Abstract
Insecticide resistance raises concerns for the control of vector-borne diseases. However, its impact on parasite transmission could be diverse when considering the ecological interactions between vector and parasite. Thus we investigated the fitness cost associated with insecticide resistance and Plasmodium falciparum infection as well as their interactive cost on Anopheles gambiae survival and fecundity. In absence of infection, we observed a cost on fecundity associated with insecticide resistance. However, survival was higher for mosquito bearing the kdr mutation and equal for those with the ace-1R mutation compared to their insecticide susceptible counterparts. Interestingly, Plasmodium infection reduced survival only in the insecticide resistant strains but not in the susceptible one and infection was associated with an increase in fecundity independently of the strain considered. This study provides evidence for a survival cost associated with infection by Plasmodium parasite only in mosquito selected for insecticide resistance. This suggests that the selection of insecticide resistance mutation may have disturbed the interaction between parasites and vectors, resulting in increased cost of infection. Considering the fitness cost as well as other ecological aspects of this natural mosquito-parasite combination is important to predict the epidemiological impact of insecticide resistance.
Malaria is a parasitic disease caused by protozoa of the genus Plasmodium, which are transmitted between vertebrate hosts through mosquito bites. In 2015, 214 million cases occurred with approximately 438,000 deaths, ~70% of which were children younger than five years of age1. To combat malaria, current strategies include anti-malarial drug treatments alongside vector control using long-lasting insecticidal bed-nets and indoor-residual spraying of insecticides. However, widespread insecticide resistance in vectors2 and concern over spreading artemisinin resistance3,4 trigger the fragility of malaria treatment and control. To assess the epidemiological impact of such adaptation, it is crucial to understand the ecological processes underlying the co-evolution of vector-parasite interactions. Genes responsible for an adaptation to a new environment are usually assumed to be detrimental in the previous environment5. The new adaptive allele may be associated with modification of physiological processes or resource availability, which often leads to decrease performance and overall fitness6,7. Such a fitness cost is a determinant factor in the evolution of an adaptation and has been well characterized for insecticide resistance in arthropods species8,9. Unraveling these costs on natural vector-parasite interactions is essential for guiding the implementation of vector control strategies10.
As a result of an intense selective pressure from insecticides to reduce vector longevity and abundance, several resistance mechanisms have been selected in mosquito vectors11. Among them two main mechanisms are responsible for high level of resistance: increased metabolism of detoxification (named metabolic resistance) and modification of the insecticide target site (named target-site resistance)11. The molecular basis of target-site resistance has been characterized in many insect species and has demonstrated conserved resistant mutations11. In several mosquito species and populations, the unique G119S mutation in the ace-1 gene (ace-1R allele) is responsible for organophosphate and carbamate resistance12, the L1014F mutation in the para-type sodium channel gene (kdr-west mutation) is responsible for pyrethroid resistance in malaria vectors from West Africa13, and the A302G mutation in the Rdl gene in An. gambiae is responsible for cyclodiene resistance14. In mosquitoes, resistance alleles spread and increase in frequency in insecticide-treated areas whereas in non-treated areas their frequency remains low, thus revealing a substantial fitness cost15. The fitness cost associated with insecticide resistance has been characterized on a large range of life history traits: such as development time, mortality and predation avoidance in larvae, flight and host-seeking activities, male reproductive success, fecundity, survival and susceptibility to infection in adults8,16,17,18,19,20,21,22,23,24,25. It remains however uncertain to which extend insecticide resistance would have an impact on vector population dynamics and parasite transmission26.
Another fitness cost that may have great impact on the coevolutionary dynamic of mosquito and parasite might be the cost that the parasite may impose to its own vector. Investigation of the fitness cost of Plasmodium infection in vector mosquitoes resulted in conflicting results. Studies carried out on non-natural vector-parasite combinations mainly showed that infection affects mosquito fitness related traits such as survival and fecundity, while studies of natural combinations of vector and parasites revealed more complex results27. Cost of infection in the natural vectorial system responsible for transmission of human malaria An. gambiae-P. falciparum was not detected in some studies28,29 whereas cost on fecundity30 and stress-dependent cost on survival was observed upon infection in other studies31,32. In the natural avian malaria system, Culex pipiens-P. relictum, a decreased fecundity and an increased survival was observed33 as well as a stress-dependent cost on survival34,35. These studies therefore showed that Plasmodium infection is more likely to imply a cost when mosquitoes experience unfavorable environmental conditions.
Most research has focused on the direct consequences of mosquito adaptation to the insecticides to assess the entomological efficacy of vector control, but they do not necessarily take into account the ecological complexity of the natural vectorial systems in order to predict its epidemiological impact. However, complex interactions have been recently identified. For instance, insecticide resistant mutations have been associated with increased vector competence in absence of insecticides22. In contrast, exposure to insecticide decrease the competence of resistant vectors for malaria parasite36. In addition, P. falciparum infection reduces the ability of resistant vectors to survive insecticide exposure37. This illustrates complex interactions between insecticide resistance, exposure to insecticide and malaria parasite transmission. However, to our knowledge, it remains unknown whether cost of infection and cost of insecticide resistance may interplay in malaria vectors.
Therefore, in this study, we aim to determine the cost of insecticide resistance and its impact on the cost of Plasmodium infection. We measured the survival and fecundity of Anopheles gambiae s.s. either susceptible or harboring the resistant kdr or ace-1R mutations (target site resistance mechanism) in absence of insecticides. The strains shared a common genetic background to avoid any confounding effect due to other resistance mechanisms or any other mutations that are not in linkage disequilibrium. We compared these life history traits between Plasmodium infected and unexposed mosquitoes from each strains and demonstrated that the cost of infection is higher in the resistant vectors than in the susceptible ones.
Results
We performed experimental infections of laboratory strains of Anopheles gambiae s.s. with wild isolates of Plasmodium falciparum using the standard membrane feeding assay protocol38 implemented by Sangaré et al.39 to obtain the corresponding non-infectious control blood. Five replicates each with a distinct blood donor were performed for each experiment (survival and fecundity, respectively). The proportion of infected mosquitoes among those that took an infectious blood meal (all experiments included) varied from 51.4% to 97.6% for Kisumu, from 58.1% to 96.9% for Acerkis, and from 61% to 100% for Kdrkis strain. Consistent with previous data22, the susceptible strain Kisumu showed the lowest susceptibility to infection (averaged across replicates, 66.4% ± 2.3) and the resistant strains were more susceptible to Plasmodium infection (Acerkis: 79.7% ± 2.0, p < 0.001 and Kdrkis: 80.2% ± 2.0, p < 0.001).
Survival
We followed 1290 female mosquitoes of the three strains from blood feeding until death, among them 649 took a gametocyte-infectious blood meal and 641 took a heat inactivated (control) blood meal through five replicates. Among the 649 blood-fed females on infectious blood, 487 were oocyst-infected after midgut dissection; the females that did not carry oocysts were removed from the following analysis. Cox proportional analysis of the survivorship on the whole data set indicated that the blood donor has significantly influenced mosquito survivorship (variance: 0.08459 ± 0.06319). The analysis revealed also a significant impact of the strain (χ2df=2 = 30.38, p < 0.001), the infection status (χ2df=1 = 27.90, p < 0.001) and the strain by infection interaction (χ2df=2 = 13.43 p = 0.0012) but heamatin (indicative of blood meal size) and wing length (proxy of body size) and their interactions were not retained in the minimal model.
Influence of insecticide resistance
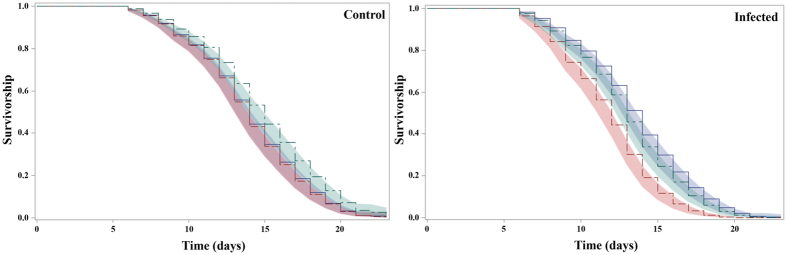
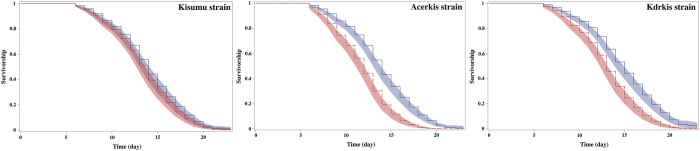
Figure 1 presents the Kaplan-Meier survival curves for each strain within each infection status (Plasmodium infected or control mosquitoes). The survival distributions were significantly different between mosquito strains in the control (i.e. unexposed to infection) (Log-Rank statistic, χ2df=2 = 14.48, p < 0.001) or in the infected group (Log-Rank statistic, χ2df=2 = 32.86, p < 0.001). Interestingly, comparison among mosquito strains unexposed to infection revealed a similar survivorship between Acerkis and Kisumu (HR = 0.96, Confidence Interval, CI: [0.78–1.17], Table 1) but a higher survivorship of Kdrkis over that of Kisumu (HR = 1.37, CI: [1.12–1.68]). However, among Plasmodium infected mosquitoes, Acerkis females had a lower survivorship than Kisumu (HR = 0.55, CI: [0.44–0.69]), while survivorship of Kdrkis and Kisumu were similar (HR = 0.90, CI: [0.72–1.12]).
Figure 1. Kaplan-Meier survival curves of the susceptible strain Kisumu (blue) the resistant strains Acerkis (red) and Kdrkis (green) within control or infected mosquitoes.
The control group corresponds to mosquitoes that fed on non-infectious blood and the infected group to mosquitoes with at least one oocyst in the midgut.
Table 1. Hazard ratio associated with insecticide resistance alleles and P. falciparum infection in An. gambiae s.s. survivorship.
| Insecticide resistance | Insecticide resistance | Median survival | Hazard ratio | Lower 95%CI | Upper 95%CI | Log-rank χ2 | p-value |
|---|---|---|---|---|---|---|---|
| Unexposed Kisumu* | 13 | — | — | — | 14.48 | p < 0.001 | |
| Unexposed Acerkis | 14 | 0.957 | 0.779 | 1.175 | |||
| Unexposed Kdrkis | 15 | 1.373 | 1.120 | 1.682 | |||
| Infected Kisumu* | 13 | — | — | — | 32.86 | p < 0.001 | |
| Infected Acerkis | 12 | 0.552 | 0.441 | 0.692 | |||
| Infected Kdrkis | 13 | 0.898 | 0.722 | 1.116 | |||
| P. falciparum infection | P. falciparum infection | Median survival | Hazard ratio | Lower 95%CI | Upper 95%CI | Log-rank χ2 | p-value |
| Unexposed Kisumu* | 13 | — | — | — | 1.540 | 0.210 | |
| Infected Kisumu | 13 | 0.897 | 0.716 | 1.122 | |||
| Unexposed Acerkis* | 14 | 49.45 | p < 0.001 | ||||
| Infected Acerkis | 12 | 0.518 | 0.420 | 0.638 | |||
| Unexposed Kdrkis* | 15 | 39.26 | p < 0.001 | ||||
| Infected Kdrkis | 13 | 0.587 | 0.481 | 0.715 |
Hazard ratio was estimated by comparing survivorship with that of a reference (indicated by an asterisk) and significance was tested using post-hoc tests with a Bonferroni correction.
Influence of P. falciparum infection
Figure 2 presents the Kaplan-Meier survival curves for control (non-exposed to Plasmodium) and infected mosquitoes within each strain. Infection did not impact survival of the susceptible Kisumu strain (Log-Rank statistic, χ2df=1 = 1.54, p = 0.21, Table 1). However survival analysis on resistant mosquitoes, separately, indicated a significant difference between infected and control groups (Log-Rank statistic, χ2df=1 = 49.45, p < 0.001 for Acerkis and χ2df=1 = 39.26, p < 0.001 for Kdrkis), revealing a cost of P. falciparum infection only in insecticide resistant mosquitoes. Survivorship of the susceptible strain Kisumu did not differ significantly between Plasmodium infected and unexposed mosquitoes (HR = 0.90, CI: [0.72–1.12], Table 1). However Plasmodium infection reduced the survivorship in both resistant strains: Acerkis and Kdrkis infected females had about twice less chance to survive at any time than the unexposed females (HR = 0.52, CI: [0.42–0.64] and 0.59, CI: [0.48–0.71] for Acerkis and Kdrkis respectively).
Figure 2. Kaplan-Meier survival curves of infected (red) and control (blue) mosquitoes within susceptible (Kisumu) or resistant (Acerkis and Kdrkis) strains of An. gambiae.
For each strain, the control group corresponds to mosquitoes that fed on non-infectious blood and the infected group to mosquitoes with at least one oocyst in the midgut.
Fecundity
Female mosquitoes of the three strains were allowed to lay eggs individually after blood feeding on infectious or non-infectious control blood (1BM, N = 688). Statistical analysis of the fecundity rate revealed a significant influence of insecticide resistant alleles (strain variable: Fdf=2,677 = 4.96, p = 0.007), the infection status (Fdf=1,677 = 9.14, p = 0.002) as well as the interaction of insecticide resistance and body size (strain:wing: Fdf=2,677 = 4.81, p = 0.008) indicating a distinct impact of body size depending in the different strains. Among females that have laid at least one egg (N = 271), only the interaction between wing length and insecticide resistance strains has significantly influenced the number of eggs (wing:strain interaction: Fdf=2,261 = 5.13, p = 0.006).
Influence of insecticide resistance
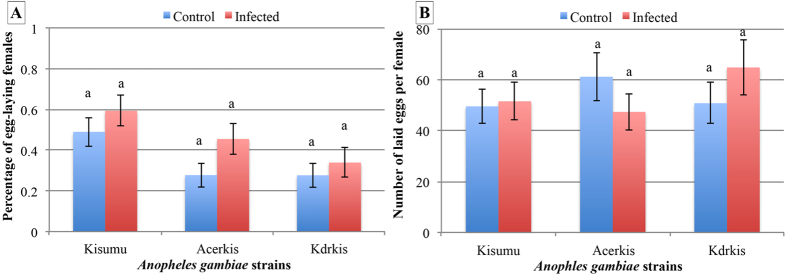
In absence of Plasmodium infection, both insecticide resistant strains had a lower fecundity rate than Kisumu (27.8% ± 5.9 for Acerkis and 27.7% ± 6.0 for Kdrkis compared to 49.0% ± 7.2 for Kisumu, p < 0.001, Table 2 and Fig. 3A). When comparing infected individuals, only the Kdrkis strain showed a lower fecundity rate compared to the susceptible strain Kisumu (33.9% ± 7.3 for Kdrkis compared to 59.4% ± 7.5 for Kisumu, p = 0.002; and 45.5% ± 7.5 for Acerkis, p = 0.065). While wing length did not affect fecundity rate in the susceptible strain, we observed an opposite relationship in the two insecticide resistant strains: in Acerkis smaller females had a greater probability to produce eggs while in Kdrkis bigger female had a greater probability to produce eggs. When comparing females that produced at least one egg (Fig. 3B), no difference in the number of laid eggs wa oserved between mosquito strains. However, the influence of body size on the number of eggs depended on the strains: in the Kdrkis strain, bigger females produced more eggs whereas no correlation was observed in the Kisumu and Acerkis strains.
Table 2. Fecundity rate and number of eggs produced of infected and control mosquitoes for each Anopheles gambiae strain.
| Insecticide resistance |
One Blood Meal |
Two blood Meals |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Infection group | Strain | Fecundity rate | p-value (compared to Kisumu) | Fecundity | p-value (compared to Kisumu) | Fecundity rate | p-value (compared to Kisumu) | Fecundity | p-value (compared to Kisumu) |
| Control | Kisumu | 0.490 (0.072) | — | 49.50 (6.79) | — | 0.668 (0.080) | — | 63.85 (8.61) | — |
| Acerkis | 0.278 (0.059) | 0.001 | 61.14 (9.39) | 0.159 | 0.644 (0.074) | 0.762 | 61.92 (8.00) | 0.828 | |
| Kdrkis | 0.277 (0.060) | 0.001 | 50.73 (8.06) | 0.874 | 0.455 (0.082) | 0.013 | 58.92 (8.23) | 0.579 | |
| Infected | Kisumu | 0.594 (0.075) | — | 51.49 (7.47) | — | 0.657 (0.081) | — | 50.68 (7.09) | — |
| Acerkis | 0.405 (0.075) | 0.045 | 47.34 (7.11) | 0.585 | 0.706 (0.070) | 0.518 | 45.13 (5.71) | 0.388 | |
| Kdrkis | 0.339 (0.073) | 0.002 | 64.76 (10.74) | 0.164 | 0.442 (0.076) | 0.008 | 47.94 (6.35) | 0.707 | |
| P. falciparum infection | One Blood Meal | Two blood Meals | |||||||
| Strain | Infection group | Fecundity rate | p-value (compared to Control) | Fecundity | p-value (compared to Control) | Fecundity rate | p-value (compared to Control) | Fecundity | p-value (compared to Control) |
| Kisumu | Control | 0.490 (0.072) | — | 49.50 (6.79) | — | 0.668 (0.080) | — | 63.85 (8.61) | — |
| Infected | 0.594 (0.075) | 0.152 | 51.49 (7.47) | 0.784 | 0.657 (0.081) | 0.903 | 50.68 (7.09) | 0.119 | |
| Acerkis | Control | 0.278 (0.059) | — | 61.14 (9.39) | — | 0.644 (0.074) | — | 61.92 (8.00) | — |
| Infected | 0.405 (0.075) | 0.055 | 47.34 (7.11) | 0.086 | 0.706 (0.070) | 0.356 | 45.13 (5.71) | 0.007 | |
| Kdrkis | Control | 0.277 (0.060) | — | 50.73 (8.06) | — | 0.455 (0.082) | — | 58.92 (8.23) | — |
| Infected | 0.339 (0.073) | 0.345 | 64.76 (10.74) | 0.152 | 0.442 (0.076) | 0.861 | 47.94 (6.35) | 0.141 | |
Estimates were compared between strains to assess the effect of insecticide resistance and between control and infected for each strain separately. Difference was tested using post-hoc tests with a Bonferroni correction.
Figure 3. Fecundity of infected and control mosquitoes for each Anopheles gambiae strain following a single blood meal.
Panel A presents the percentage of females producing eggs and panel B presents the number of eggs per females. Red bars represent infected females and blue bars represent females unexposed to P. falciparum. Error bars represent the standard error of the mean.
Influence of P. falciparum infection
Overall, Plasmodium infection increased the fecundity rate of An. gambiae, regardless of the strain (Control: 34.8% ± 6.4 vs. infected: 44.6% ± 7.4, p = 0.003). When the difference in fecundity rate was tested separately within each strain (Table 2, Fig. 3A), the difference between infected and unexposed control was not significant (Kisumu: p = 0.152; Acerkis: p = 0.055; Kdrkis: p = 0.345).
Fecundity of pre-gravid female
In the field, An. gambiae s.l. commonly take two blood meals before they lay their first batch of eggs probably due to insufficient resources for egg maturation40,41. Thus the female mosquitoes that did not produce eggs following the first blood meal were allowed to re-blood feed on rabbit blood and isolated again to lay eggs (N = 547). The analysis of the fecundity rate among these pre-gravid females demonstrated a significant influence of insecticide resistance alleles (strain: Fdf=2,537 = 4.04, p = 0.018) and the interaction between the strains and body size (strain:wing interaction: Fdf=2,537 = 4.56, p = 0.011). Among the females that have laid at least one egg (N = 300), the number of eggs was significantly influenced by Plasmodium infection (status: Fdf=1,292 = 5.45, p = 0.006) and by the interaction between body size and infection (wing:status interaction: Fdf=1,292 = 4.31, p = 0.039).
Influence of insecticide resistance
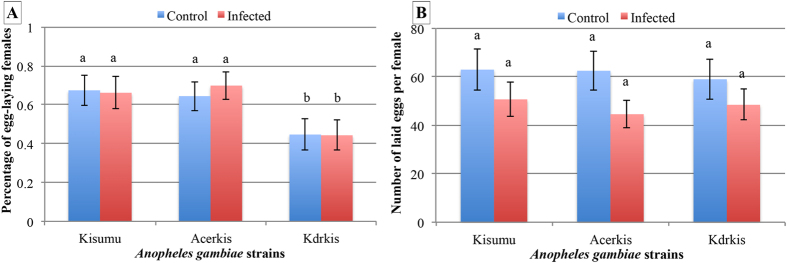
Fecundity rates were significantly lower in Kdrkis females compared to Kisumu (control: 45.5% ± 8.2 vs. 66.8% ± 8.0, p = 0.013; infected: 44.2% ± 7.6 vs. 65.7% ± 78.1, p = 0.008, Table 2, Fig. 4A). No differences were observed between the OP resistant strain Acerkis and the susceptible strain Kisumu (control: 64.4% ± 7.4 vs. 66.8% ± 8.0, p = 0.762; infected: 70.6% ± 7.0 vs. 65.7% ± 8.1, p = 0.518). In addition, no influence of insecticide resistance on the number of eggs produced was observed neither in infected nor in control mosquitoes (Table 2, Fig. 4B).
Figure 4. Fecundity of infected and control mosquitoes for each Anopheles gambiae strain following a second blood meal to complete their first gonotrophic cycle.
Panel A presents the percentage of females producing eggs and panel B presents the number of eggs per females. Red bars represent infected females and blue bars represent females unexposed to P. falciparum. Error bars represent the standard error of the mean.
Influence of P. falciparum infection
No effect of infection was observed on the fecundity rate of females that needed 2 blood meals to complete egg maturation. However, in presence of Plasmodium infection, the number of eggs produced was reduced, regardless of the strain (Control: 61.8 ± 6.5 vs. infected: 47.5 ± 5.0, p = 0.0006). In addition, bigger females produced fewer eggs in contrast to the unexposed females, for which there was no influence of the body size.
Discussion
In order to determine the cost of insecticide resistance mutations in An. gambiae s.s., we compared the survival and fecundity of strains with a common genetic background in absence of insecticides. In addition, we used natural isolates of P. falciparum to determine the cost of infection in susceptible and resistant An. gambiae. To this aim, we compared the studied life history traits between gametocyte-exposed, infected mosquito and non-exposed control counterparts but we did not include mosquito exposed and not infected as their number were limited.
We assumed that variation in the life history traits between the insecticide susceptible and the resistant strains are associated with the insecticide resistance alleles or with any tightly linked loci resulting from hitchhiking during introgression. Indeed, the genetic differences between the susceptible and the resistant strains might not be totally negligible: a portion of ~11 Mb around the resistant allele may remain different between strains after 19 backcrosses17,42. It was for instance hypothesized that the increased competence for Plasmodium parasite in insecticide resistant mosquitoes22,43 was caused by a predicted immune gene closely linked to the kdr mutation44. Then the causative mutations would be also highly linked to insecticide resistance in natural populations. Consistent to published results44, we found polymorphism on SNPs linked to the para locus in the susceptible strain Kisumu while in the resistant Kdrkis, all individuals shared the same haplotype (i.e. no polymorphism on the tested SNPs, supplementary information). Therefore, we cannot exclude the possibility that the observed differences between the two strains are derived from genetic variance rather than the resistant allele itself. However, insecticide applications would select a similar haplotype containing the resistant allele. It has been demonstrated that the same resistant allele (either ace-1R or kdr) spread among species of the An. gambiae complex in West Africa through introgression of the same allele44,45,46. Thus, we expect that the phenotypes associated with insecticide resistance observed in our strain are also true in natural mosquito populations.
In absence of Plasmodium infection, the main insecticide resistance mutations have affected differently the survival of An. gambiae. While no cost or advantage has been associated with the OP resistant allele ace-1R, in the tested conditions, the kdr resistant allele was associated with an increased survival (HR = 1.37, CI: 1.12–1.68), suggesting a selective advantage in insecticide free environment. In insect, variation in longevity could be the consequences of resource-based trade-offs and oxidative stress26. A decreased neuronal and behavioral excitability observed in mosquito and moth harboring the kdr mutation24,47 may be associated with a lower energy consumption that would benefit to survival. The higher survival rate observed in kdr mosquitoes could be also due to lower level of oxidative stress as unbalanced production of reactive oxygen species is detrimental according to the free-radical theory of ageing48,49.
In contrast, fecundity rate was reduced in both insecticide resistant strains compared to the susceptible strain Kisumu but no difference in the number of eggs per female was observed between mosquito strains. This lower fecundity rate indicated a fitness cost associated with both insecticide resistance alleles. In the field, An. gambiae s.l. commonly required two blood meals to mature and lay their first batch of eggs probably due to insufficient energetic reserves40,41. Depending on the teneral reserves accumulated during larval development, mosquito will use their first blood meal to refill their energetic stores instead of producing eggs50. The observed lower proportion of female developing eggs may be due to lower teneral reserves in insecticide resistant mosquitoes, as observed in C. pipiens bearing the ace-1R resistant allele51. Teneral reserves are positively related to nutrition and negatively related to larval rearing temperature and density52,53. As rearing conditions were similar between mosquito strains in our experimental design, one hypothesis to explain the differences in teneral reserves is the impairment of food intake and digestion and energy storage associated with insecticide resistance in mosquito larvae. Another hypothesis is that variation in larval competition could be associated with insecticide resistance leading to difference in energy stored. Further evidence with direct measurement of energetic resources is needed to test these hypotheses.
After the second blood meal to complete the first gonotrophic cycle, a larger proportion of females produced eggs with no difference between Kisumu and Acerkis strains but Kdrkis still showed a lower fecundity rate. Overall, regardless of the number of blood meal taken to complete their first gonotrophic cycle, Kdrkis females had a significantly lower fecundity rate (69% ± 6.9, p < 0.001) compared to Kisumu (87.5% ± 8.4) but not Acerkis females (75% ± 7.8, p = 0.146). Therefore, we suggest that the ace-1R allele may be associated with an energetic rather than a reproductive cost: lower teneral resources could be compensated with the first blood meal resulting in similar fecundity rate compared to the insecticide susceptible strain following subsequent blood meals. However, the kdr allele may be rather associated with a reproductive cost because additional blood meal did not abolish the reduction in fecundity rate on one hand, and on the other hand kdr mosquitoes showed an increased survival. According to Dao et al.54, reproduction can be influenced by courtship (i.e. male-female interaction), mating, quality of sperm and seminal fluid, egg development and oviposition. However, our data did not suggest a cost on egg development as the number of eggs produced was similar for all strains and all blood meals.
The effect of insecticide resistance on mosquito survival and fecundity has been also assessed in presence of infection. In presence of Plasmodium infection, ace-1R resistant allele was associated with a cost on survival when compared to the susceptible allele, while survival of kdr individuals was similar to that of the susceptible ones. In contrast, Plasmodium infection did not influence the reproductive cost (i.e. the reduction of fecundity rate) associated with insecticide resistance.
P. falciparum infection did not affect mosquito survival in the reference susceptible strain. Under similar nutritional condition (2.5% glucose), P. falciparum infection cost in An. coluzzii was found to be dependent upon blood donor: no cost was detected on some feeding assays whereas cost on survival was detected on others32. Previous studies performed on field collected An. gambiae infected with P. falciparum did not reveal any survival cost28,29. However in the present study, Plasmodium infection was associated with a survival cost in both insecticide resistant strains when compared to the corresponding Plasmodium non-exposed strains. Resistant An. gambiae provides a new physiological environment to P. falciparum in which parasites could be less adapted compared to the ancestral insecticide susceptible host. Considering that the reduction in survival was only observed in infected mosquitoes, we suggested that the insecticide resistant alleles, or closely linked alleles, may interfere directly or indirectly with important factors of the immune system55 that would increase the cost of mounting an immune response against P. falciparum.
In addition, infection influenced the fecundity of An. gambiae s.s. independently of the mosquito strain considered. After one infectious blood meal, P. falciparum infection increased the fecundity rate. It is not clear how parasite infection would lead to this observation but it is assumed that immune-challenged mosquitoes would probably invest more in reproduction in order to increase their fitness if the cost of mounting an effective immune response is very high56. When females needed two blood meals to complete their first gonotrophic cycle, Plasmodium infection did not influence the proportion of females producing eggs but the number of eggs was lower compared to females non-exposed to P. falciparum. These results suggested that pre-existing Plasmodium infection had altered egg production or development and is consistent with field data, showing that P. falciparum infection is associated with a reduction of egg production in wild caught An. gambiae30. As mounting an effective immune response is expected to be costly, infected individual may resorb eggs to produce energy for maintenance of others physiological processes57 and could explain the reduction of eggs associated with Plasmodium infection.
Although our experimental design did not allow testing the relationship between variation in reproduction and survival in infected An. gambiae mosquitoes, the hypothesis that investment in reproduction reduces survival (trade-off hypothesis58) is consistent with the observed results on both resistant strains but not on the susceptible one. Indeed, Plasmodium infection reduced survival of both insecticide resistant strains but increased the proportion of female producing eggs. This is in contrast with the results obtained in the natural avian malaria system33 where infection of C. pipiens (resistant or susceptible to insecticides) with P. relictum resulted in a reduction in fecundity (number of eggs produced) and an increased survival. Although the trade-off hypothesis was not tested directly in our study, the discrepancy may rely on the experimental design (i.e. absence of oviposition site in our survival experiment) and in the nature of the vector-parasite interaction.
From an epidemiological point, it is unknown whether the combined effects of insecticide resistance and Plasmodium infection on survival and reproduction would impact on vector population dynamics and hence on the vectorial capacity. On one hand, increased survival associated with the kdr allele may lead to the spread of this allele in natural populations of mosquitoes even in absence of insecticide selective pressure. On the other hand, cost of infection on survival in insecticide resistant mosquitoes would strongly reduce the vectorial capacity for malaria59 because insecticide resistant vectors may not survive through the extrinsic incubation period.
Moreover, Plasmodium infected mosquitoes harboring the ace-1R or the kdr allele have a reduced level of resistance to insecticides leading to a greater probability of dying after insecticide exposure than uninfected mosquitoes37. Rather than the simplistic assumption that insecticide resistance would undermine the control of vector-borne diseases, we provide evidence for more complex effects of insecticide resistance that make more difficult the prediction of its epidemiological impact. Understanding the influence of adaptive gene, not only at the species or population level, but at the ecosystem level (i.e. mosquito-parasite interaction) will provide crucial insight for the design of vector-borne disease control strategies.
The survival cost evidenced here suggests that insecticide resistance have the potential to reduce vectorial capacity while we previously showed that it increases vector competence22. This may have a great impact on transmission as considering the Ross-MacDonald equation of the vectorial capacity60, vector survival has a greater influence than vector competence. Prediction of transmission dynamics by mosquito vectors without including ecological interactions between parasites and vectors could lead to misleading results or wrong epidemiological outcomes. The impact of insecticide resistance and of malaria parasite infection appeared to be complex and therefore, its effect on others components of the vectorial capacity, like the mosquito biting behavior, needs to be assessed. In addition, there is increasing evidence for multiple insecticide resistance in An. gambiae that makes the prediction even more complex. Taking into account the ecological interactions between selected insecticide resistant alleles, mosquito and parasite life-history traits, and the environment (such as the presence of insecticides) would allow better evaluation of current vector control strategies and foster the development of new evolutionary-proof control measures.
Material and Method
Ethical Statement
All experiments were performed in accordance with the France and Burkina Faso guidelines and regulations and were approved by the Centre Muraz Institutional Ethics Committee under the ethical clearance number 003–2009/cE-cM. All human volunteers were enrolled after written informed consent from the participant and/or their legal guardians.
Mosquito strains
Three reference strains of Anopheles gambiae sensu stricto (S molecular form) were used in this study. One is the reference insecticide susceptible strain Kisumu, collected in Kenya in 195361. The two other strains were resistant to two distinct class of insecticide: the strain Acerkis, homozygous for the ace-1 G119S mutation and resistant to organophosphates and carbamates (OP/CX), and the strain, homozygous for the kdr mutation and resistant to pyrethroids (PYR) and DDT. The insecticide resistant strains were obtained by introgression of the resistant mutations (ace-1 G119S or kdr-west L1014F) allele into the Kisumu genome. The ace-1 G119S allele was obtained from a sample of a resistant An. gambiae population collected in Bobo-Dioulasso, Burkina Faso in 200262. The kdr-west allele has been obtained from pyrethroid resistant mosquitoes sampled in Kou Valley, Burkina Faso13. Kisumu and wild An. gambiae from Burkina Faso used for selection of the insecticide resistant alleles shared polymorphism for the chromosomal inversions 2Rb and 2La63,64. As the resistant alleles are located far from them22, these inversions are not expected to affect recombination at the selected loci due to low linkage disequilibrium65,66. However, it is known that selection of alleles conferring resistance may hitchhike neighboring polymorphisms and it is therefore expected that the strains differ in the regions flanking the introgressed alleles44. Insecticide resistant phenotype of all strains was regularly verified using the WHO standard vertical tube protocol67 with 0.1% bendiocarb and 4% DDT impregnated papers, and the presence/absence of the mutations were checked using the ace-1 G119S and kdr diagnostic PCR13,68. Mosquitoes were kept under standard insectary conditions (27 ± 1 °C, 70 ± 8% RH and 12:12 light and dark photoperiod) in the same secure containment facility. Larvae were reared in the same condition at a fixed density (300 first instar larvae in 700 ml of water per tray) in local spring water and were fed ad libitum with Tetramin®Baby in order to reduce variation in larval growth rate and mosquito size at emergence. After emergence, adults were fed ad libitum on a 5% glucose solution and maintained in two to three 30 × 30 × 30 cm cages. For each experiment/replicate, pupae were collected and placed in a new cage during three consecutive days. One day prior to blood exposition, mosquitoes were starved by removing the glucose solution.
P. falciparum experimental infection
Standard membrane feeding assays were performed as previously described22,38 with slight modification according to Sangare et al.39. Briefly, P. falciparum gametocyte carriers were selected by examining thick blood smears from children aged between five and eleven from two villages in southwestern Burkina Faso (Dandé and Soumousso, located 60 km north and 40 km south-east of Bobo-Dioulasso, Burkina Faso, respectively). Children with a gametocyte density of more than twenty per μl of blood were selected and a venous blood sample (8 ml) withdrawn. Blood serum was replaced with European naive AB serum to limit the potential effect of human transmission blocking immunity69. Reconstituted blood samples were divided in two batches. One batch was heated at 42 °C for 15 min to heat-inactivate gametocyte infectivity and was used as not infectious blood to produce the negative control for infection39. The other batch of blood was used without heat-treatment for infectious blood meal. Membrane feeders were filled with 500 μl of reconstituted blood and maintained at 37 °C by water jackets. Three to five day-old female mosquitoes of the three mosquito strains were allowed to feed concomitantly on infectious or heat-inactivated blood for up to 30 minutes through a Parafilm membrane. Two to three feeders were used for each mosquito strains in order to limit potential feeder effect. Unfed female mosquitoes were discarded and only fully fed mosquitoes were maintained. This procedure was repeated five times for the survival and the fecundity experiment, respectively, each feeding assay (i.e. replicate) using a different gametocyte-infected blood and different batches of each mosquito strain.
Survival experiment
After blood feeding and isolation in individual plastic tubes (Drosophila vials 25 × 95 mm), females were allowed to feed daily on a cotton-imbibed with a 2.5% glucose solution. Mortality 24 h following blood feeding did not indicate any influence of mosquito genotypes nor Plasmodium gametocytes (data not shown) and were not included in the analysis in order to disregard feeding-associated mortality. From the second day after blood feeding, dead females were removed every eight hours (more or less one hour) and dissected until all females had died. Wings and midguts were removed from freshly dead female mosquitoes. No further blood meals were given during the course of the experiment and no oviposition substrates were provided.
Fertility and fecundity experiments
After blood feeding on gametocyte-infected and control blood, distinct batches of females were isolated individually to lay eggs on a wet paper and were allowed to feed on a 2.5% glucose solution. Once they laid eggs, females were dissected to look for remaining eggs and wings and midguts were removed. Eggs on paper were counted under a binocular microscope. Females that did not lay eggs after the fifth day post-blood meal (PBM) were allowed to re-blood feed on a rabbit and were isolated to lay eggs. Females and egg papers were processed as above to measure wing length, and to count oocysts, eggs laid and non-laid eggs. Females that do not lay eggs after the 10th day after the first blood meal (fifth day after the second one) were dissected to look for developed eggs in the ovaries and to measure wing length. Oocysts were not counted on these females to avoid underestimation as some oocysts may already have released sporozoites.
Oocyst counting and measuring of wing length
Midguts were dissected in 0.4% mercurochrome stain and oocysts were counted under a light microscope on each individual female that took an infectious blood meal. The left wing of each female mosquito was cut and was pictured with a dissecting microscope (Leica EZ4D). Wing length was measured from the notch to the wing tip as previously described70. Two measures were performed independently using the ImageJ software (Wayne Rasband, rsb.info.nih.gov/ij/) and correlation between both indicated good agreements (R2 = 0.98), thus, the mean of the two measures was used.
Blood meal size determination
In the survival experiment, blood meal size was quantified retrospectively by measuring the heamatin excreted from individual females in each Drosophila tube, as described by Briegel71. Heamatin excretion was dissolved in 1 ml of a 1% LiCO3 solution and the absorbance of the resulting mixture was read at 387 nm. Blank was obtained with LiCO3 solution alone to correct measures of each individual. Heamatin content was estimated by comparing with a standard curve made with porcine serum heamatin (Sigma-Aldrich).
Statistical analyses
We examined the relationship between survival rate of mosquitoes and the presence of P. falciparum infection (categorical variable of two levels), insecticide resistance strain (categorical variable of three levels), heamatin content (proxy of blood meal size, numerical variable), wing length (proxy of body size, numerical variable) and the blood donor (categorical variable of five levels). Survival distributions of the three An. gambiae strains either infected or control (unexposed) were computed using the Kaplan-Meier method stratified with respect to the donor variable72 using the LIFETEST procedure. The differences between survival distributions were estimated using the asymptotic Log-Rank Test73 taking into account the influence the blood donor. The stratification by the variable donor, corresponding to each experimental infection, allowed having a different baseline hazard function, while the coefficients of the remaining covariates are assumed to be constant across strata.
Cox proportional hazard regression (PHREG procedure) was used to identify whether any of the five following explanatory variables: insecticide resistance strain, oocyst burden, blood donor, heamatin content (blood meal size) and wing length, could explain the observed variation in the survival of infected mosquitoes. Maximal models included all main effects and all interactions between genotypes, heamatin, wing length and the oocyst count variables, and implemented by stratifying with respect to donor. First, the best distribution of the response variable (Weibull distribution) was chosen based on the lowest Akaike Information Criterion (AIC). AIC-based stepwise selection has been performed and significance of variable of the minimum model was assessed by analyzing deviance.
Analysis of the fecundity was based on the two different response variables measured over 5 feeding assays with distinct blood donors: fecundity rate (proportion of female producing eggs) and fecundity (number of eggs) for females that produced at least one egg. Data was analyzed using a generalized mixed-effect model with a Poisson distribution for the fecundity or a binomial distribution for the fecundity rate using the GLIMMIX procedure. The maximal models included the following explanatory variables and their interactions: insecticide resistance strain, P. falciparum infection, wing length and the blood donor. The random structure with the donor gives the lowest AIC. This procedure performs a type III hypothesis for the fixed effect variables and computes the F-statistic based on Satterthwaite’s approximation. Mean percentage of egg-laying females (fecundity rate) and mean number of eggs per female (fecundity) were computed. Post-hoc tests (Lsmeans statement within the GLIMMIX procedure) were carried out to assess differences between estimates, and Bonferroni corrections were applied for multiple comparisons. All statistical analyses were performed using statistical analysis software (SAS Institute Inc., Cary, NC).
Additional Information
How to cite this article: Alout, H. et al. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci. Rep. 6, 29755; doi: 10.1038/srep29755 (2016).
Supplementary Material
Acknowledgments
We would like to thank all children and their parents for participating in this study, as well as to the local authorities for their support. We are very grateful to the IRSS staff in Burkina Faso for technical assistance. We thank the Laboratoire Mixte International LAMIVECT, Bobo Dioulasso, Burkina Faso for technical support. This study was funded by the European Community’s Seventh Framework Program (FP7/2007-2013) under grant agreements n°242095 and n°223736.
Footnotes
Author Contributions H.A. and A.C. designed the experiments. H.A. and L.A. performed the experiments. H.A. analyzed the data. A.C., V.C., F.C., L.S.D. and R.K.D. contributed to reagents/materials tools. H.A. and A.C wrote the paper. All authors read and approved the final manuscript.
References
- WHO. World Malaria Report 2015. at< http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf?ua=1> (2015).
- Corbel V. & N’Guessan R. In Anopheles mosquitoes - New insights into malaria vectors (ed. Manguin S. ) 579–633 (InTech, 2013), 10.5772/56117. [DOI] [Google Scholar]
- Mita T. & Tanabe K. Evolution of Plasmodium falciparum drug resistance: implications for the development and containment of artemisinin resistance. Jpn. J. Infect. Dis. 65, 465–75 (2012). [DOI] [PubMed] [Google Scholar]
- Ashley E. A. et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 371, 411–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A. The Genetical Theory of Natural Selection. 154, (Clarendon Press, 1930). [Google Scholar]
- Davies A. G. et al. Scalloped wings is the Lucilia cuprina Notch homologue and a candidate for the modifier of fitness and asymmetry of diazinon resistance. Genetics 143, 1321–37 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustau C. & Chevillon C. Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol. Evol. 15, 378–383 (2000). [DOI] [PubMed] [Google Scholar]
- Raymond M., Berticat C., Weill M., Pasteur N. & Chevillon C. Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica 112–113, 287–96 (2001). [PubMed] [Google Scholar]
- Roush R. T. & McKenzie J. Ecological genetics of insecticide and acaricide resistance. Ann Rev Entomol 32, 361–80 (1987). [DOI] [PubMed] [Google Scholar]
- Ferguson H. M. et al. Ecology: A Prerequisite for Malaria Elimination and Eradication. Plos Med 7, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe P., Alout H., Djogbenou L., Pasteur N. & Weill M. G. In Genetics and Evolution of Infectious Diseases (ed. Tibayrenc M. ) 363–409 (Elsevier Inc., 2011), 10.1016/B978-0-12-384890-1.00014-5. [DOI] [Google Scholar]
- Weill M. et al. Insecticide resistance in mosquito vectors. Nature 7, 7–8 (2003). [DOI] [PubMed] [Google Scholar]
- Martinez-Torres D. et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7, 179–184 (1998). [DOI] [PubMed] [Google Scholar]
- Du W. et al. Independent mutations in the Rdl locus confer dieldrin resistance to Anopheles gambiae and Anopheles arabiensis. Insect Mol Biol 14, 179–183 (2005). [DOI] [PubMed] [Google Scholar]
- Lenormand T., Bourguet D., Guillemaud T. & Raymond M. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature 400, 861–4 (1999). [DOI] [PubMed] [Google Scholar]
- Berticat C., Rousset F., Raymond M., Berthomieu A. & Weill M. High Wolbachia density in insecticide−resistant mosquitoes. Proc R Soc L. B Biol Sci 269, 1413–1416 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berticat C., Boquien G., Raymond M. & Chevillon C. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet Res 79, 41–47 (2002). [DOI] [PubMed] [Google Scholar]
- Berticat C., Duron O., Heyse D. & Raymond M. Insecticide resistance genes confer a predation cost on mosquitoes, Culex pipiens. Genet Res 83, 189–196 (2004). [DOI] [PubMed] [Google Scholar]
- McCarroll L. & Hemingway J. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem Mol Biol 32, 1345–51 (2002). [DOI] [PubMed] [Google Scholar]
- Duron O. et al. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution. 60, 303–14 (2006). [PubMed] [Google Scholar]
- Djogbénou L., Noel V. & Agnew P. Costs of insensitive acetylcholinesterase insecticide resistance for the malaria vector Anopheles gambiae homozygous for the G119S mutation. Malar. J 9, 12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alout H. et al. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One 8, e63849 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito L. P. et al. Assessing the Effects of Aedes aegypti kdr Mutations on Pyrethroid Resistance and Its Fitness Cost. PLoS One 8, e60878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop M. M. et al. Behavioral Cost and Overdominance in Anopheles gambiae. PLoS One 10, e0121755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assogba B. S. et al. An ace-1 gene duplication resorbs the fitness cost associated with resistance in Anopheles gambiae, the main malaria mosquito. Sci. Rep. 5, 14529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A., Vézilier J., Weill M., Read A. F. & Gandon S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 6, 5–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson H. M. & Read A. F. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol 18, 256–261 (2002). [DOI] [PubMed] [Google Scholar]
- Chege G. M. & Beier J. C. Effect of Plasmodium falciparum on the survival of naturally infected afrotropical Anopheles (Diptera: Culicidae). J Med Entomol 27, 454–458 (1990). [DOI] [PubMed] [Google Scholar]
- Robert V., Verhave J. P. & Carnevale P. Plasmodium falciparum infection does not increase the precocious mortality rate of Anopheles gambiae. Trans R Soc Trop Med Hyg 84, 346–347 (1990). [DOI] [PubMed] [Google Scholar]
- Hogg J. C. & Hurd H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s. l. in north east Tanzania. Parasitology 114, 325–31 (1997). [DOI] [PubMed] [Google Scholar]
- Aboagye-Antwi F. et al. Hydric stress-dependent effects of Plasmodium falciparum infection on the survival of wild-caught Anopheles gambiae female mosquitoes. Malar. J 9, 243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare I. et al. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infect. Genet. Evol. 25, 57–65 (2014). [DOI] [PubMed] [Google Scholar]
- Vézilier J., Nicot A., Gandon S. & Rivero A. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. Biol. Sci. 279, 4033–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalubin F., Delédevant A., Glaizot O. & Christe P. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. J. Anim. Ecol. 83, 850–857 (2014). [DOI] [PubMed] [Google Scholar]
- Zélé F., Nicot A., Duron O. & Rivero A. Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J. Evol. Biol. 25, 1243–1252 (2012). [DOI] [PubMed] [Google Scholar]
- Alout H. et al. Insecticide exposure impacts vector-parasite interactions in insecticide-resistant malaria vectors. Proc R Soc L. B Biol Sci 281, 20140389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alout H. et al. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J. Infect. Dis. 210, 1464–1470 (2014). [DOI] [PubMed] [Google Scholar]
- Ouédraogo A. L. et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malar. World J. 4, 17–20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare I. et al. Studying fitness cost of Plasmodium falciparum infection in malaria vectors: validation of an appropriate negative control. Malar. J 12, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M. T. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Ann. Trop. Med. Parasitol. 48, 58–74 (1954). [DOI] [PubMed] [Google Scholar]
- Takken W., Klowden M. J. & Chambers G. M. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): The disadvantage of being small. J Med Entomol 35, 639–645 (1998). [DOI] [PubMed] [Google Scholar]
- Pombi M., Stump A. D., Della Torre A. & Besansky N. J. Variation in recombination rate across the X chromosome of Anopheles gambiae. Am. J. Trop. Med. Hyg. 75, 901–903 (2006). [PubMed] [Google Scholar]
- Ndiath M. O. et al. Effects of the kdr resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: a hindrance for vector control. Malar. J. 13, 340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C. et al. The kdr-bearing haplotype and susceptibility to Plasmodium falciparum in Anopheles gambiae: genetic correlation and functional testing. Malar. J. 14, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill M. et al. The kdr mutation occurs in the Mopti form of Anopheles gambiae s.s. through introgression. Insect Mol. Biol. 9, 451–455 (2000). [DOI] [PubMed] [Google Scholar]
- Djogbénou L. et al. Evidence of introgression of the ace-1(R) mutation and of the ace-1 duplication in West African Anopheles gambiae s.s. PLoS One 3, e2172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Park Y., Brown T. M. & Adams M. E. Altered properties of neuronal sodium channels associated with genetic resistance to pyrethroids. Mol. Pharmacol. 55, 584–93 (1999). [PubMed] [Google Scholar]
- Finkel T. & Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–47 (2000). [DOI] [PubMed] [Google Scholar]
- Huang H. & Manton K. G. The role of oxidative damage in mitochondria during aging: a review. Front. Biosci. 9, 1100–17 (2004). [DOI] [PubMed] [Google Scholar]
- Hurd H., Hogg J. C. & Renshaw M. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol today 11, 411–416 (1995). [Google Scholar]
- Rivero A., Magaud A., Nicot A. & Vezilier J. Energetic Cost of Insecticide Resistance in Culex pipiens Mosquitoes. J Med Entomol 48, 694–700 (2011). [DOI] [PubMed] [Google Scholar]
- Clements A. N. The Biology of Mosquitoes: Development, nutrition, and reproduction. (Chapman & Hall, 1992). [Google Scholar]
- Timmermann S. E. & Briegel H. Larval growth and biosynthesis of reserves in mosquitoes. J Insect Physiol 45, 461–470 (1999). [DOI] [PubMed] [Google Scholar]
- Dao A. et al. Reproduction-longevity trade-off in Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 47, 769–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontas J. et al. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol Biol 14, 509–21 (2005). [DOI] [PubMed] [Google Scholar]
- Koella J. C. In Insect Infection and Immunity: Evolution, Ecology and Mechanisms (ed. Rolff J. & Reynolds S. E. ) 159–172 (Oxford University Press, 2009). [Google Scholar]
- Xue R.-D., Ali A. & Barnard D. R. Effects of forced egg-retention in Aedes albopictus on adult survival and reproduction following application of DEET as an oviposition deterrent. J. vector Ecol. 30, 45–8 (2005). [PubMed] [Google Scholar]
- Stearns S. C. The Evolution of Life Histories. (OUP Oxford, 1992). [Google Scholar]
- Smith D. L. & Ellis McKenzie F. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 3, 13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G. The epidemiology and control of malaria. (Oxford University Press, 1957). [Google Scholar]
- Shute G. T. A method of maintaining colonies of East African strains of Anopheles gambiae. Ann Trop Med Parasitol 50, 92–94 (1956). [DOI] [PubMed] [Google Scholar]
- Djogbénou L. et al. Characterization of insensitive acetylcholinesterase (ace-1R) In Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J Med Entomol 44, 805–10 (2007). [DOI] [PubMed] [Google Scholar]
- Petrarca V. & Beier J. C. Intraspecific chromosomal polymorphism in the Anopheles gambiae complex as a factor affecting malaria transmission in the Kisumu area of Kenya. Am J Trop Med Hyg 46, 229–237 (1992). [DOI] [PubMed] [Google Scholar]
- Costantini C. et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 9, 16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman D. et al. Association mapping of insecticide resistance in wild Anopheles gambiae populations: major variants identified in a low-linkage disequilbrium genome. PLoS One 5, e13140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. et al. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. Plos Path 6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Test procedures for insecticides resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides treated surfaces. (1998). at http://www.who.int/malaria/publications/atoz/who_cds_cpc_mal_98_12/en/index.html
- Weill M. et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol 13, 1–7 (2004). [DOI] [PubMed] [Google Scholar]
- Gouagna L. C. et al. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop Med Intl Heal. 9, 937–48 (2004). [DOI] [PubMed] [Google Scholar]
- Van Handel E. & Day J. F. Correlation between wing length and protein. J Am Mosq Control Assoc 5, 180–182 (1989). [PubMed] [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J Med Entomol 27, 839–850 (1990). [DOI] [PubMed] [Google Scholar]
- Kaplan E. L. & Meier P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 53, 457–481 (1958). [Google Scholar]
- Cox D. R. & Oakes D. Analysis of Survival Data. (CRC Press, 1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.