Abstract
Vaccinium myrtillus and Vaccinium vitis‐idaea are two dwarf shrubs widespread in the European Alps. We studied the hydraulics of these species hypothesizing that (1) the hydraulic architecture of dwarf shrubs differs from trees, (2) hydraulic properties reflect the species' ecological amplitude and (3) hydraulic properties vary spatially and seasonally. Key hydraulic parameters (osmotic potential, turgor loss point, xylem hydraulic conductivity, vulnerability to drought‐induced embolism, stomata closure, drought‐induced cell damage and embolism repair) and related wood anatomical traits (conduit diameter and conduit wall reinforcement) were analyzed at four sites in Tyrol, Austria. Both species exhibited low hydraulic safety as well as low hydraulic efficiency. Fifty percentage embolism accumulated at −2.08 (V. myrtillus) and −1.97 MPa (V. vitis‐idaea), 88% stomata closure was at −2.19 and −2.35 MPa, respectively. After drought, both species showed embolism repair on re‐watering. Site‐specific variation within species was low, while seasonal changes in embolism resistance and turgor loss point were observed. Results indicate that studied Vaccinium species have a high risk for embolism formation. This is balanced by refilling capacities, which are probably based on the small growth height of dwarf shrubs. V. vitis‐idaea, which occurs on drier sites, showed more efficient repair and a lower turgor loss point than V. myrtillus.
Abbreviations
- CET
Central European Time
- DW
dry weight
- FW
fresh weight
- PLC
percent loss of conductivity
- pv
pressure–volume
- PSC
percent stomata closure
- REL
relative electrolytic leakage
- WSD
water saturation deficiency
Introduction
Vaccinium myrtillus and Vaccinium vitis‐idaea (Ericaceae) are widespread dwarf shrubs in the European Alps (Polatschek 1999). V. myrtillus, the blueberry, predominantly grows in conifer forests and dwarf shrub heaths up to 2800 m. The dioecious, up to 60 cm high plant, has green, alate, multi‐branched, upright shoots, ovale light green leaves and hypogean sprouts for vegetative propagation. V. vitis‐idaea, the cowberry, has its main distribution in bright conifer forests, dwarf shrub heaths and mat grassland at altitudes up to 2300 m. This species is up to 30 cm high, has evergreen, leathery leaves with brown gland hair on the lower side and also hypogean sprouts. Both species prefer silicate, nutrient‐poor and humid soils. Although the species often co‐occur, V. myrtillus overall requires moister conditions and shady sites (Sebald et al. 1993, Polatschek 1999). V. vitis‐idaea can also be found on more arid and exposed sites, manifested in a higher light and a lower moisture ecological indicator value according to Landolt (2010).
Plant hydraulics are based on numerous and interrelated components and can be characterized by several key parameters: for example, the turgor loss point indicates how long a plant can maintain cell turgor on desiccation. It is determined by the osmotic concentration in cells and their wall elasticity (Lenz et al. 2006). Other important traits are the hydraulic efficiency and safety of water transport. The hydraulic efficiency depends on the hydraulic conductivity and on the ratio of supported tissue to conductive xylem area (Tyree and Zimmermann 2002). The hydraulic safety, i.e. the resistance to embolism formation, is important as the water columns in transpiring or drought‐stressed plants are under negative pressure (cohesion‐tension theory; Boehm 1893, Dixon and Joly 1894). At low water potential (Ψ), air seeding can cause embolism and, in consequence, severe dysfunctions of the transport system (Tyree and Zimmermann 2002). Most species try to avoid critical Ψ by stomata closure (Hacke and Sperry 2001, Cochard et al. 2002), but some species were also shown to be able to recover from embolism (e.g. Brodersen and McElrone 2013). A high drought resistance thus is often based on adjustments in cell osmotic potential, high resistance to embolism formation, rapid stomata reaction and xylem refilling. In addition, high cuticular resistance, an efficient root system, sufficient water storage capacity and tolerance of cells to low Ψ enable plants to withstand dry conditions.
There are countless reports on the drought resistance and hydraulics of trees (e.g. Zimmermann 1978, Tyree and Ewers 1991, Martinez‐Vilalta et al. 2004, McCulloh and Woodruff 2012), and comparably few studies dealt with the hydraulics of shrubs (e.g. Wheeler et al. 2005, Beikircher and Mayr 2008, 2009, Bucci et al. 2009, Mayr et al. 2010, Vilagrosa et al. 2010). In the database of Choat et al. (2012), xylem functional traits for 331 tree species and 140 shrubs are listed, and most shrubs represented were from arid environments. Shrubs show lower growth heights than trees and basitonic growth with annual formation of new shoots from the basal buds (Bresinsky et al. 2008). In consequence, shrubs differ from trees in transport distances, hydraulic resistances and related morphological and anatomical features (Gartner 1991, Tyree and Ewers 1991). Shrubs thus have different requirements for a safe and efficient water supply compared to trees and require different hydraulic designs. Several studies reported differences in hydraulic parameters between growth forms, for example in hydraulic efficiency (specific conductivity, leaf‐specific conductivity, Huber value), vulnerability to cavitation (drought‐induced embolism) and anatomical parameters (vessel length and diameter, cell wall reinforcement; Gartner 1991, Tyree and Ewers 1991, Patiño et al. 1995, Beikircher and Mayr 2008). Beikircher and Mayr (2008) compared shrub and tree‐like growth forms of Juniperus communis and found that shrub shoots were similar to tree branches in growth form and conductivity but were similar to tree stem in their vulnerability to cavitation. To our knowledge, no studies on dwarf shrubs hydraulics are available. In dwarf shrubs, the contrasting hydraulic design (compared to trees) should be even more pronounced due to their low growth height, short transport distances, small cumulative hydraulic resistances and negligible influence of gravity on water potential.
The aim of this study was a comprehensive analysis of the hydraulic architecture and drought resistance of V. myrtillus and V. vitis‐idaea. We hypothesized that (1) hydraulic key parameters and drought resistance strategies differ from trees because of the different growth pattern and lower height of dwarf shrubs, (2) interspecific differences between the species' hydraulic characteristics correspond to humidity requirements and (3) hydraulics show site‐specific and seasonal variation. We studied hydraulic parameters (osmotic potential at saturation, turgor loss point, xylem hydraulic conductivity, vulnerability to drought‐induced embolism, stomata closure and drought‐induced cell damage) and related wood anatomical characteristics (conduit diameter, hydraulic diameter and conduit wall reinforcement). Furthermore, an experiment to estimate the refilling potential of studied Vaccinium species was conducted.
Materials and methods
Sites
For sample collection and field measurements, three sites per species were chosen in Tyrol, Austria: Patscherkofel (1883 m; 47°22′N/11°47′E), Praxmar (1760 m, 47°09′N/11°07′E), Stiglreith (1368 m, 47°24′N/11°22′E; only V. vitis‐idaea) and Innsbruck (791 m, 47°27′N/11°37′E; only V. myrtillus). On all sites, widespread stands of studied dwarf shrubs can be found.
Plant sampling
For pressure–volume (pv) curve analyses, xylem hydraulic measurements and electrolyte leakage analyses, up to 30 cm long shoots were randomly selected at each site and cut on a cloudy day after a period of rainfall to avoid high xylem tensions. Cutting was performed under water to release tension. Therefore, the basal part of the shoots was packed in a plastic bag, which was filled with water. After cutting, the shoots were kept in water and covered with a dark plastic bag, transported to the laboratory and saturated for at least 24 h, to ensure a Ψ of 0 MPa. All measurements were performed between May and October 2011. While pv curves and xylem hydraulics were analyzed for all sites, electrolyte leakage, stomata closure and anatomical parameters were measured on samples of Praxmar, due to the good accessibility of this site.
Plants from Praxmar were also used for the refilling experiment. Five soil blocks (about 40 × 40 cm, 20 cm deep) with plants of V. myrtillus and V. vitis‐idaea were extracted at Praxmar at the end of August, placed in plastic boxes and transported to the greenhouse of the Botanical Garden, University of Innsbruck.
Water potential (Ψ)
Ψ was measured with a pressure chamber (model 1000 ‘upgraded to 100 bar’ pressure chamber; PMS Instrument, Albany, OR) on up to 10 cm long end shoots. Only in the refilling experiment, single leaves were used because of limited plant material. Therefore, the lamina was partially incised along the middle leaf vein for better sealing in the lid of the pressure chamber. It has to be noted that Ψ measurements during bench dehydration (vulnerability analyses) were suggested to reflect xylem Ψ, as stomata were closed and samples dehydrated slowly enabling sufficient equilibration of Ψ within samples. In contrast, the shoot Ψ in analysis of stomata closure probably differed from xylem Ψ due to the Ψ gradient caused by transpiration. Leaf Ψ measured in the refilling experiment can also not directly be compared with shoot Ψ as the latter integrates Ψ of several organs and tissues. Furthermore, Ψ determination on single leaves is difficult and thus less accurate.
pv‐curve analysis (osmotic potential, turgor loss point and cell wall elasticity)
Cell osmotic parameters were analyzed via pv‐curves (Tyree and Hammel 1972), by plotting the inverse leaf Ψ (1/Ψ) vs the relative water saturation deficiency (WSD) of drying shoots. Cut and fully hydrated shoots (Ψ = 0 MPa) were dehydrated slowly on the bench, and, in intervals, Ψ and weight were determined. WSD was calculated as
| (1) |
where FWS is the fresh weight of the saturated shoot, FWA is the actual measured fresh weight and DW is the dry weight of the shoot. For DW determination, shoots were finally dried for 48 h at 80°C in an oven. For each shoot, 1/Ψ was plotted vs WSD (9–15 values per shoot). The turgescent section was fitted with a parabolic function and the osmotic section with a linear regression according to Boyle's law using Fig.P 2006 (Fig.P Software Inc., ON, Canada). The osmotic potential at saturation (Ψo) was determined from the intersection of the linear regression with the y‐axis, the Ψ at turgor loss point (Ψtlp) from the intersection of the parabolic function and the linear regression function. The cell wall elasticity (aela) was assessed by the opening width of the parable. Elastic tissues show slow changes in Ψ on reduction of WSD and thus a wide parabolic function and a low aela. Mean ± se for all parameters were calculated from 10 to 30 curves.
Vulnerability to drought‐induced embolism and hydraulic conductivity
Saturated shoots [Ψ = 0 MPa, percent loss of conductivity (PLC) = 0] were dehydrated on the bench and embolism formation at different xylem Ψ determined. The main stem of shoots was immersed in distilled water and cut 2–3 cm from the basal end. Then, stem sections, up to 5 cm in length and 1–3 mm in diameter, were cut under water and decorticated. Sample ends were re‐cut several times for about 5 mm with a sharp wood carving knife and sealed in the silicone tubes of the apparatus.
To analyze the potential formation of artificial embolism by cutting the samples under tension, a control experiment with samples rehydrated for 30 min (Ψ > −0.5 MPa) prior to cutting was conducted as suggested by Wheeler et al. (2013). PLC values obtained with this method did not differ from results obtained without 30 min rehydration (data not shown), thus the formation of artifacts by cutting as well as artifacts by rapid refilling (Trifilò et al. 2014) can be excluded for the species used in our experiment.
Percent loss of hydraulic conductivity (PLC) in shoot samples was quantified by comparing the hydraulic conductivity before and after removal of xylem embolism by repeated high pressure flushes (Sperry et al. 1988). The flow rate was determined with a micro‐flow meter (µ‐Flow 500 mg h−1, Bronkhorst High Tech, Ruurlo, Netherlands) at a pressure of 5 kPa. Flushing (at 70 kPa; 20 min) and conductivity measurements were done with distilled, filtered (0.22 µm) and degassed water containing 0.005% (v/v) Micropur (Katadyn Products, Kemptthal, Switzerland) to prevent microbial growth. Flushing was repeated until no further increase in conductivity was observed. PLC was calculated from the ratio of initial to maximal conductivity (Sperry et al. 1988). Data (PLC vs xylem Ψ of each branch) were pooled (27–90 values) per treatment to construct vulnerability curves. Curves were fitted with an exponential sigmoidal equation according to Pammenter and Vander Willigen (1998):
| (2) |
where a is a constant related to the curve slope and ΨPLC50 the xylem Ψ at 50% loss of conductivity. Fitting of the curves was performed with Fig.P 2006. We also calculated Ψ at 12 and 88% PLC (ΨPLC12, ΨPLC88).
The specific hydraulic conductivity (ks; m2 s−1 Pa−1) was measured on fully hydrated samples and calculated as
| (3) |
where Q is the maximal volume flow rate (m3 s−1), l is the length of the sample (m), Ac is the xylem cross‐sectional area (m2; calculated from the sample diameter) and ΔP is the pressure difference between the segment ends (Pa). Calculations were corrected to 20°C to account for changes in fluid viscosity with temperature. Saturated twigs always showed a PLC near zero, confirming that sample preparation did not induce artificial embolism and ks values thus represent maximum conductivity.
Stomata closure
The stomatal conductance (gs) was measured according to Nolf et al. (2013) on a sunny day with a SC‐1 Leaf Porometer (Decagon Devices, Pullman, WA). To determine stomata closure, gs was measured on leaves of previously watered plants with maximally opened stomata (between 10:00 and 12:00 Central European Time CET). Shoots were cut and re‐positioned in the stand (similar to original position). After different periods (10 s to 2 h), gs was measured a second time on the same leaves, before shoot Ψ was determined. The percent stomata closure (PSC) was calculated from the ratio of the first to the second gs value and plotted vs respective shoot Ψ. Data (PSC vs Ψ) of all shoots were pooled (24–34 values) per treatment for construction of gs curves. Curves were fitted according to Eqn 2 with PLC substituted by PSC and ΨPLC50 corresponding to Ψ at 50% gs (ΨSC50). Stomata closure was defined as Ψ at 12% of maximum gs (ΨSC12). As stomata closure occurred within 2 h, it can be assumed that during dehydration neither light, CO2 concentration nor temperature or vapor pressure deficit changed considerably and stomata closure thus occurred in response to decreasing Ψ.
Electrolyte leakage
To determine the critical Ψ for emerging cell damage, the electrolyte leakage method was used, which analyses electrolyte leakage from the symplast to the apoplast due to cell damage. By measuring the electrical conductivity of a solution with plant samples, this leakage can be assessed, whereby the conductivity of autoclaved samples (in which all cells have been killed) enables estimating the total amount of tissue electrolytes. The comparison of conductivities of dehydrated leaves to the total conductivity provides an estimation of injuries (Leopold et al. 1981, Bajji et al. 2001). Fully hydrated shoots (Ψ = 0 MPa, PLC = 0) were dehydrated on the bench. At different shoot Ψ, round leaf segments (4 mm in diameter, about 10 per sample) were cut with a cork borer, placed in test tubes with 15 ml of distilled water and shaken for 24 h at 5°C on a horizontal gravity shaker (ST5, CAT, Germany). The electrolytic conductivity (C1) was measured at room temperature with a conductivity meter (WTW inoLab, Weilheim, Germany). Samples were then autoclaved at 120°C for 30 min (Tuttnauer autoclave steam sterilizer 2540 ELV, Syntec GmbH Wettenberg, Germany) and shaken again for 24 h at 5°C. Afterwards, the second conductivity measurement (C2) was conducted at room temperature. Electrolyte leakage caused by the cutting out of the leaf segments (C0) was determined by measuring the conductivity of full hydrated samples and had to be subtracted from C1 and C2. Relative electrolytic leakage (REL) was thus calculated as
| (4) |
Data (REL vs Ψ of each branch) of treatments were pooled (35–36 values) for construction of the curves. Curves were fitted with a sigmoid curve according to Eqn 2 with PLC substituted by REL and ΨPLC50 corresponding to Ψ at 50% cell damage (ΨEL50). In both species, maximum leakage on dehydration was lower than leakage on autoclaving. In dehydrating samples, cell contents were probably partly trapped within the tissue. The end points of fitted curves were therefore defined as 100% and all values were corrected in relation to the end point.
Wood characteristics
Shoots previously used for vulnerability measurements were soaked in ethanol/glycerol/water solution (1:1:1, v/v/v) for at least 2 weeks. Cross sections (8 µm) of 10 shoots per species were cut with a microtome (Schlittenmikrotom G.S.L. 1, Schenkung Dapples, Zürich, Switzerland), stained with Etzold solution (fuchsin‐safranine‐astrablue) and analyzed with a light microscope (Olympus BX41; Olympus Austria, Wien, Austria) interfaced with a digital camera (ProgRes CT3, Jenoptik, Jena, Germany). In randomly selected radial sectors including all growth rings, areas of all conduits (68–174 per sample) were analyzed with image analysis software (ImageJ 1.45; public domain, National Institutes of Health, MD, USA). The diameters were calculated from conduit areas assuming a circular shape, averaged per sample and the mean diameter (d) per species calculated from these values. The average hydraulic conduit diameter (dh) was calculated from the diameter of all conduits analyzed according to Sperry and Hacke (2004):
| (5) |
To characterize conduit wall reinforcement, the wall thickness to span ratio (t/b)2 (Hacke et al. 2001a) was analyzed. We measured the thickness of tangential interconduit walls (t) as well as the conduit diameter (b) for conduit pairs with average diameters within dh ± 1 µm (five conduit pairs per sample). The values were averaged per sample, and the mean (t/b)2 per species calculated from these values.
Refilling experiment
To test the ability of the plants to recover from drought‐induced embolism, a refilling experiment with controlled dehydration and subsequent re‐watering of plants in five soil blocks was conducted. Samples desiccated in the greenhouse, and leaf Ψ and gs were measured every second day at 11:00 CET (n = 5). When leaf Ψ of both species and all blocks was about −3 MPa, re‐watering was started. Every third day, the entire blocks were submersed in a water bath for 10 min to reach soil field capacity. Monitoring of leaf Ψ and gs was continued, and additional measurements of PLC were performed (n = 5 per sampling date). Samples for PLC measurements were selected randomly from the blocks, cut under water and prepared as described for vulnerability curves. The experiment was continued for 26 days.
Statistics
All values are given as mean ± se. Differences were tested (1) at an interspecific level (V. myrtillus vs V. vitis‐idaea), (2) at a site‐specific level (populations from different sites) and (3) at a time‐specific level (measurements on V. myrtillus during the growing season). For vulnerability, stomata closure and electrolyte leakage analyses, differences in thresholds were tested with Welch's test and based on mean ± se values calculated from curve fittings at n‐2 df. For ks, Ψo, Ψtlp, aela, d, dh and (t/b)2, differences were analyzed with the Bonferroni test (for data with homogeneity of variance, tested with the Levene test) or Tamhane test (no homogeneity of variance) after testing for Gaussian distribution with the Kolmogorov–Smirnov test. All tests (two‐tailed) were performed pairwise at a probability level of 5% using SPSS (version 18; SPSS, IL, USA).
Results
pv‐curve analysis
Ψo and Ψtlp were significantly more negative in V. vitis‐idaea (−1.61 and −1.88 MPa) than in V. myrtillus (−1.22 and −1.38 MPa, Table 1). aela was lower in V. myrtillus, indicating a higher cell wall elasticity of this species. Between sites, no difference in Ψo and aela but partly in Ψtlp was found in both species (Table 2). In V. myrtillus, Ψo varied from −1.08 to −1.33 MPa, Ψtlp from −1.27 to −1.55 MPa and aela from 0.015 to 0.029. V. vitis‐idaea showed a Ψo between −1.51 and −1.70 MPa, Ψtlp between −1.69 and −2.09 MPa and aela between 0.026 and 0.060. Repeated measurements on V. myrtillus during the growing season revealed a continuous decrease of Ψo, Ψtlp and aela from May to October (Table 3). The overall decline in Ψo was 0.32 MPa and in Ψtlp 0.39 MPa.
Table 1.
pv‐curve analysis and xylem hydraulic parameters of Vaccinium myrtillus and Vaccinium vitis‐idaea. Osmotic potential at saturation (Ψo; MPa), turgor loss point (Ψtlp; MPa), cell wall elasticity (aela; dimensionless), xylem water potential at 12, 50 and 88% loss of xylem conductivity (ΨPLC12, ΨPLC50, ΨPLC88; MPa) and specific xylem hydraulic conductivity (ks; 10−4 m2 s−1 MPa−1) of shoots are given. Averaged values for three sites analyzed during summer per species in Tyrol are shown. Mean ± se, different letters indicate significant differences between species, P ≤ 0.05
| V. myrtillus | V. vitis‐idaea | n | |
|---|---|---|---|
| Ψo | −1.22 ± 0.03a | −1.61 ± 0.03b | 30 |
| Ψtlp | −1.38 ± 0.03a | −1.88 ± 0.04b | 30 |
| aela | 0.024 ± 0.002a | 0.045 ± 0.004b | 30 |
| ΨPLC12 | −0.86 ± 0.19a | −0.79 ± 0.18a | 88, 90 |
| ΨPLC50 | −2.08 ± 0.06a | −1.97 ± 0.06a | 88, 90 |
| ΨPLC88 | −3.31 ± 0.07a | −3.15 ± 0.06a | 88, 90 |
| ks | 0.90 ± 0.15a | 0.84 ± 0.10a | 15 |
Table 2.
pv‐curve analysis and xylem hydraulic parameters of Vaccinium myrtillus and Vaccinium vitis‐idaea on the different study sites (Pk Patscherkofel, Pr Praxmar, Ibk Innsbruck, St Stiglreith). Osmotic potential at saturation (Ψo; MPa), turgor loss point (Ψtlp; MPa), cell wall elasticity (aela; dimensionless) and xylem water potential at 12, 50 and 88% loss of xylem conductivity (ΨPLC12, ΨPLC50, ΨPLC88; MPa) of shoots are given. Mean ± se, different letters indicate significant differences between sites, P ≤ 0.05
| V. myrtillus | V. vitis‐idaea | ||||||
|---|---|---|---|---|---|---|---|
| Pk | Pr | Ibk | Pk | Pr | St | n | |
| Ψo | −1.33 ± 0.03ab | −1.08 ± 0.05a | −1.19 ± 0.01a | −1.51 ± 0.03bc | −1.70 ± 0.03c | −1.61 ± 0.06bc | 10 |
| Ψtlp | −1.55 ± 0.03a | −1.27 ± 0.06b | −1.34 ± 0.01b | −1.69 ± 0.04a | −2.09 ± 0.04c | −1.88 ± 0.06ac | 10 |
| aela | 0.015 ± 0.002a | 0.017 ± 0.001a | 0.029 ± 0.004ab | 0.060 ± 0.010b | 0.026 ± 0.004ab | 0.052 ± 0.006b | 10 |
| ΨPLC12 | −0.84 ± 0.28a | −0.98 ± 0.39a | −0.98 ± 0.23a | −0.77 ± 0.28a | −0.90 ± 0.30a | −0.82 ± 0.27a | 27–35 |
| ΨPLC50 | −1.87 ± 0.08a | −2.31 ± 0.13b | −2.08 ± 0.07ab | −2.20 ± 0.09b | −1.89 ± 0.09a | −1.83 ± 0.09a | 27–35 |
| ΨPLC88 | −2.90 ± 0.11ab | −3.63 ± 0.14c | −3.17 ± 0.09b | −3.63 ± 0.10c | −2.87 ± 0.11a | −2.84 ± 0.08a | 27–35 |
Table 3.
pv‐curve analysis and xylem hydraulic parameters of Vaccinium myrtillus in May, August and October. Osmotic potential at saturation (Ψo; MPa), turgor loss point (Ψtlp; MPa), cell wall elasticity (aela; dimensionless), xylem water potential at 12, 50 and 88% loss of xylem conductivity (ΨPLC12, ΨPLC50, ΨPLC88; MPa) of shoots measured in Innsbruck, Tyrol are given. Mean ± se, different letters indicate significant differences between sampling dates, P ≤ 0.05
| May | August | October | n | |
|---|---|---|---|---|
| Ψo | −1.09 ± 0.02a | −1.19 ± 0.01b | −1.41 ± 0.01 c | 10 |
| Ψtlp | −1.17 ± 0.02a | −1.34 ± 0.01b | −1.56 ± 0.02c | 10 |
| aela | 0.032 ± 0.003a | 0.029 ± 0.004a | 0.027 ± 0.004a | 10 |
| ΨPLC12 | −1.35 ± 0.40a | −0.98 ± 0.23a | −1.03 ± 0.29a | 30–35 |
| ΨPLC50 | −2.90 ± 0.07a | −2.08 ± 0.07b | −2.36 ± 0.09c | 30–35 |
| ΨPLC88 | −4.45 ± 0.26a | −3.17 ± 0.09b | −3.70 ± 0.11c | 30–35 |
Vulnerability to drought‐induced embolism and hydraulic conductivity
Both species exhibited typical sigmoid vulnerability curves with nearly identical vulnerability thresholds (Fig. 1). Embolism formation started around −0.8 MPa (ΨPLC12; Table 1) and reached 50% loss of conductivity (ΨPLC50) at −2.08 MPa (V. myrtillus) and −1.97 MPa (V. vitis‐idaea). ΨPLC88 was at −3.31 and −3.15 MPa, respectively. Between sites, no difference in ΨPLC12 was found, while ΨPLC50 and ΨPLC88 differed partly in both species (Table 2). ΨPLC50 of V. myrtillus varied from −1.87 to −2.31 MPa and of V. vitis‐idaea from −1.83 to −2.20 MPa. The seasonal analysis of V. myrtillus (Table 3) revealed a noticeable decrease of the resistance to drought‐induced embolism in summer and a recovery in fall. ks was slightly (not significant) higher in V. myrtillus than in V. vitis‐idaea (Table 1).
Figure 1.
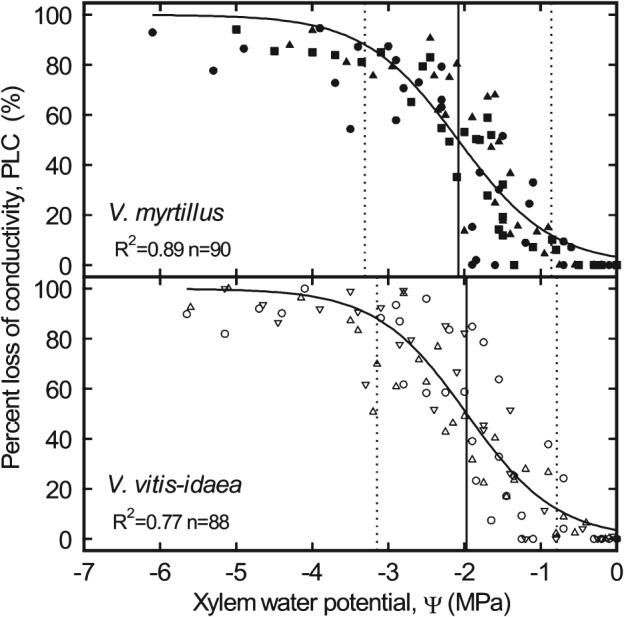
Vulnerability curves (percent loss of conductivity vs xylem water potential) of Vaccinium myrtillus (closed symbols) and Vaccinium vitis‐idaea (open symbols) measured on different sites in Tyrol: Praxmar (circles), Patscherkofel (up triangles), Innsbruck (squares) and Stiglreith (down triangles). Lines indicate water potential at 12, 50 and 88% loss of conductivity.
Stomata closure
Leaf stomatal conductance of dehydrating plants (measured on the site in Praxmar) followed sigmoid curves in both species with a slightly steeper slope in V. myrtillus (Fig. 2). Stomata closure (ΨSC12) was observed at −2.19 MPa in V. myrtillus and at −2.35 MPa in V. vitis‐idaea (Table 4).
Figure 2.
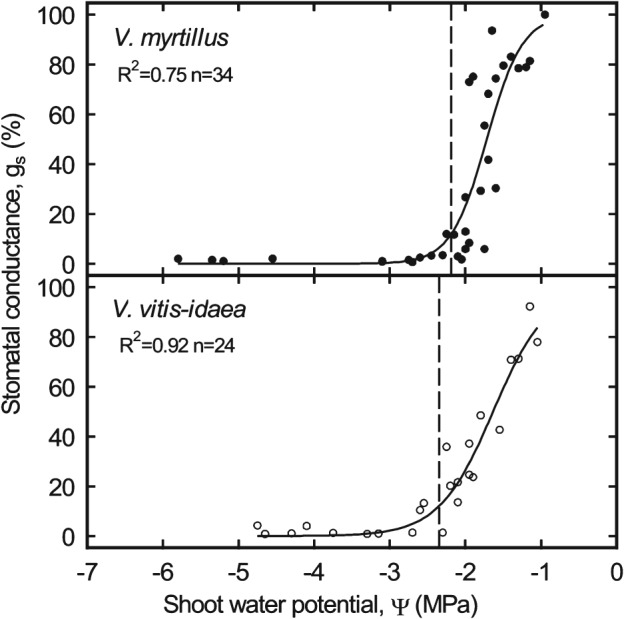
Percent stomatal conductance vs shoot water potential of Vaccinium myrtillus (closed circles) and Vaccinium vitis‐idaea (open circles) measured in Praxmar, Tyrol. Vertical lines indicate water potential at stomata closure (ΨSC12).
Table 4.
Stomata closure of Vaccinium myrtillus and Vaccinium vitis‐idaea. Shoot water potential at 12, 50 and 88% relative stomatal conductance (ΨSC12, ΨSC50, ΨSC88; MPa) measured in Praxmar, Tyrol are given. Mean ± se, different letters indicate significant differences between species, P ≤ 0.05
| V. myrtillus | V. vitis‐idaea | n | |
|---|---|---|---|
| ΨSC12 | −2.19 ± 0.15 a | −2.35 ± 0.12 a | 24, 34 |
| ΨSC50 | −1.72 ± 0.05 a | −1.64 ± 0.04 a | 24, 34 |
| ΨSC88 | −1.25 ± 0.06 a | −0.92 ± 0.04 b | 24, 34 |
Electrolyte leakage
Measurements of the relative electrolyte leakage on dehydrated leaves resulted in sigmoid curves with a distinct steeper slope in V. vitis‐idaea (Fig. 3). A first increase in electrolyte concentration was observed in both species at about −3 MPa, but V. vitis‐idaea reached 50% relative electrolyte leakage at less negative shoot Ψ (−4.03 MPa) than V. myrtillus (−4.47 MPa; Table 5).
Figure 3.
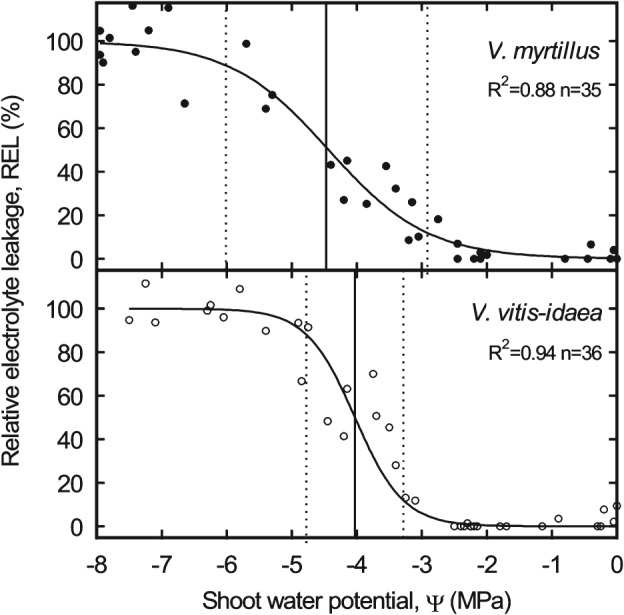
Relative electrolyte leakage vs shoot water potential of Vaccinium myrtillus (closed circles) and Vaccinium vitis‐idaea (open circles) measured on plants growing in Praxmar, Tyrol. Vertical lines indicate water potential at 12, 50 and 88% relative electrolyte leakage.
Table 5.
Drought‐induced cell damage of Vaccinium myrtillus and Vaccinium vitis‐idaea. Shoot water potential at 12, 50 and 88% relative cell damage (ΨEL12, ΨEL50, ΨEL88; MPa), analyzed with the electrolyte leakage method on plants from Praxmar, Tyrol. Mean ± se, different letters indicate significant differences between species, P ≤ 0.05
| V. myrtillus | V. vitis‐idaea | n | |
|---|---|---|---|
| ΨEL12 | −2.91 ± 0.32a | −3.28 ± 0.16a | 35, 36 |
| ΨEL50 | −4.47 ± 0.12a | −4.03 ± 0.08b | 35, 36 |
| ΨEL88 | −6.03 ± 0.08a | −4.77 ± 0.01b | 35, 36 |
Wood characteristics
Both species exhibited distinct growth rings. The frequency distribution of conduit diameters showed a bell‐shaped curve for V. vitis‐idaea, while V. myrtillus contained a noticeable higher percentage of large conduits (data not shown). No significant differences in d, dh or (t/b)2 between species were found (Table 6).
Table 6.
Xylem anatomical parameters of Vaccinium myrtillus and Vaccinium vitis‐idaea. Mean conduit diameter (d; µm), mean hydraulic diameter (dh; µm) and wall thickness to span ratio [(t/b)2; dimensionless] of plants growing in Praxmar, Tyrol are given. Mean ± se, letters indicate that no significant differences between species were found, P ≤ 0.05
| V. myrtillus | V. vitis‐idaea | n | |
|---|---|---|---|
| d | 12.41 ± 0.04a | 12.38 ± 0.07a | 10 |
| dh | 16.25 ± 0.12a | 15.67 ± 0.09a | 10 |
| (t/b)2 | 0.0218 ± 0.0002a | 0.0211 ± 0.0002a | 10 |
Refilling
Fig. 4 shows the development of leaf Ψ, gs and PLC of V. myrtillus and V. vitis‐idaea during 5 days of dehydration and the following 3 weeks of periodic watering. With the beginning of sufficient water supply, both species showed an increase in leaf Ψ and gs and a decrease in PLC, indicating refilling of embolized conduits. V. vitis‐idaea revealed a distinct earlier and more pronounced recovery of gs and hydraulic conductivity, with a decrease in PLC from about 50 to 13.29% within 1 week. In V. myrtillus, PLC decreased in the first week to 23.78%. After 3 weeks, PLC was nearly zero in both species.
Figure 4.
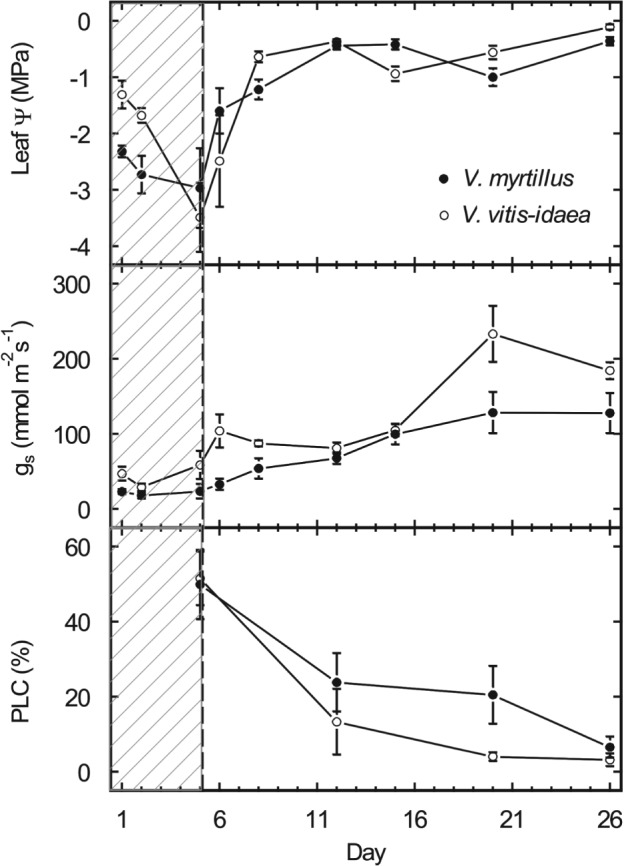
Refilling experiment with Vaccinium myrtillus (closed circles) and Vaccinium vitis‐idaea (open circles). Plants were dehydrated for 5 days until 50% loss of conductivity was reached (hatched background). Then, plants were watered periodically during 3 weeks. Leaf water potential (Ψ, MPa), Stomatal conductance (gs, mmol m−2 s−1) and percent loss of conductivity (PLC, %) during the dehydration and re‐watering period are given. Mean ± se, n = 5.
Discussion
Hydraulic architecture of dwarf shrubs
One important aspect of a plant species' strategy in the face of limited water supply is the maintenance of cell turgor. As mean Ψtlp is −2.17 ± 0.07 MPa in temperate angiosperms (Bannister 1986), obtained results (−1.38 and −1.88 MPa, see Table 1) indicate turgor loss at relatively moderate Ψ in analyzed Vaccinium species. Turgor loss is known to correspond with inhibition of photosynthesis, growth and other important cell functions (Kramer and Boyer 1995). It is a reversible process as long as Ψ does not reach critical levels. In studied species, serious cell damages appeared at about −3 MPa and thus at distinct lower Ψ than Ψtlp (Fig. 3).
Vaccinium species exhibited a quite low hydraulic efficiency (ks 0.94 and 1.11 10−4 m2 s−1 MPa−1) compared to other angiosperms (on average 17.0 ± 0.19 10−4 m2 s−1 MPa−1) and even gymnosperms (4.6 ± 0.05 10−4 m2 s−1 MPa−1; Maherali et al. 2004). Similar values were found in semi‐arid shrubs, such as Anthyllis cytisoides (0.72 ± 0.39 10−4 m2 s−1 MPa−1) or Lycium intricatum (0.99 ± 0.20 10−4 m2 s−1 MPa−1; Miranda et al. 2010). Low ks cause steep Ψ gradients in plants, but due to short transport distances in dwarf shrubs, this effect may be of minor relevance. As d and dh (Table 6) were similar to other alpine shrubs with higher ks (J. communis, Rhododendron ferrugineum and Rhododendron hirsutum; Beikircher and Mayr 2008, Mayr et al. 2010), low conductivities in Vaccinium may not only be the result of small conduits but of high pit resistance and low proportion of conducting conduits in the xylem (Tyree and Zimmermann 2002) or non‐functional conduits in the older growth rings.
The resistance to drought‐induced embolism was also relatively low in studied Vaccinium species (ΨPLC50 –2.08 and −1.97 MPa, see Table 1). In comparison, ΨPLC50 of coniferous trees and shrubs of the timberline ecotone is between −3.39 (Larix decidua) and −3.98 MPa (Picea abies; Mayr et al. 2003, 2006). However, vulnerability thresholds of V. myrtillus and V. vitis‐idaea were similar to alpine Rhododendron shrubs (−1.95 to −3.24 MPa; Mayr et al. 2010). Rhododendron ferrugineum and R. hirsutum reduce the risk of xylem embolism via stomata closure 0.89–1.57 MPa above ΨPLC50 (Mayr et al. 2010). Measurements on Vaccinium species indicated that dwarf shrubs follow a more risky strategy: although both species started to reduce stomata opening below −1 MPa, total stomata closure was not reached before −2 MPa in shoot Ψ (Table 4, Fig. 2). Studied species obviously do not rapidly close stomata on appearing drought stress and probably operate close to the point of embolism formation (Table 1, Fig. 1). It has to be noticed that shoot Ψ in gs measurements cannot directly be compared with xylem Ψ of vulnerability analyses (see Materials and Methods) and that stomata reaction on rapidly increasing desiccation may differ from slowly emerging drought stress.
In contrast, efficient stomatal control and cavitation avoidance were observed in several Mediterranean shrubs suffering from intense drought during summer. Vilagrosa et al. (2003) reported a ΨPLC50 of −4.81 MPa in Pistacia lentiscus and −6.96 MPa in Quercus coccifera. Other studies, however, revealed that some drought‐exposed shrubs from Mediterranean communities are only moderately resistant to cavitation (Miranda et al. 2010). In shrubs from arid Californian plant communities, ΨPLC50 varied from −0.5 to −9.5 MPa (Jacobsen et al. 2007). The large variation in ΨPLC50 indicates that a high cavitation resistance is only one of several strategies to cope with severe drought. Especially in shrubs, other morpho‐functional traits beyond xylem resistance may play an important role for survival.
But how can Vaccinium species survive in their natural habitat when they risk to suffer from embolism? The refilling experiment revealed that V. myrtillus and V. vitis‐idaea possess sufficient recovery mechanisms, which enable compensating the drawback of low hydraulic safety. Both species could remove severe embolization within 3 weeks after having achieved a balanced Ψ (Fig. 4). The increase in Ψ occurred during the first days of the re‐watering period and corresponded to restrictions in gs during the first 2 weeks. An increasing number of studies document the existence and importance of refilling in different plant species (e.g. Johnson et al. 2012, Ogasa et al. 2013) and propose several underlying mechanisms, including positive root pressure, secretion of osmotica and phloem unloading (e.g. Zwieniecki and Holbrook 2009, Nardini et al. 2011, Brodersen and McElrone 2013). Our experiment simulated favorable conditions like repeated rainfalls and a wet soil as often observed at higher elevation. These conditions enabled xylem repair at Ψ of or near zero and thus are not necessarily related to ‘novel refilling’ (Hacke and Sperry 2003). In this context, the low growth height of dwarf shrubs gains in importance, as hydraulic distances are minor and may facilitate xylem repair by dissolution of entrapped air in the surrounding sap.
In summary, hydraulic efficiency and safety as well as restrictive stomata regulation are obviously less important in low growing shrubs than in trees because of short transport distances, provided that they exhibit a sufficient recovery potential. A higher performance in xylem recovery of cavitation‐vulnerable species was recently also detected in temperate tree species, indicating a general coordination between these two key parameters (Ogasa et al. 2013). It should also be considered that differences in biomechanics can affect wood traits of different growth forms (Martínez‐Cabrera et al. 2011), and shrubs can sacrifice single shoots and form new ones from the basal buds. Another aspect not included in the presented study but with potential impact on the hydraulic architecture is the clonal reproduction of the analyzed species. Physiological integration and water sharing among ramets between plants in moist and dry microhabitats can increase performance of single plants and is a major advantage of clonal growth (Alpert 1990, Schenk et al. 2008).
Interspecific differences
Several hydraulic traits were nearly identical in both species, although V. vitis‐idaea can colonize drier sites. ΨPLC50 did not differ between analyzed Vaccinium species, while on a global scale higher embolism resistance was observed in drought adapted plants (Choat et al. 2012). In contrast, the remarkably more negative Ψo and Ψtlp (Table 1) as well as the better refilling capacity of V. vitis‐idaea (Fig. 4) indicate a better drought adaptation of this species.
Consistently to most pv‐curve studies (e.g. Bannister 1986, Bartlett et al. 2012), Ψtlp of V. myrtillus and V. vitis‐idaea was mainly influenced by Ψo and hardly related to cell wall elasticity (Tables 1 and 3). Ψo and Ψtlp are generally connected with the aridity of a species' natural habitat (Lenz et al. 2006). Low Ψo allows a longer water uptake under soil dehydration and with low Ψtlp, cells can maintain turgescence and physiological functions at low Ψ (Kramer and Boyer 1995). Thus, V. vitis‐idaea is able to maintain growth and photosynthesis at lower Ψ than V. myrtillus. Moreover, the efficient recovery of V. vitis‐idaea may allow an earlier increase in stomatal conductance and photosynthesis after drought events (Fig. 4). This indicates that refilling is a key hydraulic strategy of studied dwarf shrubs and that the enhanced refilling capability augments the drought resistance of V. vitis‐idaea considerably. Interestingly, the electrolyte leakage analysis revealed a faster increase of damages in desiccating shoots of V. vitis‐idaea, resulting in a less negative ΨEL50 than in V. myrtillus (Fig. 3). As damages generally occurred in both species at low Ψ, far below turgor loss and embolization, these differences in cell damage rather reflect differences in cell composition than drought resistance strategies.
Overall, the hydraulic properties and drought resistance of studied species corresponded with their habitat demands and may explain the occurrence of V. vitis‐idaea on more arid sites. Although other physiological characteristics and environmental factors, such as frost hardiness and presence of a long‐lasting snow cover, may also influence habitat requirements, we suppose that water relations play an important role.
Seasonal and site‐specific variation
V. myrtillus exhibited seasonal variation in several hydraulic parameters. The continuous decrease of Ψtlp and Ψo (Table 3) in developing leaves caused an increasing drought resistance during the growing season. In summer, this may be important to prevent turgor loss on midday. The observed decrease in embolism resistance from spring to summer may be due to the formation of new, susceptible conduits in early summer. Furthermore, repeated cavitation–refilling cycles during summer may reduce the resistance to air seeding (Hacke et al. 2001b). Interestingly, the hydraulic safety slightly increased in autumn. We suggest that older xylem parts were taken out of function leading to a shift in vulnerability.
In comparison to the seasonal variation, site‐specific differences in pv characteristics and in safety in both species were small, but partly significant. This indicates a (though rather low) intraspecific potential of hydraulic adaptation (i.e. hydraulic plasticity; Cochard et al. 1992) to local water availability.
Conclusion
Hydraulic analyses on Vaccinium species indicated a risky hydraulic strategy with low hydraulic efficiency and safety as well as stomata closure at low Ψ. The high risk of embolism formation is probably balanced by xylem repair capacities. Hydraulics thus play an important role in the ecophysiology of dwarf shrubs and help to explain species‐specific ecological amplitudes.
Author contributions
S. M originally formulated the idea, S. M. and A. G. designed the experiments, A. G. performed the measurements, analyzed the data, performed statistical analyses and wrote the manuscript, S. M. provided editorial advice.
Acknowledgements
The study was supported by the Austrian Science Fund (FWF) P20852‐B16 and I826‐B25, the alpS‐COMET‐project L03 Adapt AF II (founded by BMVIT, BMFWF, Land Tirol, Land Vorarlberg) and ‘Stipendium des Vereins zur Förderung der wissenschaftlichen Ausbildung und Tätigkeit von Südtirolern an der Landesuniversität Innsbruck’. We thank Mag. Ing. Birgit Dämon for excellent assistance and the anonymous reviewers for helpful comments on the manuscript.
The copyright line for this article was changed on 31 August 2015 after original online publication.
References
- Alpert P (1990) Water sharing among ramets in a desert population of Distichlis spicata (Poaceae). Am J Bot 77: 1648–1651 [Google Scholar]
- Bajji M, Kinet JM, Lutts S (2001) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. J Plant Growth Regul 36: 61–70 [Google Scholar]
- Bannister P (1986) Drought resistance, water potential and water content in some New Zealand plants. Flora 178: 23–40 [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta‐analysis. Ecol Lett 15: 393–405 [DOI] [PubMed] [Google Scholar]
- Beikircher B, Mayr S (2008) The hydraulic architecture of Juniperus communis L. ssp. communis: shrubs and trees compared. Plant Cell Environ 31: 1545–1556 [DOI] [PubMed] [Google Scholar]
- Beikircher B, Mayr S (2009) Intraspecific differences in drought tolerance and acclimation in hydraulics of Ligustrum vulgare and Viburnum lantana . Tree Physiol 29: 765–775 [DOI] [PubMed] [Google Scholar]
- Boehm H (1893) Capillarität und Saftsteigen. Ber Dtsch Bot Ges 11: 203–212 [Google Scholar]
- Bresinsky A, Körner C, Kadereit JW, Neuhaus G, Sonnewald U (2008) Strasburger Lehrbuch der Botanik. Spektrum Akademischer Verlag, Heidelberg: [Google Scholar]
- Brodersen CR, McElrone AJ (2013) Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front Plant Sci 4: 108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Arce ME (2009) Soil water availability and rooting depth as determinants of hydraulic architecture of Patagonian woody species. Oecologia 160: 631–641 [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez‐Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westoby M, Wright IJ, Zanne AE (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755 [DOI] [PubMed] [Google Scholar]
- Cochard H, Bréda N, Granier A, Aussenac G (1992) Vulnerability to air embolism of three European oak species (Quercus petraea (Matt) Liebl, Q. pubescens Willd, Q. robur L). Ann Sci Forest 49: 225–233 [Google Scholar]
- Cochard H, Coll L, Le Roux X, Améglio T (2002) Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol 128: 282–290 [PMC free article] [PubMed] [Google Scholar]
- Dixon HH, Joly J (1894) On the ascent of sap. Ann Bot 8: 468–470 [Google Scholar]
- Gartner BL (1991) Stem hydraulic properties of vines vs. shrubs of western poison oak, Toxicodendron diversilobum . Oecologia 87: 180–189 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS (2001) Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst 4: 97–115 [Google Scholar]
- Hacke UG, Sperry JS (2003) Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo . Plant Cell Environ 26: 303–311 [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001a) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA (2001b) Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol 125: 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Davis SD, Ewers FW (2007) Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant Cell Environ 30: 1599–1609 [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC (2012) Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different? Plant Sci 195: 48–53 [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS (1995) Water Relations of Plants and Soils. Academic Press, San Diego, CA: [Google Scholar]
- Landolt E (2010) Flora indicativa. Ökologische Zeigerwerte und biologische Kennzeichen zur Flora der Schweiz und der Alpen, 2nd Edn. Haupt Verlag, Bern, pp 176–177 [Google Scholar]
- Lenz TI, Wright IJ, Westoby M (2006) Interrelations among pressure‐volume curve traits across species and water availability gradients. Physiol Plant 127: 423–433 [Google Scholar]
- Leopold AC, Musgrave ME, Williams KM (1981) Solute leakage resulting from leaf desiccation. Plant Physiol 68: 1222–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199 [Google Scholar]
- Martínez‐Cabrera HI, Schenk HJ, Cevallos‐Ferriz SRS, Jones CS (2011) Integration of vessel traits, wood density, and height in angiosperm shrubs and trees. Am J Bot 98: 915–922 [DOI] [PubMed] [Google Scholar]
- Martinez‐Vilalta J, Sala A, Pinol J (2004) The hydraulic architecture of Pinaceae – a review. Plant Ecol 171: 3–13 [Google Scholar]
- Mayr S, Rothart B, Dämon B (2003) Hydraulic efficiency and safety of leader shoots and twigs in Norway spruce growing at the alpine timberline. J Exp Bot 54: 2563–2568 [DOI] [PubMed] [Google Scholar]
- Mayr S, Hacke U, Schmid P, Schwienbacher F, Gruber A (2006) Frost drought in conifers at the alpine timberline: xylem dysfunction and adaptions. Ecology 87: 3175–3185 [DOI] [PubMed] [Google Scholar]
- Mayr S, Beikircher B, Obkircher MA, Schmid P (2010) Hydraulic plasticity and limitations of alpine Rhododendron species. Oecologia 164: 321–330 [DOI] [PubMed] [Google Scholar]
- McCulloh KA, Woodruff DR (2012) Linking stomatal sensitivity and whole‐tree hydraulic architecture. Tree Physiol 32: 369–372 [DOI] [PubMed] [Google Scholar]
- Miranda JD, Padilla FM, Martinez‐Vilalta J, Pugnaire FI (2010) Woody species of a semi‐arid community are only moderately resistant to cavitation. Funct Plant Biol 37: 828–839 [Google Scholar]
- Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180: 604–611 [DOI] [PubMed] [Google Scholar]
- Nolf M, Pagitz K, Mayr S (2013) Physiological acclimation to drought stress in Solidago canadensis . Physiol Plant 150: 529–539 [DOI] [PubMed] [Google Scholar]
- Ogasa M, Miki NH, Murakami Y, Yoshikawa K (2013) Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiol 33: 335–344 [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Vander Willigen C (1998) A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol 18: 589–593 [DOI] [PubMed] [Google Scholar]
- Patiño S, Tyree MT, Herre EA (1995) A comparison of the hydraulic architecture of woody plants of differing phylogeny and growth form with special reference to free‐standing and hemiepiphytic Ficus species from Panama. New Phytol 129: 125–134 [DOI] [PubMed] [Google Scholar]
- Polatschek A (1999) Flora von Nordtirol, Osttirol und Vorarlberg, Band 2. Tiroler Landesmuseum Ferdinandeum, Naturwissenschaftliche Sammlungen, Innsbruck: [Google Scholar]
- Schenk HJ, Espino S, Goedhart CM, Nordenstahl M, Martinez Cabrera HI, Jones CS (2008) Hydraulic integration and shrub growth form linked across continental aridity gradients. Proc Natl Acad Sci USA 105: 11248–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald O, Seybold S, Philippi G (1993) Die Farn‐ und Blütenpflanzen Baden‐Württembergs, Band 2. Ulmer‐Verlag, Stuttgart: [Google Scholar]
- Sperry JS, Hacke UG (2004) Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. Am J Bot 91: 369–385 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11: 35–40 [Google Scholar]
- Trifilò P, Raimondo F, Lo Gullo MA, Barbera PM, Salleo S, Nardini A (2014) Relax and refill: xylem rehydration prior to hydraulic measurements favours embolism repair in stems and generates artificially low PLC values. Plant Cell Environ 37: 2491–2499 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytol 119: 345–360 [Google Scholar]
- Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and the water relations of plants by the pressure‐bomb technique. J Exp Bot 23: 267–282 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap. Springer, Berlin: [Google Scholar]
- Vilagrosa A, Bellot J, Vallejo R, Gil‐Pelegrín E (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co‐occurring Mediterranean shrubs during an intense drought. J Exp Bot 54: 2015–2024 [DOI] [PubMed] [Google Scholar]
- Vilagrosa A, Morales F, Abadía A, Bellot J, Cochard H, Gil‐Pelegrín E (2010) Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought‐resistant species. Environ Exp Bot 69: 233–242 [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter‐vessel pitting and cavitation in woody Rosaceae and other vesseled plants: a basis for a safety versus efficiency trade‐off in xylem transport. Plant Cell Environ 28: 800–812 [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36: 1938–1949 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH (1978) Hydraulic architecture of some diffuse‐porous trees. Can J Bot 56: 2286–2295 [Google Scholar]
- Zwieniecki MA, Holbrook M (2009) Confronting Maxwell's demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534 [DOI] [PubMed] [Google Scholar]


