Scheme 3.
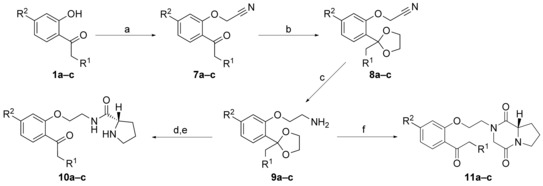
Synthesis of compounds 10 a–c and 11 a–c. Reagents and conditions: a) bromoacetonitrile, K2CO3, acetone, 50 °C, 4 d (88–100 %); b) ethylene glycol, p‐toluenesulfonic acid (pTosOH), toluene, reflux, o/n, 72–97 %; c) LiAlH4 or Red‐Al, Et2O, reflux or RT, 3–4 h, 15‐42 %; d) boc‐proline, DCC, DMAP, CH2Cl2, 2 h to o/n, 57‐78 %; e) 4 n HCl, dioxane, RT, 4 h, 97 %–quant.; f) methyl (2‐chloroacetyl)prolinate, Et3N, reflux, 2‐ethoxyethanol, 2 d, then 2 n HCl, CH3CN, RT, 4 h, 19–39 % (2 steps).
