Abstract
Many natural chemicals in food are in the nanometer size range, and the selective uptake of nutrients with nanoscale dimensions by the gastrointestinal (GI) tract is a normal physiological process. Novel engineered nanomaterials (NMs) can bring various benefits to food, e.g., enhancing nutrition. Assessing potential risks requires an understanding of the stability of these entities in the GI lumen, and an understanding of whether or not they can be absorbed and thus become systemically available. Data are emerging on the mammalian in vivo absorption of engineered NMs composed of chemicals with a range of properties, including metal, mineral, biochemical macromolecules, and lipid‐based entities. In vitro and in silico fluid incubation data has also provided some evidence of changes in particle stability, aggregation, and surface properties following interaction with luminal factors present in the GI tract. The variables include physical forces, osmotic concentration, pH, digestive enzymes, other food, and endogenous biochemicals, and commensal microbes. Further research is required to fill remaining data gaps on the effects of these parameters on NM integrity, physicochemical properties, and GI absorption. Knowledge of the most influential luminal parameters will be essential when developing models of the GI tract to quantify the percent absorption of food‐relevant engineered NMs for risk assessment. WIREs Nanomed Nanobiotechnol 2015, 7:609–622. doi: 10.1002/wnan.1333
For further resources related to this article, please visit the WIREs website.
INTRODUCTION
Many chemicals naturally found in the environment, in food, and in the human body are in the nanometer (nm) size range.1 For example, many globular proteins have a diameter of several nanometres, starch granules have substructures of approximately 30 nm, the DNA double helix has a diameter of 2 nm, and fatty acids are several nanometers in length.2, 3 Traditional food processing practices such as emulsification also generate nanosized structures such as micelles, foams, and colloids in the food matrix and many such materials have a long history of safe consumption; for example, micelles created during the homogenization of milk. Apart from food origins, there are endogenous nanoparticles (NPs) physiologically produced from ions in the mammalian gastrointestinal (GI) tract. For example, the precipitation of calcium and phosphate creates nanosized calcium phosphate particles that may be absorbed as such.4 Thus, the presence and uptake of nutrients with nanoscale dimensions is a normal physiological process.
Scientific innovation is expanding the diversity of approaches used to produce food to meet the needs of the global population. Nanotechnology, an enabling technology with applications in many divergent sectors, is being explored within the food arena to bring beneficial properties to food products and enhance nutrition. Consequently, it is necessary to develop scientific tools for the detection and risk assessment of novel engineered nanomaterials (NMs) / NPs in food.5 The NanoRelease Food Additive project, coordinated by the International Life Sciences Institute (ISLI) Research Foundation, is aiming to identify and advance NM measurement methods to support international risk assessment capacity and safe product development for engineered NMs in food. The actionable conclusions synthesized by the task groups of this project are summarized in a State of the Science report.6
A task group of the NanoRelease Food Additive project first reviewed the types of engineered NMs that may potentially be used in food.7 The presence of these substances could arise from direct incorporation during food manufacturing or from migration from food contact materials. NMs could also be incidentally present in food from environmental sources. Three broad categories of potential food‐relevant examples were defined as (1) soft/lipid‐based, such as solid lipid NPs, (2) solid non‐lipid non‐metal, such as silicon dioxide (SiO2), carbon black or cellulose NMs, and (3) solid metalloid / metal‐based, such as titanium dioxide (TiO2) or silver NPs.7 Another group of the project reviewed analytical methods that can be used to detect NMs in complex food and food contact material matrices, and their release from these matrices.8, 9, 10, 11
Following the consumption of a chemical entity, the percentage or total mass absorbed to systemic circulation can be used, along with other toxicology data, as a key parameter in the determining safe maximum levels in food.12 Therefore the NanoRelease Food Additive project also reviewed existing models of the GI tract that could be adapted to allow the assessment of digestion and bioavailability.13 The analysis concluded that in silico computational, in vitro fluid, and in vitro cell culture assays should be used, after which the necessity of ex vivo organ and in vivo animal models should be considered.
This article from the NanoRelease project summarizes GI conditions influencing the absorption of NM entities in vivo, including but not limited to engineered metal, mineral, carbohydrate, nucleic acid, protein, and lipid nanostructures. Along with a general review and examples relevant to those broad categories we have included some specific examples of TiO2, SiO2, and cellulose NMs. These three substances were selected as examples because a survey of the multistakeholder NanoRelease steering committee determined that they may have potential uses or presence in food, reflect a range of material characteristics including release potential and solubility, are of interest to various stakeholders, and are being considered for further efforts to develop measurement methods. TiO2, also known as titanium dioxide or titania, is an oxide of the transition metal element titanium. Its bulk anatase and rutile crystal forms are used as a coloring agent in food.7 SiO2, also termed silicon dioxide or silica, is an oxide of the metalloid element silicon. It is used as an anticaking agent in food. Cellulose is a biochemical polymer consisting of glucose monomers, and is a major structural component of plants, bacteria, and algae. Bulk cellulose and microcrystalline cellulose are used as anticaking agents in food.14 These chemicals can all be engineered down to nanoscale structures, and can remain intact when suspended in aqueous media or added to dry food matrices.15, 16 Examples of other pertinent chemicals are also discussed below. We reviewed uptake and evaluated evidence of whether or not the various physicochemical conditions encountered in the GI tract modify NP size and surface properties. This knowledge will be important when developing models to quantify the absorption of novel engineered NMs for risk assessment.
GI TRACT ANATOMY MEDIATING THE ABSORPTION OF NMs
Macroscopic and Microscopic Anatomy
The mammalian GI tract spans from the mouth to the anus, with an average length of approximately 5 m in humans.17 The macroscopic compartments, separated from each other by sphincters, are the buccal cavity, esophagus, stomach, small intestine, and large intestine (Figure 1). The primary functions are the maintenance of water homeostasis, the digestion, and absorption of macro‐ and micronutrients and electrolytes, trafficking of the fraction of macromolecular antigens that survive digestion, and the exclusion of pathogens.
Figure 1.
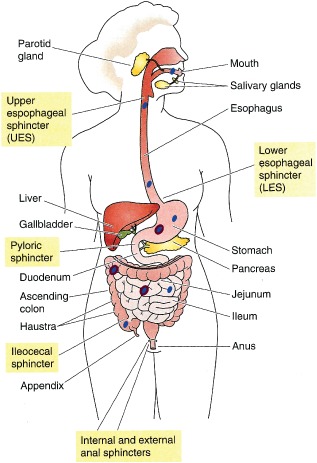
The transit of consumed particulates through the lumen of the organs of the human digestive system. The buccal cavity, esophagus, stomach, small intestine, and large intestine are separated from each other by sphincters, labeled in beige squares. Consumed particulates (shown in blue) passing through these organs may or may not remain in their native physicochemical state, and can develop a dynamic corona coating (represented in violet). (Reprinted with permission from Ref 18. Copyright 2009 Elsevier)
The continuous multilayer stratified squamous epithelium in contact with the lumen of the buccal cavity and esophagus is adapted to handle the high volume of quickly passing food. The surface area is 0.02 meters squared (m2) in the buccal cavity and likewise 0.02 m2 in the esophagus.17 There is little published information on the absorption rate of particulates through the epithelium of these two compartments. This is likely because the surface area is low, the residence time for most food matrices is short, and the intestine is more specialized for selective uptake of macromolecules in this size range. In the stomach the surface area is 0.05 m2. Despite the relatively low surface area and permeability of the gastric epithelium to macromolecules, a minimal passage of NPs is allowed.19, 20
Before particles contact epithelial cells, they must first cross the mucus barrier. Mucus is comprised of mucin glycoproteins that form viscoelastic gels resulting in an adherent unstirred layer coating the GI wall.21 In the stomach and large intestine the mucus layer is tightly bound (Figure 2). Adhesive substances are trapped, but non‐adherent materials can diffuse through this layer. In the small intestine the mucus layer is thinner and there is less interaction between lamellar strings, allowing greater access of the luminal contents to the epithelium.23, 24 This permits the uptake of nutrients, while trapping, immobilizing, and excluding larger potentially hazardous particulates, e.g., bacteria.25
Figure 2.
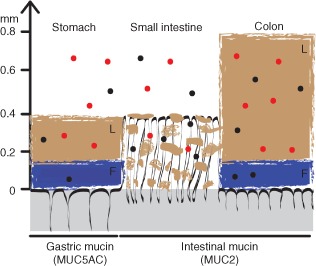
Mucus organization and nanoparticle interactions in the gastrointestinal tract. The gastrointestinal tissue is represented in grey with black folds representing the structure interfacing the lumen. The predominant mucin isotype expressed in each region is shown in parenthesis. L denotes the loosely bound outer mucus layer. Non‐interacting lamellar strands of loosely bound mucus in the small intestine are also shown in brown. F denotes the firmly attached inner mucus layer, shown in blue. Mucoadhesive nanoparticles are represented by the red circles; non‐mucoadhesive nanoparticles are represented by the black circles. (Reprinted with permission from Ref 22. Copyright 2011 National Academy of Sciences)
The small intestine consists of three consecutive parts: the duodenum, jejunum, and ileum. It is the longest segment of the GI tract, and the outermost microscopic layer is structured with villi and microvilli that project into the lumen, resulting in a very high surface area of 30 m2 in humans.17 In normal homeostasis the epithelial cell monolayer that covers the villi forms a tight but selective barrier. Microbes and most macromolecular antigens are held at bay, whereas nutrients are absorbed efficiently. The epithelial monolayer contains several specialized cell types. Enterocyte cells are responsible for nutrient absorption, while other cell types perform functions such as the secretion of mucus.26 The large intestine (colon) has haustral folds, but is shorter and lacks the villi projections seen in the small intestine, and therefore has a lower surface area of 2 m2. Columnar enterocytes in the epithelium are the predominant cell type.
Gut‐associated lymphoid tissue (GALT), distributed in localized regions of the wall of the small and large intestine, is composed of isolated follicles and of aggregated lymphoid follicles termed Peyer's patches. These tissues have a specialized epithelium containing antigen sampling microfold (M)‐cells, in addition to enterocytes, and have a much thinner mucus layer allowing direct interfacing with lumen contents.27 Overall, M‐cells represent approximately 1% of the cells lining the intestine.28 Functionally they transport particulate matter from the gut lumen across the epithelial barrier to allow sampling by antigen‐presenting cells of the immune system, which traffic through the extracellular lymph fluid on the basolateral side.29, 30
Transepithelial Absorption Mechanisms
Key to understanding NM absorption in the GI tract is an understanding of the various microscopic routes by which particulates can be taken up. Two transepithelial routes can allow the passage of substances from the lumen to the basolateral side: transcellular transport (i.e., through an epithelial cell) or paracellular transport (i.e., between adjacent epithelial cells; Figure 3).
Figure 3.
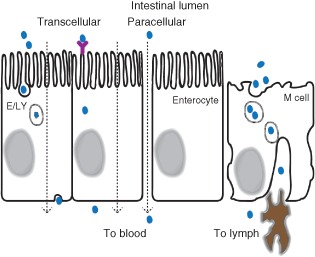
Pathways of nanoparticle absorption through the gastrointestinal tract epithelium. From left to right: vesicular endocytosis through epithelial cells where E/LY denotes endosome or lysosome; receptor‐mediated transport through epithelial cells; paracellular transport between epithelial cells; and vesicular phagocytosis through microfold (M) epithelial cells covering lymphoid aggregates, with dendritic cells below in brown. Nanoparticles are shown in blue.
Transcellular transport can be bi‐directional. When a substance is absorbed into a cell's cytoplasm through the apical membrane it can have several fates. It can (A) be secreted back into the lumen, (B) remain in the cell and accumulate, (C) degrade in the cell, or (D) be transported across the basolateral cell membrane into systemic circulation. There is limited simple diffusion of particulates into cells due to factors such as steric hindrance and immiscible solubility.28 Carrier‐mediated transport takes up specific small molecule ligands, and so is not specific to larger NMs. Vesicular transcytosis is the most efficient mechanism by which intact macromolecules can be transported into and/or across epithelial cells.31, 32 The process requires energy expenditure by the cells and, for many types of NMs, is dependent upon dynamic interactions of particles with the actin cytoskeleton, microfilaments, and microtubules.33 There are several types of vesicular transcytosis. Receptor‐mediated endocytosis begins when ligands in the contents of the intestinal lumen bind to receptor proteins (e.g., clathrin or caveolin) on the exterior of the apical cell membrane, triggering endocytosis. Because of the low endocytic activity of enterocytes, the amount of NPs translocated via this route is considered to be low.28 Nonetheless, endocytosis has been reported for NMs such as ferritin metalloprotein and tartrate‐modified ferrihydrite.34, 35, 36 Pinocytosis and macropinocytosis are non‐selective mechanisms of endocytosis. The resulting intracellular vesicles fuse with lysosomes for enzymatic degradation of the contents. Phagocytosis is the endocytosis of larger solid particles such as viruses, bacteria, or particulate matter. Lymphoid follicle and Peyer's patch M‐cells use both phagocytosis and receptor‐mediated endocytosis to sample lumen contents. It is generally believed that the majority of particle translocation through the intestinal wall occurs via this route.28, 30, 37
Paracellular transport is the passive movement of solutes across the epithelium between the tight junctions that bind cells together into a monolayer. The dimension of this paracellular space is on the order of nanometers and it varies along the intestinal tract.38, 39 In the esophagus and stomach, the epithelial barrier effectively restricts the paracellular movement of solutes, with a paracellular pore diameter of approximately 0.6 nm.40 In the small intestine the diameter is approximately 1 nm.41 However, the proximal duodenum region can permit tightly regulated permeation of molecules as large as albumin, which has a diameter of 7 nm.40 The large intestine has intermediate properties, allowing regulated passage of molecules of approximately 3 nm diameter.42 These properties are altered in several pathological circumstances (e.g., inflammation, erosion, radiation insult, etc.) in which transepithelial permeability through the paracellular pathway may be enhanced. Under normal conditions the paracellular route is generally not accessible to larger compounds. However, the normal process of persorption, whereby dead enterocytes are extruded from the epithelial layer, can create breaches in the barrier through which particulates can pass. This mechanism is unlikely to be a highly efficient pathway of NM uptake.4, 43 Nonetheless, it has been shown that 4 and 10 nm colloidal gold particles are absorbed through dead enterocytes being extruded from the intestinal villus.44
In summary, the major pathways implicated in the uptake of intact NPs across the GI wall are transcellular transport through viable M‐cells lining intestinal Peyer's patches and through the abundant intestinal enterocytes. However, degradation products released from the surface of NPs can also be absorbed by conventional mechanisms. This is important because, for some NM classes, ions released and absorbed into the body can reconstitute into NMs in tissues.45
Following the uptake of intact and degraded substances through epithelial cells to the underlying lymph fluid, they can either remain there, circulate in the lymph fluid through lymphatic ducts that eventually drain into the systemic cardiovascular circulatory system, or be absorbed directly into capillaries of the cardiovascular circulatory system.46 Buccal cavity and esophageal capillaries lead into systemic veins, whereas capillaries from the stomach and intestines pass through the liver before entering systemic circulation.47 A portion of absorbed substances can thereby be secreted back into the intestinal lumen via enterohepatic circulation through the gallbladder in bile. The remainder is distributed to systemic tissues and either metabolized in those tissues, excreted by other routes such as urine, or otherwise remains persistent.48 GI tissues, systemic blood and lymph, and peripheral tissues are examined to quantify the absorption of a consumed NM.
Examples of NM Percent Absorption
There is a limited number of published in vivo mammalian studies that quantified GI absorption following consumption of the chemical entities we selected as examples of potentially food‐relevant NMs. While the number of studies might not yet be sufficient to allow an exact numerical conclusion, they suggest that only a low percentage of administered TiO2 and SiO2 NPs are absorbed into the circulating blood and peripheral tissues in mammals. The vast majority is excreted in the feces.
Percent absorption measurements were exemplified in rats orally exposed to 500 nm rutile TiO2 particles by gavage for 10 days.49 Following necropsy, microscopy visualized particles in the intestinal Peyer's patch. Spectroscopic analysis of titanium quantified that 0.06% of the administered dose was taken up by the stomach, 0.11% was in the small intestine, 4% was in the large intestine, (and of that 2.86% was in the Peyer's patches and lymphoid tissue), and 0.02% accumulated in the blood.49 Similarly in mice 6 h after a single gavage exposure to agglomerates of 12 nm anatase TiO2 NPs, some titanium was imaged inside sections of Peyer's patches and in the regular epithelium of the small intestine.50 The titanium was observed both in the cytoplasm and below the cells. Therefore it had been absorbed. Passage of either particles and/or of dissolved titanium was speculated. The quantity in the tissue was below the 30 parts per million detection limit of the imaging technique.50 In another study following administration of agglomerates of 7 nm and of 10–25 nm amorphous SiO2 NPs to rats in their feed for 28 or 84 days, absorption through the GI tract to peripheral tissues was less than 0.25%, and may have occurred from the absorption of either intact particles and/or of silicic acid released from the particle surface.51, 52 The percent absorption decreased as dose increased, likely due to gelation of the silica particles into agglomerates in the lumen at the higher doses.
The mechanisms suggested by the TiO2 and SiO2 data include a combination of transcellular transport of intact particles via phagocytosis through M‐cells of Peyer's patches, transcellular and paracellular transport of disintegrated molecules released from the particle surface, paracellular transport of the intact particles between damaged epithelial cells, and persorption of intact particles through dead epithelial cells.50, 53, 54
As research progresses, data on the full range of novel chemical entities that may be proposed for use in food will emerge.
GASTROINTESTINAL LUMINAL FACTORS THAT INFLUENCE THE SIZE AND SURFACE PROPERTIES OF NMs
The absorption of an administered NM through mucus and epithelial cells is a function of its size, aggregation, shape, surface properties, and surface corona. All of those physicochemical properties can be impacted and modified by interaction with factors encountered in the various environments of the luminal milieu during transit through the compartments of the mammalian GI tract (Figure 1). Hydrophobic (lipid soluble) particles are generally considered to be more readily absorbed than hydrophilic (water soluble) particles.55 However, there are exceptions. A hydrophilic polyethylene glycol coating has been shown to enhance stability and bioavailability in vivo during digestion.56 Some carbohydrate‐binding plant lectin proteins such as wheat germ agglutinin have affinity for receptors on intestinal enterocytes, facilitating transport across the cellular barrier when incorporated in solid lipid NPs.57 Particle surface charge properties can also affect adhesion with the negatively charged mucus layer and thus exposure to intestinal cells.28 While mucoadhesion may trap a NP and limit its positioning for epithelial absorption, it can increase residence time and protect from digestion.58 Along with the effects of inherent surface properties, particle size is a key factor in determining mucus transit and cellular uptake.12, 59 Smaller NPs can more easily diffuse through the mucus network pores and gain access to cells. Permanent degradation, dissolution and/or aggregation during digestion can however eliminate any such nanosize specific outcomes. Various parameters in the different compartments of the GI tract can play a role in influencing digestion and absorption.
We reviewed the modifying role of the major physicochemical factors in saliva, gastric fluid, and intestinal fluids: physical forces, osmotic concentration, pH, digestive enzymes, other food and endogenous biochemicals, and commensal microbes. In conjunction, in vitro and in silico data were assessed for evidence that these factors can have effects on the integrity, aggregation, and surface properties of metal, mineral, carbohydrate, nucleic acid, protein, and lipid primary nanostructures, with a focus on specific examples of TiO2, SiO2, and cellulose NMs.
Physical Forces from Chewing and from Peristalsis during Transit
Mastication forces in the buccal cavity break down solid food matrices, resulting in a polydisperse fragment size distribution.60 Tongue forces mix the food with salivary fluid secreted from the parotid and salivary glands. Fragments with an average diameter smaller than approximately 2 mm can then be swallowed and pass down the esophagus to the stomach.61 Peristaltic waves resulting from contraction of muscles in the GI tract wall propel this digesta forward. In the stomach peristaltic forces are generally in the range of 5–20 mmHg, and occasional wave contractions can be as strong as 150 mmHg.62 These forces break down the digesta to at least the 1 mm diameter before passage from the stomach to the small intestine.60 The transit time of chyme is on the order of hours in the stomach and up to several days in the intestines.63, 64 Engineered NMs released from food matrices during this transit may be impacted by the physical forces they encounter.
To date, direct published studies were not identified on the modification of NP primary particle size distribution and aggregation from physical forces in the range of those encountered during chewing and intestinal peristalsis. However, an example of indirect in vitro fluid incubation data suggests that peristalsis‐level mixing forces can mildly modify the degradation and aggregation of cellulose NPs in a colloidal suspension.65, 66 There is a data gap on the effect of stronger forces equivalent to chewing on these and other categories of food‐relevant NPs.
Osmotic Concentration and pH
Fluid volume and solute concentration are highly variable in the GI tract compartments and fluctuate in response to ingested food.67, 68 High solute ionic strength can modify the dissolution and precipitation behavior of NMs via the salting out effect. Differently charged ions can also modify particle surface charge and zeta (ζ) potential and, therefore magnitude of electrostatic repulsion. Conversely ions can act as sandwich‐filling elements, allowing equally charged species to interact. Together these modifications of surface properties determine the growth and size of NM agglomerates.4
pH‐related environments along the GI tract change from the oral cavity to the rectum and from the lumen through the mucus layer. Saliva has a neutral pH of approximately 7.69 Although the pH in the stomach can be as low as 1, NPs in food are likely to be buffered by the food matrix, and are thus likely to be exposed to pH values in the range of 2–6 when consumed in a meal.70 The pH gradient of the mucus varies from as low as 1 in the lumen of the stomach to nearly neutral at the epithelial surface.71, 72 In the small and large intestine lumen the pH ranges from 5 to 8.73 Therefore food related NMs encounter a fluctuating pH spectrum while traveling down the GI tract.
With regard to ionic strength and pH, direct in vitro fluid and in silico computational evidence reflective of conditions encountered in the mammalian GI tract has demonstrated that these parameters have major impacts on the surface charge and aggregation of the reviewed NPs. For example in a fluid suspension, increasing the sodium chloride concentration suppressed surface electrostatic repulsion of TiO2 NPs, allowing van der Waals interactions to manifest, and the rate of aggregation to increase.74 Likewise divalent cations in water adsorbed to the surface TiO2 NPs, neutralizing their negative charge and inducing aggregation.75, 76 Similarly the theoretical inter‐NP hydrogen bonding potential bridging the surface of colloidal amorphous SiO2 NPs in an aqueous solution was calculated to be modified by the concentration of calcium and chloride ions.77 Nanocellulose fibrils also aggregated as ionic salt concentration increased, and they aggregated as pH decreased, as a result of reduced surface charge in both cases.78, 79 Increasing the pH modified the surface charge of TiO2 NPs, and therefore the rate of aggregation, the size of the aggregates and the rate of sedimentation.76, 80, 81, 82 The pH conditions are also particularly important for solid metalloid‐based NMs such as clay, as acid can partially or completely solubilize the particles and in turn the minerals can act as buffers, influencing local pH values.83
In vivo the various GI parameters are dynamic, and thus can be simulated in static or dynamic in vitro GI models to determine how NPs will change during transit through the various compartments. For example, in a simulated salivary fluid SiO2 NPs administered in water and food matrices were present as single particles and as small aggregates.84 In a gastric digestion solution containing the mammalian digestive enzyme pepsin at pH 2, TiO2 had a positive surface charge whereas SiO2 was neutral and agglomerated to significantly larger clusters.84, 85 Following subsequent simulated intestinal digestion in a solution containing of a mixture of digestive enzymes at pH 7 followed by a solution containing bile salts at pH 7, both materials were negatively charged and the SiO2 deagglomerated back to individual particles in the nano size range. The aggregation in the stomach fluid was attributed to the low pH and high ionic strength, but not to the activity of the enzymes. Clearly osmotic concentration and pH have a major impact on the surface and size of NPs.
Digestive Enzymes
The effects of endogenous enzymes such as buccal amylase, gastric pepsin, and intestinal pancreatic lipase and nucleases can have an influence on the integrity of some categories of ingested particles. For example, ‘soft lipid’ or ‘solid non‐lipid non‐metal’ biochemical macromolecule‐based NMs may be susceptible to digestion. Mammalian enzymes can also denude surface‐adsorbed biochemicals from stable particles, but re‐adsorption of novel entities will occur.4
Inorganic compounds are generally not the substrate of mammalian enzymes. Therefore the digestive impact on that category of particles is generally not studied as an independent variable.84, 85 However NPs composed of starch, lipids, protein, or nucleic acids can be influenced by digestive activity.86, 87
Endogenous Biochemicals and Food Matrix Biochemicals
Particles in the GI milieu are exposed to a range of endogenous and ingested biochemicals and surfactants that can reversibly adsorb to the particle surface, forming a corona that changes the size and surface properties.88 For example when emulsion particles consisting of soy oil coated by β‐lactoglobulin protein in water were exposed to artificial saliva, free mucin proteins in the mixture bridged the particles and caused them to flocculate out of suspension.89 Adding plant pectin protein to the coating of these emulsion particles increased their electrostatic and steric repulsive forces and stabilized the emulsion.
Bile secreted into the small intestine contains bile salts and phospholipids.90 Bile salts are among the most surface active components in the intestine, and affect colloidal behavior. They facilitate the solubilization of free fatty acids, released from triglycerides during digestion, into submicron sized mixed micelles prior to absorption, and can similarly solubilize NMs.25 For example bile salt adsorption to 500 nm emulsion droplets that were composed of bovine milk protein, caseinate, and medium‐chain triglyceride oil has been shown to significantly increase diffusion through purified intestinal mucus.24, 91 The negative charge imparted by the bile salt significantly reduced the adhesive electrostatic interactions with the negatively charged mucus network. Thus, the integrity and uptake of ‘soft lipid‐based’ NMs can particularly be modified. These surfactants may similarly bind to non‐lipid NPs, modifying their size, agglomeration, and properties.
Phospholipids are not only endogenously present in gastric secretions and in intestinal bile, but also enter the GI tract as components of food, which results in a variable concentration throughout the canal.92 These zwitterionic surfactants contribute to the solubilization of especially lipophilic compounds by decreasing the surface tension. Similarly other endogenous and dietary organic molecules including lipids, proteins, nucleic acids, carbohydrates, and dietary fibre can adsorb to the particle surface with binding strength dependent on pK and stoichiometry. There are various examples.
In aqueous fluids, exogenous surfactants and organic matter controlled size of TiO2 NP and carboxymethylcellulose NP aggregates.66, 74, 80 A corona covering composed of bile salts and/or proteins developed on the surface of TiO2 and SiO2 aggregates during incubation in simulated intestinal juices.85 In another study SiO2 NPs formed large agglomerates in intestinal fed matrix conditions, but not in intestinal fasted, gastric fed, or gastric fasted simulations.93 Combining SiO2 NPs with a low fat coffee creamer food matrix prevented their aggregation compared with incubation in water alone.94 Clearly the binding of biochemicals found in the GI lumen can greatly modify the particle surface and size.
Commensal Microbes
The GI tract harbors an extremely complex microbiota that participates in digestive function and is important in homeostasis and gut‐associated immune function.95, 96, 97 Next‐generation sequencing techniques with samples from the human gut have identified approximately 1000 different bacterial species.98 Bacteria do not have active endocytic mechanisms to take up larger particles.99 However, adherence of particles to persistent microbial biofilms in the oral and large intestine environments can occur, and microbial secretions can interact with NPs.100, 101
Relatively few studies have investigated the influence of cultured microbes and their secreted fermentation enzymes on NP integrity. Cellulase enzyme purified from anaerobic bacteria and from fungi was shown to partially digest the nanoscale architecture of cellulose microfibers.102, 103 However, this effect is specific to particles composed of that biochemical substrate. The addition of SiO2 content within carboxymethyl cellulose particles significantly reduced their hydrolysis by cellulase.104 The impact of microbes on other categories of NPs, and any reciprocal effects on the microbes, are key areas for further research.
The complexity of GI factors is increased further when we consider the natural differences that exist between individuals.
Physiological Variability and Diseases
Differences in normal physiology as well as specific diseases present altered GI environments and affect epithelial permeability. Therefore it is important to consider how such inter‐individual differences may affect the stability and movement of orally administered substances.53
The age of an individual can affect a variety of factors such as the pH at each point of the GI tract, the transit time of fluids, and the barrier function of the lining. In infants, permeability is significantly higher than in the average adult. Old age can also affect GI functions, resulting in decreases in acid and enzyme secretion, digestion, motility, and nutrient absorption.105
Gender‐specific differences can exist. In male rats for example, spherical 26 nm TiO2 NPs orally administered by gastric gavage for 90 days significantly increased the level of titanium in blood to approximately 1.25‐fold of the background levels in non‐exposed rats.106 There was no significant increase in females.
Pregnancy causes physiological changes in the digestive system.107 In addition, pregnancy can have common complications such as gastroesophageal reflux disease, peptic ulcer disease, inflammatory bowel disease, and irritable bowel syndrome. These conditions may affect the behavior and uptake of NMs, e.g., those developed for uses in food supplements to increase nutrient uptake, ease of digestion, or bioavailability.
Malnutrition greatly impacts GI physiology and structural aspects.108 The sleep cycle also affects digestive function, and stress at high levels can increase inflammatory responses and thus contribute to increased GI lining permeability.109
Inflammatory Bowel Disease is a common GI condition, which includes Crohn's disease and ulcerative colitis. The abnormal mucus layer and inflamed tissue associated with these conditions has been shown to result in increased susceptibility to the absorption of some types of NMs. For example in a mouse model of ulcerative colitis SiO2 NPs adhered to ulcerated regions of the inflamed tissue at a sixfold higher percentage than to non‐inflamed healthy tissue.110 A retrospective microscopy study with lymphoid aggregate and Peyer's patch tissues surgically resected from normal areas of the small and large intestine of human volunteers with Crohn's disease, ulcerative colitis and colonic carcinoma observed titanium oxide particles of 100–200 nm in diameter, as well as 100–700 nm particles consisting of silicon, magnesium, potassium, sodium, and iron, in phagolysosomes in macrophage cells underlying the epithelium.111 The authors postulated the source to be food additives and environmental exposures.
There can also be abnormal GI barrier function in Celiac disease and other autoimmune diseases.39, 112 Likewise gastroenteritis can increase gut permeability, for example following infection with Campylobacter jejuni, enteropathogenic Escherischa coli, or Clostridium difficilie.109, 113 The infection of human intestinal biopsies with Yersinia pseudotuberculosis significantly increased the transcellular macropinocytosis of fluorescent 200 nm and 500 nm polystyrene particles.114 GI pre‐cancerous lesions, tumors, and cancer treatments can also reduce gut barrier function.115
Clearly certain human subpopulations have differences in GI physiology that should be accounted for in the assessment of the trafficking of NMs.
CONCLUSIONS
The mammalian GI tract efficiently processes the chemical constituents and structures of food ranging from bulk materials down to atoms during transit through the buccal cavity, stomach, small intestine, and large intestine. Permeation of particulate matter through the buccal cavity epithelium is understudied, likely because the small intestine has the highest surface area and specialization for nutrient uptake. Intestinal enterocytes are the most abundant cell type in the intestinal epithelium and thus the most important barrier to absorption. M‐cells over GALTs make up less than 1% of the intestinal surface area, but are the cell type most specialized for the uptake of particulate matter. Mucus coating the epithelium is also an important barrier to absorption.
Novel engineered NMs have many promising beneficial applications in food and food contact materials. In some jurisdictional legislations those applications may require a safety assessment, particularly if the bioavailability has changed compared with a conventional bulk form. Size is a key characteristic in the bioavailability of NMs. The physicochemical properties of the NM surface also affect stability, agglomeration, interactions with mucus, interactions with the apical cell membrane, absorption, and excretion. If in vitro cellular, in vitro fluid or in silico computational models are to be adapted to accurately predict human GI digestion and absorption of novel food‐relevant engineered NMs, it is important to know which parameters are essential to incorporate in these models. Direct and indirect evidence confirms that salt concentration, pH, and biochemicals in the luminal fluid matrix are key in determining the integrity, aggregation, and surface properties of food‐relevant NPs, and therefore important in determining their absorption into systemic circulation. Physical forces, digestive enzymes, and microbes may also have impacts.
Further research is required to fill data gaps on the kinetics and absorption of the full spectrum of potentially food‐relevant NMs (Table 1). Improving our understanding of the relationship of the physicochemical aspects of NMs with the GI ecosystem will help provide data useful for risk assessment. Interlaboratory validation studies can lend strength to methods development and findings. In particular the models of the GI tract outlined as applicable by the NanoRelease Food Additive project will be useful for this purpose.13 Filling methodological gaps will allow comparison of NMs with the ionic and bulk conventional forms of chemical additives in food. Subsequently, data on the distribution of the NMs in the body along with their metabolism and excretion will complete the toxicokinetic analysis. International risk assessment of novel engineered NMs is underway as exposure, toxicokinetic and toxicology data on other key endpoints becomes available. The development of methodologies to facilitate the detection of the full range of food‐relevant NMs will facilitate risk management and public policy.
Table 1.
Knowledge Gaps on Mammalian Gastrointestinal Tract Digestive Parameters Modulating the Integrity, Surface Properties, and Absorption of Food‐Relevant Nanomaterials
| Knowledge Gap Questions |
|---|
| Among physical forces, osmotic concentration, pH, digestive enzymes, other biochemicals, and commensal microbes, which GI luminal parameters are the strongest inducers of any changes observed in the size, shape, surface properties, and surface corona of NMs? |
| Is the size, shape, and surface properties of the full range of potentially food‐relevant NMs modified in the GI luminal milieu, or only certain categories of NMs? |
| What is the difference in percent absorption through the GI tract epithelium of NMs with different physicochemical properties? |
| Through which GI tract organs and epithelial cell subsets does the absorption of NMs of different chemical makeup occur? |
| What inherent properties of NMs of different chemical makeup determine their percent absorption through mucus and epithelial cells? |
| Does the percent absorption of a given NM differ from the mass‐balanced percent absorption of the bulk or ionic form of the same chemical? |
ACKNOWLEDGMENTS
The authors are grateful to the following individuals for their expert input: Vicki Stone (Heriot‐Watt University, UK), John Milner (Agricultural Research Service, US Department of Agriculture), Bruce Hamaker (Purdue University, USA), Brian Lee (GE Global Research, USA), Jozef Kokini (University of Illinois, USA), Bevan Pearce, Joel Rotstein, Jesse Bertinato, and Rekha Mehta (Health Canada). We also thank Molly Bloom and Elyse Lee (Center for Risk Science Innovation and Application, ILSI Research Foundation, USA) for assistance with the management of the NanoRelease Food Additive project. We acknowledge the NanoRelease Food Additive Steering Committee, which operates as an independent public–private partnership (http://www.ilsi.org/ResearchFoundation/RSIA/Pages/FoodAdditiveSteeringCommittee.aspx), for convening the authors and for developing the initial framing concepts for the review. The initial steering phase of the NanoRelease Food Additive project was funded by the Pew Charitable Trusts, the US Food and Drug Administration, Health Canada, ILSI North America, the Coca‐Cola Company, the Illinois Institute of Technology's Institute for Food Safety and Health, and the ILSI Research Foundation. Substantial in‐kind support was provided by the Nanotechnology Industries Association and by the UK Medical Research Council (U105960399). This article has been reviewed in accordance with the US FDA's peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute an endorsement or recommendation for use by the US FDA. The statements made in this report do not necessarily represent the official position of the employers or affiliated organizations of the authors.
Conflict of interest: The authors have declared no conflicts of interest for this article.
REFERENCES
- 1. Florence AT. The oral absorption of micro‐ and nanoparticulates: neither exceptional nor unusual. Pharm Res 1997, 14:259–266. [DOI] [PubMed] [Google Scholar]
- 2. Langer A, Hampel PA, Kaiser W, Knezevic J, Welte T, Villa V, Maruyama M, Svejda M, Jahner S, Fischer F, et al. Protein analysis by time‐resolved measurements with an electro‐switchable DNA chip. Nat Commun 2013, 4:2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohtani T, Yoshino T, Ushiki T, Hagiwara S, Maekawa T. Structure of rice starch granules in nanometre scale as revealed by atomic force microscopy. J Electron Microsc (Tokyo) 2000, 49:487–489. [DOI] [PubMed] [Google Scholar]
- 4. Powell JJ, Faria N, Thomas‐McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun 2010, 34:J226–233. [DOI] [PubMed] [Google Scholar]
- 5. Cockburn A, Bradford R, Buck N, Constable A, Edwards G, Haber B, Hepburn P, Howlett J, Kampers F, Klein C, et al. Approaches to the safety assessment of engineered nanomaterials (enm) in food. Food Chem Toxicol 2012, 50:2224–2242. [DOI] [PubMed] [Google Scholar]
- 6. Szakal C, Roberts SM, Westerhoff P, Bartholomaeus A, Buck N, Illuminato I, Canady R, Rogers M. Measurement of nanomaterials in foods: integrative consideration of challenges and future prospects. ACS Nano 2014, 8:3128–3135. [DOI] [PubMed] [Google Scholar]
- 7. Yada RY, Buck N, Canady R, DeMerlis C, Duncan T, Janer G, Juneja L, Lin M, McClements J, Noonan G, et al. Engineered nanoscale food ingredients: evaluation of current knowledge on material characteristics relevant to uptake from the gastrointestinal tract. Comp Rev Food Sci Food Safety 2014, 13:730–744. [DOI] [PubMed] [Google Scholar]
- 8. Szakal C, Tsytsikova LDC, Duncan TV. Measurement methods for the oral uptake of engineered nanomaterials from human dietary sources: summary and outlook. Comp Rev Food Sci Food Safety 2014, 13:669–678. [DOI] [PubMed] [Google Scholar]
- 9. Noonan GO, Whelton AJ, Carlander D, Duncan TV. Measurement methods to evaluate engineered nanomaterial release from food contact materials. Comp Rev Food Sci Food Safety 2014, 13:679–692. [DOI] [PubMed] [Google Scholar]
- 10. Singh G, Stephan C, Westerhoff P, Carlander D, Duncan TV. Measurement methods to detect, characterize, and quantify engineered nanomaterials in foods. Comp Rev Food Sci Food Safety 2014, 13:693–704. [DOI] [PubMed] [Google Scholar]
- 11. Alger H, Momcilovic D, Carlander D, Duncan TV. Methods to evaluate uptake of engineered nanomaterials by the alimentary tract. Comp Rev Food Sci Food Safety 2014, 13:705–729. [DOI] [PubMed] [Google Scholar]
- 12. Elder A, Vidyasagar S, DeLouise L. Physicochemical factors that affect metal and metal oxide nanoparticle passage across epithelial barriers. WIREs Nanomed Nanobiotechnol 2009, 1:434–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lefebvre DE, Venema K, Gombau L, Valerio LG Jr, Raju J, Bondy GS, Bouwmeester H, Singh RP, Clippinger AJ, Collnot EM, et al. Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices. Nanotoxicology 2014: In Press. [DOI] [PubMed] [Google Scholar]
- 14. Hu W, Chen S, Yang J, Li Z, Wang H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr Polym 2014, 101:1043–1060. [DOI] [PubMed] [Google Scholar]
- 15. Faust JJ, Doudrick K, Yang Y, Westerhoff P, Capco DG. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol Toxicol 2014, 30:169–188. [DOI] [PubMed] [Google Scholar]
- 16. Sergent JA, Paget V, Chevillard S. Toxicity and genotoxicity of nano‐sio2 on human epithelial intestinal ht‐29 cell line. Ann Occup Hyg 2012, 56:622–630. [DOI] [PubMed] [Google Scholar]
- 17. Helander HF, Fandriks L. Surface area of the digestive tract ‐ revisited. Scand J Gastroenterol 2014, 49:681–689. [DOI] [PubMed] [Google Scholar]
- 18. Boron WF, Boulpaep EL. Medical Physiology: A Cellular and Molecular Approach. 2nd ed. Philadelphia: Elsevier; 2009, 884. [Google Scholar]
- 19. Bakhru SH, Furtado S, Morello AP, Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev 2013, 65:811–821. [DOI] [PubMed] [Google Scholar]
- 20. Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (m) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 2013, 6:666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol 2008, 1:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the muc2 mucin, whereas the outer layer is a legislator of host‐microbial interactions. Proc Natl Acad Sci USA 2011, 108(Suppl 1):4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. American journal of physiology . Gastrointest Liver Physiol 2001, 280:G922–929. [DOI] [PubMed] [Google Scholar]
- 24. Round AN, Rigby NM, Garcia de la Torre A, Macierzanka A, Mills EN, Mackie AR. Lamellar structures of muc2‐rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules 2012, 13:3253–3261. [DOI] [PubMed] [Google Scholar]
- 25. Maldonado‐Valderrama J, Wilde P, Macierzanka A, Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci 2011, 165:36–46. [DOI] [PubMed] [Google Scholar]
- 26. Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev 2009, 61:75–85. [DOI] [PubMed] [Google Scholar]
- 27. Constantinovits M, Sipos F, Molnar B, Tulassay Z, Muzes G. Organizer and regulatory role of colonic isolated lymphoid follicles in inflammation. Acta Physiol Hung 2012, 99:344–352. [DOI] [PubMed] [Google Scholar]
- 28. des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 2006, 116:1–27. [DOI] [PubMed] [Google Scholar]
- 29. Gebert A, Rothkotter HJ, Pabst R. M cells in peyer's patches of the intestine. Int Rev Cytol 1996, 167:91–159. [DOI] [PubMed] [Google Scholar]
- 30. Shakweh M, Ponchel G, Fattal E. Particle uptake by peyer's patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv 2004, 1:141–163. [DOI] [PubMed] [Google Scholar]
- 31. Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003, 422:37–44. [DOI] [PubMed] [Google Scholar]
- 32. Brayden DJ, Baird AW. Apical membrane receptors on intestinal m cells: potential targets for vaccine delivery. Adv Drug Deliv Rev 2004, 56:721–726. [DOI] [PubMed] [Google Scholar]
- 33. Catto‐Smith AG, Emselle S, Bishop RF. Changes in macromolecular transport appear early in caco‐2 cells infected with a human rotavirus. Scand J Gastroenterol 2008, 43:314–322. [DOI] [PubMed] [Google Scholar]
- 34. San Martin CD, Garri C, Pizarro F, Walter T, Theil EC, Nunez MT. Caco‐2 intestinal epithelial cells absorb soybean ferritin by mu2 (ap2)‐dependent endocytosis. J Nutr 2008, 138:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Theil EC, Chen H, Miranda C, Janser H, Elsenhans B, Nunez MT, Pizarro F, Schumann K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J Nutr 2012, 142:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pereira DI, Mergler BI, Faria N, Bruggraber SF, Aslam MF, Poots LK, Prassmayer L, Lonnerdal B, Brown AP, Powell JJ. Caco‐2 cell acquisition of dietary iron(iii) invokes a nanoparticulate endocytic pathway. PLoS One 2013, 8:e81250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kadiyala I, Loo Y, Roy K, Rice J, Leong KW. Transport of chitosan‐DNA nanoparticles in human intestinal m‐cell model versus normal intestinal enterocytes. Eur J Pharm Sci 2010, 39:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006, 55:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012, 42:71–78. [DOI] [PubMed] [Google Scholar]
- 40. Crissinger KD, Kvietys PR, Granger DN. Pathophysiology of gastrointestinal mucosal permeability. J Intern Med Suppl 1990, 732:145–154. [DOI] [PubMed] [Google Scholar]
- 41. Linnankoski J, Makela J, Palmgren J, Mauriala T, Vedin C, Ungell AL, Lazorova L, Artursson P, Urtti A, Yliperttula M. Paracellular porosity and pore size of the human intestinal epithelium in tissue and cell culture models. J Pharm Sci 2010, 99:2166–2175. [DOI] [PubMed] [Google Scholar]
- 42. Cereijido M, Anderson JM, eds. Tight Junctions. 2nd ed. Washington: CRC Press; 2001, 1–18. [Google Scholar]
- 43. Volkheimer G. Persorption of microparticles. Pathologe 1993, 14:247–252. [PubMed] [Google Scholar]
- 44. Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci 2001, 90:1927–1936. [DOI] [PubMed] [Google Scholar]
- 45. Loeschner K, Hadrup N, Qvortrup K, Larsen A, Gao X, Vogel U, Mortensen A, Lam HR, Larsen EH. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol 2011, 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cuong NV, Hsieh MF. Molecular targeting of liposomal nano‐particles to lymphatic system. Curr Cancer Drug Targets 2011, 11:147–155. [DOI] [PubMed] [Google Scholar]
- 47. Stapleton PA, Nurkiewicz TR. Vascular distribution of nanomaterials. WIREs Nanomed Nanobiotechnol 2014, 6:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garrett NL, Lalatsa A, Uchegbu I, Schatzlein A, Moger J. Exploring uptake mechanisms of oral nanomedicines using multimodal nonlinear optical microscopy. J Biophotonics 2012, 5:458–468. [DOI] [PubMed] [Google Scholar]
- 49. Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat gi tract and translocation to systemic organs after oral administration. Int J Pharm 1994, 105:157–168. [Google Scholar]
- 50. Brun E, Barreau F, Veronesi G, Fayard B, Sorieul S, Chaneac C, Carapito C, Rabilloud T, Mabondzo A, Herlin‐Boime N, et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol 2014, 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Zande M, Vandebriel RJ, Groot MJ, Kramer E, Herrera Rivera ZE, Rasmussen K, Ossenkoppele JS, Tromp P, Gremmer ER, Peters RJ, et al. Sub‐chronic toxicity study in rats orally exposed to nanostructured silica. Part Fibre Toxicol 2014, 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Kesteren PC, Cubadda F, Bouwmeester H, van Eijkeren JC, Dekkers S, de Jong WH, Oomen AG. Novel insights into the risk assessment of the nanomaterial synthetic amorphous silica, additive e551, in food. Nanotoxicology 2014: In Press. [DOI] [PubMed] [Google Scholar]
- 53. Bockmann J, Lahl H, Eckert T, Unterhalt B. Blood titanium levels before and after oral administration titanium dioxide. Pharmazie 2000, 55:140–143. [PubMed] [Google Scholar]
- 54. Janer G, Mas del Molino E, Fernandez‐Rosas E, Fernandez A, Vazquez‐Campos S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol Lett 2014, 228:103–110. [DOI] [PubMed] [Google Scholar]
- 55. McClements DJ. Edible lipid nanoparticles: digestion, absorption, and potential toxicity. Prog Lipid Res 2013, 52:409–423. [DOI] [PubMed] [Google Scholar]
- 56. Tobio M, Sanchez A, Vila A, Soriano II, Evora C, Vila‐Jato JL, Alonso MJ. The role of peg on the stability in digestive fluids and in vivo fate of peg‐pla nanoparticles following oral administration. Colloids Surf B Biointerfaces 2000, 18:315–323. [DOI] [PubMed] [Google Scholar]
- 57. Zhang N, Ping Q, Huang G, Xu W, Cheng Y, Han X. Lectin‐modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm 2006, 327:153–159. [DOI] [PubMed] [Google Scholar]
- 58. Dunnhaupt S, Barthelmes J, Hombach J, Sakloetsakun D, Arkhipova V, Bernkop‐Schnurch A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int J Pharm 2011, 408:191–199. [DOI] [PubMed] [Google Scholar]
- 59. Szentkuti L. Light microscopic observation on luminally administered dyes, dextrans, nanospheres and microspheres in the pre‐epithelial mucus gel layer of the rat distal colon. J Control Release 1997, 46:233–242. [Google Scholar]
- 60. Lentle RG, Janssen PW. Manipulating digestion with foods designed to change the physical characteristics of digesta. Crit Rev Food Sci Nutr 2010, 50:130–145. [DOI] [PubMed] [Google Scholar]
- 61. Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carriere F, Boutrou R, Corredig M, Dupont D, et al. A standardised static in vitro digestion method suitable for food ‐ an international consensus. Food Funct 2014, 5:1113–1124. [DOI] [PubMed] [Google Scholar]
- 62. Hasler WL. The use of smartpill for gastric monitoring. Expert Rev Gastroenterol Hepatol 2014, 8:587–600. [DOI] [PubMed] [Google Scholar]
- 63. Fallingborg J, Christensen LA, Ingeman‐Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. Ph‐profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Ther 1989, 3:605–613. [DOI] [PubMed] [Google Scholar]
- 64. Heller SN, Hackler LR, Rivers JM, Van Soest PJ, Roe DA, Lewis BA, Robertson J. Dietary fiber: the effect of particle size of wheat bran on colonic function in young adult men. Am J Clin Nutr 1980, 33:1734–1744. [DOI] [PubMed] [Google Scholar]
- 65. Bizmark N, Ioannidis MA, Henneke DE. Irreversible adsorption‐driven assembly of nanoparticles at fluid interfaces revealed by a dynamic surface tension probe. Langmuir 2014, 30:710–717. [DOI] [PubMed] [Google Scholar]
- 66. Tresset G, Marculescu C, Salonen A, Ni M, Iliescu C. Fine control over the size of surfactant‐polyelectrolyte nanoparticles by hydrodynamic flow focusing. Anal Chem 2013, 85:5850–5856. [DOI] [PubMed] [Google Scholar]
- 67. Jones AT, Balan KK, Jenkins SA, Sutton R, Critchley M, Roberts NB. Assay of gastricsin and individual pepsins in human gastric juice. J Clin Pathol 1993, 46:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, Weitschies W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2005, 22:971–979. [DOI] [PubMed] [Google Scholar]
- 69. Frohlich E, Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Quigley EM, Turnberg LA. Ph of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology 1987, 92:1876–1884. [DOI] [PubMed] [Google Scholar]
- 71. Ross IN, Bahari HM, Turnberg LA. Studies of the ph gradient across the mucus on rat gastric mucosa in vivo and across mucus on human gastric mucosa in vitro. Adv Exp Med Biol 1982, 144:189–191. [DOI] [PubMed] [Google Scholar]
- 72. Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv Mater 2012, 24:3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kleberg K, Jacobsen J, Mullertz A. Characterising the behaviour of poorly water soluble drugs in the intestine: application of biorelevant media for solubility, dissolution and transport studies. J Pharm Pharmacol 2010, 62:1656–1668. [DOI] [PubMed] [Google Scholar]
- 74. Zhou D, Ji Z, Jiang X, Dunphy DR, Brinker J, Keller AA. Influence of material properties on tio2 nanoparticle agglomeration. PLoS One 2013, 8:e81239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Petosa AR, Brennan SJ, Rajput F, Tufenkji N. Transport of two metal oxide nanoparticles in saturated granular porous media: role of water chemistry and particle coating. Water Res 2012, 46:1273–1285. [DOI] [PubMed] [Google Scholar]
- 76. Romanello MB, Fidalgo de Cortalezzi MM. An experimental study on the aggregation of tio2 nanoparticles under environmentally relevant conditions. Water Res 2013, 47:3887–3898. [DOI] [PubMed] [Google Scholar]
- 77. Jenkins S, Kirk SR, Persson M, Carlen J, Abbas Z. Molecular dynamics simulation of nanocolloidal amorphous silica particles: part iii. J Chem Phys 2009, 130:134702. [DOI] [PubMed] [Google Scholar]
- 78. Fall AB, Lindstrom SB, Sundman O, Odberg L, Wagberg L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27:11332–11338. [DOI] [PubMed] [Google Scholar]
- 79. Isogai A, Saito T, Fukuzumi H. Tempo‐oxidized cellulose nanofibers. Nanoscale 2011, 3:71–85. [DOI] [PubMed] [Google Scholar]
- 80. Godinez IG, Darnault CJ. Aggregation and transport of nano‐tio2 in saturated porous media: effects of ph, surfactants and flow velocity. Water Res 2011, 45:839–851. [DOI] [PubMed] [Google Scholar]
- 81. Guiot C, Spalla O. Stabilization of tio2 nanoparticles in complex medium through a ph adjustment protocol. Environ Sci Technol 2013, 47:1057–1064. [DOI] [PubMed] [Google Scholar]
- 82. Guzman KA, Finnegan MP, Banfield JF. Influence of surface potential on aggregation and transport of titania nanoparticles. Environ Sci Technol 2006, 40:7688–7693. [DOI] [PubMed] [Google Scholar]
- 83. Choy J, Choi SJ, Oh JM, Park T. Clay minerals and layered double hydroxides for novel biological applications. Appl Clay Sci 2006, 36:10. [Google Scholar]
- 84. Peters R, Kramer E, Oomen AG, Rivera ZE, Oegema G, Tromp PC, Fokkink R, Rietveld A, Marvin HJ, Weigel S, et al. Presence of nano‐sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6:2441–2451. [DOI] [PubMed] [Google Scholar]
- 85. McCracken C, Zane A, Knight DA, Dutta PK, Waldman WJ. Minimal intestinal epithelial cell toxicity in response to short‐ and long‐term food‐relevant inorganic nanoparticle exposure. Chem Res Toxicol 2013, 26:1514–1525. [DOI] [PubMed] [Google Scholar]
- 86. Jannin V, Dellera E, Chevrier S, Chavant Y, Voutsinas C, Bonferoni C, Demarne F. In vitro lipolysis tests on lipid nanoparticles: comparison between lipase/co‐lipase and pancreatic extract. Drug Dev Ind Pharm 2014: In Press. [DOI] [PubMed] [Google Scholar]
- 87. Joyce P, Tan A, Whitby CP, Prestidge CA. The role of porous nanostructure in controlling lipase‐mediated digestion of lipid loaded into silica particles. Langmuir 2014, 30:2779–2788. [DOI] [PubMed] [Google Scholar]
- 88. McClements DJ. Nanoemulsion‐based oral delivery systems for lipophilic bioactive components: nutraceuticals and pharmaceuticals. Ther Deliv 2013, 4:841–857. [DOI] [PubMed] [Google Scholar]
- 89. Benjamin O, Silcock P, Beauchamp J, Buettner A, Everett DW. Volatile release and structural stability of β‐lactoglobulin primary and multilayer emulsions under simulated oral conditions. Food Chem 2013, 140:124–134. [DOI] [PubMed] [Google Scholar]
- 90. Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci U S A 2006, 103:4333–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Macierzanka A, Rigby NM, Corfield AP, Wellner N, Bottger F, Mills EN, Mackie A. Adsorption of bile salts to particles allows penetration of intestinal mucus. Soft Matter 2011, 7:8077–8084. [Google Scholar]
- 92. Kleberg K, Jacobsen F, Fatouros DG, Mullertz A. Biorelevant media simulating fed state intestinal fluids: colloid phase characterization and impact on solubilization capacity. J Pharm Sci 2010, 99:3522–3532. [DOI] [PubMed] [Google Scholar]
- 93. Sakai‐Kato K, Hidaka M, Un K, Kawanishi T, Okuda H. Physicochemical properties and in vitro intestinal permeability properties and intestinal cell toxicity of silica particles, performed in simulated gastrointestinal fluids. Biochim Biophys Acta 1840, 2014:1171–1180. [DOI] [PubMed] [Google Scholar]
- 94. Heroult J, Nischwitz V, Bartczak D, Goenaga‐Infante H. The potential of asymmetric flow field‐flow fractionation hyphenated to multiple detectors for the quantification and size estimation of silica nanoparticles in a food matrix. Anal Bioanal Chem 2014, 406:3919–3927. [DOI] [PubMed] [Google Scholar]
- 95. Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol 2009, 28:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Young VB. The intestinal microbiota in health and disease. Curr Opin Gastroenterol 2012, 28:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Joyce SA, Gahan CG. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol 2014, 30:120–127. [DOI] [PubMed] [Google Scholar]
- 98. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez‐Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature 2012, 486:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jermy A. Evolution: bacterial endocytosis uncovered. Nat Rev Microbiol 2010, 8:534. [DOI] [PubMed] [Google Scholar]
- 100. Sintubin L, De Windt W, Dick J, Mast J, van der Ha D, Verstraete W, Boon N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol 2009, 84:741–749. [DOI] [PubMed] [Google Scholar]
- 101. Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontology 2011, 55:16–35. [DOI] [PubMed] [Google Scholar]
- 102. Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338:1055–1060. [DOI] [PubMed] [Google Scholar]
- 103. Penttila PA, Varnai A, Pere J, Tammelin T, Salmen L, Siika‐aho M, Viikari L, Serimaa R. Xylan as limiting factor in enzymatic hydrolysis of nanocellulose. Bioresour Technol 2013, 129:135–141. [DOI] [PubMed] [Google Scholar]
- 104. Alikarami M, Abbasi Z, Moradi V. Study of enzymatic degradation and water absorption of composites carboxymethyl cellulose and poly (−caprolactone) containing sio2 nanoparticle by cellulase. J Environ Sci Health A Tox Hazard Subst Environ Eng 2013, 48:1516–1521. [DOI] [PubMed] [Google Scholar]
- 105. Russell TL, Berardi RR, Barnett JL, Dermentzoglou LC, Jarvenpaa KM, Schmaltz SP, Dressman JB. Upper gastrointestinal ph in seventy‐nine healthy, elderly, North American men and women. Pharm Res 1993, 10:187–196. [DOI] [PubMed] [Google Scholar]
- 106. Cho WS, Kang BC, Lee JK, Jeong J, Che JH, Seok SH. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol 2013, 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol 2013, 27:791–802. [DOI] [PubMed] [Google Scholar]
- 108. Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: From feast to famine. Annu Rev Physiol 2006, 68:223–251. [DOI] [PubMed] [Google Scholar]
- 109. Camilleri M, Madsen K, Spiller R, Greenwood‐Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012, 24:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials 2008, 29:4554–4560. [DOI] [PubMed] [Google Scholar]
- 111. Powell JJ, Ainley CC, Harvey RS, Mason IM, Kendall MD, Sankey EA, Dhillon AP, Thompson RP. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011, 91:151–175. [DOI] [PubMed] [Google Scholar]
- 113. Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 2004, 84:282–291. [DOI] [PubMed] [Google Scholar]
- 114. Ragnarsson EG, Schoultz I, Gullberg E, Carlsson AH, Tafazoli F, Lerm M, Magnusson KE, Soderholm JD, Artursson P. Yersinia pseudotuberculosis induces transcytosis of nanoparticles across human intestinal villus epithelium via invasin‐dependent macropinocytosis. Lab Invest 2008, 88:1215–1226. [DOI] [PubMed] [Google Scholar]
- 115. Yoshida S, Matsui M, Shirouzu Y, Fujita H, Yamana H, Shirouzu K. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann Surg 1998, 227:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]


