-week-old infant with familial MEN 2B: implications for timing of prophylactic thyroidectomy. Int J Pediatr Endocrinol. 2012;2012:25. doi: 10.1186/1687-9856-2012-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michiels FM, Chappuis S, Caillou B, Pasini A, Talbot M, Monier R, et al. Development of medullary thyroid carcinoma in transgenic mice expressing the RET protooncogene altered by a multiple endocrine neoplasia type 2A mutation. Proc Natl Acad Sci USA. 1997;94:3330–3335. doi: 10.1073/pnas.94.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acton DS, Velthuyzen D, Lips CJ, Hoppener JW. Multiple endocrine neoplasia type 2B mutation in human RET oncogene induces medullary thyroid carcinoma in transgenic mice. Oncogene. 2000;19:3121–3125. doi: 10.1038/sj.onc.1203648. [DOI] [PubMed] [Google Scholar]
- 73.Johnston D, Hatzis D, Sunday ME. Expression of v-Ha-ras driven by the calcitonin/calcitonin gene-related peptide promoter: a novel transgenic murine model for medullary thyroid carcinoma. Oncogene. 1998;16:167–177. doi: 10.1038/sj.onc.1201478. [DOI] [PubMed] [Google Scholar]
- 74.Matias-Guiu X. Mixed medullary and follicular carcinoma of the thyroid. On the search for its histogenesis. Am J Pathol. 1999;155:1413–1418. doi: 10.1016/S0002-9440(10)65453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harach HR, Williams ED. Glandular (tubular and follicular) variants of medullary carcinoma of the thyroid. Histopathology. 1983;7:83–97. doi: 10.1111/j.1365-2559.1983.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 76.Wollman SH, Hilfer SR. Embryologic origin of the various epithelial cell types in the second kind of thyroid follicle in the C3H mouse. Anat Rec. 1978;191:111–121. doi: 10.1002/ar.1091910110. [DOI] [PubMed] [Google Scholar]
- 77.Hansford JR, Mulligan LM. Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet. 2000;37:817–827. doi: 10.1136/jmg.37.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vantyghem MC, Pigny P, Leteurtre E, Leclerc L, Bauters C, Douillard C, et al. Thyroid carcinomas involving follicular and parafollicular C cells: seventeen cases with characterization of RET oncogenic activation. Thyroid. 2004;14:842–847. doi: 10.1089/thy.2004.14.842. [DOI] [PubMed] [Google Scholar]
- 79.Volante M, Papotti M, Roth J, Saremaslani P, Speel EJ, Lloyd RV, et al. Mixed medullary-follicular thyroid carcinoma. Molecular evidence for a dual origin of tumor components. Am J Pathol. 1999;155:1499–1509. doi: 10.1016/S0002-9440(10)65465-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi S, Fugazzola L, De Pasquale L, Braidotti P, Cirello V, Beck-Peccoz P, et al. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumour: report of three cases with molecular analysis and review of the literature. Endocr Relat Cancer. 2005;12:281–289. doi: 10.1677/erc.1.00901. [DOI] [PubMed] [Google Scholar]
- 81.Hales M, Rosenau W, Okerlund MD, Galante M. Carcinoma of the thyroid with a mixed medullary and follicular pattern: morphologic, immunohistochemical, and clinical laboratory studies. Cancer. 1982;50:1352–1359. doi: 10.1002/1097-0142(19821001)50:7<1352::aid-cncr2820500722>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 82.Pfaltz M, Hedinger CE, Muhlethaler JP. Mixed medullary and follicular carcinoma of the thyroid. Virchows Arch A Pathol Anat Histopathol. 1983;400:53–59. doi: 10.1007/BF00627008. [DOI] [PubMed] [Google Scholar]
- 83.Gurkan E, Gurbuz Y, Tarkun I, Canturk Z, Cetinarslan B. Mixed medullary-papillary carcinoma of the thyroid: report of two cases and review of the literature. Indian J Pathol Microbiol. 2014;57:598–602. doi: 10.4103/0377-4929.142684. [DOI] [PubMed] [Google Scholar]
- 84.Papotti M, Negro F, Carney JA, Bussolati G, Lloyd RV. Mixed medullary-follicular carcinoma of the thyroid. A morphological, immunohistochemical and in situ hybridization analysis of 11 cases. Virchows Arch. 1997;430:397–405. doi: 10.1007/s004280050049. [DOI] [PubMed] [Google Scholar]
- 85.Goyal R, Nada R, Rao KL, Radotra BD. Mixed medullary and follicular cell carcinoma of the thyroid with lymph node metastasis in a 7-year-old child. Pathol Int. 2006;56:84–88. doi: 10.1111/j.1440-1827.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 86.Kovacs CS, Mase RM, Kovacs K, Nguyen GK, Chik CL. Thyroid medullary carcinoma with thyroglobulin immunoreactivity in sporadic multiple endocrine neoplasia type 2-B. Cancer. 1994;74:928–932. doi: 10.1002/1097-0142(19940801)74:3<928::aid-cncr2820740321>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 87.Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, et al. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448–1453. doi: 10.1093/jnci/89.19.1448. [DOI] [PubMed] [Google Scholar]
- 88.Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, et al. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963–971. doi: 10.1097/01.lab.0000079006.91414.be. [DOI] [PubMed] [Google Scholar]
- 89.Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro J, Silva P, et al. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol. 2004;17:819–826. doi: 10.1038/modpathol.3800124. [DOI] [PubMed] [Google Scholar]
- 90.Harmon BG, Kelley LC. Immunohistochemistry of ultimobranchial thyroid carcinomas in seven slaughtered cows and one bull. J Vet Diagn Invest. 2001;13:101–105. doi: 10.1177/104063870101300201. [DOI] [PubMed] [Google Scholar]
- 91.Reynolds L, Jones K, Winton DJ, Cranston A, Houghton C, Howard L, Ponder BA, Smith DP. C-cell and thyroid epithelial tumours and altered follicular development in transgenic mice expressing the long isoform of MEN 2A RET. Oncogene. 2001;20:3986–3994. doi: 10.1038/sj.onc.1204434. [DOI] [PubMed] [Google Scholar]
- 92.Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene. 2008;27(suppl 2):S9–S18. doi: 10.1038/onc.2009.349. [DOI] [PubMed] [Google Scholar]
- 93.Brauckhoff M, Gimm O, Hinze R, Ukkat J, Brauckhoff K, Dralle H. Papillary thyroid carcinoma in patients with RET proto-oncogene germline mutation. Thyroid. 2002;12:557–561. doi: 10.1089/105072502320288393. [DOI] [PubMed] [Google Scholar]
- 94.Melillo RM, Cirafici AM, De Falco V, Bellantoni M, Chiappetta G, Fusco A, et al. The oncogenic activity of RET point mutants for follicular thyroid cells may account for the occurrence of papillary thyroid carcinoma in patients affected by familial medullary thyroid carcinoma. Am J Pathol. 2004;165:511–521. doi: 10.1016/S0002-9440(10)63316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pueblitz S, Weinberg AG, Albores-Saavedra J. Thyroid C cells in the DiGeorge anomaly: a quantitative study. Pediatr Pathol. 1993;13:463–473. doi: 10.3109/15513819309048236. [DOI] [PubMed] [Google Scholar]
- 96.Yaday S, Singh I, Singh J, Aggarwal N. Medullary carcinoma in a lingual thyroid. Singapore Med J. 2008;49:251–253. [PubMed] [Google Scholar]
- 97.Vandernoot I, Sartelet H, Abu-Khudir R, Chanoine JP, Deladoey J. Evidence for calcitonin-producing cells in human lingual thyroids. J Clin Endocrinol Metab. 2012;97:951–956. doi: 10.1210/jc.2011-2772. [DOI] [PubMed] [Google Scholar]
- 98.Parlato R, Rosica A, Rodriguez-Mallon A, Affuso A, Postiglione MP, Arra C, et al. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol. 2004;276:464–475. doi: 10.1016/j.ydbio.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 99.Fagman H, Amendola E, Parrillo L, Zoppoli P, Marotta P, Scarfo M, et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev Biol. 2011;359:163–175. doi: 10.1016/j.ydbio.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trueba SS, Augé J, Mattei G, Etchevers H, Martinovic J, Czernichow P, Vekemans M, Polak M, Attié-Bitach T. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab. 2005;90:455–462. doi: 10.1210/jc.2004-1358. [DOI] [PubMed] [Google Scholar]