Abstract
Mean coral cover has reportedly declined by over 15% during the last 30 years across the central Great Barrier Reef (GBR). Here, we present new data that documents widespread reef development within the more poorly studied turbid nearshore areas (<10 m depth), and show that coral cover on these reefs averages 38% (twice that reported on mid- and outer-shelf reefs). Of the surveyed seafloor area, 11% had distinct reef or coral community cover. Although the survey area represents a small subset of the nearshore zone (15.5 km2), this reef density is comparable to that measured across the wider GBR shelf (9%). We also show that cross-shelf coral cover declines with distance from the coast (R2 = 0.596). Identified coral taxa (21 genera) exhibited clear depth-stratification, corresponding closely to light attenuation and seafloor topography, with reefal development restricted to submarine antecedent bedforms. Data from this first assessment of nearshore reef occurrence and ecology measured across meaningful spatial scales suggests that these coral communities may exhibit an unexpected capacity to tolerate documented declines in water quality. Indeed, these shallow-water nearshore reefs may share many characteristics with their deep-water (>30 m) mesophotic equivalents and may have similar potential as refugia from large-scale disturbances.
Coral reefs worldwide are in serious decline, with adverse changes in coral cover1,2, community structure3, habitat structural complexity4 and reef-building capacity5 occurring in many locations. Climate changes (e.g. elevated sea surface temperature events), outbreaks of coral disease and crown-of-thorns starfish, as well as localised and direct human activities (e.g. overfishing and land-use change)1 have been major drivers of this degradation6. One such stress derives from the effect of increased sediments from coastal catchments which can smother corals7,8 and attenuate the penetration of photosynthetically active radiation (PAR) through the water column9, with potential negative impacts upon coral growth, reproductive success, and disease susceptibility10. Intuitively, sediment-charged waters are therefore commonly perceived as unsuitable (or at best ‘marginal’) for coral reef development. However, recent field investigations11,12,13 have begun to highlight an increasingly diverse range of atypical reef types within Australian waters in which corals appear more resistant to external stressors (e.g. submerged reefs on the central Great Barrier Reef12 and nearshore reefs of Bonaparte Archipelago, Western Australia)13. These reefs have subsequently formed the basis of modelling studies which suggest that such ‘suboptimal’ reef habitats may act as important future climate-change refugia sites from large-scale disturbance events and may facilitate the natural genetic flow of more stress-resistant corals between reefs13,14.
Despite the potential significance of such reef-building settings, empirical data on the structure, community composition and diversity of contemporary reefs within nearshore light-limited environments (defined here as “shallow-water mesophotic” reefs, as corals experience similarly low light conditions as deep-water mesophotic coral ecosystems due to very high turbidity) remains sparse. This is largely due to difficult field working conditions especially from poor visibility. Although reef core records have provided clear evidence of prolonged (millennial duration) phases of reef-building under these conditions15,16,17, there is an assumption that contemporary reefs are in poor health. Here, we challenge these perceptions using new seafloor and benthic community data from sites in very nearshore (<10 m isobath) and highly-turbid areas of the central Great Barrier Reef (GBR), Australia. A recent analysis across the wider GBR reports that average coral cover has declined over the past 27 years (from 28% to 13.8% between 1985 and 2012), attributed to major disturbance events (e.g. coral bleaching and tropical cyclones) and outbreaks of crown-of-thorns starfish6. Although poorly quantified, similar trajectories are projected for the inner-shelf due to increased terrestrial run-off which has increased five- to ten-fold within the Burdekin river catchment since European settlement18,19, and which has been reported to have negatively impacted reefs in more distal settings along the inner- and mid-shelf boundary20. However, intra-regional variability in the magnitude and types of stresses on corals within inshore reef settings will ultimately influence their ecological health, and those located further away from river mouth outlets21 (e.g. Burdekin River), and/or where coastal development is limited, may indeed be able to support coral growth regardless of high sedimentation.
Here, in the largest examination of nearshore coral growth and reef development on the GBR to date, we use data to: 1) define the extent and composition of the nearshore reefs and coral communities within a 15 km2 area of central Halifax Bay; 2) establish the antecedent topographic controls on reef development and community structure; and 3) use these parameters to classify patterns of habitat zonation within these turbid environments. Our findings contest conventional views regarding the threshold conditions for coral recruitment and long-term reef-building in nearshore areas. In particular, our results highlight the capacity for naturally marginal marine settings to support productive and diverse reefal habitats, and provide a framework for testing hypotheses about the apparent resilience of these reefs to some of the drivers of coral community degradation associated within seemingly more optimum (high light, lower nutrient) conditions on mid- and outer shelf areas of the central GBR.
Results
Spatial extent and relative abundance of nearshore coral communities
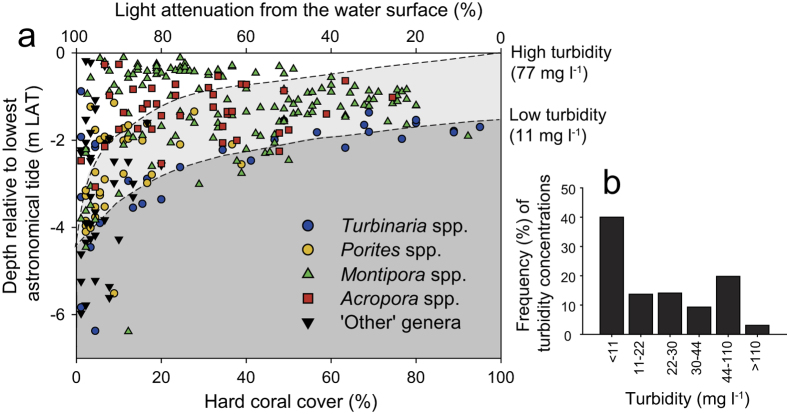
Surveys of the Paluma Shoals Reef Complex (PSRC) encompassed an area of 15.5 km2 (Fig. 1), 11% of which was occupied by hard corals. Mean (±s.d.) coral cover within the PSRC was 38 ± 24%, but locally attained coverage of up to 75% across a large area (0.012 km2). Highest mean cover (40 ± 36%) was recorded in shallow waters (<2 m below lowest astronomical tide [LAT]), and decreased rapidly with depth to 4 m below LAT, closely following light attenuation under low-turbidity scenarios (11 mg l−1; Fig. 2). Below 4 m LAT, reef framework grades into inter-reef sand/muds with very low coral cover (<2%). We also report high structural complexity of reef framework (median rugosity: 3; see Supplementary Table S1), which is a key ecological metric associated with species diversity of reef-dwelling organisms22. Despite the limited depth range of living coral, within the PSRC we recorded 21 coral genera (see Supplementary Table S2). Of these taxa, four genera were identified as dominant based on their relative mean abundance: Montipora spp. (28 ± 27%), Acropora spp. (11 ± 15%), Turbinaria spp. (8 ± 17%) and Porites spp. (4 ± 8%) (Fig. 3).
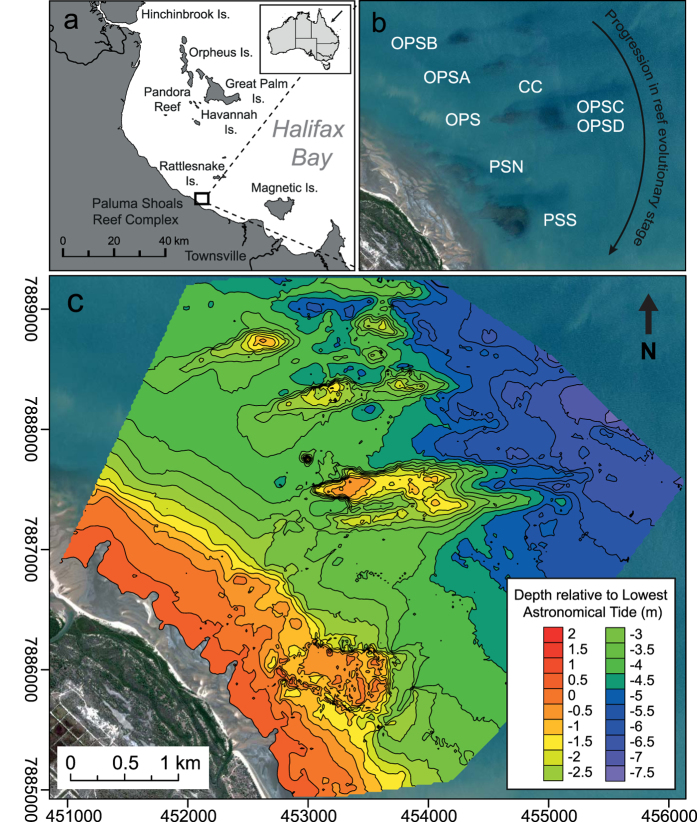
Figure 1.
(a,b) Paluma Shoals Reef Complex (PSRC) located within Halifax Bay, central Great Barrier Reef, Australia (PS: Paluma Shoals [North & South], OPS: Offshore Paluma Shoals, OPS [A,B,C,D]: Offshore Paluma Shoals [A,B,C,D], and CC: coral carpets). Australian boundaries were imported into ArcMap 10.2.2 (http://www.esri.com/) from the database of Global Administrative Areas (GADM) which is freely available for academic use (http://gadm.org/). (c) Seafloor bathymetry (co-ordinates in Australian Map Grid) of the nearshore survey area (15.5 km2) generated from single-beam acoustic survey data (contours are at 0.5 m intervals relative to lowest astronomical tide). Contour map was generated in Golden Surfer 12 (http://www.goldensoftware.com/). All maps were modified in Adobe Illustrator Version CS5 (http://www.adobe.com/). WorldView-2 satellite imagery is courtesy of the DigitalGlobe Foundation (http://www.digitalglobefoundation.org/).
Figure 2.
(a) Mean relative hard coral cover (%) versus water depth (m below LAT), calculated from 10-frame running averages of benthic cover across Paluma Shoals Reef Complex (PSRC). Symbols denote dominant coral genera at each site. Light attenuation from the water surface (%) is shown for low turbidity (11 mg l−1) and high turbidity (77 mg l−1) scenarios derived from field measurements by Browne et al.27 at Paluma Shoals. (b) Frequency (%) of turbidity concentrations (mg l−1) recorded at Paluma Shoals.
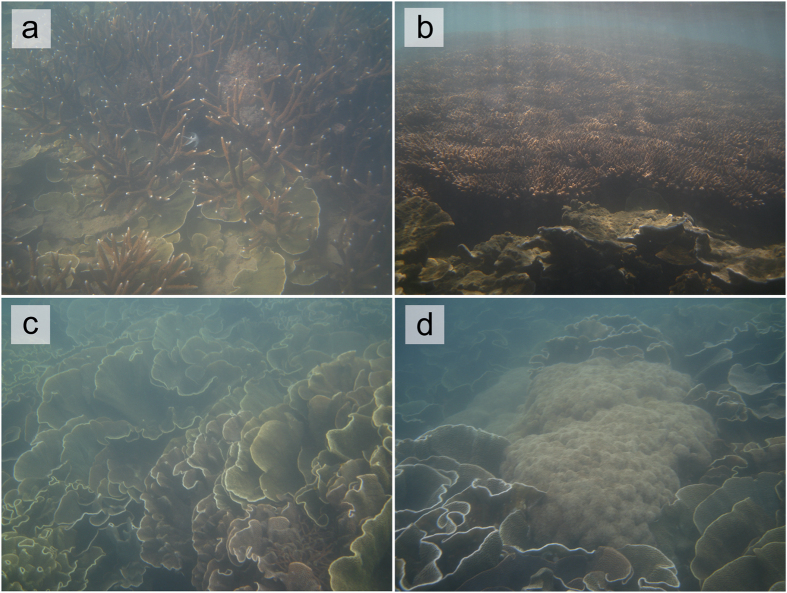
Figure 3.
Nearshore coral communities within Paluma Shoals Reef Complex (PSRC): (a) shallow water branching Acropora spp. and platy Montipora spp.; (b) tabular Acropora spp. (>1 m diameter) common in shallow water areas; (c) extensive stands of foliose Turbinaria spp. ‘coral carpets’ colonising sandy seafloor areas; (d) large Porites spp. colony on the sandy seafloor amongst Turbinaria spp.
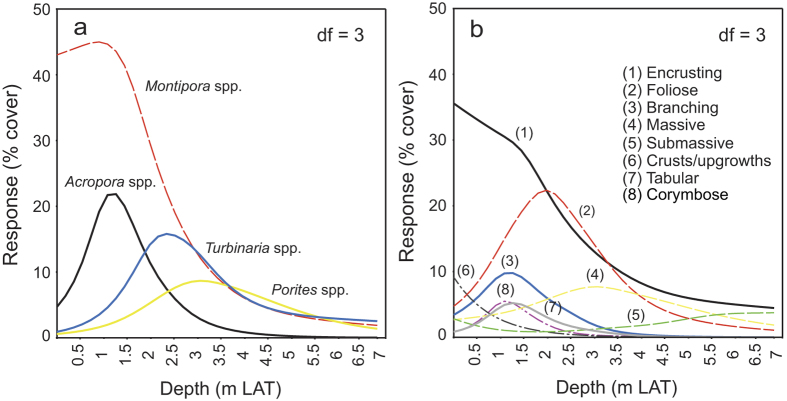
Species response curves generated for the four most common coral genera and all classified coral growth morphologies suggest that corals exhibit clear preferences in their depth distributions (p < 0.001; Fig. 4) and are similar to the patterns observed at other nearshore sites where data is available (e.g. Middle Reef)23. Acropora spp. and Montipora spp. dominate shallow water assemblages (<1.5 m LAT; see Supplementary Video S1) and the relative abundance of encrusting taxa (mostly Montipora spp.) increases considerably towards sea level, whereas branching and tabular Acropora spp. were found to occupy a normally-distributed range between 0.5–2.5 m below LAT (maximum relative cover of 22% at 1 m below LAT). Massive Porites spp. and large stands of foliose Turbinaria spp. (see Supplementary Video S2) inhabited deeper reefal areas (1.5–4 m LAT and 1.5–3.5 m LAT, respectively). Submassive colonies (e.g. Lobophyllia sp., Galaxea sp., Goniopora sp.) on the seafloor were recorded in very low relative abundances (<5%) at depth (>4 m LAT; Fig. 4).
Figure 4.
Distribution of response curves of (a) common coral genera, and (b) coral growth morphotype, with water depth (m below lowest astronomical tide) calculated by a generalised additive model (GAM) with step-wise Akaike Information Criterion (AIC) model selection (df = 3). GAM models were made in CANOCO 5 (http://www.canoco5.com/).
Seafloor bathymetry and inter-reef variability in coral assemblages
Comparative analysis of our high-resolution seafloor bathymetry with ecological datasets shows that the main areas of reef development were restricted to a series of transverse antecedent ridge-like structures (Fig. 1c). These influence both the total extent of coral growth and drive significant shifts in community assemblages. Mean (±s.d.) coral cover varied between sites (OPS: 18 ± 26%; OPSA: 22 ± 31%; OPSB: 43 ± 36%; OPSC: 64 ± 30%; OPSD: 53 ± 36%, CC: 71 ± 19%; see Supplementary Table S3). These shore-normal ridges (i.e. running broadly east-west) occur within the <6 m depth zone and are approximately 1–1.7 km long and 200 m wide. It is reasonable to speculate that these ridge structures represent a series of submarine dune ridges (with a sand/mud interior). However, past coring studies16,17,24 clearly demonstrate that most of the topography visible on the seafloor imagery is the result of vertical reef accretion, and thus the elevation of these underlying structures must be assumed to be low (<0.5 m).
We do note a clearly defined north-south gradient in ridge (i.e. reef) topography (Fig. 1b,c), ranging from: (1) shore-attached high elevation reefs with extensive emergent reef flats (Paluma Shoals; +0.5 m LAT); (2) southern reefs, with a continuous double-ridge morphology which form partially-emergent (OPS: 0 m LAT) and fully-submerged structures (OPSC & OPSD: −0.6 m LAT); (3) central reefs, a semi-continuous submerged structure with a mid-ridge depression (OPSA, −0.4 m LAT); and (4) northern reefs, formed of two submerged coalescing structures in early stages of reef development (OPSB, −0.4 m LAT). This diverse topography between reefs at different elevations is indicative of the reefs currently existing at different stages of evolutionary development25. In contrast, inter-ridge seafloor areas were relatively flat and featureless, and characterised by sands/muds with sparse coral rubble, sea whips, hydroids, and very low coral cover.
Habitat zonation of Halifax Bay
Clustering analysis of benthic substrate types and coral datasets collected from across our study area differentiated six major habitat types with each comprising different benthic associations (Fig. 5; Supplementary Fig. S1). These habitats were: (1) sand-dominated substrates (47 ± 9.2%) with massive Porites rus (24 ± 15%) on submerged reef slopes; (2) terrigenous sand/mud (91 ± 13%) covering inter-reef seafloor areas; (3) large Turbinaria spp. stands (80 ± 15%), or “coral carpets” (sensu)26, on the flanks of partially-emergent reefs; (4) high coral cover (47 ± 19%) reef ridges (incipient “crests”) characterised by Montipora spp. (55 ± 19%) and Acropora spp. framework (16 ± 16%); (5) rubble-dominated (74 ± 12%) emergent reef areas, supporting low coral cover (13 ± 10%) of mainly foliose/encrusting Montipora spp. (13 ± 11%) and branching Acropora spp. (19 ± 22%); and (6) low coral cover (11 ± 10%) seafloor areas with submassive corals (e.g. Lobophyllia sp., Galaxea sp., Goniopora sp.). A seventh habitat type (Goniastrea-dominated emergent reef flats) was identified from existing ecological assessments of Paluma Shoals North (PSN) and South (PSS)16 and is included in the final habitat map (Fig. 5). Inter-habitat mean cumulative dissimilarity was 80% (SIMPER analysis; see Supplementary Table S4), indicating that differences in benthic characteristics between the discriminated habitat types were high (i.e. only 20% similar). Soft sediment substrates (sand/mud) identified as the main discriminator for habitat dissimilarity (31%), and the relative abundance of Montipora spp. (15%), Acropora spp. (6%) and Turbinaria spp. (5%) for within coral associations. Between the two coral framework-producing habitats (4 & 5 above), there was a mean cumulative dissimilarity of 55% differentiated by the relative proportion of coral rubble (24%), hard coral cover (20%), and the relative abundance of Montipora spp. (24%).
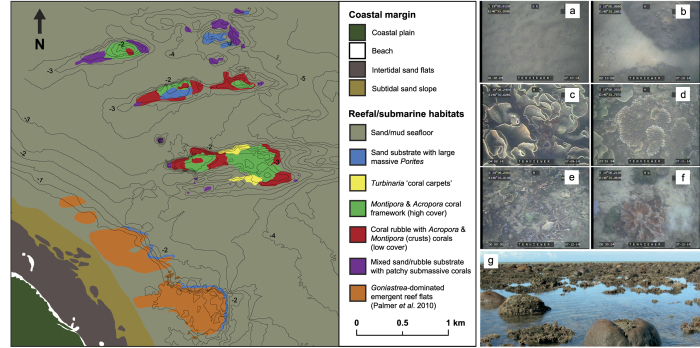
Figure 5. Habitat map of nearshore Halifax Bay, central Great Barrier Reef, Australia.
Habitat zones were delineated based on their relative benthic cover and coral community composition in ArcMap 10.2.2 (http://www.esri.com/). Bathymetric contours (at 0.5 m relative to lowest astronomical tide) are shown for topographic reference and were imported from Golden Surfer 12 (http://www.goldensoftware.com/). Ecological datasets for Paluma Shoals North and South were taken from Palmer et al.16. The final map was modified in Adobe Illustrator Version CS5 (http://www.adobe.com/). Representative images of habitat types are shown: (a) sand/mud seafloor; (b) sand-dominated substrates with massive Porites rus; (c) Turbinaria mesenterina “coral carpets”; (d) high coral cover reef crests with Montipora spp. and Acropora spp. framework; (e) rubble-dominated with low cover of Montipora spp. and Acropora spp.; (f) sand and rubble with patchy submassive corals (e.g. Lobophyllia sp., Galaxea sp., Goniopora sp.); (g) Goniastrea-dominated emergent reef flats.
Discussion
Coral reefs growing within the nearshore zone of the central GBR experience chronic high turbidity as accumulated fine sediments are resuspended by waves27,28. Because this turbidity rapidly attenuates light availability at depth9, these reefs, like their deeper mesophotic cousins, are poorly investigated systems, but it is generally assumed that coral growth and reefal development within these environments is limited by sub-optimal light conditions10. Here, however, we document large areas of topographically complex reef composed of relatively diverse (21 genera) and thriving hard coral communities in this shallow, muddy nearshore zone. Importantly, we note the presence of both large (>1 m diameter) long-lived and smaller coral colonies (10–20 cm diameter Acropora spp. and Montipora spp.) indicating recruitment is occurring within these areas. Previous reef core data from a number of locations along the GBR have shown that reef-building has occurred in isolated nearshore locations throughout the mid- to late Holocene29, and extensive stands of foliose Turbinaria spp. resembling those at PSRC have been reported elsewhere within a turbid high-latitude setting in Hervey Bay, Queensland, Australia11. However, our findings suggest that the scale and diversity of reef-building may be far more extensive than previously thought.
A key implication of this is that these low-light adapted coral communities may be better acclimated to cope with the stresses that have driven the major declines in coral cover observed on mid-outer shelf reefs since the mid-1980’s6. Therefore, and as suggested in recent global modelling projections14, turbid reef environments that are suitably flushed by tidal currents (such as PSRC) may potentially serve as important refugia sites for corals throughout many of the world’s major reef-building provinces (including eastern Australia), as high suspended sediment concentrations within the water column increase the intensity of light scatter and thus significantly reduce solar irradiance and thermal stress on corals (i.e. the direct effects of ocean warming that cause large-scale coral bleaching). These theoretical scenarios are partially supported by recent reports from the Bonaparte Archipelago, Western Australia, where diverse assemblages (60 genera) of stress-tolerant corals survive extreme environmental conditions, experiencing large fluctuations in sea surface temperature, exposure, turbidity and tidal range13, and which have begun to expand our understanding of the tolerance thresholds of reef-building corals in nearshore marginal settings. Indeed, our new data coupled with existing reef core records16,24 indicate that nearshore areas on the GBR are also capable of sustaining long-term reef development with high rates of vertical reef accretion, which continues to the present until the reefs become sea level constrained. These areas may thus harbour critical but largely-overlooked (and very poorly mapped) habitats for reef communities akin to those deep water (>30 m) mesophotic coral ecosystems that occur along the deeper outer margins of the GBR30,31.
Specifically, our surveys show high coral cover (mean: 38 ± 24%) within nearshore areas of central Halifax Bay. We note that mean hard coral cover is more than twice that of the average for the central GBR shelf (12%; AIMS long-term reef monitoring; see Supplementary Table S5). Furthermore, our ecological survey data (based on an analysis of 4000+ images from ~50 km of survey lines) indicate that these shallow-water mesophotic reefs support relatively diverse coral assemblages with some 21 coral genera identified within the PSRC, comparable to AIMS studies of inner-shelf (inshore) reefs32. Although Cyclone Yasi did disturb some intertidal reef communities in our study area33, our data from 2013 and 2014 show that coral assemblages within Halifax Bay were in good condition after the event and that subtidal branching and platy taxa were relatively unaffected. Factors linked to major declines in hard coral cover at a number of GBR mid- and outer-shelf sites6, such as partial mortality, disease scars, and crown-of thorns starfish were all largely absent within the study area.
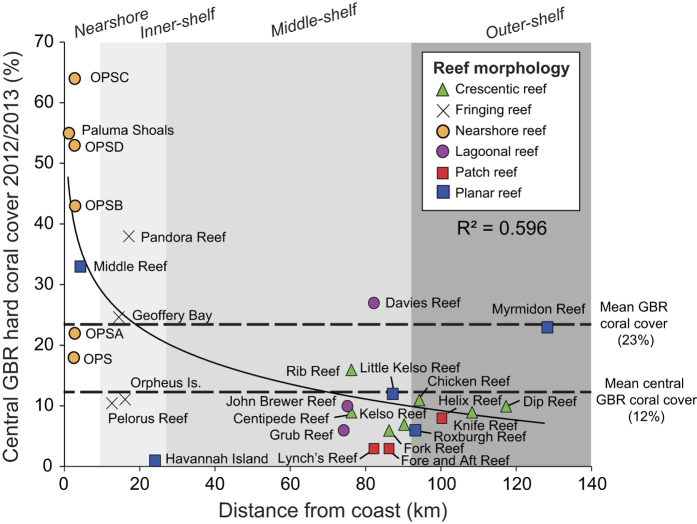
Paradoxically, these nearshore reefs that are proximal to the coast (<3 km) and located relatively close to human-modified catchments, have hard coral cover levels much greater than those measured at many ‘clear-water’ mid- and outer-shelf sites on the central GBR in recent years. Our data shows that mean cover declines rapidly with distance from the mainland coast (R2 = 0.596, F1,23 = 21.1, p < 0.001; Fig. 6). Importantly, we also observed no evidence of large-scale Acropora die-off within contemporary assemblages at PSRC following European settlement such as that reported at sites ~15 km offshore along the inner-mid-shelf boundary of the central GBR (e.g. Pelorous Island)20, and which lie outside these most turbid nearshore areas. Indeed, our surveys show that branched and tabular Acropora (A. pulchra and A. hyacinthus) are common across our sites (mean: 15 ± 17%, but up to 74% in some areas) in depths <2 m LAT (Fig. 3).
Figure 6. Hard coral cover (%) versus distance from the mainland coast (km) at reef sites on the central Great Barrier Reef (AIMS reef monitoring sector-Townsville), Australia.
Hard coral cover estimates (2010–2013) taken from AIMS long-term reef monitoring database (http://data.aims.gov.au/monmap3/cruisereport.jsp?cruise=all). Symbols denote the present reef morphology at each site.
A critical aspect to the apparent success of these nearshore corals must therefore lie in their ability to survive the low light conditions associated with high-turbidity. High suspended sediment concentrations, particularly those dark in colour typical of Halifax Bay, significantly reduce PAR to corals9. At PSRC light attenuation curves through turbid waters and the influence of local tidal range are clearly major factors driving the relative abundance of corals and in defining the limits of species distribution. We note that coral species known to be well-adapted to the physical and trophic environments of turbid coastal waters (e.g. Montipora spp., Turbinaria spp. and Porites spp.)34,35 dominated most PSRC reefs. These species typically exhibit greater autotrophic/heterotrophic plasticity as they are able to utilise organic nutrients bound within planktonic organisms and suspended particulates to offset reduced photosynthetic efficiency7,36, and often display morphotypic adaptations that enhance sediment sloughing and increase light capture (e.g. Turbinaria mesenterina)36,37. This allows corals, such as Turbinaria spp., to withstand sediment loads an order of magnitude higher than maximum background conditions38 and exploit less desirable habitats to form extensive monospecific stands11.
A strong topographic control on coral development within the PSRC is also evident in our data (Fig. 1), and is most likely associated with the physiological requirements of the reef-building corals. Bathymetric surveys showed a number of low-amplitude (1–2 m) submarine antecedent ridges that are attached at the shore and appear rhythmic in distribution, aligned in such a way that suggest they are controlled by longshore currents. These ridges appear to provide sufficient elevation to facilitate initial coral recruitment and, ultimately, reef growth above the soft-sediment seafloor at these sites. This may mitigate the likelihood of corals on the seabed from being smothered during temporary periods of fine material accumulation, and in later stages of vertical reef growth, provide increasing (with shallowing) access to light and repositioning of the corals into the resuspension zone39.
Underlying sedimentary structures built by terrigenoclastic sediments thus ironically appear fundamental in determining the location and extent of nearshore reef growth within the PSRC. Reefs rise across a depth range of 0.5 m at the shore-attached Paluma Shoals, to 4 m water depth (OPSB) and occur along a continuum of reef evolutionary states defined by their current position relative to lowest astronomical tide (LAT). These range from deeper incipient coral communities (“coral carpets”), through to juvenile (OPSC & OPSD), late-juvenile (OPSA & OPSB), mature (OPS) and senile reefs (Paluma Shoals), that are fully emplaced at sea level and emergent under low tidal conditions (based on evolutionary classifications of clear-water GBR reefs from)25. Reef structures across varying reef evolutionary stages are associated with type-specific coral cover and coral community assemblages, and share many parallels with geological records of Miocene reefs which were also influenced by terrigenoclastic sediment inputs, initiating directly on coarse-grained fluvial fan deposits40,41. In these early reef settings, reef morphology was similarly controlled by local sedimentary processes rather than independent coral framework growth42, as at PSRC.
However, the broader distribution of reefs in the most nearshore areas of the GBR shelf still remains relatively unknown. The widespread cover of hard corals within the mapped area of Halifax Bay suggests that it is highly unlikely that such reef communities are restricted to the PSRC alone. Indeed, preliminary analysis of satellite imagery shows numerous near-surface features (~2 km2 of potential reef structure between Townsville and Lucinda) at sites north of the PSRC with similar geometries to those identified here15. This indicates that coral reef development may be far more abundant within this coastal nearshore zone than previously thought. However, whether these reefs exhibit comparable coral cover or display similar levels of ecological ‘health’ as PSRC is unknown, as each reef will experience varying degrees of external stress depending on their proximity to major coastal development and/or river mouth outlets. The proportion of seafloor covered by reefal structures within our survey area (11%) is consistent with the average density of reefs on the GBR shelf (9%)25, and this thus points to a need to better map and define what has previously been a very over-looked habitat type (both in terms of seafloor topography and the ecological communities which inhabit these areas), and one which in contrast to many mid- and outer-shelf reefs on the GBR have been accreting (and continue to accrete) over contemporary time-scales24.
In this context, and based on the relationships between water depth, substrate availability and seafloor topography that we present, we suggest our data could form a useful basis as a predictive model of nearshore reef distribution by assessing suitable habitat extent for coral growth. Under future scenarios of sea-level rise, inundation of low-lying coastal plains will increase accommodation space for coral reef expansion providing new areas for turbid-zone reef growth similar to the reefs that we present here. In turn, understanding reef development within marginal environments is important as they provide close analogues to past and future reefs, and may provide an essential functional role as refugia sites from large-scale disturbance events, or as sources of genetic material (through natural gene flow) of more resistant coral types to reseed degraded reefs.
Methods
Study site
Our study focused on the wider Paluma Shoals Reef Complex (PSRC), located within Halifax Bay on the inner-shelf (<3 km from the coast) of the central Great Barrier Reef, Australia (Fig. 1a). Here, several partially-emergent reef structures have previously been identified, two of which have been cored to determine their age and growth history (Paluma Shoals [PS]15,16; Offshore Paluma Shoals [OPS]24; Fig. 1b). In addition, preliminary studies also indicated substantial sub-surface incipient reef and coral cover within proximal seafloor areas of PSRC. We hereafter refer to these discrete reef structures as Offshore Paluma Shoals A, B, C and D (OPS [A, B, C & D]; Fig. 1b). We also make a basic division of the GBR inner-shelf (<20 m isobath at ~20 km offshore)43 to differentiate these “coastal nearshore” reefs (<10 m isobath), from other inner-shelf (or “inshore”) reef settings further offshore, which experience different environmental conditions.
The PSRC is located landwards of the shore-detached inshore sediment prism (ISP), a wedge of terrigenoclastic sands that were reworked onshore during the postglacial transgression, and fine material that has accumulated at the shoreline since sea level stabilised in the mid-Quaternary highstand43. The PSRC therefore experiences naturally high turbidity (up to 385 mg l−1, with 40 days per year exceeding 88 mg l−128). As a result these nearshore reefs are extremely light-limited due to sediment laden waters, and thus we classify the PSRC as a series of shallow-water mesophotic reefs. The term “mesophotic” is conventionally used to describe coral ecosystems surviving in deep-water (>30 m) low-light conditions44, but was first applied to reefs that experience low-light from muddy waters45,46. Halifax Bay has a diurnal tidal cycle with a tidal range of 3.6 m.
Data collection and analysis
We conducted a high-resolution survey of seafloor bathymetry within the PSRC area (15.5 km2) to establish the extent and morphology of reefal structures. Because of the shallow nature of the survey area (<8 m depth) bathymetry data was collected using a single-beam echo sounder (Ceeducer Ceestar 200 kHz), coupled with a Real-Time Kinematic Global Positioning System (RTK-GPS). Data were acquired along a suite of closely spaced (every 100 m) north-south parallel lines across the location of known shoals (observed in Landsat imagery), and a series of 500 m spaced shore-perpendicular lines to provide broader context for the shoals (see Supplementary Fig. S2). Depth values were corrected for tidal variations throughout surveying in CarisTM HIPS, and reduced to lowest astronomical tide (LAT) datum using tide gauge data at the Port of Townsville. A digital elevation model of seafloor bathymetry was constructed in Surfer 12 using kriging (10 × 10 m grid size).
Towed video surveys (July 2013/2014) of the seafloor were undertaken using a drop-down video system (SeaViewer with Sea-Track™ GPS overlay), as along with poor visibility due to high turbidity, the presence of saltwater crocodiles and Irukandji jellyfish make long SCUBA transects impractical. The camera was towed 1 m above the seafloor along multiple 300 m transect lines (see Supplementary Fig. S2) to delineate the extent and composition of reefal and non-reefal areas. High-resolution transects (20–40 m spacings) supplemented areas identified as supporting hard coral communities. Still frames (1 m2) were extracted from the video at automated 8 sec intervals (n = 4420) and a digital 9-point grid overlay was added for analysis of benthic cover. Corals were classified by genera and growth morphology. Reef rugosity within each frame was assessed visually using a modified rugosity classification scheme47 (see Supplementary Table S1). Frames were depth-calibrated to LAT datum using the seafloor bathymetric model generated, and 10-frame running averages were used to determine the relative abundance (% cover) of different benthic types. Data collection and analysis produced comparable outputs (% hard coral cover/% coral genera) to those reported by the Australian Institute of Marine Science (AIMS) allowing us to directly compare our findings to AIMS survey data collected at a range of sites within the central GBR region (sector-Townsville, 2010–2013). A habitat map of the nearshore Halifax Bay study area was constructed by combining spatially-corrected benthic cover and bathymetry thematic layers with multi-spectral satellite imagery (WorldView-2 courtesy of DigitalGlobe Foundation) in ArcGIS 10.2.2.
Habitat classification and statistical analysis
Benthic data was classified into different habitat types using hierarchical agglomerative clustering with group-average sorting48,49. Non-transformed data, allowing for dominant species or substrata to exert an appropriate influence on habitat cluster groups49,50, were used to construct a Bray-Curtis similarity matrix from which nearshore reef habitats were identified (<70% similarity). Habitats were distinguished based on their relative cover of substrate type (e.g. sand, rubble, etc.) and coral genera. A similarity percentage (SIMPER) analysis was run to establish biota or substrata driving inter-habitat (dis)similarity48,49. Species responses (coral genera and growth morphology) to depth (LAT) were examined using a generalised additive model (GAM) generated in CANOCO 5 by applying a Poisson distribution (df = 3), as it provides greater flexibility to fit non-linear relationships. Model selection was determined by a step-wise Akaike Information Criterion (AIC). Regression analyses were conducted to test for differences in coral cover with water depth, as well as cross-shelf changes in mean coral cover with distance from the coast.
Additional Information
How to cite this article: Morgan, K. M. et al. Evidence of extensive reef development and high coral cover in nearshore environments: implications for understanding coral adaptation in turbid settings. Sci. Rep. 6, 29616; doi: 10.1038/srep29616 (2016).
Supplementary Material
Acknowledgments
This work was supported by NERC grant NE/J023329/1 to C.T.P and S.G.S. We thank the DigitalGlobe Foundation for providing satellite imagery, and the support and assistance of the crew of the R.V. James Kirby research vessel.
Footnotes
Author Contributions Conceived and designed the study: K.M.M., C.T.P. and S.G.S. Participated in fieldwork: K.M.M., C.T.P., S.G.S., J.A.J. and J.J.D. Analyzed the data: K.M.M. and J.A.J. Secured project funding: C.T.P. and S.G.S. Wrote the paper: K.M.M., C.T.P. and S.G.S. Co-ordinated fieldwork: S.G.S., C.T.P. and K.M.M. All authors gave final approval for publication.
References
- Jackson J. B. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001). [DOI] [PubMed] [Google Scholar]
- Bruno J. F. & Selig E. R. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2, e711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. T. et al. Regional-scale dominance of non-framework building corals on Caribbean reefs affects carbonate production and future reef growth. Glob. Chang. Biol. 21, 1153–1164 (2015). [DOI] [PubMed] [Google Scholar]
- Alvarez-Filip L., Dulvy N. K., Gill J. A., Côté I. M. & Watkinson A. R. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. Biol. Sci. 276, 3019–3025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. T. et al. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4, 1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De’ath G., Fabricius K. E., Sweatman H. & Puotinen M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 109, 17995–17999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junjie R. K., Browne N. K., Erftemeijer P. L. A. & Todd P. A. Impacts of sediments on coral energetics: partitioning the effects of turbidity and settling particles. PLoS One 9, e107195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne N. K., Precht E., Last K. S. & Todd P. A. Photo-physiological costs associated with acute sediment stress events in three near-shore turbid water corals. Mar. Ecol. Prog. Ser. 502, 129–143 (2014). [Google Scholar]
- Storlazzi C. D., Norris B. K. & Rosenberger K. J. The influence of grain size, grain color, and suspended-sediment concentration on light attenuation: Why fine-grained terrestrial sediment is bad for coral reef ecosystems. Coral Reefs 34, 967–975 (2015). [Google Scholar]
- Rogers C. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 62, 185–202. (1990). [Google Scholar]
- Butler I. R., Sommer B., Zann M. & Zhao J. -x. & Pandolfi, J. M. The impacts of flooding on the high-latitude, terrigenoclastic influenced coral reefs of Hervey Bay, Queensland, Australia. Coral Reefs 32, 1149–1163 (2013). [Google Scholar]
- Roberts T. E., Moloney J. M., Sweatman H. P. A. & Bridge T. C. L. Benthic community composition on submerged reefs in the central Great Barrier Reef. Coral Reefs 34, 569–580 (2015). [Google Scholar]
- Richards Z. T., Garcia R. A., Wallace C. C., Rosser N. L. & Muir P. R. A diverse assemblage of reef corals thriving in a dynamic intertidal reef setting (Bonaparte Archipelago, Kimberley, Australia). PLoS One 10, e0117791 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia C. & van Woesik R. Reef-coral refugia in a rapidly changing ocean. Glob. Chang. Biol. 21, 2272–2282 (2015). [DOI] [PubMed] [Google Scholar]
- Smithers S. & Larcombe P. Late Holocene initiation and growth of a nearshore turbid-zone coral reef: Paluma Shoals, central Great Barrier Reef, Australia. Coral Reefs 22, 499–505 (2003). [Google Scholar]
- Palmer S. E., Perry C. T., Smithers S. G. & Gulliver P. Internal structure and accretionary history of a nearshore, turbid-zone coral reef: Paluma Shoals, central Great Barrier Reef, Australia. Mar. Geol. 276, 14–29 (2010). [Google Scholar]
- Perry C. T. & Smithers S. G. Cycles of coral reef ‘turn-on’, rapid growth and ‘turn-off’ over the past 8500 years: a context for understanding modern ecological states and trajectories. Glob. Chang. Biol. 17, 76–86 (2011). [Google Scholar]
- McCulloch M. et al. Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421, 727–730 (2003). [DOI] [PubMed] [Google Scholar]
- Kroon F. J. et al. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 65, 167–181 (2012). [DOI] [PubMed] [Google Scholar]
- Roff G. et al. Palaeoecological evidence of a historical collapse of corals at Pelorus Island, inshore Great Barrier Reef, following European settlement. Proc. R. Soc. B Biol. Sci. 280, 20122100–20122100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- .Fabricius K. E., Logan M., Weeks S. J., Lewis S. E. & Brodie J. Changes in water clarity in response to river discharges on the Great Barrier Reef continental shelf: 2002–2013. Estuar. Coast. Shelf Sci. 173, A1–A15 (2016). [Google Scholar]
- Graham N. A. J. & Nash K. L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326 (2012). [Google Scholar]
- Browne N. K., Smithers S. G. & Perry C. T. Geomorphology and community structure of Middle Reef, central Great Barrier Reef, Australia: An inner-shelf turbid zone reef subject to episodic mortality events. Coral Reefs 29, 683–689 (2010). [Google Scholar]
- Perry C. T., Smithers S. G. & Gulliver P. Rapid vertical accretion on a ‘young’ shore-detached turbid zone reef: Offshore Paluma Shoals, central Great Barrier Reef, Australia. Coral Reefs 32, 1143–1148 (2013). [Google Scholar]
- Hopley D. The geomorphology of the Great Barrier Reef: Quaternary development of coral reefs John Wiley & Sons. (1982). [Google Scholar]
- Riegl B. & Piller W. E. Reefs and coral carpets in the northern Red Sea as models for organism-environment feedback in coral communities and its reflection in growth fabrics. Geol. Soc. London, Spec. Publ. 178, 71–88 (2000). [Google Scholar]
- Browne N. K., Smithers S. G. & Perry C. T. Spatial and temporal variations in turbidity on two inshore turbid reefs on the Great Barrier Reef, Australia. Coral Reefs 32, 195–210 (2013). [Google Scholar]
- Larcombe P., Ridd P. V., Prytz a. & Wilson B. Factors controlling suspended sediment on inner-shelf coral reefs, Townsville, Australia. Coral Reefs 14, 163–171 (1995). [Google Scholar]
- Perry C. T., Smithers S. G., Gulliver P. & Browne N. K. Evidence of very rapid reef accretion and reef growth under high turbidity and terrigenous sedimentation. Geology 40, 719–722 (2012). [Google Scholar]
- Bridge T. C. L. et al. Variability in mesophotic coral reef communities along the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 428, 63–75 (2011). [Google Scholar]
- Bridge T. C. L. et al. Diversity of Scleractinia and Octocorallia in the mesophotic zone of the Great Barrier Reef, Australia. Coral Reefs 31, 179–189 (2011). [Google Scholar]
- Sweatman H., Thompson A., Delean S., Davidson J. & Neale S. Status of near-shore reefs of the Great Barrier Reef 2004. Marine and Tropical Research Facility Research Report Series. Reef and Rainforest Research Centre Limited, Cairns (2007). [Google Scholar]
- Perry C. T., Smithers S. G., Kench P. S. & Pears B. Impacts of Cyclone Yasi on nearshore, terrigenous sediment-dominated reefs of the central Great Barrier Reef, Australia. Geomorphology 222, 92–105 (2014). [Google Scholar]
- Anthony K. R. N. Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19, 59–67 (2000). [Google Scholar]
- Browne N. K. Spatial and temporal variations in coral growth on an inshore turbid reef subjected to multiple disturbances. Mar. Environ. Res. 77, 71–83 (2012). [DOI] [PubMed] [Google Scholar]
- Anthony K. R. & Fabricius K. E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Bio. Ecol. 252, 221–253 (2000). [DOI] [PubMed] [Google Scholar]
- Anthony K. R. N., Hoogenboom M. O. & Connolly S. R. Adaptive variation in coral geometry and the optimization of internal colony light climates. Funct. Ecol. 19, 17–26 (2005). [Google Scholar]
- Sofonia J. J. & Anthony K. R. N. N. High-sediment tolerance in the reef coral Turbinaria mesenterina from the inner Great Barrier Reef lagoon (Australia). Estuar. Coast. Shelf Sci. 78, 748–752 (2008). [Google Scholar]
- Wolanski E., Fabricius K., Spagnol S. & Brinkman R. Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of Australia. Estuar. Coast. Shelf Sci. 65, 153–158 (2005). [Google Scholar]
- Karabıyıkoğlu M. et al. Facies and environmental setting of the Miocene coral reefs in the late-orogenic fill of the Antalya Basin, western Taurides, Turkey: implications for tectonic control and sea-level changes. Sediment. Geol. 173, 345–371 (2005). [Google Scholar]
- Santodomingo N. et al. A diverse patch reef from turbid habitats in the middle Miocene (East Kalimantan, Indonesia). Palaios 30, 128–149 (2015). [Google Scholar]
- Hayward A. B. Coral reefs in a clastic sedimentary environment: Fossil (Miocene, S.W. Turkey) and modern (Recent, Red Sea) analogues. Coral Reefs 1, 109–114 (1982). [Google Scholar]
- Larcombe P., Costen A. & Woolfe K. J. The hydrodynamic and sedimentary setting of nearshore coral reefs, Central Great Barrier Reef shelf, Australia: Paluma Shoals, a case study. Sedimentology 48, 811–835 (2001). [Google Scholar]
- Kahng S. E. et al. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275 (2010). [Google Scholar]
- Rosen B., Aillud G. & Bosellini F. Platy coral assemblages: 200 million years of functional stability in response to the limiting effects of light and turbidity. Proc. 9th Int. Coral Reef Symp. 1, 255–264 (2000). [Google Scholar]
- Morsilli M., Bosellini F. & Pomar L. Mesophotic coral buildups in a prodelta setting (late Eocene, southern Pyrenees, Spain): a mixed carbonate–siliciclastic system. Sedimentology 59, 766–794 (2012). [Google Scholar]
- Polunin N. & Roberts C. Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar. Ecol. Prog. Ser. 100, 167–176 (1993). [Google Scholar]
- Clarke K. R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 18, 117–143 (1993). [Google Scholar]
- Mumby P. & Harborne A. Development of a systematic classification scheme of marine habitats to facilitate regional management and mapping of Caribbean coral reefs. Biol. Conserv. 88, 155–163 (1999). [Google Scholar]
- Green E. P., Ellis A. C., Mumby P. J., Edwards A. J. & Clark C. D. The assessment of mangrove areas using high resolution multispectral airborne imagery. J. Coast. Res. 14, 443–483 (1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.