Abstract
Cytochrome P450s (P450s) are key enzymes in the synthesis of bioactive natural products in plants. Efforts to harness these enzymes for in vitro and whole-cell production of natural products have been hampered by difficulties in expressing them heterologously in their active form, and their requirement for NADPH as a source of reducing power. We recently demonstrated targeting and insertion of plant P450s into the photosynthetic membrane and photosynthesis-driven, NADPH-independent P450 catalytic activity mediated by the electron carrier protein ferredoxin. Here, we report the fusion of ferredoxin with P450 CYP79A1 from the model plant Sorghum bicolor, which catalyzes the initial step in the pathway leading to biosynthesis of the cyanogenic glucoside dhurrin. Fusion with ferredoxin allows CYP79A1 to obtain electrons for catalysis by interacting directly with photosystem I. Furthermore, electrons captured by the fused ferredoxin moiety are directed more effectively toward P450 catalytic activity, making the fusion better able to compete with endogenous electron sinks coupled to metabolic pathways. The P450-ferredoxin fusion enzyme obtains reducing power solely from its fused ferredoxin and outperforms unfused CYP79A1 in vivo. This demonstrates greatly enhanced electron transfer from photosystem I to CYP79A1 as a consequence of the fusion. The fusion strategy reported here therefore forms the basis for enhanced partitioning of photosynthetic reducing power toward P450-dependent biosynthesis of important natural products.
With the continued development of tools for engineering biosynthetic pathways into microorganisms, elucidation of routes leading to plant natural products of high value is attracting considerable interest.1−3 Cytochrome P450s (P450s) are key enzymes in specialized metabolism and are involved in the formation of terpenoids, alkaloids, cyanogenic glycosides, glucosinolates, and phenylpropanoids like flavonoids, coumarins, and stilbenes. They catalyze stereo- and regiospecific hydroxylations, epoxidations, and C–C couplings that are often difficult to accomplish by chemical synthesis. In eukaryotes, P450s reside on the endoplasmic reticulum, and plant genomes often contain several hundred P450-encoding genes.4−6 Biotechnological production of many high-value specialized metabolites thus requires heterologous expression of one or more P450s in highly active form. The yeast Saccharomyces cerevisiae remains the favored host for introduction of plant P450-dependent pathways,1 but cyanobacteria are gaining in popularity and offer unparalleled sustainability because they too are photosynthetic organisms and therefore require minimal nutrient input.7 We recently showed that the thylakoid membranes of both cyanobacteria and plants, which host the photosynthetic apparatus, can also accommodate plant P450s.8−11
Two key issues encountered when engineering P450-dependent pathways are the need for high-level functional expression of the P450 and the provision of sufficient reducing power to drive their catalytic cycle.12 This study addresses the latter. Many alternative P450 reductase systems and P450-reductase fusions have been reported, but only a few have resulted in increased activity.13 Photosynthetic hosts offer several advantages for P450-dependent pathways:2,3 Plant P450s are functionally active when targeted to thylakoid membranes and benefit from the ample supply of electrons and molecular oxygen generated by photosynthetic electron transport (Figure 1a).8,9 Cyanobacteria or plant-cell cultures may thus constitute a useful vehicle for environmentally contained, heterologous production of specialized plant metabolites of high value, such as structurally complex diterpenoids functionalized in P450-dependent reactions.14−18 However, competition from native electron sinks, such as the metabolic reactions involved in CO2 fixation and redox regulation processes,8 complicates the diversion of electrons from photosynthesis to non-native processes. In an effort to improve partitioning of electrons toward the P450, we have now designed and tested fusions that covalently connect the electron transfer protein ferredoxin (Fd) to the well-characterized P450 CYP79A1 from Sorghum bicolor (Figure 1a).
Figure 1.
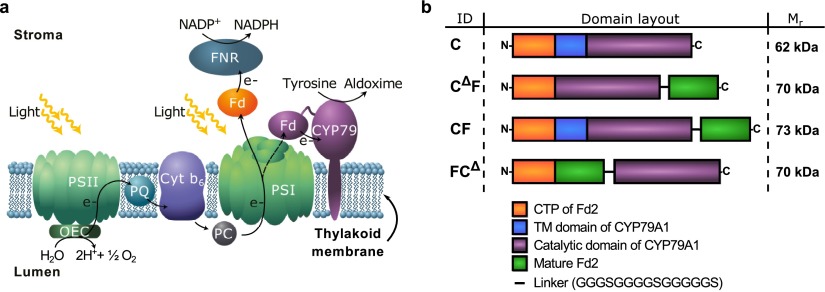
Overview of light-driven biosynthesis and fusion constructs used in this study. (a) Schematic illustration of the interfacing of photosynthetic electron transport with light-driven biosynthesis by fusing ferredoxin to CYP79A1 in the thylakoid membrane, thus directing electrons toward cytochrome P450-catalyzed hydroxylations. OEC, oxygen-evolving complex; PSII, photosystem II; PQ, plastoquinone pool; Cyt b6f, cytochrome b6f; PC, plastocyanin; PSI, photosystem I; Fd, ferredoxin; FNR, ferredoxin:NADPH-reductase. (b) Design of gene constructs that fuse CYP79A1 from Sorghum bicolor to the major Fd of Arabidopsis thaliana, Fd2.35 All constructs included an N-terminal Fd chloroplast transit peptide (CTP), which targets them to the chloroplast. The mature Fd (F) was fused via a 15-residue linker either to the full-length CYP79A1 (C) or to its heme domain lacking the transmembrane (TM) anchor (CΔ). ID, abbreviated construct names; Mr, calculated mass (not counting the transit peptide).
In the current study, we expressed and targeted P450-Fd fusions to the chloroplast in Nicotiana benthamiana. One of the fusion constructs, which could acquire photosynthetic reducing power by direct electron transfer from photosystem I without the need for a dedicated reductase, was better able to compete with endogenous electron sinks and showed higher in vivo activity than the native enzyme. This study thus represents a proof of concept that P450-Fd fusions can interface directly with electron transfer from photosystem I and divert more photosynthetic reducing power to engineered metabolism.
Results
Design of CYP79A1-Ferredoxin Fusion Proteins
The initial step in the biosynthetic pathway leading to the cyanogenic glucoside dhurrin in Sorghum bicolor(19−21) is the conversion of l-tyrosine to (E)-p-hydroxyphenylacetaldoxime, which is catalyzed by CYP79A1.21−24 We chose CYP79A1 for our ferredoxin (Fd) fusion studies because it is stable both in its native ER and in photosynthetic membranes8,9 and well characterized.21,22,24 We designed three different fusion constructs—two C-terminal fusions named CΔF and CF and one N-terminal construct FCΔ (Figure 1b). A linker length of 15 amino acids was chosen based on the distances between the C-terminus of CYP79A1 and the N-terminus of Fd derived from docking models, the presence in CYP79A1 of four C-terminal residues with predicted random-coil secondary structure not resolved in crystal structures,25 and the estimated distance from the edge of photosystem I to its Fd binding site (Supporting Figure S1). A Gly/Ser-rich sequence was chosen to avoid secondary structure and maximize the ability of the Fd domain to transfer electrons to the heme of CYP79A1 and reduce susceptibility to proteases.26 Because the native CYP79A1 (named C in Figure 1b) contains an N-terminal transmembrane domain that normally anchors it to the ER membrane, N-terminal fusions to Fd were constructed with CYP79A1 lacking this domain, to avoid placing Fd and CYP79A1 domains on opposite sides of the thylakoid membrane. An additional C-terminal Fd fusion with the same truncated CYP79A1 variant was therefore assembled to control for differences due to a lack of the transmembrane anchor.
Chloroplast Sublocalization of Fusion Proteins
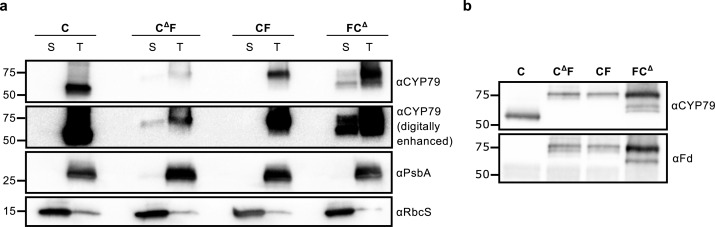
We used Agrobacterium-mediated infiltration of N. benthamiana leaves to generate plants that transiently expressed the CYP79A1 constructs. Subsequently, we fractionated intact chloroplasts prepared from infiltrated leaves and subjected each fraction to immunoblot analysis with an anti-CYP79 antibody to verify that the Fd transit peptide targeted fusion proteins to thylakoid membranes (Figure 2a). As expected, the fusions were found mainly in thylakoid fractions, though both FCΔ and CΔF were also detectable in the stromal fraction.
Figure 2.
CYP79A1-ferredoxin fusion enzymes localized to thylakoid membranes of tobacco chloroplasts and containing both CYP79A1 and ferredoxin epitopes. (a) Immunoblot analysis of chloroplasts isolated from tobacco leaves 5 days after agroinfiltration and fractionated into stroma (S) and thylakoid (T) fractions. Membranes were probed with antibodies against either CYP79A1, the D1 protein of photosystem II (PsbA, a thylakoid marker), or a RuBisCo small subunit (RbcS, a stroma marker). Mobilities of protein standards of known mass (in kDa) are indicated on the left. The immunoblot probed with the CYP79A1 antibody is shown as the original exposure and in a digitally enhanced version that emphasizes weak signals. (b) Immunoblot analysis of thylakoids isolated from tobacco expressing fusion constructs as in a, probed with antibodies against CYP79A1 or ferredoxin (Fd).
Immunoblot analyses performed with an antibody raised against ferredoxin showed clear signals for CΔF, CF, and FCΔ constructs, but not the unfused construct C (Figure 2b), thus confirming that the translated fusion proteins contained both CYP79A1 and Fd domains. The CΔF, CF, and FCΔ proteins comprised 0.2–2.6% of total thylakoid protein, based on SDS-PAGE densitometry (Supporting Figure S2c). The C protein could not be quantified by this approach because it comigrated with the abundant β-subunit of ATP synthase. A high pigment background (due to chlorophylls and carotenoids) in the isolated thylakoid membranes interfered with standard determination of concentrations of the P450s by CO difference spectra, and we therefore measured their relative levels by immunoblot analysis (Supporting Figure S2a,b and Supporting Table S1).
Fusion with Fd Directs Electrons to CYP79A1 in the Absence of Soluble Fd
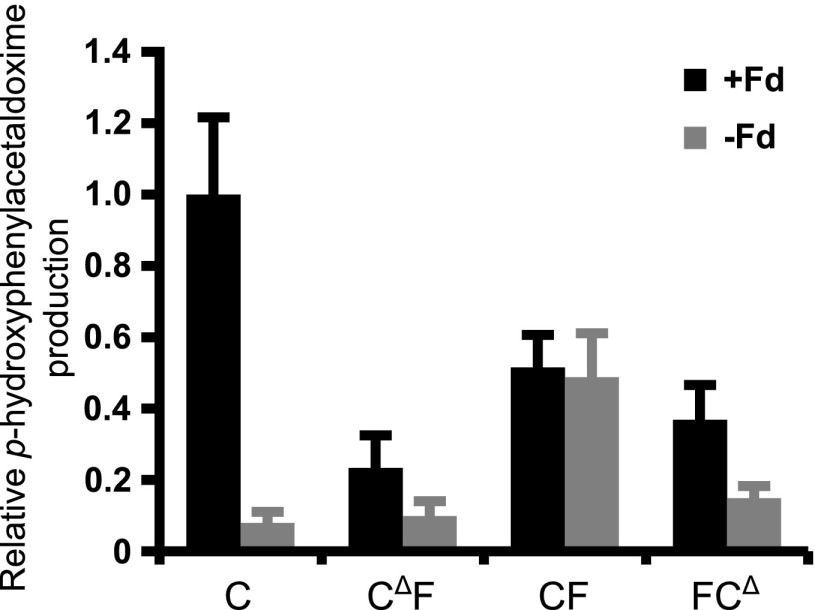
We next performed enzyme assays using thylakoid preparations containing C, CΔF, CF, or FCΔ protein to investigate whether the Fd domain fused to CΔF, CF, and FCΔ mediated direct electron transfer from photosystem I to the heme of the CYP79A1 domain. In vitro assays were carried out to assess whether added soluble Fd was required for electron transfer between photosystem I and the P450s (Figure 3). Only the CF fusion protein harboring the full-length CYP79A1 protein was found to exhibit substantial activity in the absence of added soluble Fd. Direct electron transfer between Fd and CYP79A1 domains also operated in assays in which the Fd domain was reduced in a light-independent manner by the enzyme ferredoxin:NADP+ reductase (FNR) in the presence of NADPH (Supporting Figure S3). Based on these findings, we focused subsequent experiments on characterizing the function of the CF enzyme.
Figure 3.
Comparison of in vitro light-driven enzyme activity from N. benthamiana thylakoids harboring CYP79A1 enzyme variants. Assays were carried out in the presence or absence of 8.3 μM soluble Fd (+Fd or – Fd) as an electron carrier. Bars show average activities of four separate thylakoid preparations per construct, normalized to their relative protein levels as determined from immunoblotting experiments. Error bars indicate ± SD of biological replicates.
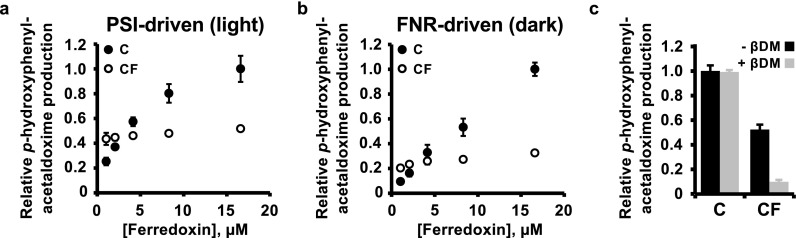
Dependence of Activity on Soluble Ferredoxin
Because the activity of CF was unchanged by the addition of soluble Fd in our initial experiments, we performed light-driven activity assays with varying concentrations of soluble Fd to compare the fusion protein’s dependence on soluble Fd with that of the unfused C enzyme. Increasing the Fd concentration from 1 to 16.6 μM caused only a modest 1.2-fold increase in the activity of the CF enzyme, compared to a 4-fold increase and clear saturation in the activity of C (Figure 4a). To test whether the reduced activity of CF was due to suboptimal interaction with photosystem I, we performed activity assays with varying Fd concentrations under dark conditions, using a soluble electron donor system composed of NADPH and FNR (Figure 4b). As in the light-driven assays (Figure 4a), the >15-fold increase in Fd concentration only modestly enhanced CF activity (by 1.6-fold), while the activity of C increased 10-fold and did not saturate (Figure 4b). Overall activity was 3–4 times lower for both C and CF in this assay compared to the light-driven assay. To investigate the importance of thylakoid integrity for the activity of C and CF enzymes, we compared light-driven activities of C and CF in intact and solubilized thylakoid membranes. Solubilization of the membrane reduced the activity of the CF enzyme by 80% but did not affect that of C (Figure 4c).
Figure 4.
Effects of soluble ferredoxin and detergent on in vitro enzyme activity of C and CF proteins. (a) Light-dependent enzyme activity of thylakoids harboring either C or CF, driven by electron transfer from soluble or fused ferredoxin reduced by photosystem I. (b) Enzyme activity of dark-incubated thylakoids harboring C or CF, driven by electron transfer from soluble or fused ferredoxin reduced by 0.6 μM FNR in the presence of 0.5 mM NADPH. (c) Light-dependent activity of intact (-βDM) or solubilized (+βDM) thylakoids harboring C or CF in the presence of 8.3 μM soluble Fd. Experimental values are means of four technical repeats each, normalized to relative protein levels determined by immunoblot, with error bars showing ± SD. βDM, n-dodecyl β-D-maltoside.
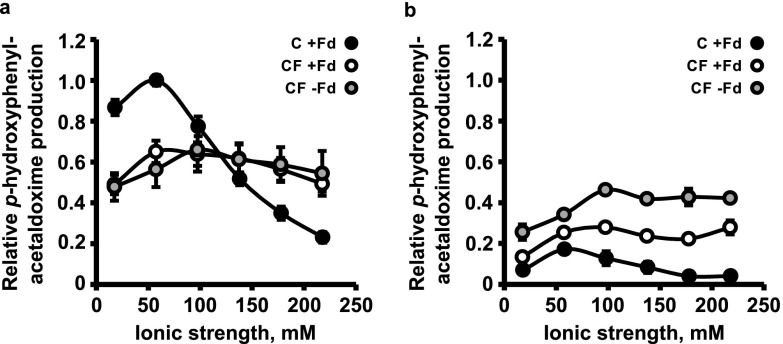
Dependence of Activity on Ionic Strength
Because Fd interacts electrostatically with redox partners,27 we examined the effect of the ionic strength (I) on the interaction between Fd and the enzymes C and CF. The activity of C showed a marked bell-shaped dependence on I, reaching a maximum at I = 60 mM, whereas CF was largely unaffected by I (Figure 5a). To test whether fusion with Fd enabled CF to acquire more electrons from photosystem I even in the presence of a competing sink, we examined the effect of I on the light-driven activity in the presence of FNR and the acceptor NADP+. FNR and NADP+ competed strongly with C and CF for reduced ferredoxin, as the catalytic activities of both enzymes were reduced in their presence. However, CF retained more activity (up to 57% (78%) of its peak activity in the presence (absence) of soluble ferredoxin, respectively) than C (18% of maximal or less) under these conditions (Figure 5b).
Figure 5.
Effect of ionic strength on light-driven enzyme activity of tobacco thylakoids containing C (+soluble Fd) or CF (±soluble Fd). (a) Dependence of C and CF activity on ionic strength, adjusted with NaCl. (b) Dependence on ionic strength of C and CF activity measured in the presence of 0.6 μM FNR and 1.63 mM NADP+ acting as a competing electron sink for reduced Fd. Data are plotted relative to the maximum activity of C obtained in a. Curves show mean activities from (a) four or (b) two technical replicates, normalized to the relative levels of C and CF proteins. Error bars indicate ± SD.
In Vivo Activity of C and CF
Because in vitro activities of C and CF depended greatly on assay conditions, we examined the in vivo activity of both chloroplast-targeted proteins by quantifying reaction products extracted directly from tobacco. The aldoxime produced by ER-localized CYP79A1 was previously shown to undergo glycosylation in tobacco plants.28 To test whether chloroplast-produced p-hydrophenylacetaldoxime was glycosylated in our transient transfection experiments, we treated extracts of infiltrated leaves with a commercial β-glucosidase mixture and observed the release of large amounts of p-hydroxyphenylacetaldoxime (Supporting Figure S4).
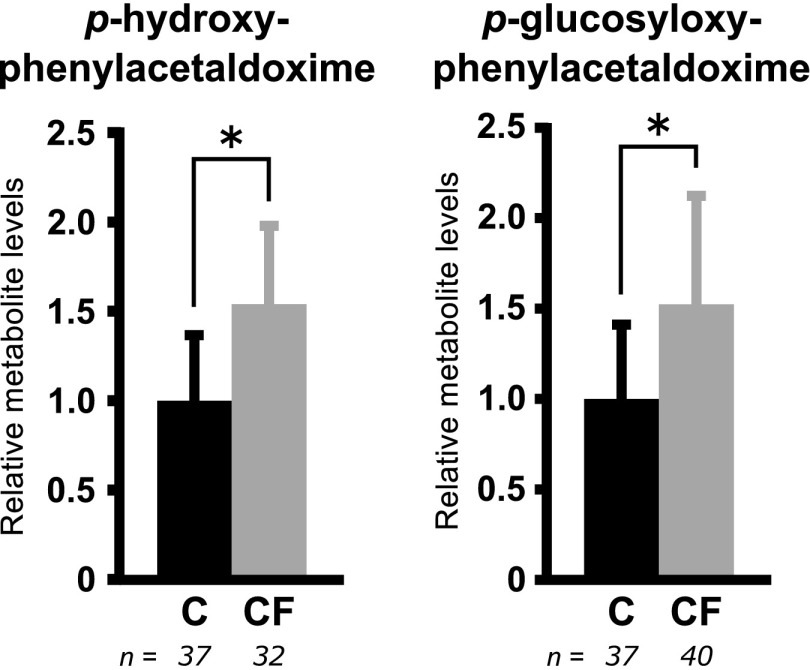
We also synthesized the glucoside p-glycosyloxyphenylacetaldoxime (see Supporting Information, Materials and Methods), which allowed us to quantify both free and glycosylated aldoxime using LC-MS/MS. We found amounts of p-glucosyloxyphenylacetaldoxime to be linearly correlated with, and on average 100-fold higher (w/w) than, those of p-hydroxyphenylacetaldoxime (Supporting Figure S5). To ensure that differences in the average metabolite levels detected in C- and CF-expressing plants were not caused by differences in expression of C and CF enzymes, we also quantified the relative amounts of both proteins in total protein extracts from infiltrated leaves by immunoblotting and found average CF expression to be 40% that of C (Supporting Figure S6). The expression-normalized production of free and glycosylated p-hydroxyphenylacetaldoxime showed that the specific activity of CF is 50% higher than that of C (Figure 6).
Figure 6.
Relative amounts of p-hydroxyphenylacetaldoxime and p-glucosyloxyphenylacetaldoxime extracted from N. benthamiana leaves 5 days post infiltration with Agrobacterium strains bearing plasmids encoding either C or CF, as quantified by LC-MS/MS and normalized to relative protein expression as determined by immunoblot. Equal numbers of leaf samples were analyzed for p-hydroxyphenylacetaldoxime and p-glucosyloxyphenylacetaldoxime in C (n = 37) and CF (n = 40), but p-hydroxyphenylacetaldoxime was below the level of detection in some CF samples. Error bars ± SD. *Statistical significance (p < 0.05) according to two-tailed unpaired t-tests.
Discussion
This study reports the light-driven activity of three fusions between CYP79A1 and Fd and confirms the establishment of functional electron transfer from photosystem I to the heme domain in the CF enzyme. We expected the CΔF and FCΔ fusions to be soluble proteins, but both associated with thylakoid membranes, possibly via hydrophobic interactions with the F-G loop of CYP79A125 and/or electrostatic Arg and Lys interactions with membrane phospho- or sulfolipids.29,30 The CΔF fusion was barely detectable in isolated chloroplasts, but readily so in thylakoid membrane preparations (Figure 2). The FCΔ fusion protein showed the highest expression in both chloroplast and thylakoid preparations, but both it and CΔF gave rise to additional immunospecific bands below the main one (Figure 2b and Supporting Figure S2a). Neither C nor CF, which include the full-length CYP79A1 sequence, appeared susceptible to proteolysis and produced single bands at the expected positions on immunoblots (62 and 73 kDa). We conclude that truncation of CYP79A1 increases its susceptibility to chloroplast proteases, and this might explain the reduced activities of CΔF and FCΔ. Other possible explanations are poor interaction between Fd and P450 domains, aberrant incorporation of heme or 2Fe–2S clusters into the truncated proteins, or poor interaction with the PsaC, -D, and -E subunits of photosystem I, which would limit electron transfer from its FB [4Fe–4S] cluster (see Supporting Figure S1).31
Cytochrome P450 concentrations are commonly determined by inhibiting the dithionite-reduced enzyme with CO to yield a characteristic reduced-plus-CO vs reduced difference spectrum.32 We were unable to detect characteristic peaks at 450 or 420 nm because the high spectral background from pigments in the photosynthetic membrane swamps the weak signal expected from the P450:CO adduct (data not shown). Though CO spectra can also measure enzyme inactivation, the presence of other hemoproteins compromises their accuracy.32 Since thylakoid membranes contain many hemoproteins, in particular the Cytochrome b6f complex, the method is unlikely to be accurate in this matrix. We instead measured activities in several independent thylakoid preparations and found them to be highly consistent overall, indicating that inactivation of the cytochromes does not cause major variation in this case.
The CF fusion was preferentially reduced by electrons transferred to its fused Fd domain by photosystem I ,and only a minor fraction of its reducing power was derived from soluble Fd (Figure 7a). The most likely explanations for this preference are steric hindrance by the Fd domain limiting access of soluble Fd to the heme-proximal surface and much faster kinetics of electron transfer from photosystem I to the CF enzyme due to reduced dependence on diffusion. While our evidence does not exclude other possibilities, a number of observations support this interpretation. First, the addition of high concentrations of soluble Fd resulted in only limited enhancement of CF activity (Figure 4a). Second, the same trend was observed when FNR and NADPH were used to reduce Fd, with activities of C and CF being equal at ∼4 μM soluble Fd in both experiments (Figure 4a,b). Since FNR is a soluble enzyme, it is not affected by steric hindrance in the spatially highly organized environment of the thylakoid, which consists of ∼70% (mol/mol) protein33 and allows only limited diffusion.34 Third, CF activity depends on colocalization with photosystem I in the thylakoid membrane, even when soluble Fd is present (Figure 4c). Solubilization of the membrane effectively causes its constituent proteins to be diluted, which is consistent with a decrease in activity of CF if it can only be reduced via direct interaction of its Fd domain with photosystem I. In contrast, the concentration of soluble Fd (8.3 μM) is unchanged by solubilization of the membrane, and therefore so also is the activity of the C protein. Finally, the higher specific activity of CF relative to unfused C under in vivo conditions (Figure 6) indicates that the differences observed between C and CF in vitro relate to the specific assay conditions used. Since the concentrations of Fd available to CF and C differ in principle, membrane colocalization of CF with photosystem I must compensate for its inability to interact with soluble Fd.
Figure 7.
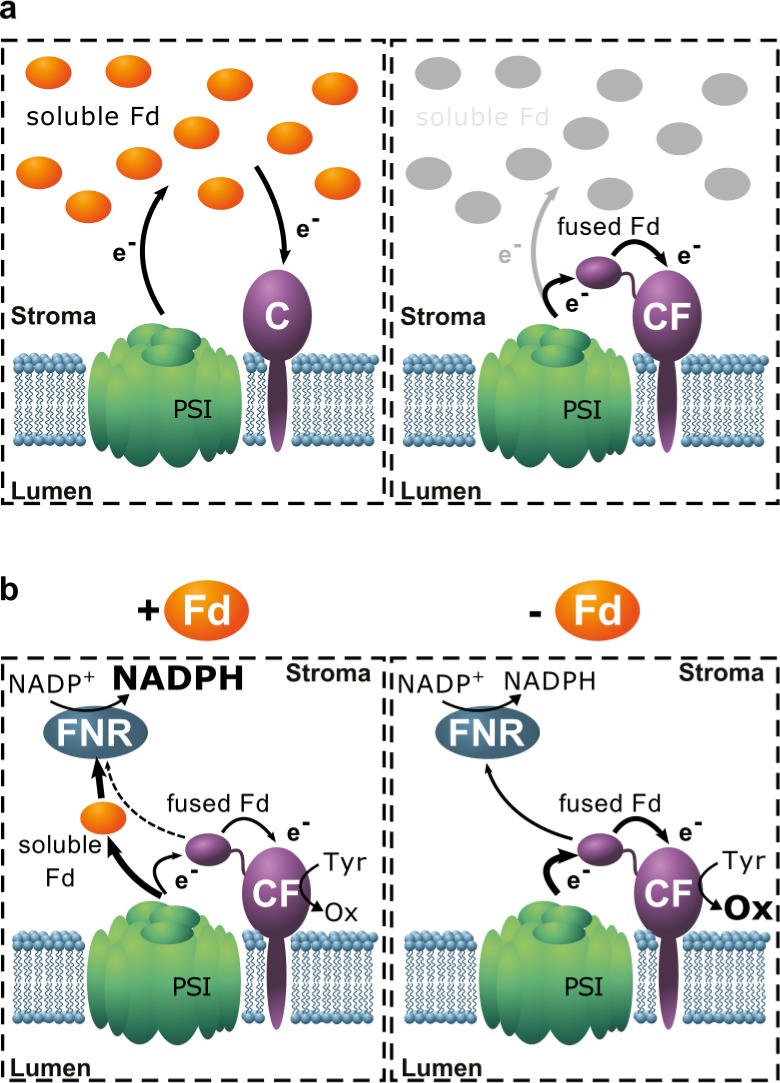
Electron transfer from photosystem I to CF independently of soluble Fd and competition between soluble and fused Fd for electrons in the presence of FNR. (a) Electron transfer chains involved during catalysis by C (left), where electrons flow from photosystem I (PSI) through the soluble Fd pool to the P450, and CF (right), where electrons flow from photosystem I via the Fd domain fused to the P450. (b) Electron flow toward FNR/NADP+ vs CF in the presence of soluble Fd (left). When soluble Fd is absent (right), more electrons flow through the fused Fd domain of CF and consequently end up being used for hydroxylation of Tyr; i.e., intramolecular electron transfer is faster than transfer to FNR.
Unfused C protein showed typical saturation behavior as Fd concentrations were increased in light- but not in FNR-driven assays (Figure 4a,b). This difference probably results from slower reduction of Fd by FNR, because of thermodynamically uphill electron transfer from NADPH (Em = −320 mV) to Fd (Em = −433 mV).35 From its saturation curve in light-driven assays we estimated the KM of C for Fd to be 5.0 ± 0.6 μM. For comparison, FNR—which consumes the majority of reduced Fd in vivo—has a KM for Fd of 2.8 μM.35 Such a high affinity toward an unnatural interaction partner can be rationalized by similarities between cytochrome P450 reductases (POR) and chloroplast Fd’s. POR contains a small FMN-binding domain responsible for electron transfer to P450s, which is structurally homologous to the small electron carrier protein flavodoxin (Fld).36 Since Fld and Fd act interchangeably in some photosynthetic organisms,37 both carry highly negatively charged surfaces, and both interact electrostatically with photosystem I and FNR,38 their ability to support catalysis by many P450s8−11,39−41 is consistent with the hypothesis that these small electron carrier proteins evolved to interact with proteins through charge and surface shape complementarity in a relatively unspecific manner.42
The light-driven activity of C showed a bell-shaped dependence on ionic strength (I) (Figure 5a). Similar I dependences have been reported for Fd-dependent FNR activity and for reduction of plastocyanin by cytochrome c, and the increase in activity observed likely reflects increased dissociation rates due to suppression of nonspecific electrostatic interactions.43−45 In contrast, the activity of the CF protein showed less dependence on ionic strength, probably because the covalent association of the interacting partners obviates the need for long-range electrostatic steering. The CF fusion is better able to compete with FNR for electrons (Figure 5b), but reduced CF activity in the presence of soluble Fd shows that the latter competes for interaction with photosystem I (Figure 7b).
This work has important implications in relation to the choice of assay for assessment of P450-reductase fusions. Such enzymes are often evaluated in comparison with a stoichiometric reductase:P450 mixture, which can be misleading, since in vivo reductase:P450 ratios are often far from 1,46 and the activities of fusion enzymes tend to increase linearly with concentration, while differing reductase:P450 ratios show nonlinear effects on rates.47 The choice of ionic strength is likewise important, because high ionic strengths would favor intramolecular electron transfer by less electrostatic-reliant fusion enzymes (Figure 5). In this study, we showed that our CF fusion can acquire more reducing power in the presence of a strong competing electron sink than unfused C, which partly explains its increased in vivo activity. We should note, however, that in vivo concentrations of FNR and Fd (10–100 μM and 300–800 μM, respectively, reported for chloroplasts and cyanobacteria)48−50 would be difficult or impossible to replicate in vitro. Consequently, we stress the importance of backing up in vitro comparisons with in vivo experiments whenever possible.
Since light-driven cytochrome P450 pathways have key applications in live cell-culture production systems, we compared the in vivo activities of unfused C and the CF fusion. A preliminary time course analysis of p-hydroxyphenylacetaldoxime content in leaf extracts at 1–5 days post infiltration showed levels peaking on day 3, with no significant change on the fourth and fifth days (data not shown). The levels of C and CF proteins detected 5 days post infiltration indicated that the plants converted p-hydrophenylacetaldoxime to other compounds, and this was confirmed by enzymatic deglycosylation of extracted metabolites (Supporting Figure S4). We found free aldoxime to accumulate to 100-fold lower levels than, and be linearly correlated with, its glucoside (Supporting Figure S6), which implies highly active glycosylation machinery for detoxification of foreign compounds, such as oximes.51,52 By quantifying both, we demonstrated that the catalytic activity of the fusion enzyme is superior to that of unfused CYP79A1 (Figure 6), thanks to its covalent association with Fd, thus confirming that the thylakoid membrane possesses the plasticity required to allow interaction of heterologous enzymes with photosystem I.
This study represents the first report of enhanced light-driven activity of a P450-reductase fusion in vivo. Improved light-driven hydrogenase activity through Fd fusion in vitro was previously demonstrated.53 We did not explore the effect of linker length or composition in the present study, but both were recently found to influence electron transfer rates in fusions between the E. coli flavodoxin reductase and Fld.54 Though five residues were sufficient to support activity of a CYP11A1-adrenodoxin fusion,55 both studies found higher activity with longer linkers.54,55 Assembly of a complex between the model P450cam and its reductase system (putidaredoxin and putidaredoxin reductase) by an alternative approach whereby they were fused with subunits of the PCNA trimer gave a 100-fold rate increase, and this could be further increased by optimizing the linker, further demonstrating the rate enhancements achievable by proper linking of P450 and reductase.56,57 Consequently, we consider it likely that our reported CF fusion can be improved further by exploring alternate linker designs.
Conclusion
This study reports the in vitro and in vivo effects of introducing a P450-ferredoxin fusion protein into the thylakoid membranes of chloroplasts. The fusion CF could obtain electrons directly from photosystem I, and its activity was affected little by soluble Fd. The fusion enzyme also retained higher activity in the presence of competing electron sinks, probably because it is colocalized with photosystem I in the thylakoid membrane. Our P450-ferredoxin fusion approach thus enables direct coupling of photosynthetic electron transfer to P450s involved in desired biosynthetic pathways introduced into higher plant chloroplasts, algae, or cyanobacteria. To our knowledge, this is the first report of a fusion between a ferredoxin and a eukaryotic P450 involved in specialized metabolism. The CF fusion protein had a higher specific activity than unfused CYP79A1 in tobacco leaves, but was less abundant following transient expression. Minimal interaction between the fusion enzyme and soluble Fd makes the catalytic activity achieved by the fusion enzyme in vivo especially remarkable, since its fused Fd domain is present in substoichiometric amounts relative to free Fd. Evolution has tuned the distribution of photosynthetic reducing power to balance maximal biomass accumulation with the necessary redox regulation of metabolic processes. Thus, successful exploitation of photosynthetic organisms for light-driven production of high-value specialized metabolites or biofuels such as H2 will require strategies that modulate the distribution of reducing power. Our work indicates that Fd fusions constitute a transferable approach to channeling of photosynthetic reducing power into non-native pathways.
Acknowledgments
The authors are indebted to G. T. Hanke (Queen Mary University of London) for valuable discussions and input regarding Fd and its interactions, and to C. Crocoll for technical assistance. We thank L. M. Lassen (DTU) for help with illustrations. The research leading to these results received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Program FP7/2007-2013/under REA Grant Agreement No. 317184. This material reflects only the views of the authors and the Union is not liable for any use that may be made of the information therein. The authors also acknowledge financial support from the VILLUM Center of Excellence in Plant Plasticity, the Center for Synthetic Biology (bioSYNergy) funded by the UCPH Excellence Programme for Interdisciplinary Research, the Innovation Fund Denmark (formerly the Danish Council for Strategic Research) Programme Commission on Strategic Growth Technologies (for “Plant Power: Light-Driven Synthesis of Complex Terpenoids Using Cytochromes P450” (Grant No. 12-131834), and the European Research Council [Advanced Grant “Light-driven Chemical Synthesis using Cytochrome P450s” (ERC-2012-ADG_20120314, Project No: 323034, Project Acronym: LightdrivenP450s)].
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.6b00190.
Materials and methods, Supporting Tables S1 and S2, Supporting Figures S1–S6 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- O’Connor S. E. (2015) Engineering of Secondary Metabolism. Annu. Rev. Genet. 49, 71–94. 10.1146/annurev-genet-120213-092053. [DOI] [PubMed] [Google Scholar]
- Lassen L. M.; Nielsen A. Z.; Ziersen B.; Gnanasekaran T.; Møller B. L.; Jensen P. E. (2013) Redirecting Photosynthetic Electron Flow into Light-Driven Synthesis of Alternative Products Including High-Value Bioactive Natural Compounds. ACS Synth. Biol. 3, 1. 10.1021/sb400136f. [DOI] [PubMed] [Google Scholar]
- Møller B. L. (2014) Disruptive innovation: channeling photosynthetic electron flow into light-driven synthesis of high-value products. Synth. Biol. 1, 330. 10.1039/9781849737845-00330. [DOI] [PubMed] [Google Scholar]
- Schuler M. A. (2015) P450s in Plants, Insects, and Their Fungal Pathogens, in Cytochrome P450, pp 409–449, Springer International Publishing, Cham, Switzerland. [Google Scholar]
- Gleadow R. M.; Møller B. L. (2014) Cyanogenic Glycosides: Synthesis, Physiology, and Phenotypic Plasticity. Annu. Rev. Plant Biol. 65, 155–185. 10.1146/annurev-arplant-050213-040027. [DOI] [PubMed] [Google Scholar]
- Renault H.; Bassard J.; Hamberger B.; Werck-Reichhart D. (2014) Cytochrome P450-mediated metabolic engineering: current progress and future challenges. Curr. Opin. Plant Biol. 19, 27–34. 10.1016/j.pbi.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Oliver J. W. K.; Atsumi S. (2014) Metabolic design for cyanobacterial chemical synthesis. Photosynth. Res. 120, 249–261. 10.1007/s11120-014-9997-4. [DOI] [PubMed] [Google Scholar]
- Nielsen A. Z.; Ziersen B.; Jensen K.; Lassen L. M.; Olsen C. E.; Møller B. L.; Jensen P. E. (2013) Redirecting Photosynthetic Reducing Power toward Bioactive Natural Product Synthesis. ACS Synth. Biol. 2, 308–315. 10.1021/sb300128r. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk A.; Gnanasekaran T.; Nielsen A. Z.; Zulu N. N.; Mellor S. B.; Luckner M.; Thøfner J. F. B.; Olsen C. E.; Mottawie M. S.; Burow M.; Pribil M.; Feussner I.; Møller B. L.; Jensen P. E. (2016) Metabolic engineering of light-driven cytochrome P450 dependent pathways into Synechocystis sp. PCC 6803. Metab. Eng. 33, 1–11. 10.1016/j.ymben.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Gnanasekaran T.; Vavitsas K.; Andersen-Ranberg J.; Nielsen A. Z.; Olsen C. E.; Hamberger B.; Jensen P. E. (2015) Heterologous expression of the isopimaric acid pathway in Nicotiana benthamiana and the effect of N-terminal modifications of the involved cytochrome P450 enzyme. J. Biol. Eng. 9, 24. 10.1186/s13036-015-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanasekaran T.; Karcher D.; Nielsen A. Z.; Martens H. J.; Ruf S.; Kroop X.; Olsen C. E.; Motawie M. S.; Pribil M.; Møller B. L.; Bock R.; Jensen P. E. (2016) Transfer of the cytochrome P450-dependent dhurrin pathway from Sorghum bicolor into Nicotiana tabacum chloroplasts for light-driven synthesis. J. Exp. Bot. 67, 2495. 10.1093/jxb/erw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. T.; Lauchli R.; Arnold F. H. (2011) Cytochrome P450: Taming a wild type enzyme. Curr. Opin. Biotechnol. 22, 809–817. 10.1016/j.copbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi S. J.; Gilardi G. (2013) Chimeric P450 enzymes: Activity of artificial redox fusions driven by different reductases for biotechnological applications. Biotechnol. Appl. Biochem. 60, 102–110. 10.1002/bab.1086. [DOI] [PubMed] [Google Scholar]
- Englund E.; Andersen-Ranberg J.; Miao R.; Hamberger B.; Lindberg P. (2015) Metabolic Engineering of Synechocystis sp. PCC 6803 for Production of the Plant Diterpenoid Manoyl Oxide. ACS Synth. Biol. 4, 1270–8. 10.1021/acssynbio.5b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formighieri C.; Melis A. (2014) Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 240, 309–324. 10.1007/s00425-014-2080-8. [DOI] [PubMed] [Google Scholar]
- Lindberg P.; Park S.; Melis A. (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 12, 70–79. 10.1016/j.ymben.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Angermayr S. A.; Gorchs Rovira A.; Hellingwerf K. J. (2015) Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 33, 352–361. 10.1016/j.tibtech.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Nielsen A. Z., Mellor S. B., Vavitsas K., Wlodarczyk A. J., Gnanasekaran T., Perestrello Ramos H de Jesus M., King B. C., Bakowski K., and Jensen P. E. (2016) Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J. Accepted Article, DOI: 10.1111/tpj.13173. [DOI] [PubMed] [Google Scholar]
- Jones P. R.; Møller B. L.; Høj P. B. (1999) The UDP-glucose: p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. J. Biol. Chem. 274, 35483–35491. 10.1074/jbc.274.50.35483. [DOI] [PubMed] [Google Scholar]
- Kahn R. A.; Bak S.; Svendsen I.; Halkier B. A.; Møller B. L. (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol. 115, 1661–70. 10.1104/pp.115.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen O.; Koch B.; Halkier B. a; Møller B. L. (1994) Isolation of the heme-thiolate enzyme cytochrome P-450TYR, which catalyzes the committed step in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Proc. Natl. Acad. Sci. U. S. A. 91, 9740–9744. 10.1073/pnas.91.21.9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen O.; Koch B.; Halkier B. A.; Møller B. L. (1995) Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of L-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J. Biol. Chem. 270, 3506–3511. 10.1074/jbc.270.8.3506. [DOI] [PubMed] [Google Scholar]
- Clausen M.; Kannangara R. M.; Olsen C. E.; Blomstedt C. K.; Gleadow R. M.; Jørgensen K.; Bak S.; Motawie M. S.; Møller B. L. (2015) The bifurcation of the cyanogenic glucoside and glucosinolate biosynthetic pathways. Plant J. 84, 558–573. 10.1111/tpj.13023. [DOI] [PubMed] [Google Scholar]
- Kahn R. a; Fahrendorf T.; Halkier B. a; Møller B. L. (1999) Substrate specificity of the cytochrome P450 enzymes CYP79A1 and CYP71E1 involved in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch. Biochem. Biophys. 363, 9–18. 10.1006/abbi.1998.1068. [DOI] [PubMed] [Google Scholar]
- Jensen K.; Osmani S. A.; Hamann T.; Naur P.; Møller B. L. (2011) Homology modeling of the three membrane proteins of the dhurrin metabolon: Catalytic sites, membrane surface association and protein–protein interactions. Phytochemistry 72, 2113–2123. 10.1016/j.phytochem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Reddy Chichili V. P.; Kumar V.; Sivaraman J. (2013) Linkers in the structural biology of protein-protein interactions. Protein Sci. 22, 153–167. 10.1002/pro.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama K. (2004) Structure and function of plant-type ferredoxins. Photosynth. Res. 81, 289–301. 10.1023/B:PRES.0000036882.19322.0a. [DOI] [PubMed] [Google Scholar]
- Bak S.; Olsen C. E.; Halkier B. A.; Møller B. L. (2000) Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in Dhurrin biosynthesis. Plant Physiol. 123, 1437–1448. 10.1104/pp.123.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk B. C.; Tomasiak T. M.; Keniya M. V.; Huschmann F. U.; Tyndall J. D. a.; O’Connell J. D.; Cannon R. D.; McDonald J. G.; Rodriguez A.; Finer-Moore J. S.; Stroud R. M. (2014) Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc. Natl. Acad. Sci. U. S. A. 111, 3865–3870. 10.1073/pnas.1324245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headlam M. J.; Wilce M. C. J.; Tuckey R. C. (2003) The F-G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. Biochim. Biophys. Acta, Biomembr. 1617, 96–108. 10.1016/j.bbamem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Andersen B., Koch B., Scheller H. V., Okkels J. S., and Møller B. L. (1990) Nearest Neighbour Analysis of the Photosystem I Subunits in Barley and Their Binding of Ferredoxin, in Current Research in Photosynthesis, pp 1631–1634, Springer, Netherlands, Dordrecht. [Google Scholar]
- Guengerich F. P.; Martin M. V.; Sohl C. D.; Cheng Q. (2009) Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 4, 1245–1251. 10.1038/nprot.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J. P.; Boekema E. J. (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta, Bioenerg. 1706, 12–39. 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Kirchhoff H.; Mukherjee U.; Galla H.-J. (2002) Molecular architecture of the thylakoid membrane: lipid diffusion space for plastoquinone. Biochemistry 41, 4872–4882. 10.1021/bi011650y. [DOI] [PubMed] [Google Scholar]
- Hanke G. T.; Kimata-Ariga Y.; Taniguchi I.; Hase T. (2004) A post genomic characterization of Arabidopsis ferredoxins. Plant Physiol. 134, 255–264. 10.1104/pp.103.032755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine M. J. I., Scrutton N. S., Munro A. W., Gutierrez A., Roberts G. C. K., and Wolf C. R. (2005) Electron transfer partners of cytochrome P450. Cytochrome P450 Struct. Mech. Biochem. Third ed., pp 115–148, DOI: 10.1007/0-387-27447-2_4. [DOI] [Google Scholar]
- Zhao Q.; Modi S.; Smith G.; Paine M.; McDonagh P. D.; Wolf C. R.; Tew D.; Lian L. Y.; Roberts G. C.; Driessen H. P. (1999) Crystal structure of the FMN-binding domain of human cytochrome P450 reductase at 1.93 A resolution. Protein Sci. 8, 298–306. 10.1110/ps.8.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann G. M.; Hauswald M.; Jensen A.; Knapp E. W. (2000) Structural alignment of ferredoxin and flavodoxin based on electrostatic potentials: implications for their interactions with photosystem I and ferredoxin-NADP reductase. Proteins: Struct., Funct., Genet. 38, 301–309. . [DOI] [PubMed] [Google Scholar]
- Jensen K.; Jensen P. E.; Møller B. L. (2011) Light-driven cytochrome p450 hydroxylations. ACS Chem. Biol. 6, 533–539. 10.1021/cb100393j. [DOI] [PubMed] [Google Scholar]
- Jensen K.; Johnston J. B.; de Montellano P. R. O.; Møller B. L. (2012) Photosystem I from plants as a bacterial cytochrome P450 surrogate electron donor: terminal hydroxylation of branched hydrocarbon chains. Biotechnol. Lett. 34, 239–245. 10.1007/s10529-011-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi G.; Zöllner A.; Lisurek M.; Velázquez-Campoy A.; Pinto S.; Gómez-Moreno C.; Hannemann F.; Bernhardt R.; Medina M. (2009) Cyanobacterial electron carrier proteins as electron donors to CYP106A2 from Bacillus megaterium ATCC 13368. Biochim. Biophys. Acta, Proteins Proteomics 1794, 1635–1642. 10.1016/j.bbapap.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Crowley P. B.; Carrondo M. A. (2004) The Architecture of the Binding Site in Redox Protein Complexes: Implications for Fast Dissociation. Proteins: Struct., Funct., Genet. 55, 603–612. 10.1002/prot.20043. [DOI] [PubMed] [Google Scholar]
- Hurley J. K.; Morales R.; Martínez-Júlvez M.; Brodie T. B.; Medina M.; Gómez-Moreno C.; Tollin G. (2002) Structure-function relationships in Anabaena ferredoxin/ferredoxin:NADP(+) reductase electron transfer: insights from site-directed mutagenesis, transient absorption spectroscopy and X-ray crystallography. Biochim. Biophys. Acta, Bioenerg. 1554, 5–21. 10.1016/S0005-2728(02)00188-3. [DOI] [PubMed] [Google Scholar]
- Meyer T. E.; Zhao Z. G.; Cusanovich M. a; Tollin G. (1993) Transient kinetics of electron transfer from a variety of c-type cytochromes to plastocyanin. Biochemistry 32, 4552–9. 10.1021/bi00068a010. [DOI] [PubMed] [Google Scholar]
- Kovalenko I. B.; Abaturova A. M.; Riznichenko G. Y.; Rubin A. B. (2011) Computer simulation of interaction of photosystem 1 with plastocyanin and ferredoxin. BioSystems 103, 180–7. 10.1016/j.biosystems.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W.; Franklin M. R.; Cohen B.; Shigamatzu A.; Hildebrandt A. G. (1971) Biochemical and genetic factors influencing drug metabolism. Influence of hepatic microsomal mixed function oxidation reactions on cellular metabolic control. Metab., Clin. Exp. 20, 187–99. 10.1016/0026-0495(71)90091-6. [DOI] [PubMed] [Google Scholar]
- Sibbesen O.; De Voss J. J.; Ortiz de Montellano P. R. (1996) Putidaredoxin Reductase-Putidaredoxin-Cytochrome P450 cam Triple. J. Biol. Chem. 271, 22462–22469. 10.1074/jbc.271.37.22462. [DOI] [PubMed] [Google Scholar]
- Okutani S.; Hanke G. T.; Satomi Y.; Takao T.; Kurisu G.; Suzuki A.; Hase T. (2005) Three maize leaf ferredoxin:NADPH oxidoreductases vary in subchloroplast location, expression, and interaction with ferredoxin. Plant Physiol. 139, 1451–1459. 10.1104/pp.105.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moal G.; Lagoutte B. (2012) Photo-induced electron transfer from photosystem i to NADP+: Characterization and tentative simulation of the in vivo environment. Biochim. Biophys. Acta, Bioenerg. 1817, 1635–1645. 10.1016/j.bbabio.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Antal T. K.; Kovalenko I. B.; Rubin A. B.; Tyystjärvi E. (2013) Photosynthesis-related quantities for education and modeling. Photosynth. Res. 117, 1–30. 10.1007/s11120-013-9945-8. [DOI] [PubMed] [Google Scholar]
- Riechers D. E.; Kreuz K.; Zhang Q. (2010) Detoxification without intoxication: herbicide safeners activate plant defense gene expression. Plant Physiol. 153, 3–13. 10.1104/pp.110.153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. L. (2010) Plant science. Dynamic metabolons. Science 330, 1328–9. 10.1126/science.1194971. [DOI] [PubMed] [Google Scholar]
- Yacoby I.; Pochekailov S.; Toporik H.; Ghirardi M. L.; King P. W.; Zhang S. (2011) Photosynthetic electron partitioning between [FeFe]-hydrogenase and ferredoxin:NADP+-oxidoreductase (FNR) enzymes in vitro. Proc. Natl. Acad. Sci. U. S. A. 108, 9396–401. 10.1073/pnas.1103659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkes P. J.; Biemann S.; Bokel A.; Eickholt M.; Girhard M.; Urlacher V. B. (2015) Design and improvement of artificial redox modules by molecular fusion of flavodoxin and flavodoxin reductase from Escherichia coli. Sci. Rep. 5, 12158. 10.1038/srep12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strushkevich N.; MacKenzie F.; Cherkesova T.; Grabovec I.; Usanov S.; Park H.-W. (2011) Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. U. S. A. 108, 10139–10143. 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H.; Nagamune T. (2010) Molecular Assembly of P450 with Ferredoxin and Ferredoxin Reductase by Fusion to PCNA. ChemBioChem 11, 1517–1520. 10.1002/cbic.201000226. [DOI] [PubMed] [Google Scholar]
- Haga T.; Hirakawa H.; Nagamune T. (2013) Fine tuning of spatial arrangement of enzymes in a PCNA-mediated multienzyme complex using a rigid poly-L-proline linker. PLoS One 8, e75114. 10.1371/journal.pone.0075114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.