Abstract
The defects of articular cartilage in the knee joint are a common degenerative disease and currently there are several established techniques to treat this problem, each with their own advantages and shortcomings. Autologous chondrocyte implantation is the current gold standard but the technique is expensive, time-consuming and most versions require two stage procedures and an arthrotomy. Autologous collagen induced chondrogenesis (ACIC) is a single-stage arthroscopic procedure and we developed. This method uses microfracture technique with atelocollagen mixed with fibrin gel to treat articular cartilage defects. We introduce this ACIC techniques and its scientific background.
Keywords: Knee arthroscopy, Cartilage defect, Atelocollagen, ACIC, Single stage, Cartilage repair
1. Introduction
Articular cartilage is a hyaline cartilage, which is avascular, aneural, and low in metabolic activity. Repairing of cartilage damage resulting from trauma or degeneration has been a challenging clinical problem.1
Currently, there are several established techniques to treat this problem, each with their own advantages and shortcomings. Microfracture is technically simple and cost-effective but has been repeatedly shown to produce predominantly fibrous cartilage, especially for large lesions.2 Osteochondral grafting is effective but limited by the size and location of the lesions.3 Autologous chondrocyte implantation is the current gold standard but the technique is expensive, time-consuming and most versions require two stage procedures and an arthrotomy.4, 5
In recent years, research has shown successful use of cell free collagen type I gels and scaffolds, combined with marrow stimulation techniques to treat articular cartilage defects.6, 7, 8, 9, 10, 11, 12, 13 Atelocollagen is a highly purified type I collagen obtained following the treatment of skin dermis with pepsin and removal of telopeptide. This makes it non-immunogenic with physical properties similar to natural, insoluble collagen; an ideal scaffold for tissue regeneration.
2. Basic science – cell survival and chondrogenic differentiation of mesenchymal stem cells in the fibrin and atelocollagen mixture
2.1. Culture of human mesenchymal stem cells (hMSCs)
Human mesenchymal stem cells (hMSCs) in passage 2 and derived from bone marrow (Promocell, Germany) were cultured for two more passages using Promocell's MSC growth medium and according to the manufacturer's instructions. A proliferation medium consisting of Dulbecco's modified Eagle's medium-low glucose (DMEM-LG, PAA, Austria), 20% fetal bovine serum (FBS, PAA, Austria), 100 U/mL penicillin, and 100 μg/mL streptomycin was used for further passages unless otherwise specified. Cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2. After washing with Hank's Balanced Salt Solution without calcium or magnesium (HBSS, PAA, Austria), 0.25% Trypsin/1 mM EDTA (Gibco-Invitrogen, USA) was used to detach cells for subculture. hMSCs expanded up to the 4th passages were used for further experiments.
2.2. Encapsulation of MSCs in collagen gel beads
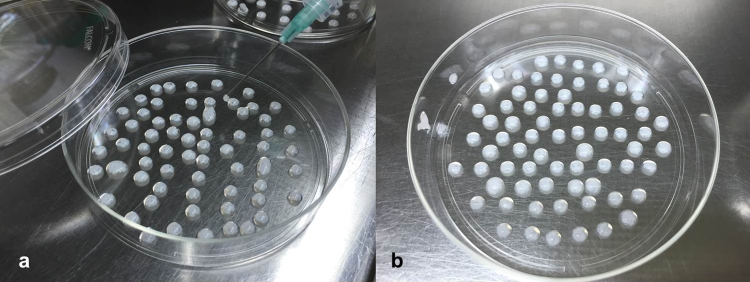
Mesenchymal stem cells at a final concentration of 1 × 106 cells/mL were mixed with porcine type I atelocollagen solution (UBIOSIS, Seongnam, South Korea). The mixture was then dropped onto a sterile Petri dish (Fig. 1a). The drops were incubated for 45 min at room temperature in order to induce collagen gelling (Fig. 1b). The beads produced were then collected by adding warmed culture media to detach them from dishes. The beads were distributed in four, 50-mL, conical centrifuge tubes (BD-Falcon, USA). The volume of each drop was 60 μL and 100,000 cells were included in each drop. About 60 drops were added to each 50 mL tube.
Fig. 1.
(a) Dropping atelocollagen and fibrin mixture on the Petri dish. (b) Gelling and bead formation after incubation.
Proliferation medium was added to each tube and was incubated for 2 days in a 5% CO2 incubator (Hercules, USA) at 37 °C in order to allow the cells to adapt to the bead culture environment. Beads in the four tubes were allocated to two groups, i.e. the control group and the chondrogenic differentiation (CH) group. The control beads were cultured in proliferation medium, while the CH beads were cultured in chondrogenic differentiation medium during the differentiation culture period. The chondrogenic differentiation medium consisted of Dulbecco's modified Eagle's medium–high glucose (DMEM-HG, PAA, Austria) containing 10 ng/mL transforming growth factor beta 3 (TGF-β3), 25 μg/mL ascorbic acid, 25 μM ascorbic acid-2-phosphate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1% (v/v) ITS plus (all from Sigma–Aldrich, USA). The media were changed twice weekly for 3 weeks.
2.3. Analysis of chondrogenic phenotype differentiation
MSC beads were washed twice with HBSS after removal of the culture medium by suction. The beads were then incubated at 37 °C in a 5% CO2-humidified atmosphere.
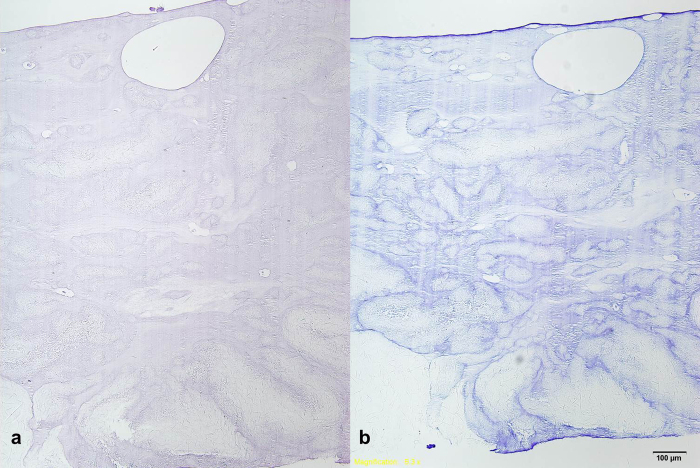
10 mg/mL safranin-O (Sigma–Aldrich) in distilled water was used to detect glycosaminoglycan in the samples (Fig. 2a). Toluidin blue solution was applied for 12 h at room temperature after washing the fixed beads with distilled water and 3% acetic acid (Fig. 2b).
Fig. 2.
Analysis of chondrogenic differentiation. (a) Safranin-O staining of collagen gel bead. (b) Toluidin blue staining of collagen gel bead shows chondrogenic matrix formation.
2.4. Microstructures in the MSC-collagen gel beads
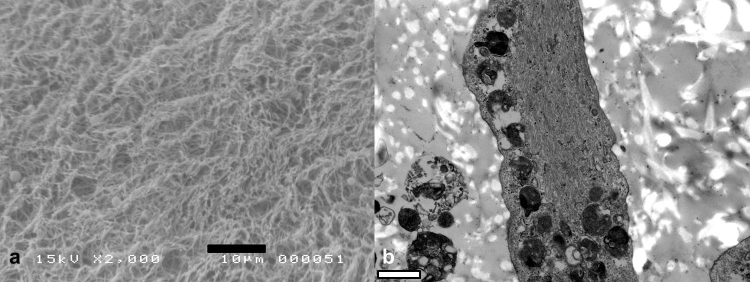
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to analyze the microstructural morphologies in MSC-collagen gel beads cultured under the chondrogenic condition. Briefly, beads were fixed in 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1-M phosphate buffer overnight, were then washed in 0.1 M phosphate buffer, and were then post-fixed with 1% osmium tetroxide in the same buffer for 1 h. The beads were dehydrated using a graded ethyl alcohol series and acetone and were then embedded in Epon 812 (epoxy resin) for TEM processing. Ultrathin sections (70–80 nm) were obtained using an ultramicrotome (Leica Ultracut UCT, Germany), were double-stained with uranyl acetate/lead citrate, and were then examined using a JEM 1010 unit (JEOL, Tokyo, Japan) at 60 kV. After dehydration, specimens were transferred to hexametyldisilazane (HMDS) and allowed to air dry for SEM processing. All samples were coated with gold using a sputter coater and were examined using a JSM-5410LV unit (JEOL, Japan). The SEM was operated at an accelerating voltage of 15 kV (Fig. 3).
Fig. 3.
(a) Scanning electron microscopy of the MSC-collagen gel beads. (b) Transmission electron microscopy of MSC-collagen gel beads cultured in a chondrogenic differentiation condition for 21 days revealed newly-biosynthesized collagen fibers incorporated cells were visible in the TEM pictures of collagen-MSC hydrogel beads.
2.5. Cell distribution and viability in the collagen gel beads
Fluorescent dyes were used to distinguish viable cells from dead cells in the slices of incubated MSC-collagen beads. In brief, the beads were cut into 1-mm sections using a blade after 4 days of cultivation in the proliferation medium. The sections were incubated in the proliferation medium containing 2 μM Calcein acetoxymethylester (Sigma, USA) and 4 μM Ethidium homodimer (Sigma, USA) for 30 min before examination using fluorescence microscopy.
Regenerative medicine approaches based on decellularized extracellular matrix (ECM) scaffolds and tissues are rapidly expanding. This article presents the encouraging 4-year clinical and radiological results using acellular matrix. This is a single stage athroscopic technique using microdrilling and implantation of a cell-free atelocollagen with fibrin gel. This results in growth of hyaline-like cartilage with minimal morbidity to the patient (Fig. 4).
Fig. 4.

Biochemical staining for the sections from MSC-collagen gel beads. Fluorescent dye staining of the sections from 4-day incubated beads (original magnification, ×40). Viable cells were shown green and dead cells were shown red by staining with Calcein acetoxymethylester and Ethidium Homodimer, respectively.
3. Materials and methods
3.1. Patients
This is a cohort of symptomatic patients with demonstrable lesions who were treated with the described technique. The inclusion criteria were as follows:
-
•
Age 18–65 years
-
•
Demonstrated isolated ICRS/Outerbridge grade III/IV lesions in the knee, 2–8 cm2
-
•
Fit to give informed consent and undergo surgery with subsequent rehabilitation.
The exclusion criteria were:
-
•
Malalignment of the knee exceeding 5° of valgus or varus.
-
•
Generalized osteoarthritic changes in the knee.
3.2. Surgical technique
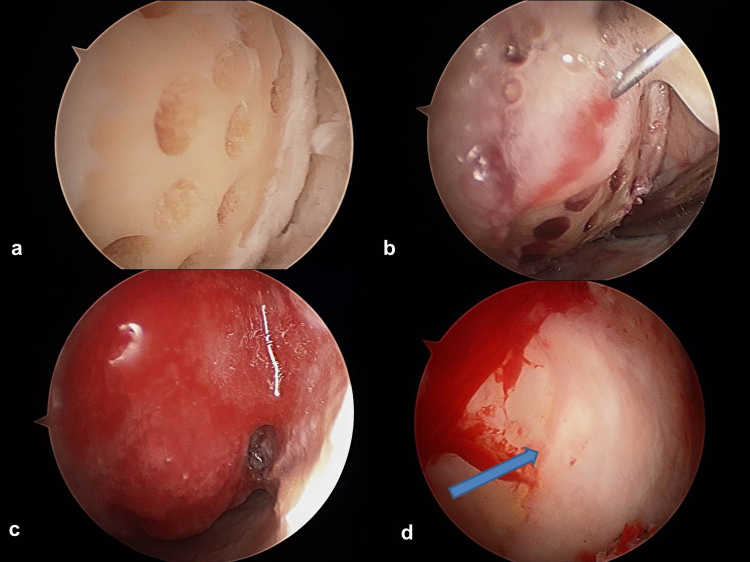
All procedures were performed under general anesthesia. The standard anterolateral and anteromedial arthroscopic portals were used to evaluate the knee using normal saline under pressure (approximately systolic blood pressure). After assessing the defects, the lesions were debrided down to the subchondral bone using a curette and shaver. A stable shoulder was established at the margin of the defect. Sclerotic subchondral bone is removed by a gentle bur. Microdrilling was performed using the 45° angled drill (Powerpick drill; Arthrex Ltd., Sheffield, United Kingdom). Drill holes were made at 3-mm intervals to a depth of 6 mm (Fig. 5a).
Fig. 5.
ACIC arthroscopic procedure. (a) Microdrilling. (b) Atelocollagen mixed with fibrin injection to the prepared defect. (c) Stable attachment of atelocollagen (Coltrix) mixture. (d) 2nd look arthroscopic finding of post-operative 1 year.
The second part of the procedure was performed under dry arthroscopic conditions using carbon dioxide (CO2) insufflation. Carbon dioxide was introduced at 20 mmHg pressure with a flow rate of 20 L/min using a Wolf cannula (Karl Storz GmbH, Tuttlingen, Germany) and disposable tubing with a filter (Insufflation tubing with Wolf adaptor; Leonhard Lang UK Ltd., Stroud, United Kingdom) through a superolateral portal (Fig. 6).
Fig. 6.
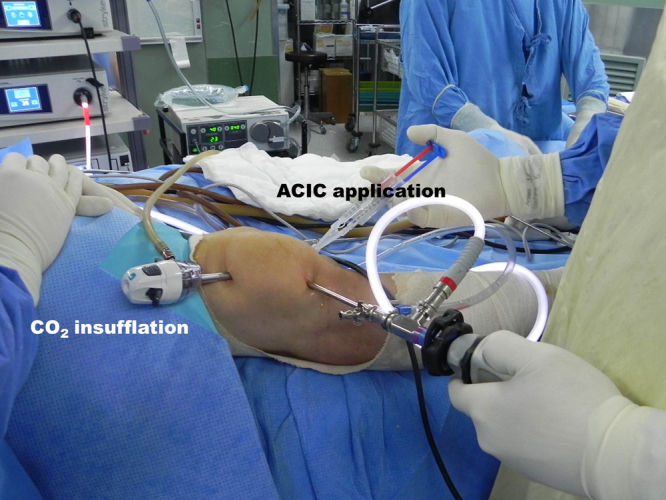
Arthroscopic setting for ACIC operation.
To make the joint completely dry, residual normal saline in the joint was aspirated using gentle suction with a 20-mL syringe and an angled suction tube (Exmoor, Taunton, United Kingdom). The lesions were dried using cotton buds, which were introduced through the joint using plastic tubing. For patellar and trochlear lesions, a patellar clamp (AO or Lewin bone clamp [DePuy Synthes Ltd., Hertfordshire, United Kingdom]) was applied to lift the patella and further open the joint.
For the injection procedure, two 1-mL syringes and a Y-shaped mixing catheter connected to a 20-gauge needle (inner diameter, 0.9 mm; length, 90 mm) were used. One syringe was filled with 1 mL of fibrinogen (Tisseel; Baxter, Thetford, United Kingdom), and the other syringe was filled with 0.9 mL of atelocollagen (Coltrix™, Ubiosis, Seongnam, Korea) and 0.1 mL of thrombin (50 IU) (Fig. 7). The 20-gauge needle was inserted through 1 of the portals or an appropriate separate portal to access the lesion.
Fig. 7.
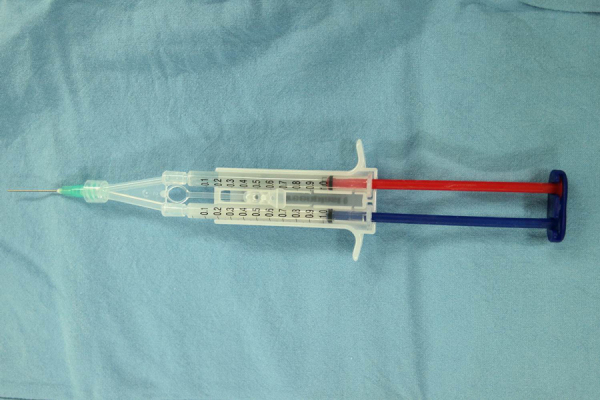
Injection preparation with one syringe of thrombine mixed with atelocollagen and the other syringe of fibrinogen.
Under arthroscopic vision, the gel was applied into the defect. The CO2 pressure and the adhesiveness of the gel allowed attachment even against gravity, especially to patellar defects. Once the first layer of gel was applied and after a delay of 1–2 min, the gel was injected under the first layer to achieve full defect filling. The gel usually hardened within 5 min, and then it was shaped in situ using a McDonalds dissector (Bolton Surgical Ltd., Sheffield, United Kingdom) (Fig. 5b and c).
Once this was established, the CO2 was switched off, and the knee was insufflated with normal saline. The stability of the graft was further ascertained by moving the knee through a full range of motion several times followed by visual inspection. The skin was closed with sutures or steri-strips depending on the oozing from the arthroscopic portals.
3.3. Rehabilitation
All patients followed the same standardized regimen to optimize results. All patients had CPM soon after surgery and were advised to partially weight bear on the affected leg for 6 weeks post-operatively, gradually increasing loads with full weight bearing at the end of 6 weeks. Additionally, patients with patello-femoral lesions, flexion was restricted with a range movement removable brace to 20° in the first 2 weeks and gradually increased to 90° over 6 weeks. Return to contact sports and running was allowed at 9–12 months.
3.4. Assessment
Clinical improvement was assessed with the Lysholm, IKDC and KOOS score. Radiologically, all patients were assessed with a specific cartilage magnetic resonance imaging (MRI) protocol using fast spin-echo and double-echo steady-state sequences preoperatively and post-operatively. A 1.5-T MRI unit (Siemens Avanto; Siemens Healthcare, Inc., Erlangen, Germany) and a dedicated multichannel knee coil were used. High field strength MRI (1.5 or 3 T) resolution has been shown to identify most chondral lesions.14, 15, 16 Structural properties of the lesions, pre- and post-operatively were quantified using the MOCART score17; with 0 being the worst score and 100 the best. Qualitative assessment of the repair tissue was carried out with T2* mapping and d-GEMRIC at 12 months and at 18 months respectively.
4. Results
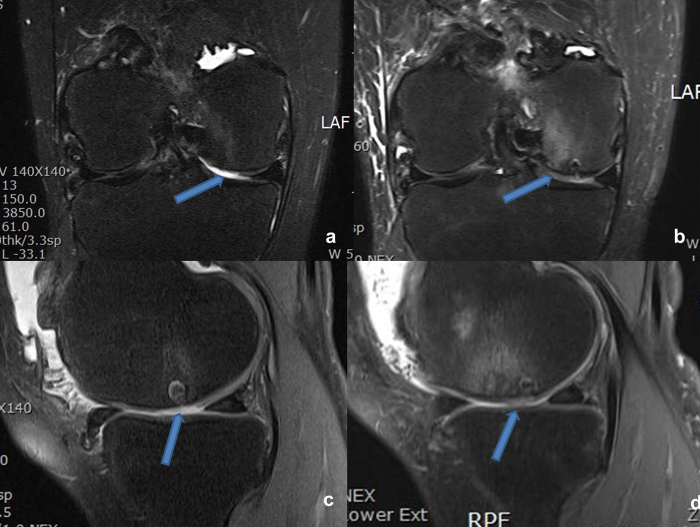
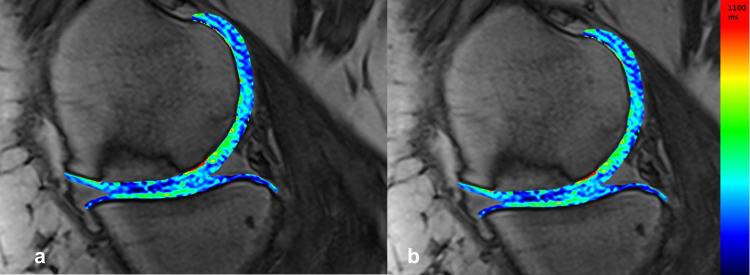
There were 30 patients in this study. The lesions were located on the MFC, LFC, trochlea or patella, ranging from 2 to 8 cm2. At 4-year follow-up, Lysholm score was 80.4, as compared to 50.8 pre-operatively (p < 0.05). KOOS (symptomatic) was 88.2, as compared to 64.7 pre-operatively (Fig. 5d). IKDC (subjective) was 78.6, up from 39 preoperatively. Average MOCART score for all lesions was 72. The mean T2* relaxation-times for the repair tissue and native cartilage were 26.4 and 29.9 respectively (Fig. 8, Fig. 9).
Fig. 8.
MRI findings of 60 years old woman with articular cartilage defect of right medial femoral condyle. (a and c) Preoperative MRI and arrow indicating lesion. (b and d) Postoperative 1 year of MRI and arrow indication repaired lesion.
Fig. 9.
(a and b) On the T2* map, one can observe similar T2* values in the cartilage repair tissue (34 ± 6 ms) in comparison to the surrounding weight-bearing native cartilage (36 ± 6 ms)
5. Discussion
When this technique was first published,7 it was, to the best of our knowledge, the first report of an arthroscopic single-stage method using CO2 insufflation and atelocollagen gel for cartilage repair (ACIC-Shetty–Kim). The authors demonstrated good clinical results at 2-year follow-up after ACIC. However, other centers have also reported successful cartilage repair using collagen gel or collagen scaffolds in the knee.6, 8, 9, 10, 11, 12, 13 The technique described here is different in its use of CO2 for insufflation instead of air to inflate the joint. Carbon dioxide has an excellent safety profile and is widely used in general abdominal surgery.18, 19, 20 In fact, animal studies have shown no adverse effects on continuous venous infusion of CO2.21
Certain cartilage repair procedures use collagen membranes which are transplanted directly onto the defect. The defect has to be sufficiently deep to achieve a satisfactory press-fit. In shallower defects the membrane needs to be sutured for stability. The atelocollagen and fibrin mixture, by virtue of its gel form and clotting properties, remains stable at shallow defects. Collagen is the connective tissue protein that helps maintain tissue morphology, and atelocollagen is a highly purified type I collagen that was obtained following the treatment of pepsin from the skin dermis. For this technique, the collagen was obtained from porcine skin. By removing the telopeptide, which is the immunologically active component, purified atelocollagen is sourced. This immunologically inactive product is used for wound healing, vessel prosthesis, bone substitute and hemostasis.
The authors advise against the use of a circular punch or large diameter drill to debride the defect. Instead, the surrounding native articular cartilage can be preserved using a curette and shaver during debridement. For this technique, the shape of defect is immaterial as the collagen and fibrin mixture can be molded to fit any shape. It is important to remove the subchondral sclerotic bone using a gentle burr without destroying the subchondral plate.
Once implanted, the fibrin and collagen mixture can retain its shape in the defect approximately 5 min after injection due to the reaction between the thrombin and fibrinogen in the 2-way syringe. Additionally the joint can be taken through its range of motion to further sculpt the implant. Of late, there is ample evidence supporting fibrin as a suitable material for cartilage repair surgery.22, 23
Chen et al. have demonstrated that microdrilling is preferable to microfracture, as it forms better channels to bone marrow stroma, facilitating stem cell recruitment.24 These channels also increase the adhesive force of the graft to the defect and also the rotational stability during knee range of motion.
Our technique shows encouraging clinical results at 4-year follow-up. Morphological MRI and MOCART scores have demonstrated good cartilage growth; these are comparable to other similar studies.6, 25, 26 A quantitative MRI evaluation with T2-mapping and delayed gadolinium enhanced (d-GEMRIC) scan of cartilage has also suggested hyaline-like repair tissue with significant benefit to patients (Fig. 9).
6. Conclusion
A single-stage arthroscopic procedure using microdrilling combined with atelocollagen and fibrin gel appears to be an effective cartilage regeneration method.
Conflicts of interest
The authors have none to declare.
Acknowledgements
This research was co-supported by the Global High-tech Biomedicine Technology Development Program of the National Research Foundation (NRF) & Korea Health Industry Development Institute (KHIDI) funded by the Korean Government (MSIP&MOHW) (2015M3D6A1065464) and Clinical Research Laboratory Foundation of Uijeongbu St. Mary's Hospital, The Catholic University of Korea.
References
- 1.Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Hjelle K., Solheim E., Strand T., Muri R., Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 3.Bedi A., Feeley B.T., Williams R.J., III Management of articular cartilage defects of the knee. J Bone Jt Surg Am. 2010;92(4):994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 4.Knutsen G., Engebretsen L., Ludvigsen T.C. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Jt Surg Am. 2004;86(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M., Lindahl A., Nilsson A. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Efe T., Theisen C., Fuchs-Winkelmann S. Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1915–1922. doi: 10.1007/s00167-011-1777-5. [DOI] [PubMed] [Google Scholar]
- 7.Shetty A.A., Kim S.J., Bilagi P., Stelzeneder D. Autologous collagen-induced chondrogenesis: single-stage arthroscopic cartilage repair technique. Orthopedics. 2013;36(5):e648–e652. doi: 10.3928/01477447-20130426-30. [DOI] [PubMed] [Google Scholar]
- 8.Benthien J.P., Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 9.Dhollander A.A., De Neve F., Almqvist K.F. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):536–542. doi: 10.1007/s00167-010-1337-4. [DOI] [PubMed] [Google Scholar]
- 10.de Girolamo L., Bertolini G., Cervellin M., Sozzi G., Volpi P. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41(11):1172–1177. doi: 10.1016/j.injury.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Benthien J.P., Behrens P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76(2):260–263. [PubMed] [Google Scholar]
- 12.Gille J., Schuseil E., Wimmer J. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 13.Kusano T., Jakob R.P., Gautier E. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC) Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109–2115. doi: 10.1007/s00167-011-1840-2. [DOI] [PubMed] [Google Scholar]
- 14.Roemer F.W., Crema M.D., Trattnig S., Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260(2):332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 15.Kojima K.Y., Demlow T.A., Szumowski J., Quinn S.F. Coronal fat suppression fast spin echo images of the knee: evaluation of 202 patients with arthroscopic correlation. Magn Reson Imaging. 1996;14(9):1017–1022. doi: 10.1016/s0730-725x(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 16.Marlovits S., Striessnig G., Resinger C.T. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Marlovits S1, Singer P., Zeller P., Mandl I., Haller J., Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Hirai F., Beppu T., Nishimura T. Carbon dioxide insufflation compared with air insufflation in double-balloon enteroscopy: a prospective, randomized, double-blind trial. Gastrointest Endosc. 2011;73(4):743–749. doi: 10.1016/j.gie.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Nonaka S., Saito Y., Takisawa H. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24(7):1638–1645. doi: 10.1007/s00464-009-0824-5. [DOI] [PubMed] [Google Scholar]
- 20.Dellon E.S., Hawk J.S., Grimm I.S., Shaheen N.J. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69(4):843–849. doi: 10.1016/j.gie.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 21.Corson S.L., Hoffman J.J., Jackowski J., Chapman G.A. Cardiopulmonary effects of direct venous CO2 insufflation in ewes. A model for CO2 hysteroscopy. J Reprod Med. 1988;33(5):440–444. [PubMed] [Google Scholar]
- 22.Kim M.K., Choi S.W., Kim S.R., Oh I.S., Won M.H. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):528–534. doi: 10.1007/s00167-009-0905-y. [DOI] [PubMed] [Google Scholar]
- 23.Domayer S.E., Welsch G.H., Nehrer S. T2 mapping and dGEMRIC after autologous chondrocyte implantation with a fibrin-based scaffold in the knee: preliminary results. Eur J Radiol. 2010;73(3):636–642. doi: 10.1016/j.ejrad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Chen H., Sun J., Hoemann C.D. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;27(11):1432–1438. doi: 10.1002/jor.20905. [DOI] [PubMed] [Google Scholar]
- 25.Dhollander A.A., Verdonk P.C., Lambrecht S. The combination of microfracture and a cell-free polymer-based implant immersed with autologous serum for cartilage defect coverage. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1773–1780. doi: 10.1007/s00167-011-1763-y. [DOI] [PubMed] [Google Scholar]
- 26.Krusche-Mandl I., Schmitt B., Zak L. Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthr Cartil. 2012;20(5):357–436. doi: 10.1016/j.joca.2012.01.020. [DOI] [PubMed] [Google Scholar]