Abstract
Background
The effect of long-term N-carbamylglutamate (NCG) treatment on the rate and severity of decompensations due to propionic aciduria (PA) and methylmalonic aciduria (MMA) is unknown. This paper presents clinical experience from a single-centre cohort of patients with PA and MMA who received continuous long-term treatment with NCG.
Methods
The effect of oral NCG treatment (initial dose: 50 mg/kg/day) was investigated in patients with PA or MMA who were experiencing frequent progressive episodes of metabolic decompensation, who had pathological levels of ammonia, and who were referred to the Division of Metabolic Diseases, University Hospital of Padova between August 2014 and December 2015. Clinical and biochemical data, including the number of metabolic decompensations, lactic acid, uric acid and plasma ammonia levels, protein intake and body weight, were collected before and after the initiation of NCG treatment.
Results
Eight patients with PA (n = 4) and MMA (n = 4) aged 2–20 years were treated with NCG (50 mg/kg/day) for 7–16 months. Metabolic decompensation episodes decreased in number and severity, with three of the patients having no episodes (pre-treatment: 24 episodes; post-treatment: 9 episodes). After NCG treatment, all episodes were treated at home and none required hospitalisation, lactic acid values were 1.3–2.1 mmol/L and uric acid values were 0.21–0.36 mmol/L. Significant reductions in blood ammonia levels after NCG initiation were observed in five patients, whereas levels were reduced or maintained in the normal range in the remainder. Over the treatment period, patients had an increase in natural protein intake of 20–50% and gained 0–6.5 kg in bodyweight.
Conclusion
These observations suggest that, in addition to short-term benefits for the acute treatment of hyperammonaemia, NCG may be effective and well tolerated as a long-term treatment in patients with severe PA and MMA, and that further prospective studies are warranted.
Keywords: Metabolic decompensation, Methylmalonic aciduria, N-carbamylglutamate, Organic acidurias, Propionic aciduria
1. Introduction
Propionic aciduria (PA) and methylmalonic aciduria (MMA) are rare, autosomal recessive metabolic disorders caused by deficiency of propionyl-CoA carboxylase and methylmalonyl-CoA mutase, respectively. PA and MMA often present in the neonatal period with acute sepsis-like metabolic decompensation, involving metabolic acidosis, keto and lactic acidosis, and hyperammonaemia. Metabolic decompensation and hyperammonaemia are recurrent features over the course of these diseases, with potentially life-threatening consequences, including brain damage and renal failure [1].
Blood ammonia levels are normally regulated by the conversion of ammonia to urea in the liver, followed by urinary excretion. Although the underlying mechanism of hyperammonaemia in organic aciduria is still debated, it is well documented that propionyl-CoA and methylmalonyl-CoA accumulate in patients with PA and MMA. The excess propionyl-CoA and methylmalonyl-CoA in patients with PA and MMA act to inhibit N-acetylglutamate synthase (NAGS) [2], which catalyses the formation of N-acetylglutamate (NAG), an activator required by carbamoyl-phosphate synthase-1 (CPS-1) [2], [3], [4]. Inhibition of CPS-1 leads to the build-up of ammonia, which cannot then enter the urea cycle [3]. Both compounds further inhibit the urea pathway by depleting hepatic acetyl-CoA, which is required for NAG synthesis [5]. Inhibition of the tricarboxylic acid (TCA) cycle is also deemed to contribute to the observed increase in ammonia in patients with PA and MMA [6].
During acute decompensation episodes, the prompt normalisation of blood ammonia levels is essential to avoid neurological damage and associated complications. Therefore, first-line treatment in patients with MMA and PA undergoing an acute decompensation includes the reduction of catabolism and the promotion of anabolism by the administration of a protein-restricted high-calorie diet. Guidelines by Baumgartner et al. suggest stopping protein intake during episodes of hyperammonaemia and treating the patient with intravenous glucose to stop catabolism [7]. Ammonia-scavenging drugs such as sodium benzoate, sodium phenylbutyrate, and arginine hydrochloride, which have high renal clearance, are also often used to accelerate the elimination of ammonia in the urine [2], [5].
A number of studies have shown that N-carbamylglutamate (NCG) effectively and rapidly reduces hyperammonaemia when added to classical treatment for emergent acute metabolic decompensation, both in neonates and older patients with PA and MMA [3], [8]. One short trial (3 days in duration) in seven patients with PA evidenced the beneficial effects of NCG on ureagenesis and reductions in plasma ammonia and glutamine [9]. However, the effect of long-term NCG treatment on the rate and severity of decompensations due to PA and MMA is unknown. Here, we present the clinical experience from a single-centre cohort of patients with PA and MMA who received continuous long-term treatment with NCG.
2. Methods
2.1. Study population
This study investigated the effects of oral NCG in patients with PA or MMA referred to the Division of Metabolic Diseases, University Hospital of Padova between August 2014 and December 2015. Patients were diagnosed by urinary organic acid analysis, and the diagnosis was confirmed by molecular analysis. All patients included were experiencing frequent progressive episodes of metabolic decompensation, had pathological levels of ammonia and had received a standard management regimen comprising protein restriction, with or without supplementary amino acids, and carnitine prior to initiation of long-term NCG (Carbaglu®, Orphan Europe).
2.2. Treatment
NCG was initiated at a dose of 50 mg/kg/day in patients who were experiencing frequent progressive episodes of metabolic decompensation and who had pathological levels of ammonia. Written informed consent to receive long-term NCG treatment was obtained from all patients and/or a legal representative, for those who were minors, prior to commencement of therapy. NCG was prescribed on a case-by-case basis, and in accordance with local health regulations.
2.3. Clinical measurements and observations
This was a retrospective, observational review of patient medical records to assess the clinical experience of patients with PA and MMA treated with long-term NCG. Patient demographics and characteristics were collected prior to the initiation of NCG treatment, and included age, gender, diagnosis, age at diagnosis, body weight, genetic mutation, the presence of neurological involvement (dystonia or epilepsy), renal damage, pancreatitis and heart disease. Medical records were reviewed for the following clinical and biochemical characteristics: number of metabolic decompensations, protein intake, body weight, and levels of plasma ammonia, amino acids, lactic acid and uric acid, before and after initiation of NCG. Mean ammonia levels measured during the monitoring follow-up and acute decompensation episodes are reported for 3 years prior to long-term NCG initiation and 1 year afterwards. For the purpose of this study, all other assessments were reported for 1 year prior to the initiation of treatment for all patients, regardless of time from diagnosis.
3. Results
3.1. Patient overview and demographics
Eight patients (aged 2–20 years) were included in the study: four with PA and four with MMA (Table 1). One patient was the sibling of a male with PA and was diagnosed at birth. All other patients were diagnosed within the first few weeks after birth with one exception: one patient was 11 months old at diagnosis. Protein intake was low for each patient (1–1.5 g/kg/day) at the initial assessment for this retrospective analysis. An overview of each patient's clinical history is provided in Table 2.
Table 1.
Patient characteristics and demographics prior to initiating long-term NCG treatment at the University Hospital of Padova.
| Patient | Sex | Diagnosis | Age (years) | Age at diagnosis (years) | Genetics | Neurological impairment (mental retardation/dystonia) | Renal damage | Pancreatitis | Heart disease | Protein intake (g/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | PA | 20 | Neonatal | c.1111C > T (p.Q3 71X) / c.1625–1628delT (p.Q544fs) | +/− | − | No | Dilated CMP | 1.0 |
| 2 | F | PA | 11 | Neonatal | c.937C > T (p.R313X) / nf | +/− | − | No | Dilated CMP | 1.3 |
| 3 | M | PA | 9 | Neonatal | c.937C > T (p.R313X) / nf | ++/++ | − | No | No | 1.1 |
| 4 | M | PA | 3 | Neonatal | c.69_78del (p.Gln23Hisfs*2) / c.1788G > A (p.Trp596*) | ++/− | − | No | No | 1.5 |
| 5 | F | MMA Mut0 | 12 | Neonatal | c.643G > A (p.G215S) / c.2179C > T (p.R727X) | −/− | ++ | No | No | 1.4 |
| 6 | F | MMA Mut0 | 6 | 11 months | c.1844C > T (p.P615L) / c.1844C > T (p.P615L) | +/++ | ++ | Yes | No | 1.3 |
| 7 | M | MMA Mut- | 2 | Neonatal | c.655A > T (p.N219Y) / c.2197del4ins5 (p.A732WfsX5) | −/− | + | No | No | 1.0 |
| 8 | F | MMA Mut0 | 12 | Neonatal | c.1844C > T (p. P615L) / c.1953T > C (p. L618P) | −/++ | ++ | Yes | No | 1.1 |
+ = mild; ++ = severe; CMP, cardiomyopathy; MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria; nf, not found.
Table 2.
Overview of patients treated with N-carbamylglutamate at the University Hospital of Padova.
| Patient | Overview |
|---|---|
| 1(PA) | • 20-year-old male with developmental delay, born at term after an uncomplicated pregnancy, labour and delivery |
| • Patient presented with severe metabolic acidosis in the first days of life associated with hyperammonaemia and hyperglycinaemia, leading to a diagnosis of PA confirmed by organic acid analysis | |
| • A protein-restricted diet with amino acids supplementation, carnitine was initiated | |
| • Clinical course characterised by frequent and severe episodes of decompensation, mostly requiring hospital admission for intravenous fluid therapy | |
| • Patient more stable since adolescence, but in last 3 years developed stable dilated cardiomyopathy, with ammonia levels of 80-140 μmol/l | |
| • Following an episode of vomiting with food refusal, mild hyperammonaemia and acidosis requiring hospitalisation 13 months ago, patient initiated on NCG therapy, 50 mg/kg/day | |
| 2(PA) | • 11-year-old male, born at term |
| • Patient presented with acute metabolic acidosis and hyperammonaemia at 5 days old | |
| • Treated with a low protein diet, amino acid mixture and carnitine, which brought progressive clinical improvement | |
| • Frequent mild to moderate episodes of metabolic acidosis over disease course, with ammonia levels of 80-120 μmol/l | |
| • After a prolonged period of constipation, with plasma ammonia at 100 μmol/l, patient initiated on NCG therapy, 50 mg/kg/day | |
| 3(PA) | • 9-year-old sister of patient 2, diagnosed with propionic aciduria at birth as a result of her affected brother, therefore avoiding any decompensation episodes |
| • A low protein diet and carnitine was initiated | |
| • At the age of 3 years she had meningitis with severe metabolic decompensation and hyperammonaemia requiring haemodialysis and severe neurological impairment | |
| • Several subsequent hospital admissions, primarily for food refusal, persistent vomiting with ketosis and hyperammonaemia (up to 200 μmol/l); after her last episode (13 months ago) patient initiated on NCG therapy, 50 mg/kg/day | |
| 4(PA) | • 3-year-old male, born at term after an uncomplicated pregnancy, labour and delivery |
| • Patient presented with severe hyperammonaemia and metabolic acidosis that required haemodialysis | |
| • Diagnosed with PA by organic acid analysis, and started treatment with a low protein diet with amino acids supplementation and carnitine | |
| • Multiple hospital admissions during first two years of life, typically for acute exacerbations due to catabolic states, during which his plasma ammonia level rose up to 200 μmol/l | |
| • Following a severe episode with persistent hyperammonaemia (160 μmol/l) 14 months ago, patient initiated on NCG therapy, 50 mg/kg/day | |
| 5 (MMA) | • 12-year-old female who presented at birth with metabolic decompensation and hyperammonaemia (400 μmol/l) requiring haemodialysis and ventilation support |
| • Medical history characterised by progressive clinical deterioration with increasing episodes of vomiting, acidosis and loss of appetite | |
| • Patient’s kidney function fluctuated over the years, but she has not required dialysis | |
| • Owing to persistent mild acidosis and ammonia level of around 100 μmol/l, patient initiated on NCG therapy, 50 mg/kg/day | |
| 6 (MMA) | • 6-year-old female with slight mental retardation, who presented at the age of 11 months, after an episode of gastroenteritis, with severe metabolic acidosis and hyperammonaemia requiring haemodialysis and ventilation |
| • Responded well to a protein-restricted diet supplemented with carnitine but not to vitamin B12 | |
| • Patient suffering with frequent episodes of metabolic acidosis and hyperammonaemia some of these requiring admission to the ICU; complicated with severe basal ganglia damage | |
| • Patient initiated on NCG therapy, 50 mg/kg/day | |
| 7 (MMA) | • 2-year-old male who presented with metabolic acidosis and severe hyperammonaemia in the first days of life, requiring haemodialysis and ventilator support |
| • Vomiting and poor feeding were present in the first months of life, despite a gastrostomy, requiring multiple admissions for intravenous fluids | |
| • Hyperammonaemia was always present during metabolic acidosis. He also has kidney failure | |
| • Following a recent admission (6 months ago) patient was initiated on NCG therapy, 50 mg/kg/day | |
| 8 (MMA) | • 12-year-old female who presented with neonatal metabolic acidosis and hyperammonaemia requiring therapy and intubation in ICU |
| • Patient began treatment with a protein-restricted diet with amino acids mixture and carnitine | |
| • In the first years of life, she had several episodes of metabolic decompensation with hyperammonaemia (< 100 μmol/l) and vomiting requiring intravenous fluid therapy. Two years ago she presented with severe dystonia episodes refractory to pharmacological treatment, which required subthalamic deep brain stimulation | |
| • Patient required hospitalisation owing to food refusal and frequent vomiting prior to NCG treatment; despite fluid treatment and a low protein diet her ammonia levels increased to 140 μmol/l | |
| • Five months ago, patient was initiated on NCG therapy, 50 mg/kg/day |
ICU, intensive care unit; MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria.
3.2. Decompensation episodes
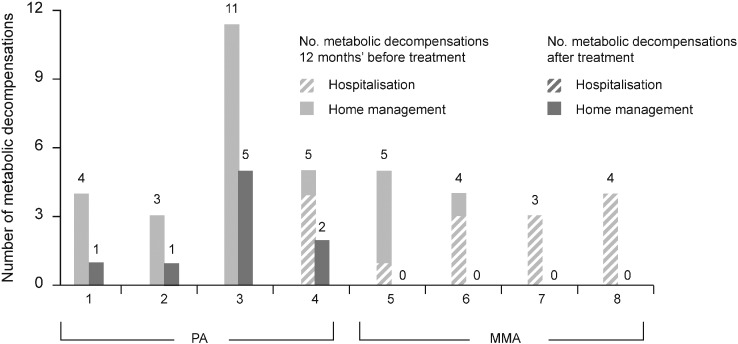
During the year prior to the initiation of continuous treatment with NCG all patients experienced at least three decompensation episodes, and one patient with PA experienced 11. All the patients with MMA and one patient with PA required hospitalisation for a metabolic decompensation during this period. Following treatment with NCG (50 mg/kg/day) for 7–16 months, metabolic decompensation episodes decreased in number and in the severity of ketoacidosis; three patients had no episodes, and none required hospitalisation for metabolic decompensation (Fig. 1). One patient was hospitalised owing to a recurring gastroenteritis; however, the patient did not present with metabolic acidosis or hyperammonaemia. When patients were assessed by age and severity of decompensation episodes, no pattern in the frequency of episodes was observed either before or after NCG treatment.
Fig. 1.
Metabolic decompensations before and after long-term treatment with NCG (50 mg/kg/day) in patients with PA and MMA.
MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria.
3.3. Clinical outcome
Improvements in attention, exercise tolerance, appetite, and strength following continuous NCG treatment were reported for all patients. Over the treatment period, patients had an increase in natural protein intake of 20–50% and gained 0–6.5 kg in body weight (Table 3). This was partly due to the decrease in decompensation episodes and an improvement in appetite.
Table 3.
Change in protein intake and bodyweight per patient following long-term treatment with NCG (50 mg/kg/day) compared with pretreatment values.
| Patient | Diagnosis | Calendar month of treatment initiation | NCG treatment duration (months) | Pre-treatment protein (synthetic protein) intake (g/kg/day) | Increase in natural protein intake (%) | Weight gain (kg) |
|---|---|---|---|---|---|---|
| 1 | PA | September | 9 | 1.0 (0.5) | 50 | 1.5 |
| 2 | PA | November | 10 | 1.5 (0.4) | 20 | nc |
| 3 | PA | November | 10 | 1.2 (0.4) | 50 | 2.8 |
| 4 | PA | May | 12 | 1.5 (0.5) | 50 | 6.5 |
| 5 | MMA | October | 5 | 1.0 | 20 | 0.6 |
| 6 | MMA | March | 5 | 1.3 | – | nc |
| 7 | MMA | February | 4 | 1.0 | 20 | 0.6 |
| 8 | MMA | March | 3 | 1.1 | – | nc |
MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria; nc, no change.
Patient 1, the oldest patient in this cohort at 20 years of age, presented with dilated cardiomyopathy, a mild reduction of systolic function (ejection fraction 39.7%), elevated creatine phosphokinase (CPK) (350–500 U/L; normal value: < 220 U/L) and a CPK-MB of 4.3% (normal value:<3%), which required medication with beta-blocker drugs. He was asthenic and easily fatigued. After 12 months of NCG treatment, cardiac function improved, with an increase in ejection fraction of ~ 43.0% and normalisation of CPK. Interestingly, owing to a delay in drug supply, this patient had a half dose of NCG for 2 months during which his CPK rose to 373 U/L. These symptoms dissipated when full-dose treatment was reinstated.
3.4. Biochemical profiles
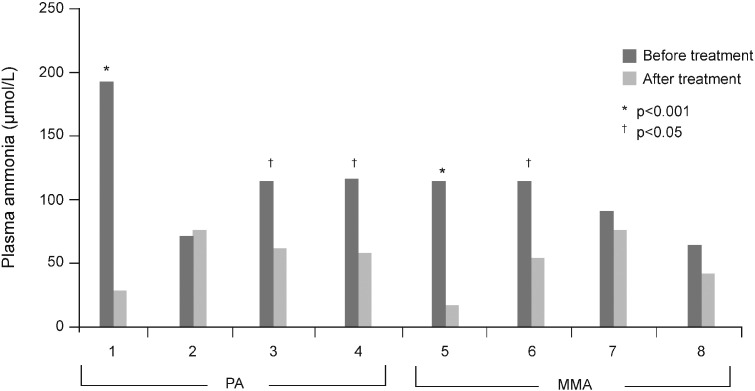
Significant reductions in plasma ammonia compared with pre-treatment levels were observed in five patients (two patients with MMA and three with PA) (Fig. 2); none of these patients presented with decompensation episodes during the period of NCG follow-up. Levels were reduced or maintained in the normal range in the remainder, with the exception of one patient whose levels increased slightly post-NCG initiation. In patient 1, where NCG treatment was reduced owing to the shortage of the drug, there was a slight increase in the level of ammonia (80 μmol/L); this normalised after the reintroduction of the drug at the dose of 50 mg/kg/day.
Fig. 2.
Plasma ammonia levels before and after long-term treatment with NCG (50 mg/kg/day) in patients with PA and MMA.
MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria.
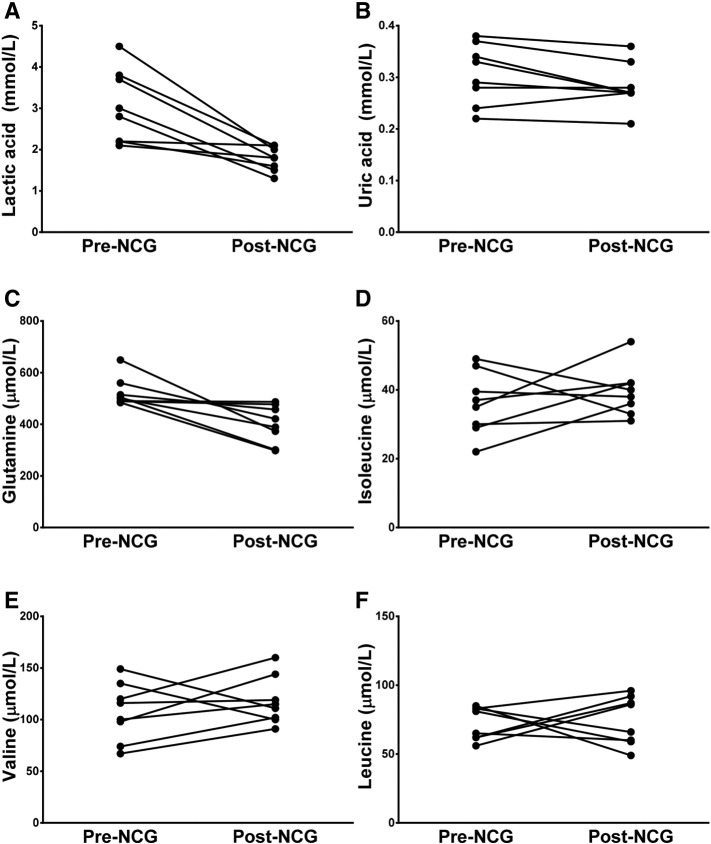
Reductions in lactic acid and uric acid were observed in five patients following treatment compared with pre-treatment levels (Fig. 3). After NCG treatment initiation, lactic acid values ranged from 1.3 to 2.1 mmol/L (normal range: 0.56–1.40 mmol/L) and uric acid values ranged from 0.21 to 0.36 mmol/L (normal range: 0.26–0.45 mmol/L) [10]; these data were not statistically significant. Amino acid analyses were performed during the routine monitoring of disease progression (Fig. 3). In all patients, no statistically significant modifications were observed in the profile of glycine and glutamine after the initiation of NCG treatment, whereas in branched-chain amino acids (leucine, isoleucine and valine), normalisation of isoleucine was observed in three patients, possibly due to the increase in protein intake.
Fig. 3.
Changes in plasma levels of: A) lactic acid, B) uric acid, C) glutamine, D) isoleucine, E) valine, and F) leucine levels after long-term treatment with NCG (50 mg/kg/day) in patients with PA and MMA.
MMA, methylmalonic aciduria; NCG, N-carbamylglutamate; PA, propionic aciduria.
4. Discussion
Because long-term survival in patients with PA and MMA has improved over recent years, long-term complications have become increasingly apparent and pose new challenges in terms of patient care. The efficacy of NCG in the acute treatment of patients with organic acidurias experiencing metabolic decompensation is well established [3], [6], [8], [11], [12], [13], [14]. However, to our knowledge, this is the first report of the long-term use of NCG in patients with PA or MMA.
Long-term management of both PA and MMA is based on reducing the production of methylmalonic or propionic acid by restricting natural protein, maintaining an optimal calorie intake, supplementing with carnitine and reducing intestinal production of propionate by metronidazole [15]. In this study, long-term continuous administration of NCG was shown to stabilise metabolic control, reducing the number and severity of episodes of metabolic decompensation in patients with PA and MMA spanning a broad range of ages, and eliminating the need for hospitalisation. Patients were selected for long-term treatment with NCG if they had frequent metabolic decompensations and elevated ammonia levels. In seven out of eight patients, the ammonia concentration was decreased or maintained within the normal range after treatment initiation, and improvements in clinical outcomes were reported for all patients. Management of ammonia in these patients improved appetite, and patients were able to increase their natural protein intake. Subsequently, patients gained up to 6.5 kg in weight over the treatment period and, in addition to increased appetite, reported improvements in attention, exercise tolerance and strength.
Dietary management including a protein-restricted diet is often the first-line treatment in the management of decompensation episodes in patients with PA and MMA. However, there are limitations to the effectiveness of this approach because an extremely restricted diet should not generally be implemented for longer than 48 h [4], [16]. As observed previously in the treatment of NAGS deficiency, long-term continuous administration of NCG may allow a faster discontinuation of a protein-restricted diet and consequently limit muscular catabolism and subsequent hyperammonaemia [17].
The authors of a number of studies have reported the efficacy of NCG in reducing elevated serum ammonia levels in patients with PA and MMA during acute metabolic decompensation [3], [4], [5], [6], [8], [9], [12], [13], [14], [18], and several investigators have attributed this to NCG stimulation of ureagenesis [8], [18], [19], [20]. However, all of these studies related to brief treatment (of a few hours up to 3 days) focused on the rapid normalisation of ammonia in the acute decompensation state. In this analysis, long-term treatment with NCG, at a dose determined on the basis of a previous long-term study of patients with NAGS deficiency [21], markedly reduced ammonia levels in five out of eight patients, with only one patient with PA experiencing a slight increase in ammonia levels. The relationship between NCG and ammonia was clear; moreover, all patients experienced a reduction and stabilisation in the number of metabolic decompensations following treatment. There were no decompensation crises in patients with MMA, and none of the patients with PA required hospitalisation during long-term treatment with NCG, suggesting clinical improvement even with sub-optimal improvement in ammonia levels.
Although the frequency of metabolic decompensation decreases with increasing age [22], it is worth noting that in our retrospective analysis, no pattern was observed in the frequency of decompensation episodes in the period before or after NCG treatment when patients were assessed by age or severity of episodes. However, as stated previously, when the number and severity of episodes in the 12 months before and post long-term NCG initiation were assessed, all patients experienced a reduction and stabilisation in the number of metabolic decompensations following treatment. Ammonia concentration also decreased for the majority of patients.
Hyperammonaemia is a frequent corollary of metabolic decompensation in patients with organic acidurias; indeed, elevated ammonia and pathological changes in the acid–base balance reflecting metabolic acidosis are the two most reliable biochemical indicators of metabolic decompensation in patients with PA and MMA [23], [24]. The underlying mechanism of hyperammonaemia in organic aciduria is debated. The most quoted hypothesis is that the increase of ammonia is secondary to the inhibition of an early step in the urea cycle [3], [25], [26]. Inhibition of the TCA cycle is deemed to contribute to a greater or lesser degree [14], and hyperammonaemia has also been suggested to relate primarily to the inability to maintain adequate concentrations of glutamate precursors through a dysfunctional TCA cycle [27]. The metabolic sequelae are exacerbated by the fact that patients with PA and MMA frequently refuse food, leading to a decrease in glucose availability and an increase in protein catabolism, with a consequent build-up of toxic metabolites and propionate produced by bacteria in the gut [14].
In this analysis, a decrease in ammonia levels was the most prominent result of long-term NCG treatment. Ammonia levels are measured routinely in clinical practice in patients with PA and MMA, and the data are reported here in that context. Post long-term NCG initiation, ammonia levels were in the normal range, indicating that NCG can enhance the rate of ureagenesis in organic acidurias, presumably by increasing the rate of carbamoyl phosphate synthesis in the urea cycle [28]. The effects of NCG therapy are not apparent when patients are treated for acute symptoms, but become noticeable after long-term intervention.
Because this is an observation of patients in clinical practice, the plasma amino acid data were also measured during the routine follow-up of these patients. No significant change was observed in plasma glycine levels during the treatment, even though the ammonia levels normalised. The independent nature of the two findings can be explained by the mode of action of NCG, because NCG is not expected to alter the hepatic concentration of propionyl-CoA, which inhibits the glycine cleavage system [29]. The decrease in glutamine observed in five patients who also had an increase in natural protein intake could have been due to the effect of NCG in augmenting ureagenesis and consequently decreasing plasma glutamine [28]. We hypothesised that the normalisation of the branched-chain amino acid profiles (leucine, isoleucine and valine) observed in some of these patients may have been due to the increased protein intake and decreased metabolic decompensation episodes reported for long-term NCG treatment. Because decompensation episodes are frequently observed in the long-term follow-up of patients with PA and MMA, we suggest that this is a direct effect of long-term NCG administration. Five patients with elevated plasma lactic acid levels experienced fluctuating reductions as metabolic decompensation declined following NCG treatment, whereas the remainder stayed within the normal range (0.5–2.2 mmol/L) [10]. Plasma uric acid levels decreased slightly, but not significantly, following NCG treatment.
In this analysis, NCG was more efficacious in patients with PA compared with those with MMA, possibly because the treatment of MMA was less prolonged. There were indications of stabilisation in cardiac function during long-term NCG treatment in the patient with the most severe heart condition, and stabilisation of heart deficits in other patients. This would be consistent with a beneficial effect of NCG on mitochondrial cardiomyopathy [30]. No improvement in kidney function in the four MMA patients or the neurological status of the three patients with severe dystonia was apparent; the relatively limited duration of treatment could account for this. Importantly, the long-term use of NCG did not appear to be associated with any side effects, and all patients or their parents chose to continue treatment owing to improvements in clinical outcome.
Limitations of this analysis include the small sample size, and the lack of formal statistical analysis and study controls. We would stress that this is a retrospective observational analysis of patients in clinical practice; therefore, the results are preliminary and provisional, and require further verification and exploration. Nevertheless, it is worth noting that, to the best of our knowledge, conventional treatments of organic aciduria with carnitine and metronidazole are also based on observational data and have not been proven effective in clinical trials. This is of particular interest because metronidazole treatment does have associated adverse events.
In conclusion, the present findings suggest that, in addition to its short-term benefits for the acute treatment of hyperammonaemia, NCG appears to be effective and well tolerated as a long-term treatment in patients with severe PA and MMA. Prolonged benefits for general clinical outcomes were reported for all patients in this single-centre cohort, and five out of eight patients had lower ammonia levels after NCG initiation. Therefore, further studies on the long-term treatment of NCG in patients with PA and MMA are warranted.
Acknowledgements
The authors would like to thank COMETA ASMME (Associazione Studio Malattie Metaboliche Ereditarie) for providing a grant for this research, Simone Boniface from Springer Healthcare Communications for preparing the initial draft of the manuscript, and Nina C Kennard from iS LifeScience for editorial assistance throughout the development of the manuscript. Editorial assistance was funded by Orphan Europe.
References
- 1.Kölker S., Valayannopoulos V., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J. Inherit. Metab. Dis. 2015;38:1059–1074. doi: 10.1007/s10545-015-9840-x. [DOI] [PubMed] [Google Scholar]
- 2.Coudé F.X., Sweetman L., Nyhan W.L. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J. Clin. Invest. 1979;64:1544–1551. doi: 10.1172/JCI109614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippi L., Gozzini E., Fiorini P., Malvagia S., la Marca G., Donati M.A. N-carbamylglutamate in emergency management of hyperammonemia in neonatal acute onset propionic and methylmalonic aciduria. Neonatology. 2010;97:286–290. doi: 10.1159/000255168. [DOI] [PubMed] [Google Scholar]
- 4.Schofield J.P., Cox T.M., Caskey C.T., Wakamiya M. Mice deficient in the urea-cycle enzyme, carbamoyl phosphate synthetase I, die during the early neonatal period from hyperammonemia. Hepatology. 1999;29:181–185. doi: 10.1002/hep.510290112. [DOI] [PubMed] [Google Scholar]
- 5.Daniotti M., la Marca G., Fiorini P., Filippi L. New developments in the treatment of hyperammonemia: emerging use of carglumic acid. Int. J. Gen. Med. 2011;4:21–28. doi: 10.2147/IJGM.S10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vianey-Saban C., Acquaviva-Bourdain C., Levrat V., Boyer S., Forest I., Fouilhoux A. Role of N-carbamylglutamate in undiagnosed neonatal hyperammonaemia. In: Bachmann C., Haberle J., Leonard J., editors. Pathophysiology and Management of Hyperammonaemia. Int Symposium. 2006. pp. 114–128. [Google Scholar]
- 7.Baumgartner M.R., Hörster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abacan M., Boneh A. Use of carglumic acid in the treatment of hyperammonaemia during metabolic decompensation of patients with propionic acidaemia. Mol. Genet. Metab. 2013;109:397–401. doi: 10.1016/j.ymgme.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Ah Mew N., McCarter R., Daikhin Y., Nissim I., Yudkoff M., Tuchman M. N-carbamylglutamate augments ureagenesis and reduces ammonia and glutamine in propionic acidemia. Pediatrics. 2010;126:e208–e214. doi: 10.1542/peds.2010-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burtis C., Ashwood E., Bruns D., editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. fourth ed. Elsevier Inc; 2006. [Google Scholar]
- 11.Gebhardt B., Dittrich S., Parbel S., Vlaho S., Matsika O., Bohles H. N-carbamylglutamate protects patients with decompensated propionic aciduria from hyperammonaemia. J. Inherit. Metab. Dis. 2005;28:241–244. doi: 10.1007/s10545-005-5260-7. [DOI] [PubMed] [Google Scholar]
- 12.Kasapkara C.S., Ezgu F.S., Okur I., Tumer L., Biberoglu G., Hasanoglu A. N-carbamylglutamate treatment for acute neonatal hyperammonemia in isovaleric acidemia. Eur. J. Pediatr. 2011;170:799–801. doi: 10.1007/s00431-010-1362-9. [DOI] [PubMed] [Google Scholar]
- 13.Levrat V., Forest I., Fouilhoux A., Acquaviva C., Vianey-Saban C., Guffon N. Carglumic acid: an additional therapy in the treatment of organic acidurias with hyperammonemia? Orphanet J. Rare Dis. 2008;3:2. doi: 10.1186/1750-1172-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soyucen E., Demirci E., Aydin A. Outpatient treatment of propionic acidemia-associated hyperammonemia with N-carbamoyl-l-glutamate in an infant. Clin. Ther. 2010;32:710–713. doi: 10.1016/j.clinthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Wendler U., de Baulny B. Branched-chain organic acidurias/acidemias. In: Fernandos J., Saudubray J.-M., van den Berghe G., Walter J.H., editors. Inborn Metabolic Diseases. fifth ed. Springer; Heidelberg: 2012. pp. 245–262. [Google Scholar]
- 16.Häberle J., Boddaert N., Burlina A., Chakrapani A., Dixon M., Huemer M. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guffon N., Gessler P., Galloway P., Martinez-Pardo M., Meli C., Mulder M.F. Treatment of NAGS deficiency: retrospective data on 23 patients treated with carglumic acid over 16 years (abstract) Mol. Genet. Metab. 2011;102:286–287. [Google Scholar]
- 18.Tuchman M., Caldovic L., Daikhin Y., Horyn O., Nissim I., Nissim I. N-carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr. Res. 2008;64:213–217. doi: 10.1203/PDR.0b013e318179454b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ah Mew N., Payan I., Daikhin Y., Nissim I., Nissim I., Tuchman M. Effects of a single dose of N-carbamylglutamate on the rate of ureagenesis. Mol. Genet. Metab. 2009;98:325–330. doi: 10.1016/j.ymgme.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mew N.A., Yudkoff M., Tuchman M. Stable isotopes in the diagnosis and treatment of inherited hyperammonemia. J. Pediatr. Biochem. 2014;4:57–63. doi: 10.3233/JPB-140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Häberle J. Role of carglumic acid in the treatment of acute hyperammonemia due to N-acetylglutamate synthase deficiency. Ther. Clin. Risk Manag. 2011;7:327–332. doi: 10.2147/TCRM.S12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grünert S.C., Müllerleile S., De Silva L., Barth M., Walter M., Walter K. Propionic acidemia: clinical course and outcome in 55 pediatric and adolescent patients. Orphanet J. Rare Dis. 2013;8:6. doi: 10.1186/1750-1172-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwickler T., Haege G., Riderer A., Hörster F., Hoffmann G.F., Burgard P. Metabolic decompensation in methylmalonic aciduria: which biochemical parameters are discriminative? J. Inherit. Metab. Dis. 2012;35:797–806. doi: 10.1007/s10545-011-9426-1. [DOI] [PubMed] [Google Scholar]
- 24.Zwickler T., Riderer A., Haege G., Hoffmann G.F., Kölker S., Burgard P. Usefulness of biochemical parameters in decision-making on the start of emergency treatment in patients with propionic acidemia. J. Inherit. Metab. Dis. 2014;37:31–37. doi: 10.1007/s10545-013-9621-3. [DOI] [PubMed] [Google Scholar]
- 25.Lévesque S., Lambert M., Karalis A., Melancon S., Russell L., Braverman N. Short-term outcome of propionic aciduria treated at presentation with N-carbamylglutamate: a retrospective review of four patients. JIMD Rep. 2012;2:97–102. doi: 10.1007/8904_2011_54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coudé F.X., Grimber G., Parvy P., Kamoun P. N-acetyl glutamate synthetase in human liver: regulation of activity by l-arginine and N-acetylglutamate. Biochem. Biophys. Res. Commun. 1981;102:1016–1020. doi: 10.1016/0006-291x(81)91639-9. [DOI] [PubMed] [Google Scholar]
- 27.Filipowicz H.R., Ernst S.L., Ashurst C.L., Pasquali M., Longo N. Metabolic changes associated with hyperammonemia in patients with propionic acidemia. Mol. Genet. Metab. 2006;88:123–130. doi: 10.1016/j.ymgme.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Ah Mew N., McCarter R., Daikhin Y., Nissim I., Yudkoff M., Tuchman M. N-carbamylglutamate augments ureagenesis and reduces ammonia and glutamine in propionic acidemia. Pediatrics. 2010;126:e208–e214. doi: 10.1542/peds.2010-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayasaka K., Narisawa K., Satoh T., Tateda H., Metoki K., Tada K. Glycine cleavage system in ketotic hyperglycinemia: a reduction of H-protein activity. Pediatr. Res. 1982;16:5–7. doi: 10.1203/00006450-198201001-00002. [DOI] [PubMed] [Google Scholar]
- 30.Meyers D.E., Basha H.I., Koenig M.K. Mitochondrial cardiomyopathy: pathophysiology, diagnosis, and management. Tex. Heart Inst. J. 2013;40:385–394. [PMC free article] [PubMed] [Google Scholar]