Abstract
Various approaches to treat articular cartilage have been widely investigated due to its poor intrinsic healing capacity. Stem cell-based therapy could be a promising approach as an alternative to chondrocyte-based therapy and some of these therapies have been already applied in clinical condition. This review discusses the current development of stem cell-based therapies in cartilage repair, specifically focusing on scaffold-free approaches.
Keywords: Stem cell-based therapy, Scaffold free, Cartilage repair, Mesenchymal stem cell
1. Introduction
It is widely accepted that chondral injuries usually do not heal spontaneously. Therefore, a variety of approaches have been tested to improve cartilage healing.
Bone marrow stimulations, such as microfracture and subchondral drilling, are commonly applied first-line treatments for symptomatic small articular cartilage defects.1, 2, 3 These procedures establish a communication of the cartilage defect with the bone marrow, by focal perforation of the subchondral plate allowing bone marrow cells to migrate into the chondral lesion and to stimulate formation of fibrocartilaginous tissue.4 Repair tissues generated by bone marrow stimulation are not hyaline cartilage but fibrocartilaginous tissue, which is biochemically and biomechanically inferior to native hyaline cartilage.5, 6 Therefore, decrease in long-term clinical outcomes have been reported.6, 7, 8
Osteochondral autograft transfer (OAT) (or mosaicplasty) is another long-standing surgery. In this procedure, one or more cylindrical osteochondral autografts from a non-weight-bearing area of articular cartilage are transferred to the chondral lesions. It had demonstrated positive clinical benefits for young patients with an active lifestyle.9 Lynch et al. reported in a systemic review that compared to microfracture, OAT/mosaicplasty offers patients better clinical outcomes, with a higher rate of return to sport and maintenance of their sports activity. When compared with autologous chondrocyte implantation (ACI), improvement of clinical outcomes was not conclusive; however, at 10-year follow-up, a greater failure rate was found to be present in the OAT/mosaicplasty group. They also suggested that OAT/mosaicplasty procedures might be more appropriate for lesions that are smaller than 2 cm2 with the known risk of failure between 2 and 4 years.10 Pareek et al. concluded in a systemic review that OAT showed successful outcomes in 72% of patients at mean follow-up of 10.2 years and concomitant surgical procedures negatively correlated with failure rate.11
Autologous chondrocyte implantation (ACI) was firstly demonstrated by Brittberg et al.12 During ACI procedure, chondrocytes are isolated from the cartilage specimen harvested from non-weight-bearing area in the knee joint, and then, chondrocytes were culture expanded in vitro for subsequent implantation to the chondral lesions. Cultured chondrocytes were covered with autologous periosteal patch in the first-generation ACI and collagen type I/III membrane in the second-generation ACI. First-generation ACI has been shown to be associated with symptomatic chondral hypertrophy that requires subsequent shaving at a greater rate than second-generation ACI.13 Both, first- and second-generation ACI are technically demanding because these procedure require suturing of the patch to the adjacent cartilage. Third-generation ACI (matrix-associated autologous chondrocyte implantation (MACI)) is transplantation of cultured chondrocytes to the lesion with biomaterials made of either synthetic or natural polymers as the scaffolds.14, 15 MACI is technically less challenging because it does not require suturing technique and therefore is easy for surgeons to handle. Recently, Oussedik et al. reported in a systematic review that MACI has been shown to be more effective than microfracture16 and Goyal et al. reported in another systematic review that either the second- or third-generation ACI procedure demonstrated better clinical outcomes than did the first generation, but with weak evidence.17 Within each ACI procedure, the best technique has not been proven due to the great variety of techniques, absence of long-term follow-up, and heterogeneity of outcome measures.16
Both of the OAT and ACI has limitation regarding the sacrifice of the undamaged cartilage and the donor site morbidity.9, 18 In addition, dedifferentiation of chondrocytes during in vitro culture is a major concern about the ACI. Culture expansion of chondrocytes in a 2D environment is thought to lead to alterations in cellular phenotype, thereby compromising repair efficacy.19, 20 Tissue engineering approaches using chondrocytes also have the same limitations.21
After all, “gold standard” for the cartilage repair is still lacking, and therefore, stem cell therapy for cartilage repair has caught researcher's and clinician's attention as a next-generation therapy over the past decade.
2. Stem cell therapy for cartilage repair
Transplantation of autologous mesenchymal stem cells (MSC) is an attractive strategy to repair articular cartilage compared with the transplantation of articular chondrocytes.22 MSCs have a potent differentiation capacity to the mesodermal lineage (chondrocytes, osteoblasts, and adipocytes). MSC can be isolated from various tissues, such as bone marrow, synovium, adipose tissue, and skeletal muscle.23, 24, 25, 26, 27 The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy had defined the standard criteria for uniform characterization of MSCs: they must be plastic-adherent cells when maintained in standard culture conditions; they must express CD105, CD73, and CD90; they must lack surface expression of CD45, CD34, CD14 (CD11b), CD79α (CD19), and HLA-DR; and must be capable of differentiating to cells of the mesodermal lineage.28, 29, 30
As an alternative options for a cell source, allogeneic MSCs31 or induced pluripotent stem (iPS) cells32, 33 may also be considered. However, there have not been much evidence about clinical safety of these cells, and thus further studies are needed to apply these cells in clinical condition. Regarding the safety of MSC, Wakitani et al. who transplanted autologous bone marrow MSC to repair articular cartilage in their clinical trial firstly in the world,34 reported long-term safety of MSC followed for up to 11 years and 5 months.35
3. Type of MSC
In 1966, Friedenstein proved that bone marrow includes progenitor cells that can generate connective tissue-forming cells.23 In the 1980–1990s, many researchers extended these observations and demonstrated that the cells identified by Frirdenstein had multipotency to differentiate into osteoblasts, chondrocytes, and adipocytes.36, 37, 38, 39 Caplan et al. named these cells as mesenchymal stem cells.24 It is notable that bone marrow MSCs are most widely studied as a cell source of stem cell therapy and applied clinically.30, 40, 41 However, aspiration of bone marrow is an invasive and painful procedure, often requiring anesthesia and often with attendant morbidity.42
In 2001, Zuk et al. identified adipose tissue-derived MSCs from lipoaspirates, which have multilineage potential.26 However, several investigators demonstrated that the chondrogenic capacity of adipose tissue-derived MSCs is not as extensive as that of bone marrow MSCs.43, 44, 45, 46
In 2001, De Bari et al. identified synovial MSCs from human synovium.25 There have been several reports demonstrating that synovial MSCs have the greatest chondrogenic potential compared with MSCs from the other tissues.47, 48, 49 In addition, multipotency of synovial MSCs is not influenced by donor age or cell passages, and synovial MSCs have less senescence and great proliferative capacity.25, 47 Koizumi et al. reported that synovial MSCs from patients with osteoarthritis or rheumatoid arthritis are no less appropriate for repairing cartilage than those from trauma patients.50 To harvest synovial membrane, arthroscopic surgery is needed, but the procedure is less invasive than bone marrow aspiration because arthroscopic surgeons usually remove and discard synovial membrane to get clear vision during arthroscopy. Thus, synovial MSCs could be an attractive cell source for tissue engineering in cartilage repair.46
4. With or without exogenous 3-dimensional scaffold
In 1998, Wakitani et al. transplanted autologous bone marrow MSC to repair articular cartilage, which was the first clinical trial ever reported in the world.34 They transplanted MSC suspension in collagen gel and covered it with autologous periosteum. Thereafter, other researchers have reported good clinical outcomes using MSC suspension.51, 52, 53 Nejadnik reported direct comparison of first-generation ACI with transplantation of bone marrow MSC suspension in the same surgical procedure and concluded that using MSCs in cartilage repair is as effective as chondrocytes to repair articular cartilage.54 However, in these techniques, the transplanted MSCs do not contain extracellular matrix (ECM) and therefore it must be difficult to maximize the cellular function of transplanted cells because appropriate 3-dimensional (3D) environment is an essential factor to optimize cell proliferation and chondrogenic differentiation.55
In this regard, 3D scaffolds have been investigated to enhance repair of the chondral lesions. Additional advantage of using 3D scaffolds is better handling to deliver MSC to the chondral lesion and possible barrier effect against fibroblast invasion of the graft that may otherwise induce fibrous repair.56 As an alternative to transplantation of MSC in cell suspension, various 3D scaffolds, such as synthetic polymers,57, 58 natural polymers extracted from different species,59, 60 collagen,61 fibrin,62 and hyaluronan63 have been investigated to transplant MSCs.
However, there are still several issues regarding the long-term safety and feasibility of these materials. Synthetic polymers, such as polyglycolic acid57 and poly(lactic-co-glycolic acid),58 may have potential problems associated with residual and degradation in situ64, 65 that can be the risk factor to cause subsequent inflammation. Biological materials have possibility to transmit infectious agents like bacteria, virus, and prion, which initiate immunological reactions.66, 67 For the above reasons, such materials should ideally be avoided to minimize unknown risk throughout the treatment procedure, and in this regard, cell delivery system without use of exogenous scaffold would be an excellent alternative.
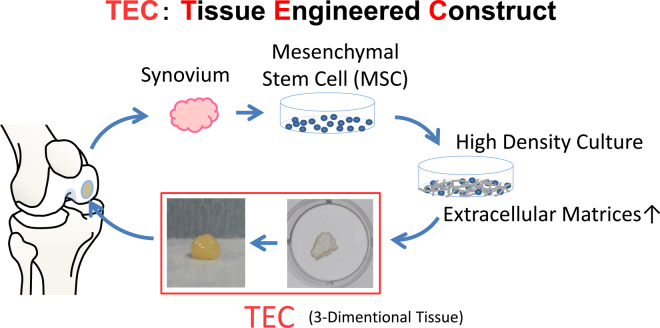
To address these problems, we have developed a 3D tissue-engineered construct (TEC) without the use of exogenous scaffolds. TEC is composed of synovial MSCs and ECMs synthesized by the cells. Plasticity and adhesiveness of the TEC enable scaffold-free transplantation (Fig. 1). Such a new, exogenous scaffold-free MSC-based therapy could be considered as the next-generation construct for cartilage regeneration. In this review, we discuss the feasibility and effectiveness of the TEC methodology repair and the advancement and problem in stem cell therapy for cartilage repair.
Fig. 1.
Schematic representation of the TEC-mediated cartilage repair.
5. Development of the basic TEC
When MSCs were cultured to confluence in the basic growth medium (DMEM with 10% FBS), they did not deposit abundant collagenous matrices, because ascorbic acid is an essential cofactor to the formation of triple helix structure of collagen. In contrast, when Asc-2P was added to the medium, collagen synthesis significantly promoted. Subsequently, the monolayer cell–matrix complex cultured in growth medium added with Asc-2P became a stiff sheet-like structure, which could be easily detached from the substratum by applying gentle shear stress using pipette. After detachment, the monolayer cell–matrix complex immediately started active contraction and evolved into a thick 3D tissue. Such tissue contraction was partially, but significantly, inhibited by addition of dihydrocytochalasin B, an actin polymerization inhibitor, or Y-27632, a Rho kinase inhibitor, in a dose-dependent manner. These observations indicate that active contraction of the TEC is associated with cytoskeletal contraction.
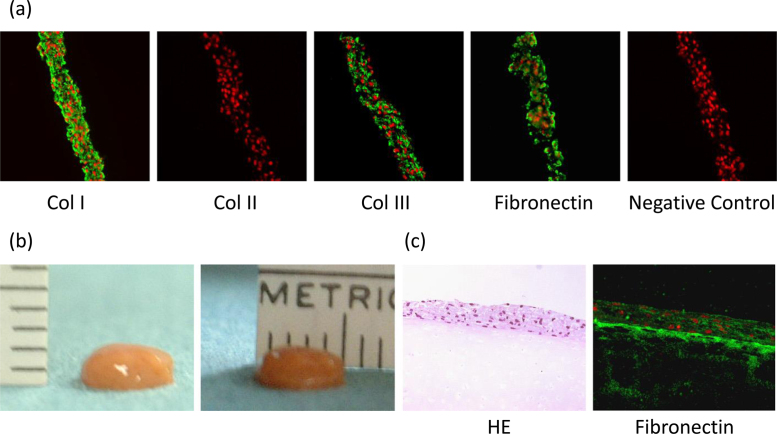
Immunohistochemical evaluation showed that the TEC was rich in type I and III collagen and lacked expression of type II collagen. Besides that, fibronectin and vitronectin were also abundant in the TEC (Fig. 2a). It is notable that all the molecules detected within the TEC were diffusely distributed without obvious polarity to the matrix organization. As cell–matrix complex folded and contracted, TEC could change its morphology into one spherical body with several millimeters thickness or any other shape, because of its plasticity (Fig. 1, Fig. 2). This contracted cell–matrix complex was termed a tissue-engineered construct (TEC) derived from MSCs.68, 69
Fig. 2.
Development of the tissue-engineered construct. (a) Immunohistochemical analysis of the basic TEC stained with type I collagen (Col I), type II collagen (Col II), type III collagen (Col III), fibronectin, and negative IgG (control). Bar = 100 mm. (b) Macroscopic view of the TEC, which was integrated to one spherical body. The diameter of this TEC was 5 mm and the thickness was 2 mm. (c) Microscopic view of HE staining (left side) and fibronectin staining (right side) of the cultured porcine chondral fragment for 7 days after the implantation of the basic TEC on the injured surface. Bar = 100 mm.
Cited from Ref. 69.
6. Adhesive property of the TEC
To evaluate the adhesive property of TEC, basic porcine TECs were transplanted on the partial thickness defect created on the thawed fresh-frozen porcine chondral fragments. Just five minutes after transplantation, the TEC had adhered to the chondral fragments. During seven days of ex vivo culture of the TEC–chondral complexes, TEC adhered stably for the entire time. Histology at day 7 showed integration of the TEC to the bottom of the chondral defect on the fragments (Fig. 2c). Immunohistochemical finding revealed expression of fibronectin at the boundary surface between the TEC and the bottom of the chondral defect on the fragments (Fig. 2c).69
7. Chondrogenic differentiation potential of the TEC
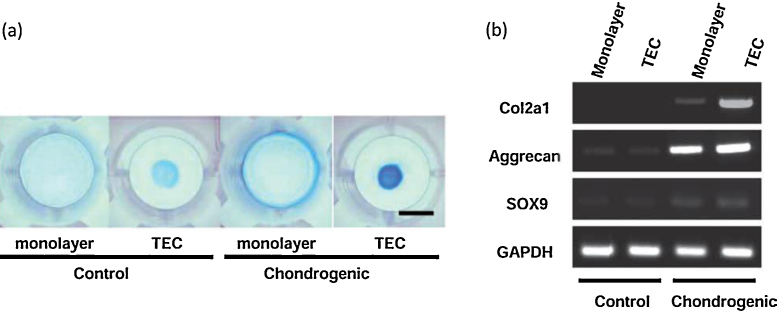
Human basic TECs were replated on the bottom of the culture dishes and then subsequent chondrogenic differentiation was performed in a chondrogenic medium containing BMP2. Such chondrogenic-differentiated TEC showed increased GAG synthesis and deposition as evidenced by intense alcian blue staining (Fig. 3a). Semiquantitative RT-PCR of cartilage-specific markers, type II collagen (COL2A1), aggrecan (ACAN), and SOX9 revealed the cartilage phenotype of the chondrogenic-differentiated TEC. In contrast, undifferentiated basic TEC, as well as monolayer cell cultures, showed no expression of the cartilage-specific marker, type II collagen (Fig. 3b). These observations indicate that TEC provided appropriate 3D microenvironment to the MSC to differentiate into chondrogenic lineage.68
Fig. 3.
Chondrogenic differentiation potential of the TEC. (a) Alcian blue staining of monolayer cultured MSCs or a basic TEC in control medium or in the chondrogenic medium containing 500 ng/mL BMP2 for 14 days. Bar = 1 cm. (b) RT-PCR analysis of monolayer cultures or TEC for chondrogenic marker genes, type II collagen (COL2A1), aggrecan (ACAN), SOX9, and GAPDH.
Cited from Ref. 68.
8. Implantation of in vitro generated basic TEC into porcine chondral defects in vivo
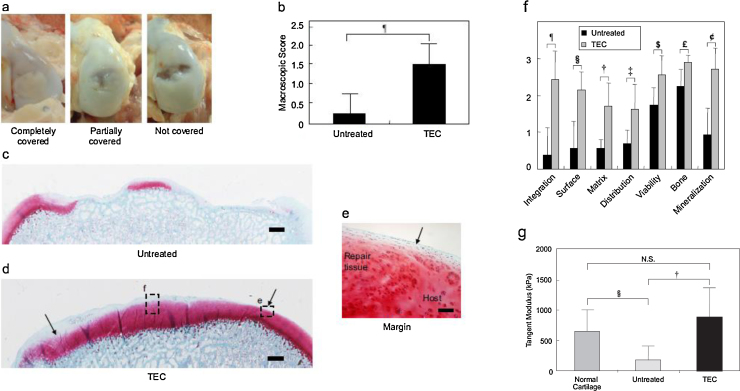
Porcine basic TECs were prepared as an allograft. The medial femoral condyles of the 4-month-old pigs were exposed, and chondral defects of 8.5 mm diameter and 2.0 mm depth were made. Then, the basic TECs were transplanted without suture or glue. At 6 months post-transplantation, the mean macroscopic score for the TEC-treated group was significantly lower than that for the untreated group (Fig. 4b), where a higher score is suggestive of a failure. Histological evaluation revealed that the chondral lesions in the nontreatment control group showed apparent osteoarthritic changes (Fig. 4c) while patients in the TEC-treated group were repaired with hyaline cartilaginous tissue exhibiting good integration to the adjacent native cartilage (Fig. 4d and e). Regarding the histological score, all assessment category for the TEC-treated group was significantly higher than those for the untreated group (Fig. 4f). These data indicated that the TEC maintains good tissue integration to the adjacent cartilage matrix, and the repair tissue exhibits chondrogenic differentiation without any evidence of central necrosis up to 6 months after sutureless transplantation.
Fig. 4.
Macroscopical and histological assessment of in vivo TEC implantation on chondral defect. (a) Macroscopic view of porcine chondral lesion treated with or without the TEC at 6 months after implantation. When treated with the TEC, 4 of 8 defects are completely covered with repair tissue (left side) and the others are partially covered (middle). Without the TEC, most of the chondral lesions have little tissue coverage (right side). (b) Macroscopic score of the chondral lesion treated with (TEC, N = 8) or without (untreated, N = 4) TEC at 6 months. ¶: p = 0.017. (c, d) Safranin O staining of chondral lesion treated with (c) or without (d) the TEC at 6 months after operation. Bar = 1 mm. (e) Light magnification view in the area enclosed by dotted rectangle in (d) at the margin area. Bar = 100 mm. Note that the defect treated with the TEC is completely filled with repair tissue with good tissue integration to the adjacent cartilage and with restoration of smooth surface (arrow). In contrast, the chondral defect in the control group (c) shows osteoarthritic change with loss of cartilage and destruction of subchondral bone. Modified ICRS Visual Histological Assessment Scale of repair tissue treated with (TEC; N = 8) and without (untreated; N = 4) TEC. ¶: p = 0.009, §: p = 0.008, †: p = 0.010, ‡: p = 0.026, $: p = 0.037, £: p = 0.011, ¢: p = 0.006.
Cited from Ref. 69.
Biomechanical analysis also revealed that the repaired tissue by the TEC transplantation exhibited modulus similar to the properties of native articular cartilage (Fig. 4g). To our knowledge, this study was the first demonstration of a successful MSC-based therapy for the repair of chondral lesions in a clinically relevant injury model without breaching the subchondral plate.69
9. Clinical trials to repair chondral defect using a TEC derived from human synovial MSCs
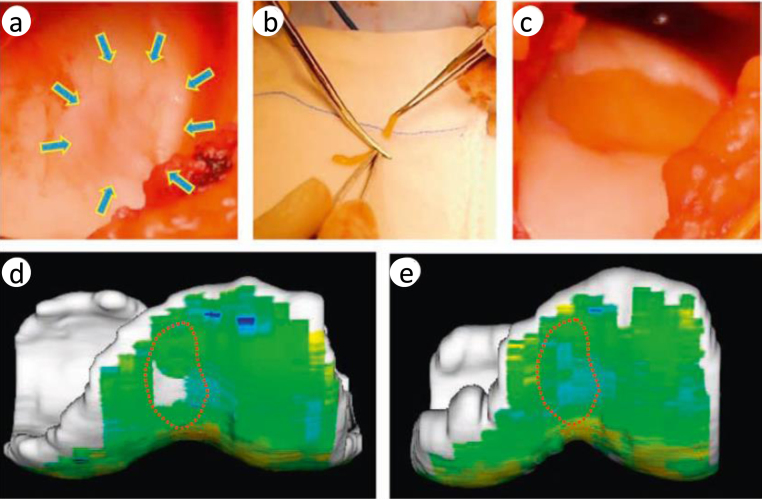
Based on the results of the preclinical studies discussed above, we have stepped forward to a clinical study at Osaka University Hospital, which has a current good manufacturing practice-grade cell processing center, and submitted the application of the “first-in-men” clinical trial to the Ministry of Health and Labour of Japan in 2011 (UMIN ID: UMIN000008266; Authorization number: HM1201). We got the approval in 2012, and the clinical trial was initiated in 2013 after the preparation of good clinical practice-based protocols. Patients who suffered from symptomatic chondral lesions of the knee, and who met the inclusion criteria (isolated chondral lesion ≤5 cm2, 20–60 years of age, with normal alignment), have been registered. Approximately 1 g of synovium was obtained arthroscopically from the knee aseptically, and MSCs were isolated and expanded in the cell processing center. 3–5 weeks after harvest of synovium, the TECs were prepared for autologous implantation. Affected chondral lesion was exposed by mini-arthrotomy, and then chondral lesion was debrided so as to not breach the subchondral plate (Fig. 5a). Before transplantation, the TEC was trimmed and the shape of the TEC was adjusted to match that of the chondral lesion (Fig. 5b). Transplantation was completed within 5–10 min, without any suture or glue (Fig. 5c). After the transplantation of the TEC, joint capsule was closed temporary and knee joint was passively flexed and extended several times to confirm stable attachment of the TEC to the lesion.
Fig. 5.
Arthroscopic and magnetic resonance imaging (MRI) analyses of repair tissue following implantation of a tissue-engineered construct (TEC) to repair human chondral defects in clinical trial. (a) A ICRS grade III lesion in the medial femoral chondyle after debridement. (b) Adjustment of the size of the TEC to match the lesion size just before implantation. (c) Implanted TEC into the lesion. (d, e) T2 mapping of the lesion at the femoral groove. (d) Before implantation and (e) 6 months after implantation.
Cited from Ref. 70.
As a postoperative treatment, immobilization of the knee joint was done in a brace for 2 weeks, and then, range-of-motion exercises and muscle exercises were initiated. Full weight bearing was allowed 6 to 8 weeks after transplantation surgery. Return to strenuous activity was allowed approximately 12 months following transplantation. The duration of follow-up was 1 year, and the primary end point of this study was to evaluate the adverse effects. The secondary end point was the assessment of effectiveness, including clinical scores (visual analog score [VAS], Knee Injury and Osteoarthritis Outcome Score [KOOS], Lysholm Knee Questionnaire, and Tegner Activity Scale), MRI (conventional and quantitative, such as T2-weighted mapping) at 3, 6, and 12 months, and histological assessment of a biopsy sample at 12 months. The preliminary results indicated that TEC transplantation restored normal joint function by completely covering the cartilage defect with cartilage-like repair tissue (Fig. 5d) with high, T2-weighted mapping profile (Fig. 5e).70 This clinical study was completed in March 2015 and we will report the outcomes of this clinical trial in the near future.
10. Nonhomologous use of stem cell therapy
The present review has discussed the feasibility of exogenous scaffold-free TEC generated by synovial MSCs for effective stem cell therapy to repair articular cartilage. The strategy discussed above is nonhomologous use of stem cell therapy, that is, transplantation of cells or tissue that is different from target tissue. Therefore, transplanted cells have to differentiate into chondrocytes according to the host microenvironment and differentiated chondrocyte has to generate cartilage matrices to achieve cartilage repair.
Nonhomologous use of stem cell therapies for cartilage repair has been investigated and some of the therapies are already clinically applied. Orth et al. reviewed outcomes of nonhomologous use of stem cell therapies for cartilage repair in 2014. In this review, although many studies reported clinical improvement, the repair tissue was hyaline-like tissue or fibrocartilage and none of these studies achieved pure hyaline cartilage restoration. Therefore, long-term outcome may be compromised due to the inferior mechanical property of fibrocartilage. We precisely evaluated the morphological and mechanical property of the repair cartilage generated by TEC in porcine cartilage defect model. Although macroscale compressive and lubrication properties were comparable to uninjured cartilage, microindentation evaluation showed that the surface stiffness of the repair tissue by TEC was significantly lower than that of native articular cartilage. Morphological observation showed that the superficial zone of the repair tissue by TEC was more fibrocartilaginous, in contrast to the middle or deep zones that were of more hyaline cartilaginous morphology. Then, histological scores were compared between superficial, middle, and deep zones of repair tissue by TEC and superficial zone was significantly compromised.
To overcome such limitation of nonhomologous use of stem cell therapy, various approaches have been investigated to achieve better quality of repair cartilage, including improvement of culture condition (growth factor, hypoxia, co-culture), introduction of gene therapy, 3D printing, and iPS (induced pluripotent stem) cells. Clinical application of these procedures is expected after confirmation of safety and ethical issue.
11. Homologous use of stem cell therapy
Homologous use of stem cell therapy is the strategy to transplant artificial hyaline cartilage generated by stem cells to the chondral lesion. MSCs can be differentiated into chondrocyte using three-dimensional culture at high density, such as micromass cultures71 or pellet cultures,72 and differentiated chondrocyte deposits cartilage matrices to generate hyaline cartilage. However, these methods cannot be directly applied to most clinical situations because of limitations in the mass size of the materials.55 Bhardwaj et al. reviewed current strategy to generate tissue-engineered cartilage in 2015 and mentioned that despite tremendous growth and progress in the field of cartilage tissue engineering, the properties and structure of native cartilage have not been entirely mimicked by any tissue-engineered replacement till date.21
It is notable that recently Yamashita et al. succeeded in generating hyaline cartilaginous tissue from iPS cells without use of exogenous scaffold. iPS cells are an attractive cell source because of its unlimited self-renewal capacity. The average diameter of the hyaline cartilaginous particles is 1.4 ± 0.5 mm and they were transplanted to the chondral defect of the miniature pig. Although only short-term results were evaluated, the particles integrated with the native cartilage.33 However, there have not been much evidence using these cells in terms of safety, and thus further studies are likely necessary.
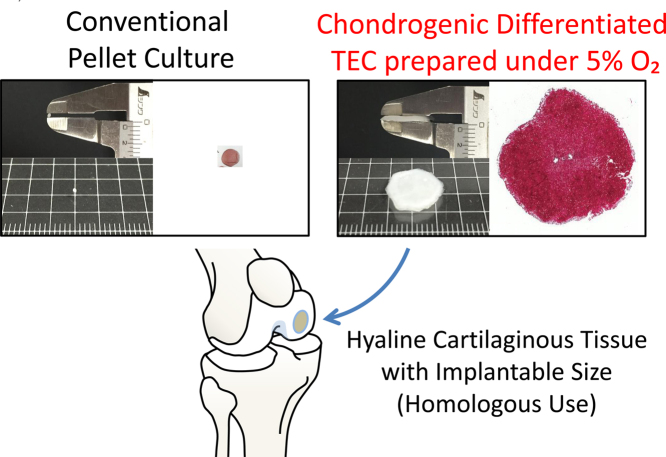
We recently demonstrated the generation of pure hyaline cartilaginous tissue of approximately 1 cm in diameter by differentiation of basic TEC. While chondrogenic-differentiated TEC cultured under conventional normal oxygen was a mixture of hyaline-like and fibrocartilaginous tissue, chondrogenic-differentiated TEC cultured under low oxygen tension was pure hyaline cartilaginous tissue without fibrous tissue.73 This was the first demonstration of in vitro development of a hyaline-like cartilaginous tissue of an implantable size to chondral defect that was generated by human MSCs without the use of exogenous scaffolds. The low oxygen tension culture at physiological range is a safe procedure with low cost, and thus, may be a clinically relevant option to repair cartilage.
These homologous uses of stem cell therapies are expected to overcome the limitations of nonhomologous use of stem cell therapies and thus further studies are necessary (Fig. 6).
Fig. 6.
Schematic representation of homologous use of stem cell therapy using chondrogenic-differentiated TEC under low oxygen tension.
12. Conclusion
Stem cell therapy for cartilage repair is a promising method of cell-based therapy without use of chondrocytes, which has limitation regarding the dedifferentiation and donor site morbidity. In the majority of stem cell-based cartilage repair, exogenous scaffolds made of chemical or animal-derived biomaterials are widely used to provide an appropriate three-dimensional environment for subsequent cell proliferation and differentiation. However, exogenous scaffold-free approach has advantage in terms of long-term safety. We have developed novel exogenous scaffold-free TEC-mediated cartilage repair as discussed above. Native ECM within the TEC, synthesized by MSCs, must play an important role as internal 3D scaffolds, providing MSCs appropriate microenvironment to differentiate into chondrocyte and generate cartilage matrices. TEC can be generated by MSC from other tissue, and differentiate into mesenchymal lineage, and thus, TEC methodology could be introduced to variety of therapeutic approaches in regenerative medicine.
Conflicts of interest
The authors have none to declare.
References
- 1.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001:S362. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 2.Gomoll A.H., Farr J., Gillogly S.D., Kercher J., Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470. [PubMed] [Google Scholar]
- 3.Moran C.J., Barry F.P., Maher S.A., Shannon F.J., Rodeo S.A. Advancing regenerative surgery in orthopaedic sports medicine: the critical role of the surgeon. Am J Sports Med. 2012;40:934. doi: 10.1177/0363546511426677. [DOI] [PubMed] [Google Scholar]
- 4.Gomoll A.H., Madry H., Knutsen G. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18:434. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae D.K., Yoon K.H., Song S.J. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22:367. doi: 10.1016/j.arthro.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Kreuz P.C., Steinwachs M.R., Erggelet C. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarth Cartil. 2006;14:1119. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Gobbi A., Nunag P., Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 8.Kon E., Gobbi A., Filardo G., Delcogliano M., Zaffagnini S., Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 9.Gross A.E., Kim W., Las Heras F., Backstein D., Safir O., Pritzker K.P. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch T.S., Patel R.M., Benedick A., Amin N.H., Jones M.H., Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015 doi: 10.1016/j.arthro.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Pareek A., Reardon P.J., Maak T.G., Levy B.A., Stuart M.J., Krych A.J. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016 doi: 10.1016/j.arthro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 13.Gooding C.R., Bartlett W., Bentley G., Skinner J.A., Carrington R., Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Bright P., Hambly K. A systematic review of reporting of rehabilitation in articular-cartilage-repair studies of third-generation autologous chondrocyte implantation in the knee. J Sport Rehabil. 2014;23:182. doi: 10.1123/JSR.2013-0045. [DOI] [PubMed] [Google Scholar]
- 15.Gobbi A., Kon E., Berruto M. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37:1083. doi: 10.1177/0363546509331419. [DOI] [PubMed] [Google Scholar]
- 16.Oussedik S., Tsitskaris K., Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy. 2015;31:732. doi: 10.1016/j.arthro.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Goyal D., Goyal A., Keyhani S., Lee E.H., Hui J.H. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29:1872. doi: 10.1016/j.arthro.2013.07.271. [DOI] [PubMed] [Google Scholar]
- 18.Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97:418. doi: 10.1016/j.otsr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Ma B., Leijten J.C., Wu L. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarth Cartil. 2013;21:599. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Caron M.M., Emans P.J., Coolsen M.M. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarth Cartil. 2012;20:1170. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Bhardwaj N., Devi D., Mandal B.B. Tissue-engineered cartilage: the crossroads of biomaterials, cells and stimulating factors. Macromol Biosci. 2015;15:153. doi: 10.1002/mabi.201400335. [DOI] [PubMed] [Google Scholar]
- 22.Cucchiarini M., Venkatesan J.K., Ekici M., Schmitt G., Madry H. Human mesenchymal stem cells overexpressing therapeutic genes: from basic science to clinical applications for articular cartilage repair. Biomed Mater Eng. 2012;22:197. doi: 10.3233/BME-2012-0709. [DOI] [PubMed] [Google Scholar]
- 23.Friedenstein A.J., Piatetzky S., II, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381. [PubMed] [Google Scholar]
- 24.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 25.De Bari C., Dell’Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arth Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Zuk P.A., Zhu M., Mizuno H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski R.J., Deasy B.M., Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 28.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz E.M., Le Blanc K., Dominici M. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 30.Orth P., Rey-Rico A., Venkatesan J.K., Madry H., Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning Adv Appl. 2014;7:1. doi: 10.2147/SCCAA.S42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimomura K., Ando W., Tateishi K. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31:8004. doi: 10.1016/j.biomaterials.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Tsumaki N., Okada M., Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2014 doi: 10.1016/j.bone.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita A., Morioka M., Yahara Y. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015;4:404. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakitani S., Mitsuoka T., Nakamura N., Toritsuka Y., Nakamura Y., Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transpl. 2004;13:595. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 35.Wakitani S., Okabe T., Horibe S. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 36.Haynesworth S.E., Goshima J., Goldberg V.M., Caplan A.I. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 37.Ashton B.A., Allen T.D., Howlett C.R., Eaglesom C.C., Hattori A., Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980:294. [PubMed] [Google Scholar]
- 38.Bab I., Passi-Even L., Gazit D. Osteogenesis in in vivo diffusion chamber cultures of human marrow cells. Bone Miner. 1988;4:373. [PubMed] [Google Scholar]
- 39.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz E.M., Prockop D.J., Fitzpatrick L.A. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 41.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S.Y., Miwa M., Sakai Y., Kuroda R., Niikura T., Kurosaka M. Osteogenic potential of cells in vitro derived from haemarthrosis of the knee induced by injury to the anterior cruciate ligament. J Bone Joint Surg Brit Vol. 2006;88:129. doi: 10.1302/0301-620X.88B1.16795. [DOI] [PubMed] [Google Scholar]
- 43.Mehlhorn A.T., Niemeyer P., Kaiser S. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12:2853. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 44.Afizah H., Yang Z., Hui J.H., Ouyang H.W., Lee E.H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 45.Puetzer J.L., Petitte J.N., Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev. 2010;16:435. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 46.Inui A., Iwakura T., Reddi A.H. Human stem cells and articular cartilage regeneration. Cells. 2012;1:994. doi: 10.3390/cells1040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arth Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 48.Jones B.A., Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura H., Muneta T., Nimura A., Yokoyama A., Koga H., Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 50.Koizumi K., Ebina K., Hart D.A. Synovial mesenchymal stem cells from osteo- or rheumatoid arthritis joints exhibit good potential for cartilage repair using a scaffold-free tissue engineering approach. Osteoarth Cartil. 2016 doi: 10.1016/j.joca.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Sekiya I., Muneta T., Horie M., Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473:2316. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K.B., Wang V.T., Chan Y.H., Hui J.H. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid – a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singapore. 2012;41:511. [PubMed] [Google Scholar]
- 53.Skowronski J., Skowronski R., Rutka M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane – results. Ortop Traumatol Rehabil. 2013;15:69. doi: 10.5604/15093492.1012405. [DOI] [PubMed] [Google Scholar]
- 54.Nejadnik H., Hui J.H., Feng Choong E.P., Tai B.C., Lee E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 55.De Bari C., Dell’Accio F., Luyten F.P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arth Rheum. 2004;50:142. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 56.Iwasa J., Engebretsen L., Shima Y., Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol. 2009;17:561. doi: 10.1007/s00167-008-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vunjak-Novakovic G., Martin I., Obradovic B. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 58.Andriano K.P., Tabata Y., Ikada Y., Heller J. In vitro and in vivo comparison of bulk and surface hydrolysis in absorbable polymer scaffolds for tissue engineering. J Biomed Mater Res. 1999;48:602. doi: 10.1002/(sici)1097-4636(1999)48:5<602::aid-jbm3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Guo J.F., Jourdian G.W., MacCallum D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 60.Lahiji A., Sohrabi A., Hungerford D.S., Frondoza C.G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 61.Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 62.Homminga G.N., Buma P., Koot H.W., van der Kraan P.M., van den Berg W.B. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64:441. doi: 10.3109/17453679308993663. [DOI] [PubMed] [Google Scholar]
- 63.Brun P., Cortivo R., Zavan B., Vecchiato N., Abatangelo G. In vitro reconstructed tissues on hyaluronan-based temporary scaffolding. J Mater Sci Mater Med. 1999;10:683. doi: 10.1023/a:1008960413362. [DOI] [PubMed] [Google Scholar]
- 64.Daniels A.U., Andriano K.P., Smutz W.P., Chang M.K., Heller J. Evaluation of absorbable poly(ortho esters) for use in surgical implants. J Appl Biomater. 1994;5:51. doi: 10.1002/jab.770050108. [DOI] [PubMed] [Google Scholar]
- 65.van der Elst M., Klein C.P., de Blieck-Hogervorst J.M., Patka P., Haarman H.J. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20:121. doi: 10.1016/s0142-9612(98)00117-3. [DOI] [PubMed] [Google Scholar]
- 66.Yang C., Hillas P.J., Baez J.A. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18:103. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 67.Martin M.J., Muotri A., Gage F., Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 68.Ando W., Tateishi K., Katakai D. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14:2041. doi: 10.1089/ten.tea.2008.0015. [DOI] [PubMed] [Google Scholar]
- 69.Ando W., Tateishi K., Hart D.A. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28:5462. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura N., Hui J., Koizumi K. Stem cell therapy in cartilage repair – culture-free and cell-based methods. Oper Tech Orthop. 2014;24:54. [Google Scholar]
- 71.Denker A.E., Nicoll S.B., Tuan R.S. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-beta 1. Differ Res Biol Divers. 1995;59:25. doi: 10.1046/j.1432-0436.1995.5910025.x. [DOI] [PubMed] [Google Scholar]
- 72.Lennon D.P., Haynesworth S.E., Arm D.M., Baber M.A., Caplan A.I. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dynam. 2000;219:50. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1037>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 73.Yasui Y., Chijimatsu R., Hart D.A. Preparation of scaffold-free tissue-engineered constructs derived from human synovial mesenchymal stem cells under low oxygen tension enhances their chondrogenic differentiation capacity. Tissue Eng Part A. 2016;22:490. doi: 10.1089/ten.tea.2015.0458. [DOI] [PubMed] [Google Scholar]