Abstract
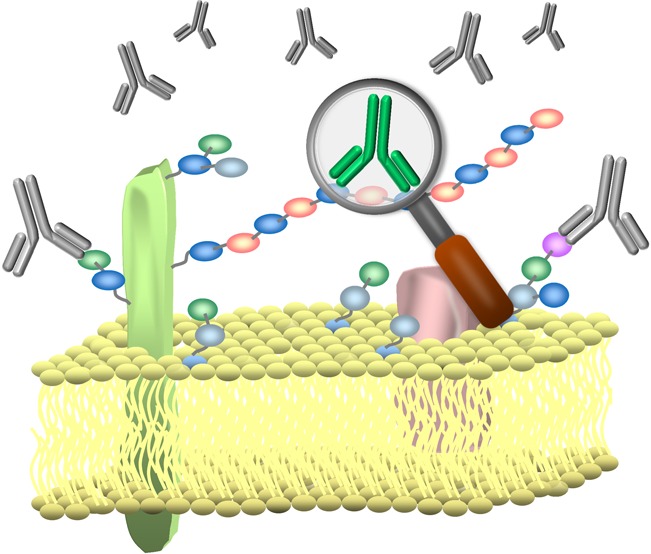
Antibodies are used extensively for a wide range of basic research and clinical applications. While an abundant and diverse collection of antibodies to protein antigens have been developed, good monoclonal antibodies to carbohydrates are much less common. Moreover, it can be difficult to determine if a particular antibody has the appropriate specificity, which antibody is best suited for a given application, and where to obtain that antibody. Herein, we provide an overview of the current state of the field, discuss challenges for selecting and using antiglycan antibodies, and summarize deficiencies in the existing repertoire of antiglycan antibodies. This perspective was enabled by collecting information from publications, databases, and commercial entities and assembling it into a single database, referred to as the Database of Anti-Glycan Reagents (DAGR). DAGR is a publicly available, comprehensive resource for anticarbohydrate antibodies, their applications, availability, and quality.
Monoclonal antibodies have transformed biomedical research and clinical care. In basic research, these proteins are used widely for a myriad of applications, such as monitoring/detecting expression of biomolecules in tissue samples, activating or antagonizing various biological pathways, and purifying antigens. To illustrate the magnitude and importance of the antibody reagent market, one commercial supplier sells over 50 000 unique monoclonal antibody clones. In a clinical setting, antibodies are used frequently as therapeutic agents and for diagnostic applications. As a result, monoclonal antibodies are a multibillion dollar industry, with antibody therapeutics estimated at greater than $40 billion annually, diagnostics at roughly $8 billion annually, and antibody reagents at $2 billion annually as of 2012.1
Carbohydrates are one of the major classes of biomolecules found in living organisms, and antibodies to carbohydrates are useful for many applications. Carbohydrates are critical for numerous biological processes such as cell–cell adhesion, protein folding, protein trafficking, and cell signaling. Moreover, aberrant glycosylation can contribute to a variety of disease states such as cancer and congenital disorders of glycosylation. Antibodies are critical for locating and monitoring expression of carbohydrates and defining their biological roles (Figure 1). Carbohydrates are also valuable targets for diagnostics and therapeutics. Unfortunately, the development and availability of carbohydrate binding monoclonal antibodies lag severely behind that of antiprotein/peptide monoclonal antibodies, in terms of both quantity and quality. In fact, a recent report by the National Academy of Sciences on the current state of glycoscience cited the lack of glycan-specific antibodies as a key barrier for advancing the field.2 Even with the antibodies that are available, it can be difficult to determine if a particular antibody has the appropriate specificity, which antibody is best suited for a given application, and where to obtain that antibody. Although the shortage of antiglycan antibodies and lack of information are generally appreciated by specialists, the true extent of the problem and the needs of the field are unclear.
Figure 1.
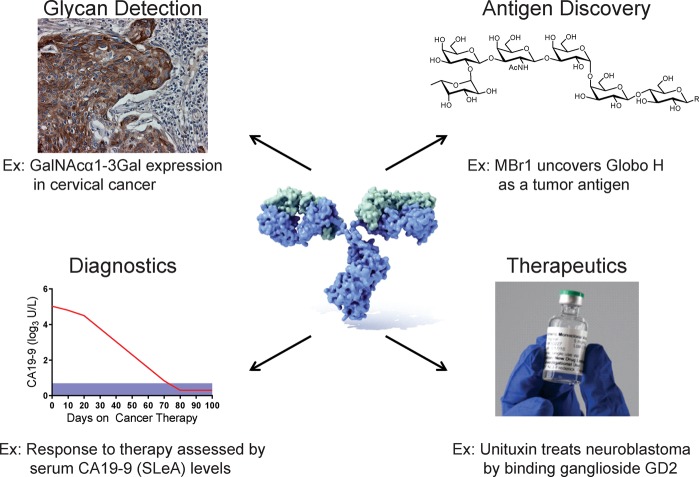
Applications of anticarbohydrate antibodies in research and clinical therapy. Antiglycan antibodies have been used in the detection and discovery of glycoantigens in various tumor samples. Antiglycan antibodies are also used as diagnostic tools (CA19–9 levels in pancreatic cancer patients) and as therapeutics, such as Unituxin (ch14.18) in the treatment of neuroblastoma.
This perspective will provide an overview of the current state of the field of monoclonal antibodies to carbohydrates as well as offer perspective on the immediate and long-term needs. This effort was motivated by the development of a database of carbohydrate-binding reagents, referred to as the Database for Anti-Glycan Reagents (DAGR). The database contains information collected from publications, commercial entities, and other existing databases. It is publicly accessible (https://ccr2.cancer.gov/resources/Cbl/Tools/Antibody/), searchable, and provides opportunities for the community to add information. We anticipate it will become a useful resource for specialists and nonspecialists alike.
Carbohydrates in Nature
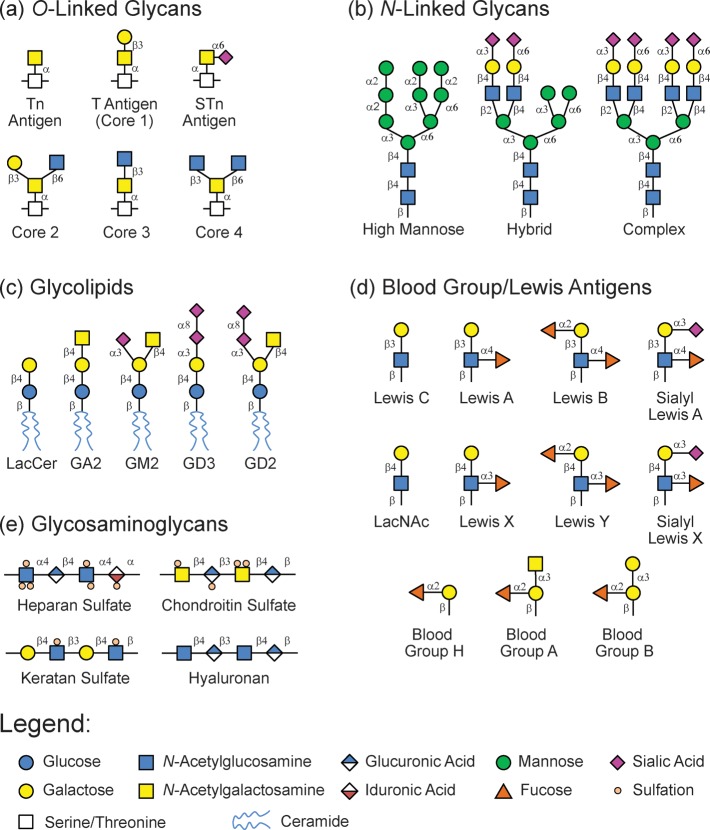
Carbohydrates are composed of monosaccharide residues connected together via glycosidic linkages to produce oligosaccharides and polysaccharides. Although the full repertoire of carbohydrate structures in nature is unknown, substantial diversity exists.3 Monosaccharide building blocks have multiple hydroxyls that can serve as attachment sites, and the glycosidic bond between residues can have either alpha or beta stereochemistry, leading to a wide variety of potential connectivities between two monosaccharide residues. Moreover, individual monosaccharide units can be glycosylated at multiple positions at the same time, leading to branching of the carbohydrate chain. Glycans can be further diversified via postglycosylational modifications such as sulfation, phosphorylation, and acylation.4 Finally, glycans in nature are often attached to other biomacromolecules such as proteins to produce glycoproteins and lipids to produce glycolipids.5,6 The carrier molecule of a particular glycan can influence biological activity and recognition.7−9 Some of the common mammalian glycan biosynthetic families are shown in Figure 2.
Figure 2.
Cartoon representations of the major mammalian biosynthetic carbohydrate families.
Many carbohydrates are large and heterogeneous, containing a variety of subdomains within the full glycan molecule. A particular biological activity or recognition motif frequently resides within a specific subdomain of a carbohydrate, and the portion of a glycan that forms the binding region is referred to as the epitope or glycan determinant. A protein binding pocket can typically accommodate a glycan that is two to six residues long within the longest linear portion.10−12 The linear portion can have branches stemming from the linear backbone. Therefore, glycan determinants have been described as oligosaccharide domains with a longest linear portion that is two to six residues long. It has been estimated that there are over 7000 unique carbohydrate determinants within the mammalian glycome.12 The number of glycan determinants in bacteria, fungi, and plants is unknown.
Carbohydrates play many key roles in biology. Glycans on proteins can have a major impact on properties such as bioactivity, folding, trafficking, stability, and half-life. For example, O-GlcNAcylation, the modification of a protein with a single GlcNAc residue on serine or threonine, occurs on hundreds of intracellular proteins and can regulate protein function in a manner similar to phosphorylation. On the cell surface, glycans can modulate the location and residency times of other proteins and can directly mediate cell–cell interactions. For example, l-selectin on the surface of leukocytes interacts with carbohydrates on endothelial cells to mediate rolling and extravasation from the blood vessel into the surrounding tissue during inflammation. Cell surface glycans are also involved in pathogenic processes, such as adhesion of bacteria to mammalian cells and metastasis. While glycans are essential in many ways, the effects and roles of most carbohydrates are not well understood.
Carbohydrates are extremely difficult to study. Many of the techniques commonly used to identify and monitor proteins are not amenable to carbohydrates. The primary challenge is that carbohydrate structure is not genetically encoded. As a result, one cannot selectively knock-in, knock-out, or mutate a particular glycan determinant in a cell or organism without affecting numerous other glycans. Furthermore, one cannot genetically attach tags, such as green fluorescent protein (GFP), to locate and track a particular glycan. The complexity of carbohydrates, both in structure and presentation, coupled with the fact that carbohydrates are not encoded genomically makes the need for well-characterized antibody reagents particularly acute.
Antibodies to Carbohydrates
Antibodies to carbohydrates are essential for basic research (Figure 1). Due to the difficulties of direct detection of glycans from complex biological samples, the vast majority of studies rely on antibodies to detect and monitor expression of carbohydrate antigens/determinants using techniques such as immunohistochemical staining, Western blotting, and ELISA. Furthermore, antiglycan antibodies are useful for modulating biological processes mediated by glycans, as agonists/antagonists, and for purification of glycans/glycoproteins. Additionally, antibodies are useful for discovering carbohydrates relevant to disease states. For example, antibody MBr1 played a key role in the identification of Globo H as a tumor associated carbohydrate antigen. For these applications, access to high-quality, well-characterized antibodies is vital.
Antibodies to carbohydrates have also found usage in clinical applications. For example, anti-GD2 antibodies are currently part of the standard-of-care in the treatment of neuroblastoma,13−15 with the anti-GD2 antibody Unituxin (ch14.18) being granted FDA approval in March 2015. A number of other antiglycan antibodies are in clinical trials for treating cancer, such as antibodies to GD3, GM2, fucosyl-GM1, and Lewis Y.16−24 Antiglycan antibodies are also used for diagnostic applications. For example, antibodies that target Sialyl Lewis A (CA19.9) are currently used in detection and monitoring of several cancers.25−27
Challenges for Identifying Antiglycan Antibodies
Although there are quite a few antiglycan antibodies that have been published and/or are commercially available, finding a high quality antibody for a particular study can be challenging, especially for nonspecialists. First, companies, individual investigators, and organizations may use different clone names for the same antibody, making it difficult to identify the desired antibody. Second, the nomenclature used to describe the carbohydrate antigen/epitope is highly variable. For example, an antibody to the Lewis Y antigen might be described as binding “Lewis Y,” “LeY,” “Ley,” “blood group Lewis Y,” “Le(Y),” and/or “Fucα1–2Galβ1–4(Fucα1–3)GlcNAc.” In addition, common names for carbohydrate antigens often describe a family of structurally related targets. A prime example is the Sialyl Tn or STn antigen (Figure 3). The term “Sialyl Tn antigen” refers to a structure containing a sialic acid alpha-linked to the 6-position of a GalNAc residue, which is alpha-linked to either a serine or threonine of a polypeptide chain. The sialic acid can be a 5-N-acetylated neuraminic acid (Neu5Ac), a 5-N-glycoylyl neuraminic acid (Neu5Gc), or an O-acetylated variant of Neu5Ac or Neu5Gc. Furthermore, the Sialyl Tn antigen can be found as a single unit or as part of a cluster, wherein there are multiple STn residues in close proximity on a polypeptide chain. Collectively, this produces a family of structures that are all generally referred to as the STn antigen. Taken together, these difficulties highlight the need for a collective database of available options.
Figure 3.
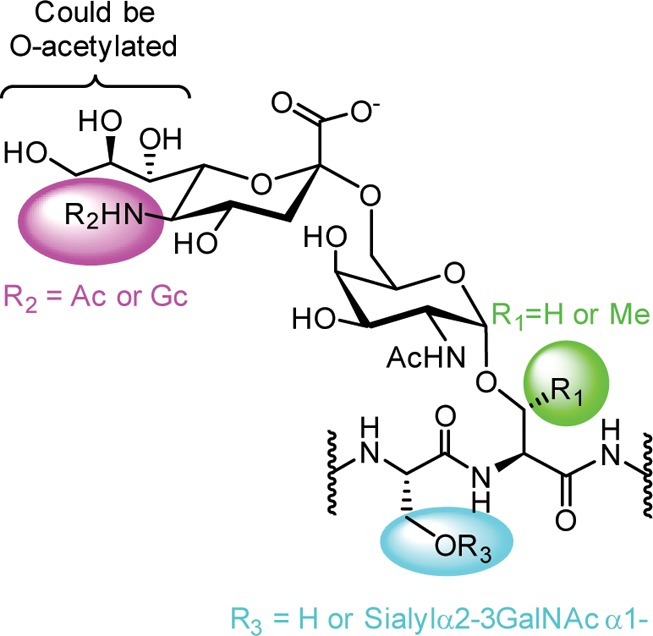
Structural variations of the sialyl Tn (STn) antigen. An antibody targeting the sialyl Tn (STn) antigen may target one of many structurally similar glycans. For example, the nitrogen on the sialic acid residue can be modified with an acetyl group (Ac) or a glycolyl group (Gc). Additionally, O-acetylated variations can occur at numerous hydroxyl positions. The GalNAc residue can be attached to either serine or threonine. Finally, there may be a single STn residue on a peptide chain or there maybe additional STn residues in close proximity on the peptide chain (clustered STn). All of these structures could be described generally as STn.
Last, some antibodies bind mixed epitopes composed of both carbohydrate and the carrier molecule to which the carbohydrate is attached, such as peptide or lipid portions of glycoproteins and glycolipids. For example, antibody SM3 binds Tn glycopeptides and interacts with both peptide and carbohydrate portions.28 Antibodies with mixed epitopes are especially difficult to identify and characterize.
Overview of Antiglycan Antibodies That Have Been Produced
To evaluate the spectrum of antiglycan monoclonal antibodies, information from existing databases (e.g., GlycoEpitope, Consortium for Functional Glycomics), publications, and commercial suppliers was collected and combined into a single database. This database, referred to as the Database for Anti-Glycan Reagents (DAGR), also includes other glycan reagents, such as plant lectins, but this perspective only covers the antibodies. Besides the names and glycan targets of the antibodies, information regarding host species, isotype, and immunogen used to raise the antibody was also collected. Antibodies with mixed epitopes (i.e., antiglycopeptide reagents) and antibodies only suspected of binding carbohydrates were excluded from the DAGR.
All Glycan Binding Antibodies
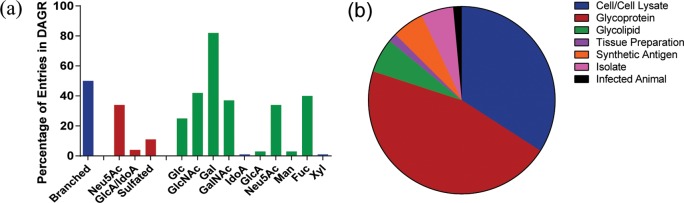
DAGR currently has 1120 unique monoclonal antibody entries (see Table 1). These numbers do not take into account antibody quality/specificity. The majority of antibodies are IgM (∼58%), but a significant number of IgG antibodies (∼40%) have been produced. IgA represent about 1.5% of the antiglycan antibodies. Murine is by far the most common host organism for generating these antibodies, but some human, rat, and rabbit antibodies are also present. For listings in which immunogens were available, antibodies were primarily generated by immunization with natural materials, such as whole cells/cell lysates, tissue/cell preparations, natural glycoproteins, or natural glycolipids/glycolipid fractions (see Figure 4). Some other approaches include immunization with a synthetic antigen,29 isolation from infected animals, and isolation from human patients.
Table 1. Overview of the DAGR.
| DAGR Entries |
isotype
information1 |
host information1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| selected epitope families | total | comm. available | unique epitopes | IgM | IgG | IgA | Mu. | Rat | Hu. | Rab. |
| O-linked | 77 | 16 | 10 | 42 | 25 | 1 | 63 | 2 | 3 | 0 |
| N-linked | 25 | 0 | 8 | 1 | 19 | 0 | 3 | 1 | 15 | 2 |
| glycolipid | 259 | 47 | 80 | 141 | 96 | 0 | 211 | 10 | 20 | 5 |
| blood group | 155 | 77 | 22 | 84 | 36 | 1 | 110 | 1 | 1 | 0 |
| Lewis antigen | 197 | 69 | 31 | 92 | 75 | 0 | 164 | 5 | 0 | 0 |
| glycosaminoglycan | 41 | 23 | 11 | 15 | 17 | 1 | 33 | 1 | 6 | 0 |
| plant glycan | 197 | 172 | 13 | 88 | 82 | 6 | 144 | 32 | 1 | 0 |
| total2 | 1116 | 417 | 247 | 542 | 387 | 12 | 811 | 67 | 66 | 8 |
Information is provided as available. Complete information (isotype, host, immunogen) is not available for all antibodies.
The “total” row represents the whole database, not a summation of hanging columns.
Figure 4.
Graphical representations of the DAGR entries. (a) Antibodies that target branched glycan structures (blue) are well represented in the DAGR. Antibodies targeting sialic acid charged epitopes are well represented; however sulfated or uronic acid containing glycan epitopes are conspicuously underrepresented (red). Common monosaccharides are present in a significant percentage of the epitopes targeted (green). (b) Cell/cell lysate (blue) and glycoprotein (red) immunizations are the preferred means of generating antibodies to glycans. Glycolipid (green), tissue preparation (purple), and synthetic antigen (orange) are other immunizations strategies. Antibodies to glycans have also been prepared as human isolates (pink) and from infected animals (black).
Overall, coverage of glycan diversity space is quite minimal (see Figure 4). Although there are over 1000 antibodies in the database, many of them target the same glycan determinant; as a result, the 1120 antibodies only target 247 different glycan epitopes. Antibodies to most mammalian biosynthetic families are represented, but only a small percentage of determinants in each family have a matching antibody. Of particular note, roughly 70–75% of the ∼7000 determinants in the mammalian glycome are sulfated,12 but only about 10% of the antibodies in the database recognize a sulfated determinant. Moreover, a large number of these are not well-defined in terms of their epitope, such as antibodies that bind heparan sulfate. Given the abundance of sulfated glycans and the importance of sulfation for biological activity, new antibodies in this area could be particularly valuable.
Commercially Available Antibodies
Approximately one-third (417) of the anticarbohydrate antibodies in our database are currently available from commercial entities. For comparison, one online distributor sells 287 unique monoclonal antibodies to the protein tubulin and 269 unique monoclonal antibodies to CD4. Thus, there are more commercially available monoclonal antibodies to these two proteins than all the commercially available antibodies to carbohydrates combined. Antibody isotype proportions, host organism usage, and immunogen family usage are comparable for commercial antibodies and the full group. As described in more detail below, commercial antibodies cover a much narrower set of glycan families and epitopes.
Antibodies to O-glycans
O-linked glycans (Figure 2a) are defined as glycans that are attached via an oxygen to a polypeptide chain. The attachment site is frequently the side chain of serine or threonine, but it can also be tyrosine, hydroxylysine, or hydroxyproline.30−32 The most abundant type of O-linked glycosylation in mammals involves attachment of a GalNAc residue to the side chain of a serine or threonine of a polypeptide chain. The GalNAc residue is typically modified further to produce eight core disaccharide or trisaccharide units, referred to as cores 1–8, which can be extended in a variety of ways to produce a complex assortment of O-glycans.33 In cancerous tissues, O-glycans are often truncated, resulting in a variety of tumor associated carbohydrate antigens such as the Tn antigen (GalNAcα1-Ser/Thr), STn, (Siaα2–3GalNAcα1-Ser/Thr), and the TF antigen (Galβ1–3GalNAcα1-Ser/Thr).34 Other O-linked modifications include O-GlcNAc, O-xylose, O-fucose, O-glucose, and O-mannose.35−39 Collectively, there are estimated to be around 750 O-glycan determinants.12
Our database has 77 entries that are defined under the family of antibodies to O-linked carbohydrates, 16 of which are commercially available. However, these antibodies target an extremely small set of unique epitopes. Fifty-nine of 77 total antibodies, as well as 14 of the 16 commercially available, target Tn, sialyl Tn, or TF. Though these three glycans are relevant in numerous disease states, antibodies to these glycans are overrepresented.
Many important O-glycan structures lack a corresponding antibody. For example, the majority of the eight core glycans that are connected to serine/threonine via GalNAc do not have a corresponding antibody. This is especially surprising considering previous observations of core 2-type glycan overexpression and core 3 and core 4 glycan suppression on tumors.40−44 Additionally, there are very few antibodies to other types of O-linked glycans, such as O-fucose, O-xylose, and O-glucose. Furthermore, linkage to serine versus threonine can have a significant effect on conformational preferences and flexibility of the O-glycan, and the attachment site may influence biological activity. Antibodies that discriminate based on the attachment amino acid residue might help to address this question.
Antibodies to N-glycans
The N-linked family (Figure 2b) contains glycans attached to a protein/peptide via a nitrogen atom. N-linked glycans occur predominantly on the nitrogen atom of the asparagine side chain as part of an Asn-X-Ser/Thr- tripeptide; however, glycans attached to arginine via N-glycosidic bonds are known.45N-linked glycans are highly influential post-translational modifications in glycobiology, especially with regard to the structure and function of glycoproteins. N-linked glycans are generally grouped into three families: high mannose, complex, and hybrid (see Figure 2b). All three are usually composed of a branched pentasaccharide core, [Manα1–6(Manα1–3)Manβ1–4GlcNAcβ1–4GlcNAcβ1−], referred to as Man3. The high mannose family of glycans has varying numbers of additional mannose residues attached to the terminal Manα1–6 and Manα1–3 residues of Man3. Complex N-glycans have additional GlcNAc residues attached to the terminal Manα1–6 and Manα1–3 residues of Man3. These residues can be modified further with a variety of other glycans, such as LacNAc repeats and sialylation. Hybrid N-glycans have a mixture of mannose and GlcNAc residues, resulting in one or more branches with high mannose type glycans and one or more branches with complex type glycans. Finally, the chitobiose core (4GlcNAcβ1–4GlcNAcβ1−) of N-glycans can be modified by fucosylation. Collectively, there are estimated to be around 2000 N-glycan determinants in the mammalian glycome.12
Despite their importance in biology, antibodies to N-linked glycans are rare. Our database has only 25 entries that are defined as antibodies to N-linked glycans. Fifteen of these antibodies were obtained by isolation from HIV patients.46−48 None of these entries are commercially available, but several of them are available through consortia, such as the National Institutes of Health AIDS Reagent program. Along with the low numbers of antibodies, specificity is also an issue. Most of these antibodies recognize families of structurally related glycans rather than a single distinct N-glycan. For example, a number of these antibodies recognize multiple high mannose glycans, including Man7, Man8, and Man9.
The lack of antibodies to N-linked glycans and the specificity problems are not entirely surprising. N-glycans are naturally present in standard host species used to produce antibodies. Thus, they have very low immunogenicity. Moreover, many N-linked glycans are highly homologous in structure, making selective recognition a challenging problem. Given the dearth of antibodies to N-glycans and their fundamental importance in biology, development of new and better antibodies to these glycans would be very useful.
Antibodies to Glycolipids (including Glycosphingolipids)
Glycolipids (Figure 2c) are composed of carbohydrates that are glycosidically linked to a ceramide tail that is buried within the lipid membrane of the cell. Glycolipids are found ubiquitously throughout mammalian and nonmammalian systems and are involved in many biological processes, such as cell–cell interactions.49,50 The glycan core structure is predominantly a disaccharide with the sequence Galβ1–4Glcβ–. From this core, significant structural diversity is generated by variations in glycosidic linkages, saccharide composition, and branching. There are estimated to be approximately 500 glycolipids in the mammalian glycome.12
Antibodies to glycolipids are one of the more abundant and readily accessible families of antiglycan antibodies. The DAGR lists 259 antibodies directed against the glycan determinants of glycolipids, representing nearly 27% of listings. These antibodies target 80 unique glycan epitopes, a substantially greater number than any other glycan family. Antibodies to GD1b (19 entries), GD2 (20 entries), and GD3 (24 entries) are especially popular given the significance of these glycans in disease states, such as neuroblastoma and melanoma. Antibodies to GM1 and its variants, such as asialo- or fucosyl-GM1, are also well represented (28 combined entries). Of the 259 antibodies, 47 are commercially available targeting about 30 unique epitopes.
While there are good antibodies to glycolipids, there is considerable room for new additions in this area. First, antibodies to glycolipids often bind families of related structures, rather than a single distinct glycolipid structure. Therefore, antibodies with improved selectivity could be useful. Second, most glycolipids do not have a corresponding antibody. Of particular scarcity are antibodies that target unique sialic acid variants of glycolipids such as 5- or 9-Gc modified neuraminic acid, O-acetylated variants of sialic acids, and variants that contain 2-keto-3-deoxynonic acid (KDN) rather than neuraminic acid. Given their significance in disease states and fundamental biological processes,51,52 development of new antibodies to glycolipids will continue to be a high priority for the foreseeable future.
Antibodies to Lewis and Blood Group Antigens
Lewis and blood group antigens are a family of glycan determinants that are often found at the terminal, outermost end of a glycan chain (see Figure 2d). These capping motifs can be found on N-linked glycans, O-linked glycans, and glycolipids.
Lewis Antigens
The Lewis antigens are a carbohydrate blood group system found on the surface of red blood cells, cells of epithelial origin, and some secreted glycoproteins.53 Lewis antigens are involved in various biological processes and have altered expression in several disease states, such as cancer.54,55 The biosynthetic precursor for type 1 Lewis antigens is the type 1 chain or Lewis C (LeC; Galβ1–3GlcNAcβ). The addition of fucose residues and/or sialic acid (see Figure 2d) can produce Lewis A (LeA), Lewis B (LeB), and sialyl Lewis A (SLeA). The biosynthetic precursor for type 2 Lewis antigens is the type 2 chain, LacNAc (Galβ1–4GlcNAcβ). The addition of fucose residues and/or sialic acid can produce Lewis X (LeX), Lewis Y (LeY), and sialyl Lewis X (SLeA). Lewis antigens can also be sulfated.
There are currently 197 antibodies to Lewis antigens in our database, 69 of which are commercially available. However, these antibodies target less than 30 unique epitopes. For some Lewis antigens, there are ample antibodies for the same target; this includes Lewis A (19 entries), Lewis B (8 entries), Lewis X (42 entries), and Lewis Y (22 entries). Another 15 antibodies bind to a combination of these antigens. Antibodies to certain sialylated and sulfated Lewis antigens are also available in abundance, with 30 antibodies to sialyl-Lewis A, eight antibodies to sialyl-Lewis X, five antibodies to sulfo sialyl-Lewis X, and three antibodies to sialyl-Lewis C; an additional five antibodies bind a combination of sialyl-Lewis A and sialyl-Lewis C.
ABH Blood Group Antigens
The ABH blood group antigens are the glycans that define ABO blood types in humans. They are found on the surface of a variety of cell types and on secreted glycoproteins, but they are most well-known for their abundant expression on red blood cells. The precursor carbohydrate in this group is blood group H (BG-H; Fucα1–2Gal). Individuals of blood type A have an α1,3N-acetylgalactosaminyltransferase that will attach a GalNAc residue to the 3 position of Gal to produce the blood group A antigen [BG-A; GalNAcα1–3(Fucα1–2)Gal]. Individuals of blood type B express an α1,3galactosyltransferase that will attach a Gal residue to the 3 position of Gal to produce the blood group B antigen [BG-B; Galα1–3(Fucα1–2)Gal]. Type AB individuals express both transferases and can produce both BG-A and BG-B. As discussed in more detail later, BG-A, BG-B, and BG-H can be attached to a variety of glycan carrier chains.
Antibodies to ABH blood group antigens are indispensable tools in clinical, diagnostic, and basic research applications. For example, antibodies to BG-A and BG-B are used frequently for blood typing. As a result, blood group antibodies are some of the most accessible and well-characterized antiglycan antibodies produced to date. Currently, there are 155 antibodies to blood group antigens in the database; 77 of these antibodies are commercially available. To illustrate the abundance of ABH antibodies, there are 72 antibodies to BG-A, and 29 of these are commercially available. Thus, there are more antibodies to BG-A in the database than to N-linked glycans and glycosaminoglycans combined.
Antibodies to Glycosaminoglycans
The glycosaminoglycan family of carbohydrates (Figure 2e) is comprised of long, unbranched polysaccharides that possess repeating disaccharide subunits. A large degree of heterogeneity is introduced into these repeats through variations in sulfation patterns and uronic acid epimerization.56 These structures are categorized into four groups based on disaccharide repeats: heparin/heparan sulfate, chondroitin/dermatan sulfate, keratan sulfate, and hyaluronan. Heparan sulfate is important in numerous physiological functions including blood coagulation processes, inflammatory processes, cell growth and differentiation, and cell–cell interactions.57,58 Chondroitin and keratan sulfates are typically isolated from cartilage and have been shown to interact with some glycosaminoglycan binding proteins.59−63 Hyaluronan is a nonsulfated, extracellular matrix glycosaminoglycan that interacts with several matrix and cell surface hyaluronan binding proteins.64,65 It is estimated that nearly half of the mammalian glycan determinants are glycosaminoglycans.12
Despite the importance of glycosaminoglycans in numerous signaling pathways, antibodies to these structures are underrepresented in both developed and commercially available entities. Antibodies to glycosaminoglycans represent less than 4% of the DAGR database, covering a tiny fraction of glycosaminoglycan determinants. Along with low availability, selectivity is also a serious concern. Nearly all antibodies to glycosaminoglycans in the database are defined broadly, such as antiheparan sulfate or antichondroitin sulfate. The exact glycan sequences recognized by these antibodies are typically not known. This lack of information is a major concern, given that there are numerous structural possibilities. For example, there are 64 structures that could be described as a keratan sulfate-pentasaccharide, nearly 1000 structures described as a chondroitin sulfate-pentasaccharide, and over 2900 structures described as a heparan sulfate-pentasaccharide.
One key difficulty in developing quality antibodies to glycosaminoglycans is limited access to pure, structurally defined oligosaccharides for antibody generation and characterization. Isolation from natural sources is extremely challenging. Chemical or chemoenzymatic syntheses of defined oligosaccharides are improving66−70 but are still generally slow and costly. Regardless, glycosaminoglycans have considerable importance in physiology and new antibodies to defined structures would be extremely useful for unraveling the glycosaminoglycan interactome.
Antibodies to Nonmammalian Glycans
Beyond the mammalian biosynthetic families, there are numerous other types of glycans including bacterial, fungal, algal, and plant glycans. For example, bacteria produce a variety of polysaccharides, such as peptidoglycan, lipopolysaccharides, capsules, and exopolysaccharides. Plants also produce an assortment of polysaccharides, including cellulose, hemicelluloses, pectins, β-glucans, gums, and starches. There are approximately 200 antibodies that target nonhuman glycan determinants. The vast majority of these recognize plant glycans. There are approximately 170 antibodies in this group that are available through commercial sources or other organizations. In particular, over 150 of these antibodies are available through CarboSource, a DOE sponsored resource for plant science reagents. Though quite useful, the exact recognition sequences for most of these antibodies are not well-defined. Beyond antibodies to plant glycans, there are very few antibodies to glycans from other nonmammalian organisms, such as bacteria, fungi, and algae. Thus, there are countless opportunities for the development of new antibodies in this area.
Challenges for Selecting an Antiglycan Antibody
Once a researcher has located one or more antibodies to their target of interest, the next step is determining which, if any, antibody is suitable for their experiment. Antibody performance and quality are critical when selecting an appropriate antibody for a given application and interpreting results. Besides general factors such as appropriate host animal and isotype, specificity is a fundamental consideration. Assessing the specificity of an antiglycan antibody presents unique challenges, especially for nonspecialists. Information may be lacking, hard to find, or difficult to interpret. Additionally, different suppliers of the same antibody may have different perceptions and information regarding the applicability of an antibody toward a particular experimental protocol. Below, several key considerations are discussed in more detail.
Multivalent Binding
Multivalent complex formation (Figure 5a) is imperative for many carbohydrate-binding antibodies.71,72 Interactions between a single binding site and a single glycan determinant (Figure 5b) are usually weak, with equilibrium dissociation constants (KD) in the micromolar to low millimolar range. To achieve tight binding, antibodies engage two or more of their binding sites with two or more glycan determinants to form a multivalent complex. Multivalent binding interactions can have much higher functional affinity (avidity) and enhanced or even altered specificity relative to the monovalent binding interaction.73,74
Figure 5.
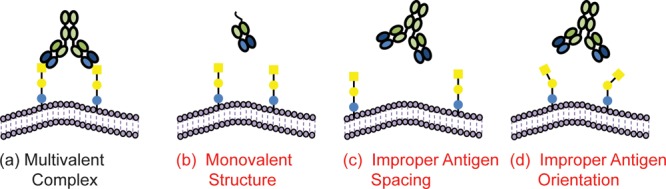
Multivalency influence on binding affinity and specificity. For many antiglycan antibodies, formation of a multivalent complex (a) is required for a high avidity interaction. Antibody formats with a single binding site (b) often have poor affinity and/or specificity. If the target epitope is present with the wrong spacing (c) or orientation (d) to form a multivalent complex, an antiglycan antibody may not bind the sample of interest. These factors must be considered when selecting an antibody and interpreting results.
To form a multivalent complex, the spacing and orientation of the glycans must be matched with the spacing and orientation of the binding sites (Figure 5c,d). The requirement for appropriate geometry imposes additional restrictions on binding, a factor that must be considered when evaluating specificity. Antibodies can bind a glycan presented in certain contexts but not others. For example, some monoclonal antibodies will bind glycans at both high and low density equally well, while other antibodies will only bind high density glycans.75 Thus, depending on the presentation, an antibody that binds a particular glycan may not always bind that glycan.
Methods to Evaluate Specificity
There are a variety of techniques used for evaluating specificity. One approach is to evaluate binding to panels of cells and/or glycoproteins. For example, recognition of ABH blood group antigens is often evaluated by comparing binding of red blood cells from type A, type B, and/or type O donors. As another example, specificity for sialyl Tn or Tn has often been studied using natural glycoproteins with well-defined glycan composition, such as ovine submaxillary mucin and its desialylated counterpart, asialo-ovine submaxillary mucin. The advantage of this approach is that it investigates recognition of glycans in a natural context. In most cases, however, suitable panels of cells and glycoproteins are not available.
A complementary strategy involves evaluating binding with structurally defined, homogeneous glycans. One common approach is monosaccharide/oligosaccharide inhibition studies. While relatively easy to implement, many inhibition studies use soluble monovalent ligands, which may not provide information that is relevant for multivalent binding events. A second approach is to assess binding to a multivalent surface using techniques such as ELISA, SPR, and glycan microarrays. Of these methods, glycan microarrays, offer the highest throughput.76 They are composed of numerous glycan determinants, or fragments of determinants, immobilized in a spatially defined arrangement. Binding to all the carbohydrates can be assessed in parallel while using miniscule amounts of each carbohydrate. When using multivalent surfaces, features of presentation such as glycan density, linker length and flexibility, and glycan accessibility can have a significant effect on recognition.
Antibody Specificity
In general, information about specificity is still fairly limited for antiglycan antibodies. Many of the antibodies that are reported to be specific for a single glycan determinant can bind other glycans. For example, in a study of 27 commercially available antiglycan antibodies, about half of them bound at least one determinant other than the listed glycan.77 With the growing use of glycan microarrays for routine profiling of antibodies, information about specificity, especially at the molecular level, is improving significantly. To facilitate evaluation of specificity, DAGR includes references to papers that analyze specificity of monoclonal antibodies and web links to glycan array data.
As researchers learn more about antibody recognition, it is becoming apparent that traditional descriptions of specificity are usually incomplete. Many of the carbohydrate sequences/structures that are well-known in the literature and referred to as “antigens” or “determinants” are motifs that describe only part of the full determinant. The blood group A antigen is a prime example. BG-A is defined as a trisaccharide with the sequence GalNAcα1–3(Fucα1–2)Galβ. It can be attached to the nonreducing end of a carbohydrate chain in six different ways to produce six different BG-A tetrasacharides (e.g., BG-A1 through BG-A6). The type of attachment can have a substantial impact on recognition by antibodies. For example, some BG-A antibodies will bind BG-A2 [GalNAcα1–3(Fucα1–2)Galβ1–4GlcNAcβ−] but not BG-A3 [GalNAcα1–3(Fucα1–2)Galβ1–3GalNAcα1−].75,77,78 Thus, defining antibodies as BG-A binders does not fully designate target specificity. Moreover, type 1–6 only refers to the primary attachment residue. The full glycan determinant may extend beyond the initial attachment site. For example, within the proposed list of determinants in the mammalian glycome, ∼150 different determinants carry the BG-A trisaccharide as the nonreducing terminal trisaccharide. While ABH antibodies are some of the best characterized antibodies in the field, these antibodies have only been tested with a small fraction of the glycan determinants that carry the target sequences/motif. Therefore, more comprehensive analyses of specificity are needed, even for the most extensively studied antiglycan antibodies.
Database for Anti-Glycan Reagents (DAGR)
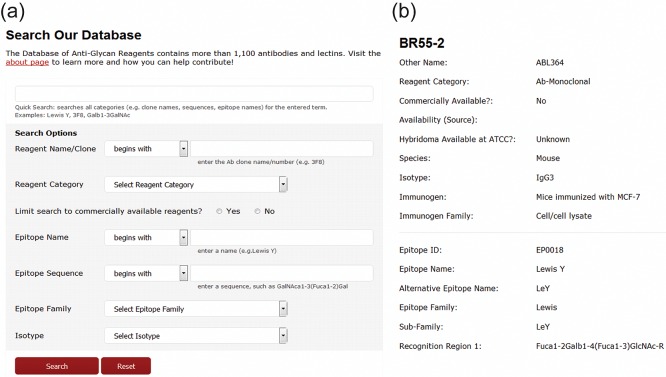
The Database for Anti-Glycan Reagents is designed as a comprehensive resource for specialists and nonspecialist of glycobiology (Figure 6). Within the database, we attempted to provide as much information as possible, including host, immunogen, isotype, biochemical applications, and commercial availability. Citations to key references regarding development and use of the antibodies are also incorporated. The DAGR database was designed with the user in mind and the understanding that carbohydrate nomenclature can be very confusing. As such, we have attempted to be as accommodating as possible with the Website search algorithms. We have included different common names and abbreviations for a given epitope or determinant to account for the variations in nomenclature usage. Individuals also have the opportunity to search based on commercial availability. Finally, where possible, glycan array data have been listed with antibodies. Currently, about 20% of the antibodies have a link or reference to glycan array data.
Figure 6.
Snapshots of the Database for Anti-Glycan Reagents (DAGR) interface. (a) The advanced search option allows the user to search based upon a variety of criteria such as clone name, epitope name, sequence or family, and commercial availability. (b) Following the selection of a particular reagent, available information on immunogen, host species, and hybridoma availability are provided. References to significant literature in antibody production (not shown) are also provided.
Although the current database has considerable content, new antibodies are continually produced, and some previously produced antibodies are likely to have been inadvertently missed. To enable expansion and improvement of the database, researchers can submit new antibody reagents and new information for existing antibodies to the database through the Website. Additional citations and general comments/advice can also be submitted.
Concluding Remarks
Glycobiology is a challenging field for experts and a daunting one for nonspecialists. Access to high quality antibodies is essential for advancing the field and exploiting the potential of glycans as therapeutic and diagnostic targets. In addition, improved access to antibodies is a crucial step for expanding the number of researchers willing and able to study glycobiology. Our hope is that this perspective will provide encouragement for developing new and better antiglycan antibodies. Furthermore, we anticipate that the Database of Anti-Glycan Reagents (DAGR) developed in conjunction with this perspective will enable researchers from any discipline to more readily find antibodies and information about those antibodies. As such, we anticipate that all members of the research community find the DAGR resource beneficial to their current and future research.
Acknowledgments
We would like to thank W. Shea Wright and Matthew Gildersleeve for assisting in collecting data for the database. We also would like to thank Nancy Brandt, Ben Shorb, and Archana Shrestha for developing the Database of Anti-Glycan Reagents website. This work was supported by the Intramural Research Program of the National Cancer Institute, Nationl Institutes of Health.
The authors declare no competing financial interest.
References
- (2012) Biologic drugs set to top 2012 sales, Nat. Med. 18, 636. 10.1038/nm0512-636a [DOI] [PubMed] [Google Scholar]
- (2012) In Transforming Glycoscience: A Roadmap for the Future, National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Bertozzi C. R., and Rabuka D. (2009) Structural Basis of Glycan Diversity, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., and Etzler M. E., Eds.), 2nd ed., Cold Spring Harbor, New York. [PubMed] [Google Scholar]
- Muthana S. M.; Campbell C. T.; Gildersleeve J. C. (2012) Modifications of glycans: biological significance and therapeutic opportunities. ACS Chem. Biol. 7, 31–43. 10.1021/cb2004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T.; Childs R. A. (1985) Carbohydrate structures of glycoproteins and glycolipids as differentiation antigens, tumor-associated antigens and components of receptor systems. Trends Biochem. Sci. 10, 24–29. 10.1016/0968-0004(85)90012-X. [DOI] [Google Scholar]
- Bush C. A.; Martin-Pastor M.; Imbery A. (1999) Structure and conformation of complex carbohydrates of glycoproteins, glycolipids, and bacterial polysaccharides. Annu. Rev. Biophys. Biomol. Struct. 28, 269–293. 10.1146/annurev.biophys.28.1.269. [DOI] [PubMed] [Google Scholar]
- Borgert A.; Heimburg-Molinaro J.; Song X. Z.; Lasanajak Y.; Ju T. Z.; Liu M.; Thompson P.; Ragupathi G.; Barany G.; Smith D. F.; Cummings R. D.; Live D. (2012) Deciphering structural elements of mucin glycoprotein recognition. ACS Chem. Biol. 7, 1031–1039. 10.1021/cb300076s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyabu N.; Hinou H.; Matsushita T.; Izumi R.; Shimizu H.; Kawamoto K.; Numata Y.; Togame H.; Takemoto H.; Kondo H.; Nishimura S. I. (2009) An essential epitope of anti-MUC1 monoclonal antibody KL-6 revealed by focused glycopeptide library. J. Am. Chem. Soc. 131, 17102–17109. 10.1021/ja903361f. [DOI] [PubMed] [Google Scholar]
- Wandall H. H.; Blixt O.; Tarp M. A.; Pedersen J. W.; Bennett E. P.; Mandel U.; Ragupathi G.; Livingston P. O.; Hollingsworth M. A.; Taylor-Papadimitriou J.; Burchell J.; Clausen H. (2010) Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 70, 1306–1313. 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A.; Bezer A. E. (1958) The effect of variation in molecular weight on the antigenicity of dextran in man. Arch. Biochem. Biophys. 78, 306–318. 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. (1954) Some configurational requirements and dimensions of the combining site on an antibody to a naturally occurring antigen. J. Am. Chem. Soc. 76, 3709–3713. 10.1021/ja01643a034. [DOI] [Google Scholar]
- Cummings R. D. (2009) The repertoire of glycan determinants in the human glycome. Mol. BioSyst. 5, 1087–1104. 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- Cheung N. K. V.; Cheung I. Y.; Kramer K.; Modak S.; Kuk D.; Pandit-Taskar N.; Chamberlain E.; Ostrovnaya I.; Kushner B. H. (2014) Key role for myeloid cells: phase II results of anti-GD2 antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int. J. Cancer 135, 2199–2205. 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N. K.; Kushner B. H.; Yeh S. D.; Larson S. M. (1998) 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: a phase II study. Int. J. Oncol. 12, 1299–1306. 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- Yu A. L.; Gilman A. L.; Ozkaynak M. F.; London W. B.; Kreissman S. G.; Chen H. X.; Smith M.; Anderson B.; Villablanca J. G.; Matthay K. K.; Shimada H.; Grupp S. A.; Seeger R.; Reynolds C. P.; Buxton A.; Reisfeld R. A.; Gillies S. D.; Cohn S. L.; Maris J. M.; Sondel P. M. (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334. 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N.; Mintzer D.; Cordoncardo C.; Welt S.; Fliegel B.; Vadhan S.; Carswell E.; Melamed M. R.; Oettgen H. F.; Old L. J. (1985) Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase-I trial in patients with malignant-melanoma. Proc. Natl. Acad. Sci. U. S. A. 82, 1242–1246. 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. M.; Lee F. T.; Hopkins W.; Cebon J. S.; Wheatley J. M.; Liu Z. Q.; Smyth F. E.; Murone C.; Sturrock S.; MacGregor D.; Hanai N.; Inoue K.; Yamasaki M.; Brechbiel M. W.; Davis I. D.; Murphy R.; Hannah A.; Lim-Joon M.; Chan T.; Chong G.; Ritter G.; Hoffman E. W.; Burgess A. W.; Old L. J. (2001) Specific targeting, biodistribution, and lack of immunogenicity of chimeric anti-GD3 monoclonal antibody KM871 in patients with metastatic melanoma: results of a phase I trial. J. Clin. Oncol. 19, 3976–3987. [DOI] [PubMed] [Google Scholar]
- Livingston P. O.; Hood C.; Krug L. M.; Warren N.; Kris M. G.; Brezicka T.; Ragupathi G. (2005) Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol. Immunother. 54, 1018–1025. 10.1007/s00262-005-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezicka F. T.; Olling S.; Nilsson O.; Bergh J.; Holmgren J.; Sorenson S.; Yngvason F.; Lindholm L. (1989) Immunohistological detection of fucosyl-GM1 ganglioside in human-lung cancer and normal-tissues with monoclonal-antibodies. Cancer Res. 49, 1300–1305. [PubMed] [Google Scholar]
- Nilsson O.; Brezicka F. T.; Holmgren J.; Sorenson S.; Svennerholm L.; Yngvason F.; Lindholm L. (1986) Detection of a ganglioside antigen associated with small-cell lung carcinomas using monoclonal-antibodies directed against fucosyl-GM1. Cancer Res. 46, 1403–1407. [PubMed] [Google Scholar]
- Scott A. M.; Tebbutt N.; Lee F. T.; Cavicchiolo T.; Liu Z. Q.; Gill S.; Poon A. M. T.; Hopkins W.; Smyth F. E.; Murone C.; MacGregor D.; Papenfuss A. T.; Chappell B.; Saunder T. H.; Brechbiel M. W.; Davis I. D.; Murphy R.; Chong G.; Hoffman E. W.; Old L. J. (2007) A phase I biodistribution and pharmacokinetic trial of humanized monoclonal antibody Hu3s193 in patients with advanced epithelial cancers that express the Lewis-Y antigen. Clin. Cancer Res. 13, 3286–3292. 10.1158/1078-0432.CCR-07-0284. [DOI] [PubMed] [Google Scholar]
- Posey J. A.; Khazaeli M. B.; Bookman M. A.; Nowrouzi A.; Grizzle W. E.; Thornton J.; Carey D. E.; Lorenz J. M.; Sing A. P.; Siegall C. B.; LoBuglio A. F.; Saleh M. N. (2002) A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin. Cancer Res. 8, 3092–3099. [PubMed] [Google Scholar]
- Pai L. H.; Wittes R.; Setser A.; Willingham M. C.; Pastan I. (1996) Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat. Med. 2, 350–353. 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- Ross H. J.; Hart L. L.; Swanson P. M.; Rarick M. U.; Figlin R. A.; Jacobs A. D.; McCune D. E.; Rosenberg A. H.; Baron A. D.; Grove L. E.; Thorn M. D.; Miller D. M.; Drachman J. G.; Rudin C. M. (2006) A randomized, multicenter study to determine the safety and efficacy of the immunoconjugate SGN-15 plus docetaxel for the treatment of non-small cell lung carcinoma. Lung Cancer 54, 69–77. 10.1016/j.lungcan.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeck S.; Haas M.; Laubender R. P.; Kullmann F.; Klose C.; Bruns C. J.; Wilkowski R.; Stieber P.; Holdenrieder S.; Buchner H.; Mansmann U.; Heinemann V. (2010) Application of a time-varying covariate model to the analysis of CA 19–9 as serum biomarker in patients with advanced pancreatic cancer. Clin. Cancer Res. 16, 986–994. 10.1158/1078-0432.CCR-09-2205. [DOI] [PubMed] [Google Scholar]
- Hess V.; Glimelius B.; Grawe P.; Dietrich D.; Bodoky G.; Ruhstaller T.; Bajetta E.; Saletti P.; Figer A.; Scheithauer W.; Herrmann R. (2008) CA 19–9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 9, 132–138. 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- Sawada R.; Sun S. M.; Wu X.; Hong F.; Ragupathi G.; Livingston P. O.; Scholz W. W. (2011) Human monoclonal antibodies to sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor activity. Clin. Cancer Res. 17, 1024–1032. 10.1158/1078-0432.CCR-10-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Saez N.; Castro-Lopez J.; Valero-Gonzalez J.; Madariaga D.; Companon I.; Somovilla V. J.; Salvado M.; Asensio J. L.; Jimenez-Barbero J.; Avenoza A.; Busto J. H.; Bernardes G. J.; Peregrina J. M.; Hurtado-Guerrero R.; Corzana F. (2015) Deciphering the non-equivalence of serine and threonine O-glycosylation points: Implications for molecular recognition of the Tn antigen by an anti-MUC1 antibody. Angew. Chem., Int. Ed. 54, 9830–9834. 10.1002/anie.201502813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broecker F.; Anish C.; Seeberger P. H. (2015) Generation of Monoclonal Antibodies against Defined Oligosaccharide Antigens. Methods Mol. Biol. 1331, 57–80. 10.1007/978-1-4939-2874-3_5. [DOI] [PubMed] [Google Scholar]
- Zarschler K.; Janesch B.; Pabst M.; Altmann F.; Messner P.; Schaffer C. (2010) Protein tyrosine O-glycosylation - a rather unexplored prokaryotic glycosylation system. Glycobiology 20, 787–798. 10.1093/glycob/cwq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsson E.; Malmstrom V.; Reis S.; Engstrom A.; Burkhardt H.; Holmdahl R. (1994) T cell recognition of carbohydrates on type II collagen. J. Exp. Med. 180, 745–749. 10.1084/jem.180.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M. J.; O’Neill M.; Leykam J.; Orlando R. (1995) Tandem mass spectrometry and structural elucidation of glycopeptides from a hydroxyproline-rich plant cell wall glycoprotein indicate that contiguous hydroxyproline residues are the major sites of hydroxyproline O-arabinosylation. J. Biol. Chem. 270, 2541–2549. 10.1074/jbc.270.6.2541. [DOI] [PubMed] [Google Scholar]
- Marino K.; Bones J.; Kattla J. J.; Rudd P. M. (2010) A systematic approach to protein glycosylation analysis: a path through the maze. Nat. Chem. Biol. 6, 713–723. 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P.; Dabelsteen S.; Madsen F. B.; Francavilla C.; Kopp K. L.; Steentoft C.; Vakhrushev S. Y.; Olsen J. V.; Hansen L.; Bennett E. P.; Woetmann A.; Yin G.; Chen L.; Song H.; Bak M.; Hlady R. A.; Peters S. L.; Opavsky R.; Thode C.; Qvortrup K.; Schjoldager K. T.; Clausen H.; Hollingsworth M. A.; Wandall H. H. (2014) Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. U. S. A. 111, E4066–4075. 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M.; Laremore T. N.; Linhardt R. J. (2010) Proteoglycomics: recent progress and future challenges. OMICS 14, 389–399. 10.1089/omi.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney D. J.; Lin A. I.; Haltiwanger R. S. (1997) The O-linked fucose glycosylation pathway. Evidence for protein-specific elongation of O-linked fucose in Chinese hamster ovary cells. J. Biol. Chem. 272, 19046–19050. 10.1074/jbc.272.30.19046. [DOI] [PubMed] [Google Scholar]
- Shao L.; Luo Y.; Moloney D. J.; Haltiwanger R. S. (2002) O-Glycosylation of EGF repeats: identification and initial characterization of a UDP-glucose: protein O-glucosyltransferase. Glycobiology 12, 763–770. 10.1093/glycob/cwf085. [DOI] [PubMed] [Google Scholar]
- De Groot P. W.; Ram A. F.; Klis F. M. (2005) Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 42, 657–675. 10.1016/j.fgb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Strahl-Bolsinger S.; Gentzsch M.; Tanner W. (1999) Protein O-mannosylation. Biochim. Biophys. Acta, Gen. Subj. 1426, 297–307. 10.1016/S0304-4165(98)00131-7. [DOI] [PubMed] [Google Scholar]
- Tsuboi S.; Fukuda M. (2001) Roles of O-linked oligosaccharides in immune responses. BioEssays 23, 46–53. . [DOI] [PubMed] [Google Scholar]
- Tsuboi S.; Sutoh M.; Hatakeyama S.; Hiraoka N.; Habuchi T.; Horikawa Y.; Hashimoto Y.; Yoneyama T.; Mori K.; Koie T.; Nakamura T.; Saitoh H.; Yamaya K.; Funyu T.; Fukuda M.; Ohyama C. (2011) A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 30, 3173–3185. 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur F.; Dole K.; Yang J.; Matta K. L.; Myerscough N.; Corfield A.; Paraskeva C.; Brockhausen I. (1994) O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. Eur. J. Biochem. 222, 415–424. 10.1111/j.1432-1033.1994.tb18880.x. [DOI] [PubMed] [Google Scholar]
- Vavasseur F.; Yang J. M.; Dole K.; Paulsen H.; Brockhausen I. (1995) Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R β3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology 5, 351–357. 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- Tsuboi S.; Hatakeyama S.; Ohyama C.; Fukuda M. (2012) Two opposing roles of O-glycans in tumor metastasis. Trends Mol. Med. 18, 224–232. 10.1016/j.molmed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. G.; Lomako J.; Lomako W. M.; Whelan W. J.; Meyer H. E.; Serwe M.; Metzger J. W. (1995) β-Glucosylarginine: a new glucose-protein bond in a self-glucosylating protein from sweet corn. FEBS Lett. 376, 61–64. 10.1016/0014-5793(95)01247-6. [DOI] [PubMed] [Google Scholar]
- Falkowska E.; Le K. M.; Ramos A.; Doores K. J.; Lee J. H.; Blattner C.; Ramirez A.; Derking R.; van Gils M. J.; Liang C. H.; McBride R.; von Bredow B.; Shivatare S. S.; Wu C. Y.; Chan-Hui P. Y.; Liu Y.; Feizi T.; Zwick M. B.; Koff W. C.; Seaman M. S.; Swiderek K.; Moore J. P.; Evans D.; Paulson J. C.; Wong C. H.; Ward A. B.; Wilson I. A.; Sanders R. W.; Poignard P.; Burton D. R. (2014) Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40, 657–668. 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley J. M.; Wrin T.; Korber B.; Zwick M. B.; Wang M.; Chappey C.; Stiegler G.; Kunert R.; Zolla-Pazner S.; Katinger H.; Petropoulos C. J.; Burton D. R. (2004) Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78, 13232–13252. 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. M.; Huber M.; Doores K. J.; Falkowska E.; Pejchal R.; Julien J. P.; Wang S. K.; Ramos A.; Chan-Hui P. Y.; Moyle M.; Mitcham J. L.; Hammond P. W.; Olsen O. A.; Phung P.; Fling S.; Wong C. H.; Phogat S.; Wrin T.; Simek M. D.; Koff W. C.; Wilson I. A.; Burton D. R.; Poignard P. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470. 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar R. L. (2004) Glycolipid-mediated cell-cell recognition in inflammation and nerve regeneration. Arch. Biochem. Biophys. 426, 163–172. 10.1016/j.abb.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Lopez P. H.; Schnaar R. L. (2009) Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 19, 549–557. 10.1016/j.sbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D. H.; Bertozzi C. R. (2005) Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discovery 4, 477–488. 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Hakomori S.; Zhang Y. M. (1997) Glycosphingolipid antigens and cancer therapy. Chem. Biol. 4, 97–104. 10.1016/S1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- Yuriev E.; Farrugia W.; Scott A. M.; Ramsland P. A. (2005) Three-dimensional structures of carbohydrate determinants of Lewis system antigens: implications for effective antibody targeting of cancer. Immunol. Cell Biol. 83, 709–717. 10.1111/j.1440-1711.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- Zhang S. L.; Zhang H. S.; CordonCardo C.; Reuter V. E.; Singhal A. K.; Lloyd K. O.; Livingston P. O. (1997) Selection of tumor antigens as targets for immune attack using immunohistochemistry. 2. Blood group-related antigens. Int. J. Cancer 73, 50–56. . [DOI] [PubMed] [Google Scholar]
- Sakamoto J.; Furukawa K.; Cordoncardo C.; Yin B. W. T.; Rettig W. J.; Oettgen H. F.; Old L. J.; Lloyd K. O. (1986) Expression of Lewisa, LewisB, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell-lines. Cancer Res. 46, 1553–1561. [PubMed] [Google Scholar]
- Gandhi N. S.; Mancera R. L. (2008) The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 72, 455–482. 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Capila I.; Linhardt R. J. (2002) Heparin-protein interactions. Angew. Chem., Int. Ed. 41, 391–412. . [DOI] [PubMed] [Google Scholar]
- Esko J. D.; Selleck S. B. (2002) Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471. 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Knudson C. B.; Knudson W. (2001) Cartilage proteoglycans. Semin. Cell Dev. Biol. 12, 69–78. 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- Nandini C. D.; Sugahara K. (2006) Role of the sulfation pattern of chondroitin sulfate in its biological activities and in the binding of growth factors. Adv. Pharmacol. 53, 253–279. 10.1016/S1054-3589(05)53012-6. [DOI] [PubMed] [Google Scholar]
- Deepa S. S.; Umehara Y.; Higashiyama S.; Itoh N.; Sugahara K. (2002) Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors - Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 277, 43707–43716. 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- Rogers C. J.; Clark P. M.; Tully S. E.; Abrol R.; Garcia K. C.; Goddard W. A.; Hsieh-Wilson L. C. (2011) Elucidating glycosaminoglycan-protein-protein interactions using carbohydrate microarray and computational approaches. Proc. Natl. Acad. Sci. U. S. A. 108, 9747–9752. 10.1073/pnas.1102962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner E.; Meli L.; Kwon S. J.; Dordick J. S.; Linhardt R. J. (2013) FGF-FGFR signaling mediated through glycosaminoglycans in microtiter plate and cell-based microarray platforms. Biochemistry 52, 9009–9019. 10.1021/bi401284r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. B.; Knudson W. (1993) Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 7, 1233–1241. [PubMed] [Google Scholar]
- Toole B. P. (1990) Hyaluronan and its binding proteins, the hyaladherins. Curr. Opin. Cell Biol. 2, 839–844. 10.1016/0955-0674(90)90081-O. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Masuko S.; Takieddin M.; Xu H.; Liu R.; Jing J.; Mousa S. A.; Linhardt R. J.; Liu J. (2011) Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science 334, 498–501. 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.; Dickinson D. M.; Li L.; Masuko S.; Suflita M.; Schultz V.; Nelson S. D.; Bhaskar U.; Liu J.; Linhardt R. J. (2014) Fluorous-assisted chemoenzymatic synthesis of heparan sulfate oligosaccharides. Org. Lett. 16, 2240–2243. 10.1021/ol500738g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis P. L.; Liu J.; Linhardt R. J. (2013) Chemoenzymatic synthesis of glycosaminoglycans: re-creating, re-modeling and re-designing nature’s longest or most complex carbohydrate chains. Glycobiology 23, 764–777. 10.1093/glycob/cwt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong C.; Venot A.; Dhamale O.; Boons G. J. (2013) Fluorous supported modular synthesis of heparan sulfate oligosaccharides. Org. Lett. 15, 342–345. 10.1021/ol303270v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Xu Y.; Chen M.; Weiwer M.; Zhou X.; Bridges A. S.; DeAngelis P. L.; Zhang Q.; Linhardt R. J.; Liu J. (2010) Chemoenzymatic design of heparan sulfate oligosaccharides. J. Biol. Chem. 285, 34240–34249. 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen M.; Choi S. K.; Whitesides G. M. (1998) Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem., Int. Ed. 37, 2755–2794. . [DOI] [PubMed] [Google Scholar]
- Lundquist J. J.; Toone E. J. (2002) The cluster glycoside effect. Chem. Rev. 102, 555–578. 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- Gordon E. J.; Sanders W. J.; Kiessling L. L. (1998) Synthetic ligands point to cell surface strategies. Nature 392, 30–31. 10.1038/32073. [DOI] [PubMed] [Google Scholar]
- Liang R.; Loebach J.; Horan N.; Ge M.; Thompson C.; Yan L.; Kahne D. (1997) Polyvalent binding to carbohydrates immobilized on an insoluble resin. Proc. Natl. Acad. Sci. U. S. A. 94, 10554–10559. 10.1073/pnas.94.20.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. L.; Campbell C.; Li Q. A.; Gildersleeve J. C. (2010) Multidimensional glycan arrays for enhanced antibody profiling. Mol. BioSyst. 6, 1583–1591. 10.1039/c002259d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L.; Cummings R. D.; Smith D. F.; Huflejt M.; Campbell C. T.; Gildersleeve J. C.; Gerlach J. Q.; Kilcoyne M.; Joshi L.; Serna S.; Reichardt N. C.; Parera Pera N. P.; Pieters R. J.; Eng W.; Mahal L. K. (2014) Cross-platform comparison of glycan microarray formats. Glycobiology 24, 507–517. 10.1093/glycob/cwu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manimala J. C.; Roach T. A.; Li Z.; Gildersleeve J. C. (2007) High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology 17, 17C–23C. 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- Gildersleeve J. C.; Wright W. S. (2016) Diverse molecular recognition properties of blood group A binding monoclonal antibodies. Glycobiology 26, 443. 10.1093/glycob/cwv171. [DOI] [PMC free article] [PubMed] [Google Scholar]