Introduction
Immune checkpoint inhibitors show remarkable antitumor activity across multiple tumor types, with approval of programmed death–1 (PD-1) inhibitors for non–small cell lung cancer and melanoma. A recent clinical trial found median progression-free survival of 11.5 months for combined nivolumab plus ipilimumab therapy for metastatic melanoma compared with 6.9 months (nivolumab alone) or 2.9 months (ipilimumab alone).1 Nivolumab, a fully human IgG4 PD-1 antagonist antibody, and ipilimumab, a fully human IgG1 cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) antagonist antibody, act by relieving suppression of antitumor T cells.
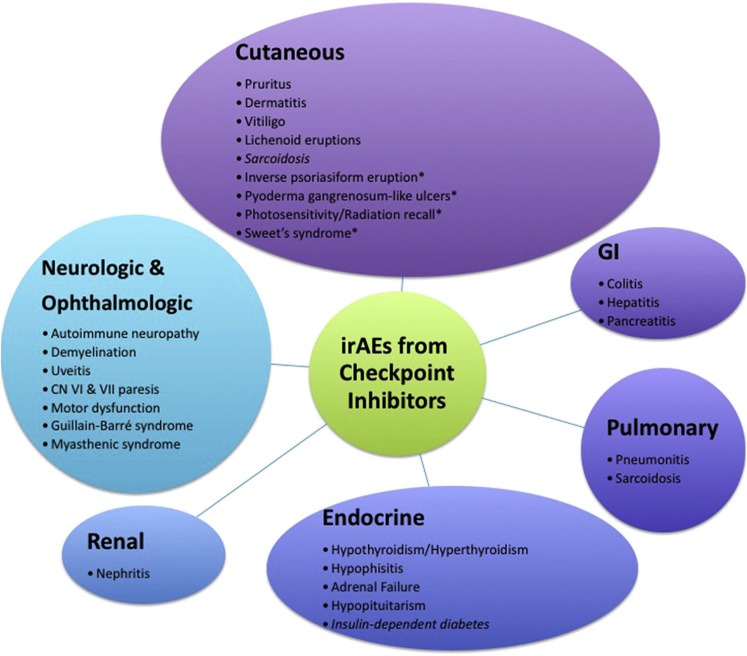
CTLA-4 inhibition (ipilimumab) potentiates early T-cell activation during antigen presentation. PD-1 inhibition (nivolumab) acts primarily on T cells that have already been activated and subsequently suppressed via PD-1 signaling at sites of T cell destination. Both CTLA-4 and PD-1 signaling normally dampen the immune system to protect against excessive inflammation and development of autoimmunity; however, in the setting of malignancy, they can be co-opted by tumors to allow immune evasion. Blocking such signaling has profound antitumor effects in some patients. Adverse effects associated with immune checkpoint inhibitor therapy are termed immune-related adverse events (irAEs) (Fig 1). Here we discuss a rare irAE, sarcoidosis, in the setting of combined ipilimumab/nivolumab therapy for lung adenocarcinoma.
Fig 1.
The spectrum of immune-related adverse events reported with checkpoint inhibitor therapy. The irAEs are graded based on severity from grade 1 to 4. Grade 2 to 3 reactions are typically managed by temporarily withholding medication with or without systemic corticosteroids. Grade 4 or grade 3 reaction that recurs is indication for discontinuing medication. Cutaneous irAEs can be managed with topical steroids if mild but may require systemic corticosteroids with long tapers to prevent recurrence. (*) indicates irAE reported in the literature in single case report. CN, Cranial nerve; GI, gastrointestinal.
Report of a case
A 60-year-old white woman with lung adenocarcinoma metastatic to her lymph nodes and brain, receiving ipilimumab plus nivolumab for 7 months, presented to her oncologist with nausea, vomiting, aphasia, and confusion. She was admitted to the hospital with differential diagnoses including progressive brain metastases, radiation necrosis at an intracranial site of prior radiosurgery, or an adverse reaction to ipilimumab or nivolumab.
This patient was diagnosed with lung cancer 9 months prior after experiencing dysarthria and worsening balance. Brain imaging found a left frontal lobe lesion. Chest imaging uncovered a right upper lobe lung mass with subcarinal and paratracheal lymphadenopathy. Bronchoscopic biopsy confirmed poorly differentiated lung adenocarcinoma, with histology findings negative for epidermal growth factor receptor, anaplastic lymphoma kinase (ALK) gene rearrangement, Kirsten rat sarcoma viral oncogene homolog (KRAS) gene mutation and human homolog of v-ROS avian UR2 sarcoma virus oncogene (ROS1) rearrangement. She subsequently underwent uncomplicated gamma knife surgery to the left frontal lobe brain lesion.
She then initiated combination immunotherapy with ipilimumab every 6 weeks plus nivolumab every 2 weeks (dosing for both drugs was 1 mg/kg) according to trial protocol. Two weeks into treatment a mildly pruritic maculopapular rash developed; this was diagnosed by her oncologist as ipilimumab-associated cutaneous eruption and treated using oral diphenhydramine and topical triamcinolone 0.1% cream with resolution and no interruption in immunotherapy. Follow-up imaging per study protocol at 2 months found regression of cancer outside the brain but also found progression of the left frontal lobe lesion or treatment effect (ie, radiation necrosis). Immunotherapy was held, and she underwent intracranial resection, which found primarily radiation necrosis. She restarted immunotherapy 2 weeks after surgery, receiving a total of 3 ipilimumab and 10 nivolumab treatments prior to her current presentation.
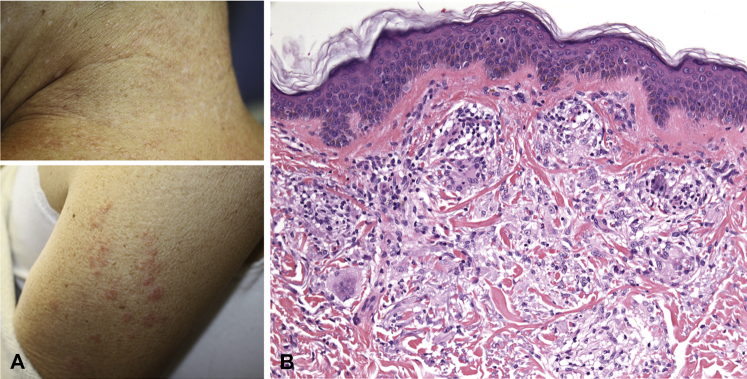
At presentation to the emergency room, her vital signs were stable, but she was in diabetic ketoacidosis (serum glucose, 766 mg/dL) and was transferred to the intensive care unit, where an insulin drip was started and proved effective. On hospital day 7, the dermatology department was consulted to evaluate a rash on the neck. On examination, the patient had greater than fifty 1- to 3-mm skin-colored to pink firm papules, some coalescing into annular plaques (Fig 2, A). Her bilateral malar cheeks also had approximately 10 thin pale pink 2- to 4-mm papules. Some papules had umbilicated appearance. There was no apple jelly color appreciated on diascopy. No cervical or axillary lymphadenopathy or lacrimal gland hyperplasia was appreciated. Skin biopsy of lesion on the posterior neck revealed a dermal granulomatous infiltrate consistent with sarcoidosis (Fig 2, B). A small focus of polarizable material was seen. This finding was noted in a previous case of ipilimumab-associated cutaneous sarcoidosis.2 A diagnosis of cutaneous sarcoidosis was made and was considered an irAE secondary to ipilimumab, nivolumab, or the combination. She was treated with topical clobetasol 0.05% ointment twice daily for 2 weeks with some improvement but not resolution of the lesions. Brain magnetic resonance imaging during the hospitalization found interval increase in the left frontal lobe metastasis, with sustained response to immunotherapy outside the brain. Subsequent intracranial resection found metastatic lung adenocarcinoma. The patient declined further treatment and died 3 months later.
Fig 2.
Sarcoidosis in the setting of immune-related therapy for lung cancer. A, Distributed on the posterior neck and upper arms are greater than fifty 1- to 3-mm skin-colored to pink firm papules, in some areas coalescing into annular plaques. B, Dermal inflammatory infiltrate including relatively scant lymphocytes and epithelioid histiocytes arranged in well-defined granulomas. Giant cells are seen. There are no prominent epidermal changes. (Hematoxylin-eosin stain.)
Discussion
Sarcoidosis is a rare irAE, with only 11 cases reported to date (Table I). Most occurred during ipilimumab treatment of metastatic melanoma, with a single report (limited detail) of sarcoidosis with anti–PD-L1 antibody treatment. None have been reported during treatment of lung cancer. Of these cases, sarcoidosis occurred predominantly in the lung (67% of the time) and less so in the skin (25%). The timing of sarcoidosis onset ranged anywhere from 2 cycles to 10 cycles of therapy. There is evidence supporting a positive association between irAEs and clinical response, specifically for vitiligo during melanoma treatment13; however, there does not seem to be a correlation between development of sarcoidosis and treatment response (Table I).
Table I.
Patients who have sarcoidosis associated with immune checkpoint inhibitor therapy for malignancy
| Patient demographics | Cancer diagnosis (location of metastases) | Location of sarcoidosis | Drug | Time into treatment, dose | Response to treatment | Study |
|---|---|---|---|---|---|---|
| 67 y, F | Melanoma (liver) | Lung | Ipilimumab | 5 courses, q3w, 10 mg/kg | Stable disease | Eckert et al, 20093 |
| 49 y, M | Melanoma (cutaneous, LN) | Lung (hilar and mediastinal LAD) | Ipilimumab | 6 courses q3w, 3 mg/kg | Complete remission | Vogel et al, 20124 |
| — | Prostate | Lung (aveolar) | Ipilimumab | GVAX + ipi, 5 mg/kg q4w | — | van den Eertwegh et al, 20125 |
| 52 y, F | Melanoma (lung, LN) | Lung | Ipilimumab | 2 courses, q3w, 3 mg/kg | Progression of disease | Wilgenhof et al, 20126 |
| 63 y, M | Melanoma (lung, liver, LN) | Lung | Ipilimumab | 4 cycles, q3w, 3 mg/kg | Regression of mets | Berthod et al, 20127 |
| — | Melanoma | — | Anti–PD-L1 Ab | 10 mg/kg, q2w | — | Brahmer et al, 20128 |
| 57 y, M | Melanoma (LN) | Lung, skin | Ipilimumab | 10 mg/kg, q3w x 4, then q12w x 2 | — | Tissot et al, 20139 |
| 55 y, M | Melanoma (LN) | Lung, skin | Ipilimumab | 2 courses 10 mg/kg | Developed cutaneous mets | Reule and North, 20132 |
| 37 y, M | Melanoma (LN, bone) | Lung, CNS (sella turcica) | Ipilimumab | 4 courses, q3w, 3 mg/kg | Sustained partial response | Murphy, 201410 |
| M | Melanoma | Spleen | Ipilimumab | — | Sustained partial response | Andersen et al, 201411 |
| 74 y, M | Melanoma (liver) | Granulomatous interstitial nephritis | Ipilimumab | 3 courses, 3 mg/kg | Toumeh et al, 201512 | |
| 60 y, F | Lung adenocarcinoma (LN, brain) | Skin | Ipilimumab + nivolimab | 10 cycles, 1 mg/kg | Progression of disease | Current case |
CNS, Central nervous system; LAD, lymphadenopathy; LN, lymph node; mets, metastases.
Sarcoidosis can be systemic, involving lung, skin, central nervous system, or kidney.2, 9 Clinicians should be aware of the association between immunotherapy and sarcoidosis, because systemic sarcoidosis can be mistaken for progression of malignancy on clinical examination and in imaging studies. Fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT), for example, can highlight inflammatory cells in sarcoidosis and be misinterpreted as cancer progression. This finding has the potential to adversely impact decisions on patient treatment and management. The diagnosis of sarcoidosis was delayed in many of the cases analyzed in Table I because systemic involvement was thought to represent progression of malignancy. In our patient, when her brain magnetic resonance image showed an enlarging left frontal lobe mass, systemic sarcoidosis was considered in the differential diagnosis and led to the recommendation for definitive tissue sampling; unfortunately, in this instance, malignancy was confirmed.
Our patient experienced 2 other adverse events related to immunotherapy: macular papular cutaneous eruption, also called ipilimumab-associated cutaneous eruption (discrete, erythematous, minimally scaly, pruritic papules that can coalesce into thin plaques, most often involving the trunk and extremities) and insulin-dependent diabetes (in the setting of PD-1 inhibition may develop over a time ranging from 1 week to 5 months8, 14). The rash usually occurs early in treatment, 3 to 4 weeks after the first dose, as occurred for our patient, and responds to topical steroids.15 The rash can worsen with subsequent cycles and be associated with a significant increase in peripheral eosinophilia (neither occurred in this patient).
Conclusion
With increased use of immune checkpoint inhibitors for cancer treatment, physicians are seeing a variety of irAEs (Fig 1). In some cases, the irAE provides insight into disease pathogenesis. For example, validating findings in mice, it appears that PD-1 inhibition unmasks genetic susceptibility to diabetes.16 The pathomechanism of sarcoidosis is poorly understood, but dysregulated cellular immunity could play a key role through a T helper–1 T-cell–mediated response to an unknown antigen (possibly mycobacterial),17, 18 and this response could be potentiated by checkpoint inhibitors. It is not clear without further scientific research whether specifically the ipilimumab or nivolumab (or both) contributed to the pathogenesis of sarcoidosis in our patient. If ipilimumab drives sarcoidosis alone, as it may be in melanoma cases, our observation then suggests the PD-1 pathway is either not involved, or not able to abrogate, the CTLA-4 pathway effect.
Footnotes
Funding sources: None.
Conflicts of interest: None decalred.
References
- 1.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reule R.B., North J.P. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J Am Acad Dermatol. 2013;69(5):e272–e273. doi: 10.1016/j.jaad.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Eckert A., Schoeffler A., Dalle S., Phan A., Kiakouama L., Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218(1):69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 4.Vogel W.V., Guislain A., Kvistborg P., Schumacher T.N., Haanen J.B., Blank C.U. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol. 2012;30(2):e7–e10. doi: 10.1200/JCO.2011.37.9693. [DOI] [PubMed] [Google Scholar]
- 5.van den Eertwegh A.J., Versluis J., van den Berg H.P. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 6.Wilgenhof S., Morlion V., Seghers A.C. Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitor. Anticancer Res. 2012;32(4):1355–1359. [PubMed] [Google Scholar]
- 7.Berthod G., Lazor R., Letovanec I. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30(17):e156–e159. doi: 10.1200/JCO.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer J.R., Tykodi S.S., Chow L.Q. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tissot C., Carsin A., Freymond N., Pacheco Y., Devouassoux G. Sarcoidosis complicating anti-cytotoxic T-lymphocyte-associated antigen-4 monoclonal antibody biotherapy. Eur Respir J. 2013;41(1):246–247. doi: 10.1183/09031936.00107912. [DOI] [PubMed] [Google Scholar]
- 10.Murphy K.P., Kennedy M.P., Barry J.E., O'Regan K.N., Power D.G. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol Res Treat. 2014;37(6):351–353. doi: 10.1159/000362614. [DOI] [PubMed] [Google Scholar]
- 11.Andersen R., Norgaard P., Al-Jailawi M.K., Svane I.M. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. Oncoimmunology. 2014;3(8):e954506. doi: 10.4161/21624011.2014.954506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toumeh A., Sakhi R., Shah S., Arudra S.K., De Las Casas L.E., Skeel R.T. Ipilimumab-Induced Granulomatous Disease Occurring Simultaneously With Disease Progression in a Patient With Metastatic Melanoma. Am J Ther. April 30, 2015 doi: 10.1097/MJT.0000000000000266. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Teulings H.E., Limpens J., Jansen S.N. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 14.Hughes J., Vudattu N., Sznol M. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J.N. Dermatologic adverse events to chemotherapeutic agents, Part 2: BRAF inhibitors, MEK inhibitors, and ipilimumab. Semin Cutan Med Surg. 2014;33(1):40–48. doi: 10.12788/j.sder.0061. [DOI] [PubMed] [Google Scholar]
- 16.Kochupurakkal N.M., Kruger A.J., Tripathi S. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9(2):e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen E.S., Moller D.R. Etiologies of Sarcoidosis. Clin Rev All Immunol. 2015;49(1):6–18. doi: 10.1007/s12016-015-8481-z. [DOI] [PubMed] [Google Scholar]
- 18.Beutler B.D., Cohen P.R. Sarcoidosis in Melanoma Patients: Case Report and Literature Review. Cancers. 2015;7(2):1005–1021. doi: 10.3390/cancers7020821. [DOI] [PMC free article] [PubMed] [Google Scholar]