Abstract
Purpose of review
The exposome concept proposes a comprehensive assessment of environmental exposures from the prenatal period onwards. However, determining exposure timing especially over the prenatal period is a major challenge in environmental epidemiologic studies.
Recent findings
For decades teeth have been used to estimate long-term cumulative exposure to metals. Recently developed high-dimensional analytical methods that combine sophisticated histological and chemical analysis to precisely sample tooth layers that correspond to specific life stages have the potential to reconstruct the exposome in the second and third trimesters of prenatal development and during early childhood.
Summary
A retrospective temporal exposomic approach that precisely measures exposure intensity and timing during prenatal and early childhood development would substantially aid epidemiologic investigations, particularly case–control studies of rare health outcomes.
Keywords: teeth, exposome, fetal, environmental, metals, organics, stress, diet
Introduction
The ‘Exposome’ concept was introduced in 2005 to address the disparity between the genomic sciences, where rapid technological advances provided an expanse of high-precision analyses, and the environmental exposure sciences that measured a small fraction of the thousands of environmental exposures individuals experience [1]. The exposome concept encompasses lifecourse environmental exposures (including lifestyle factors), from the prenatal period onwards [1]. It is important to consider that the exposome includes not only external exposures but also internal factors (e.g. inflammation, infection, and the microbiome) [2]. While the definition of the exposome evolves (see Miller and Jones [3]), the fundamental concept of the exposome continues to gain momentum internationally. Notably, in the US, the Human Exposome Project supported by the National Institutes of Health and, in Europe, the HELIX, HEALS and EXPOsOMICS projects, as well as projects at various academic institutions are examining specific aspects of the exposome.
Challenges to Uncovering the Fetal Exposome
There are many challenges that must be overcome before the exposome concept can be taken from a theoretical foundation to wide-spread practical application, as was the case with the genomic sciences [2]. Foremost, the exposome, unlike the genomic sequence, is highly variable and dynamic, and continues to evolve throughout the individual’s lifetime [1, 4]. Longitudinal birth cohort studies that collect biomarkers of environmental chemical exposure during pregnancy and then follow offspring into later life would provide the strongest evidence to assess the impact of exposures during key developmental windows in humans. However, the expense and time required for such studies are major barriers to investigating lower frequency conditions with long latency periods. Another important barrier to studying the fetal exposome is that maternal biomarkers do not necessarily provide accurate measures of fetal exposure for all chemicals. Reliance on maternal biomarkers of fetal exposure fails to account for variability in placental transport and metabolism, potentially overlooking the significant interplay at the maternal-fetal interface [5–7]. Umbilical cord blood has been successfully collected at birth in epidemiologic studies and has provided valuable exposure information [8–11]. However, for compounds with a short half-life in blood, cord blood levels can only provide information on the latter part of the third trimester.
The Tooth Exposome
To overcome the need of large sample sizes and have a direct measure of the fetal environment, we propose that the exposome biomarkers would benefit from two attributes - be retrospective and incorporate temporal signatures. This has recently been referred to as the ‘retrospective temporal exposome’ [12]. For health outcomes that occur at lower frequencies, this biomarker would be applied in population-based case-control designs. Unlike contemporary approaches that are cross-sectional, such biomarkers would provide time-series exposure data similar to that obtained from a longitudinal study, whilst doing so retrospectively.
In this overview, we propose the use of teeth as a matrix that provides an opportunity to retrospectively reconstruct the dynamic exposome. We also identify the limitations of the use of teeth, which future work will hopefully address. Key aspects of the well-defined incremental formation of teeth and its relevance to exposure assessment have been detailed previously [13 15].
Components of the Exposome that are Measurable in Tooth Matrix Biomarkers
i. Metallomics
Metals have been measured in teeth for many decades, with lead being the most studied toxicant in teeth [16–18]. Because many metal toxicants accumulate preferentially in bone, early studies considered teeth as a useful biomarker for measuring long-term exposure [16–18]. Most notable are studies on lead, as the skeletal compartment comprises the major depository of total body burden and is also a potential source of internal exposure due to release of lead during bone remodeling, such as occurs in pregnancy or osteoporosis [19]. Several studies have shown that children living in lead contaminated locations have higher lead levels in their deciduous teeth than children from lower exposure environments [17, 18, 20–22]. The suitability of teeth as exposure biomarkers for other metals was also explored (cadmium, for example [23]).
Over the last two decades, microspatial sampling combined with sophisticated histological analysis has provided a means to uncover the timing of metal uptake, including prenatal exposure, from teeth biomarkers [24–29]. However, detailed validation against environmental samples and other biomatrices has only been performed in the last five years. For validation of Mn, there was a significant positive association of levels in parts of dentine formed in the second trimester with Mn loading in floor dust sampled during the second trimester of pregnancy [30]. That study also showed that Mn levels in dentine adjacent the neonatal line was strongly associated with cord blood Mn concentrations, both biomarkers reflecting Mn uptake close to the time of birth. Another study undertook detailed validation of tooth Pb measurements against maternal pregnancy blood levels and also bone lead levels postpartum [13]. Of note for metals analysis is the application of laser ablation-based mass spectrometry that allows measurement of multiple metal targets in the same scan as shown in Figure 1a.
Figure 1.
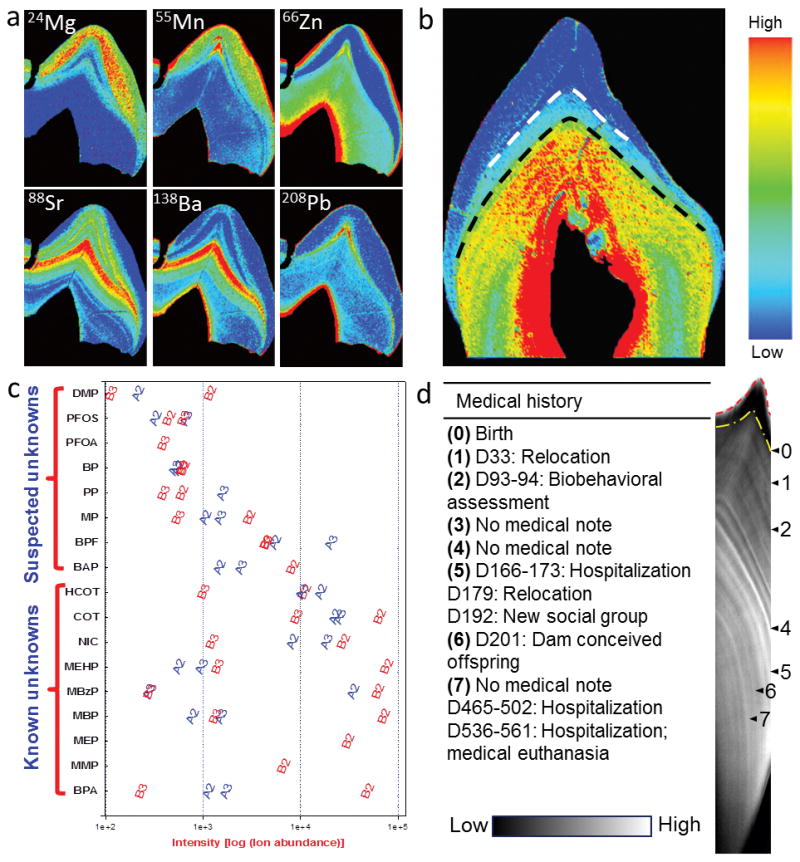
Components of the exposome measureable using tooth matrix biomarkers. (a) Metallomics: elemental bio-imaging of teeth using laser ablation-based mass spectrometry provides detailed spatial distribution of multiple metals across enamel and dentine. (b) Dietary transitions: recently developed biomarkers using barium signatures distinguish breast milk intake (between dashed lines) from introduction of infant formula (below black dashed line). (c) Targeted and untargeted organics analysis in teeth reveals exposure to multi-class organic chemicals within (2nd versus 3rd trimester) and between (Child A versus Child B) children. QTOFMS was operated in a dual electrospray ionization mode (positive and negative modes). Study design and results are presented in Andra et al [12]. Abbreviations for the chemicals on Y-axis: BPA, bisphenol A; MMP, mono-methyl phthalate; MEP, mono-ethyl phthalate; MBP, mono-nbutyl phthalate; MBzP, mono-benzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; NIC, nicotine; COT, cotinine; HCOT, hydroxycotinine; BAP; bisphenol AP; BPF, bisphenol F; MP, methyl paraben; PP, propyl paraben; BP, butyl paraben; PFOA, pentadecafluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; and DMP, dimethylphosphate. (d) Uncovering historical exposure to external stressors: Raman spectroscopic analysis of macaque tooth dentine shows signatures that correspond to various medical events.
ii. Dietary components and essential nutrients
Trace element and stable isotope signatures in teeth (and bone) have been used for several decades to reconstruct major diet transitions in past populations, such as terrestrial versus marine resource exploitation [64]. Typically, the ratio of a non-essential element to a chemically similar essential element is used to determine the trophic steps up a food chain [65]. The most common ratios used to assess diet in past populations are Sr/Ca and Ba/Ca. Due to the process known as biopurification, Sr/Ca and Ba/Ca ratios decrease during metabolic processes that involve Ca leading to a decrease in these ratios in consumers relative to diet [66]. The ratio decrease between diet and tooth values are relatively constant so that the diet of past populations can be compared against known herbivores and carnivores to identify the relative importance of plant and animal products in the diet [67]. Similarly, stable isotope ratios of light elements carbon and nitrogen also show partitioning through metabolic processes used to identify trophic level [68]. Carbon and nitrogen isotopes are typically measured from dentine collagen and reflect the major protein sources within a diet [69]. Recently, oxygen isotope values measured from the skeletal remains of Richard III were interpreted as an increase in wine intake [70]. Due to the sensitivity and sample preparation restrictions of the techniques used for stable isotope analysis, the temporal resolution is limited.
Stable isotopes have been used to reconstruct weaning practices based on the child being one trophic level higher than mother [71–74], however several drawbacks have been identified in relation to assumptions of trophic offsets [68, 72, 75]. The study of trace elements rather than isotopes offers the advantage of better temporal resolution. Barium was recently identified as a sensitive biomarker in teeth to infant diet transitions from exclusive breastfeeding to the introduction of infant formula and process of weaning [76]. In children’s teeth, Ba levels in dentine rose after birth and remained relatively steady for the duration of exclusive breastfeeding, rising again at the introduction of infant formula (Figure 1b). The distinction in diet is made possible due to differences in Ba dietary exposure. A similar pattern was observed in teeth from captured macaques that showed a decrease in Ba over the process of weaning. Strontium has also been used to determine weaning patterns in past populations and nonhuman primates [77, 78] but Ba is considered a more sensitive marker of diet [74, 76, 79].
The isotopic partitioning of other elements (Mg, Fe, Cu and Zn) from diet to tissue is also under investigation with the promise that the use of multiple elemental and isotopic systems will provide more robust results [65]. The isotopic composition of teeth (and bones) can also provide information on migration and habitat conditions [64]. The precise determination of diet components in mixed diets remains a challenge via stable isotope ratio analysis [64].
iii. Environmental organics: Targeted exposome analysis
Current prenatal exposome approaches do not allow proper characterization of timing of exposure to an organic contaminant or mixtures and association with health effects at a later life stage [31]. Correlations between targeted organic contaminant concentrations in maternal and fetal matrices collected at various stages of pregnancy and child birth suggest inconsistent associations and mixed outcomes in assessing prenatal exposures [9]. It is well acknowledged that a unifying bio-matrix to assess perinatal exposome for xenobiotics is needed [32]. Teeth offer a unique advantage of accurate fetal organic chemical exposure assessment on a temporal scale [33]. This is not true for conventional biomarkers such as maternal bio-matrices due to variations in placental transport or for cord blood due to short half-lives of many chemicals [34, 35].
Teeth analysis was explored for the presence and quantification of various organic chemicals, contaminants and metabolites such as analgesics, pesticides and plastics additives [36], anesthetics [37], antibiotics [38, 39], illegal drugs [37, 40], metabolites of alcohol [41] and tobacco [42–44], and organochlorines [45–48]. The major limitation of the methodologies employed in these studies was to grind and analyze whole teeth (that constitutes the tissue and blood vessels within the pulp chamber) leading to exposure misclassification because of differential deposition of organic chemicals and contaminants in different tooth compartments. Andra and colleagues [12, 33] have demonstrated micro-spatial organic chemical measurements of specific growth rings in dentine that correspond to trimester-specific fetal developmental windows (Figure 1c). For example, mono-benzyl phthalate was quantified in dentine layers formed during the second and third trimester using liquid chromatography coupled tandem mass spectrometry (LC-MS/MS) targeted analysis [33]. This work needs to be expanded to encompass a comprehensive validation of the dentine-phthalate biomarkers against data available from conventional bio-matrices such as maternal urine during pregnancy and at birth, and newborn and childhood urine.
iv. Environmental organics: Untargeted exposome profiling
Analyzing bio-matrices for measuring the totality of exposures to organic pollutants is (a) to assess a fraction of the vast and complex internal chemical milieu made of exogenous sources and endogenous responses, [49] and (b) a component of the top-down approach for scaling human exposome [50]. Advances in high-resolution mass spectrometers (MS), such as fouriertransform MS, [51] hybrid ion trap-orbitrap MS, [52] quadrupole time-of-flight MS, [53, 54] allow increased metabolic detection [55] and capture a wider, untargeted chemical space in the exposome [56, 57]. The power of measuring environmental organics exposome as a tool to evaluate health risks is gaining attention spanning several scientific domains [1, 4, 58, 59]. The blood exposome was the first effort directed towards incorporating literature data for about 1600 exo- and endogenous chemicals into identifying associated metabolic pathways and disease etiologies [60]. Other emerging exposome approaches that consider measuring organics with distinct features are (a) tooth exposome that utilizes a novel bio-matrix, [12] (b) volatolomics that use a specific physical fraction (viz., exhaled breath or volatile organic compounds pool), [61, 62], and (c) pregnancy exposome that relies on collective data from multiple matrices and multiple sampling points during prenatal and child birth phase [63].
Recently, we reconstructed the prenatal and early childhood exposure to multiple organic chemical classes using teeth [12]. We performed global screening of small molecules in trimester-specific formed dentine layers from deciduous teeth using liquid chromatography coupled quadrupole time-of-flight mass spectrometry (QTOF-LC/MS) metabolomics approach. QTOF-LC/MS analyses show unique and differential chemical signatures of environmental exposure that are individual and development-stage dependent. The results of this study (a) revealed more than 12,000 unique chemical signatures in trimester-specific dentine layers, (b) indicate high inter- and intra-child variability in screened chemical profiles, (c) show novel ‘known unknowns’ and ‘suspected unknowns’ compounds, (d) demonstrate exposure misclassification error that can cause misleading inferences about causality, and (e) most importantly, the reconstruction of exposure was done 7 to 10 years after prenatal and early childhood exposure. An example to demonstrate inter- and intra-individual differences in chemical fingerprints on a temporal prenatal scale is shown in Figure 1c. In future, we will develop a hybrid approach for tooth exposome-wide measures for reconstructing fetal exposures. First, we will apply discovery methods that employ QTOF-MS detection after liquid and/or gas chromatography separation to generate large datasets, and full mass spectra scan plus MS/MS fragmentation of organic compounds and biomolecules. Second, we will perform targeted analysis of the relevant biomarkers after accurate mass identification of compounds from above, and library searching combined with advanced chemometrics and bioinformatics data mining tools. Finally, the findings will be validated in multiple matrices and exposure cases from an exposome perspective.
v. Stress signatures
Formation of the neonatal line (NL) is thought to be due to disturbances in the cells which lay down the tooth matrix for mineralization [80] and it’s width has been related to the difficulties in delivery [81]. Other accentuated growth lines have been observed, largely in enamel, which are similarly believed to be due to the disruption of tooth matrix deposition due to external stressors, both physical and social [82–84]. Several studies have compared the timing of these accentuated lines with clinical events such as injuries, bouts of dehydration/diarrhea, and hospitalizations [85–87] and other events such as weaning [88, 89], and separation from dam [90]. These accentuated lines are typically identified and aged using light microscopy. However, the response to external stressors involves complex mechanisms and this technique cannot identify the biological systems or pathways impacted. Additionally, light microscopy methods are subjective, and highly dependent on operator expertise, quality of sample preparation and microscopy technique.
Recently a novel multi-tiered approach was presented that enables the identification of specific stress impacted systems using objective techniques to identify different signals in teeth and overlaying these with temporal mapping [90]. Firstly, elemental signals in teeth observed through laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) imaging indicated disruptions specific to bone remodeling. Second, it was demonstrated that molecular markers of specific homeostatic pathways activated in response to external stressors could be targeted by direct antibody labelling on thin sections of teeth. The final approach used Raman spectroscopy to chemically image teeth at high resolution that revealed both fine-scale regular rhythms and accentuated lines that corresponded to medical events (Figure 1d). Though this study was performed on a small biomedical animal model, it demonstrates the potential of these methods to measure an individual’s stress response to external stimuli across a population experiencing the same challenges.
Conclusion
Tooth matrix biomarkers provide an opportunity to incorporate the intensity and timing of exposure in environmental health studies. Recent advances in technology allow high dimension analyses of a large range of targets in a single scan, which takes us closer to the ideal of the exposome concept of capturing the entirety of exposures over a life-time. In-depth validation and recognition of the limitations of dental tissues, including missing information during the first trimester, are important considerations for future development of tooth matrix biomarkers.
Key Points.
Fetal and early postnatal development comprise critical developmental periods when environmental stressors may disrupt life-long health trajectories
Reconstructing the exposome during this time period is a major challenge in epidemiologic research due to the need for large sample sizes and long follow-ups
Tooth matrix biomarkers incorporate the intensity and timing of exposure can overcome some of these challenges
Acknowledgments
Financial support and sponsorship
This work is supported by National Institutes of Environmental Health Sciences.
Footnotes
Conflicts of interest
MA is supported by National Institutes of Environmental Health Sciences grants DP2ES025453 and R00ES019597. The remaining authors have no conflicts of interest.
References
- 1.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 2.Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science. 2010;330(6003):460–1. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. 2014;137(1):1–2. doi: 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41(1):24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- 5.Needham L, et al. Partition of Environmental Chemicals between Maternal and Fetal Blood and Tissues. Environmental Science and Technology. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudge C, et al. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11(7):1322–1330. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 7.Yoon M, et al. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol Sci. 2009;112(1):44–58. doi: 10.1093/toxsci/kfp198. [DOI] [PubMed] [Google Scholar]
- 8.Aylward LL, et al. Relationships of chemical concentrations in maternal and cord blood: a review of available data. J Toxicol Environ Health B Crit Rev. 2014;17(3):175–203. doi: 10.1080/10937404.2014.884956. [DOI] [PubMed] [Google Scholar]
- 9.Cooke GM. Biomonitoring of human fetal exposure to environmental chemicals in early pregnancy. J Toxicol Environ Health B Crit Rev. 2014;17(4):205–24. doi: 10.1080/10937404.2014.898167. [DOI] [PubMed] [Google Scholar]
- 10.Delvaux I, et al. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res. 2014;132:24–32. doi: 10.1016/j.envres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–7. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12**.Andra SS, et al. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: Towards a retrospective temporal exposome. Environ Int. 2015;83:137–45. doi: 10.1016/j.envint.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M, et al. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9(5):e97805. doi: 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulson B, Wilson D. History of lead exposure in children revealed from isotopic analyses of teeth. Arch Environ Health. 1994;49(4):279–83. doi: 10.1080/00039896.1994.9937480. [DOI] [PubMed] [Google Scholar]
- 15.Rabinowitz MB, Leviton A, Bellinger D. Relationships between serial blood lead levels and exfoliated tooth dentin lead levels: models of tooth lead kinetics. Calcif Tissue Int. 1993;53(5):338–41. doi: 10.1007/BF01351840. [DOI] [PubMed] [Google Scholar]
- 16.Altshuller LF, et al. Deciduous teeth as an index of body burden of lead. The Journal of Pediatrics. 1962;60(2):224–229. doi: 10.1016/s0022-3476(62)80040-7. [DOI] [PubMed] [Google Scholar]
- 17.Needleman HL, Shapiro IM. Dentine lead levels in asymptomatic Philadelphia school children: subclinical exposure in high and low risk groups. Environ Health Perspect. 1974;7:27–31. doi: 10.1289/ehp.74727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Needleman HL, et al. Subclinical lead exposure in philadelphia schoolchildren. Identification by dentine lead analysis. New England Journal of Medicine. 1974;290(5):245–248. doi: 10.1056/NEJM197401312900504. [DOI] [PubMed] [Google Scholar]
- 19.Hu H. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environmental Health Perspectives. 1998;106(Suppl 4):961–967. doi: 10.1289/ehp.98106s4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMichael AJ, et al. Tooth lead levels and IQ in school-age children: the Port Pirie Cohort Study. Am J Epidemiol. 1994;140(6):489–99. doi: 10.1093/oxfordjournals.aje.a117275. [DOI] [PubMed] [Google Scholar]
- 21.Ewers U, et al. Lead in deciduous teeth of children living in a non-ferrous smelter area and a rural area of the FRG. Int Arch Occup Environ Health. 1982;50(2):139–51. doi: 10.1007/BF00378076. [DOI] [PubMed] [Google Scholar]
- 22.Grobler SR, Theunissen FS, Maresky LS. Evidence of undue lead exposure in Cape Town before the advent of leaded petrol. S Afr Med J. 1996;86(2):169–71. [PubMed] [Google Scholar]
- 23.Begerow J, et al. Internal lead and cadmium exposure in 6-year-old children from western and eastern Germany. Int Arch Occup Environ Health. 1994;66(4):243–8. doi: 10.1007/BF00454362. [DOI] [PubMed] [Google Scholar]
- 24.Ericson JE. Enamel lead biomarker for prenatal exposure assessment. Environ Res. 2001;87(3):136–40. doi: 10.1006/enrs.2001.4283. [DOI] [PubMed] [Google Scholar]
- 25.Ericson JE, et al. Measurements of manganese with respect to calcium in histological enamel cross sections: toward a new manganese biomarker. Environ Res. 2001;86(1):46–50. doi: 10.1006/enrs.2000.4240. [DOI] [PubMed] [Google Scholar]
- 26.Arora M, et al. Spatial distribution of lead in the roots of human primary teeth. J Trace Elem Med Biol. 2004;18(2):135–9. doi: 10.1016/j.jtemb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Arora M, et al. Spatial distribution of lead in enamel and coronal dentine of wistar rats. Biol Trace Elem Res. 2005;105(1–3):159–70. doi: 10.1385/BTER:105:1-3:159. [DOI] [PubMed] [Google Scholar]
- 28.Arora M, et al. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011 doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Arora M, et al. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci Total Environ. 2006;371(1–3):55–62. doi: 10.1016/j.scitotenv.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 30**.Arora M, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46(9):5118–25. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grandjean P, et al. Life-Long Implications of Developmental Exposure to Environmental Stressors: New Perspectives. Endocrinology. 2015;156(10):3408–15. doi: 10.1210/EN.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoeters GE, et al. Biomonitoring and biomarkers to unravel the risks from prenatal environmental exposures for later health outcomes. Am J Clin Nutr. 2011;94(6 Suppl):1964s1969s. doi: 10.3945/ajcn.110.001545. [DOI] [PubMed] [Google Scholar]
- 33.Andra SS, Austin C, Arora M. Tooth matrix analysis for biomonitoring of organic chemical exposure: Current status, challenges, and opportunities. Environ Res. 2015;142:387–406. doi: 10.1016/j.envres.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis RM, et al. The placental exposome: placental determinants of fetal adiposity and postnatal body composition. Ann Nutr Metab. 2013;63(3):208–15. doi: 10.1159/000355222. [DOI] [PubMed] [Google Scholar]
- 35.Robinson O, Vrijheid M. The Pregnancy Exposome. Current Environmental Health Reports. 2015;2(2):204–213. doi: 10.1007/s40572-015-0043-2. [DOI] [PubMed] [Google Scholar]
- 36.Camann DE, et al. Acetaminophen, pesticide, and diethylhexyl phthalate metabolites, anandamide, and fatty acids in deciduous molars: potential biomarkers of perinatal exposure. J Expo Sci Environ Epidemiol. 2013;23(2):190–6. doi: 10.1038/jes.2012.71. [DOI] [PubMed] [Google Scholar]
- 37.Kanjanawattana S, et al. Determination of lidocaine in dental pulp by high-performance liquid chromatography. J Endod. 2001;27(1):31–5. doi: 10.1097/00004770-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Fos P, FL, Llena C, Anadon A. Analysis of clarithromycin in dental pulp with and without inflammation. International Dentistry SA. 2011;13(2):50–54. [Google Scholar]
- 39.Schussl Y, et al. Concentrations of amoxicillin and clindamycin in teeth following a single dose of oral medication. Clin Oral Investig. 2014;18(1):35–40. doi: 10.1007/s00784-013-0958-7. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini M, et al. Development and validation of a gas chromatography-mass spectrometry assay for opiates and cocaine in human teeth. J Pharm Biomed Anal. 2006;40(3):662–8. doi: 10.1016/j.jpba.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Zeren C, et al. Demonstration of ethyl glucuronide in dental tissue samples by liquid chromatography/electro-spray tandem mass spectrometry. J Forensic Leg Med. 2013;20(6):706–10. doi: 10.1016/j.jflm.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Algar O, et al. Nicotine concentrations in deciduous teeth and cumulative exposure to tobacco smoke during childhood. Jama. 2003;290(2):196–7. doi: 10.1001/jama.290.2.196. [DOI] [PubMed] [Google Scholar]
- 43*.Pascual JA, et al. A simple and reliable method for the determination of nicotine and cotinine in teeth by gas chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17(24):2853–5. doi: 10.1002/rcm.1279. [DOI] [PubMed] [Google Scholar]
- 44*.Marchei E, et al. Ultrasensitive detection of nicotine and cotinine in teeth by high-performance liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2008;22(16):2609–12. doi: 10.1002/rcm.3636. [DOI] [PubMed] [Google Scholar]
- 45.Jan J, et al. Distribution of organochlorine pollutants in ovine dental tissues and bone. Environ Toxicol Pharmacol. 2006;21(1):103–7. doi: 10.1016/j.etap.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Jan J, Ursic M, Vrecl M. Levels and distribution of organochlorine pollutants in primary dental tissues and bone of lamb. Environ Toxicol Pharmacol. 2013;36(3):1040–5. doi: 10.1016/j.etap.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Jan J, Vrbic V. Polychlorinated biphenyls cause developmental enamel defects in children. Caries Research. 2000;34(6):469–473. doi: 10.1159/000016625. [DOI] [PubMed] [Google Scholar]
- 48.Jan J, et al. Bioconcentration of lipophilic organochlorines in ovine dentine. Arch Oral Biol. 2001;46(12):1111–1116. doi: 10.1016/s0003-9969(01)00079-6. [DOI] [PubMed] [Google Scholar]
- 49.Athersuch TJ, Keun HC. Metabolic profiling in human exposome studies. Mutagenesis. 2015 doi: 10.1093/mutage/gev060. [DOI] [PubMed] [Google Scholar]
- 50.Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21(1):5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 51.Soltow Q, et al. High-performance metabolic profiling with dual chromatography-Fouriertransform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9(1):132–143. doi: 10.1007/s11306-011-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamin E, et al. Untargeted profiling of pesticide metabolites by LC–HRMS: an exposomics tool for human exposure evaluation. Analytical and Bioanalytical Chemistry. 2014;406(4):11491161. doi: 10.1007/s00216-013-7136-2. [DOI] [PubMed] [Google Scholar]
- 53.Diaz R, et al. Target and non-target screening strategies for organic contaminants, residues and illicit substances in food, environmental and human biological samples by UHPLC-QTOF-MS. Analytical Methods. 2012;4(1):196–209. [Google Scholar]
- 54.Fan R, et al. Reliable screening of pesticide residues in maternal and umbilical cord sera by gas chromatography-quadrupole time of flight mass spectrometry. Science China Chemistry. 2014;57(5):669–677. [Google Scholar]
- 55.Athersuch TJ. The role of metabolomics in characterizing the human exposome. Bioanalysis. 2012;4(18):2207–2212. doi: 10.4155/bio.12.211. [DOI] [PubMed] [Google Scholar]
- 56.Athersuch T. Metabolome Analyses in Exposome Studies: Profiling Methods for a Vast Chemical Space. Arch Biochem Biophys. 2015 doi: 10.1016/j.abb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Rappaport SM. Biomarkers intersect with the exposome. Biomarkers. 2012;17(6):483–489. doi: 10.3109/1354750X.2012.691553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wild CP, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ Mol Mutagen. 2013;54(7):480–99. doi: 10.1002/em.21777. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura J, et al. The endogenous exposome. DNA Repair. 2014;19:3–13. doi: 10.1016/j.dnarep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Rappaport SM, et al. The Blood Exposome and Its Role in Discovering Causes of Disease. Environ Health Perspect. 2014 doi: 10.1289/ehp.1308015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pleil JD, Stiegel MA. Evolution of environmental exposure science: using breath-borne biomarkers for “discovery” of the human exposome. Anal Chem. 2013;85(21):9984–90. doi: 10.1021/ac402306f. [DOI] [PubMed] [Google Scholar]
- 62.Broza YY, Zuri L, Haick H. Combined volatolomics for monitoring of human body chemistry. Sci Rep. 2014;4:4611. doi: 10.1038/srep04611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson O, et al. The Pregnancy Exposome: Multiple Environmental Exposures in the INMASabadell Birth Cohort. Environ Sci Technol. 2015;49(17):10632–41. doi: 10.1021/acs.est.5b01782. [DOI] [PubMed] [Google Scholar]
- 64.Makarewicz CA, Sealy J. Dietary reconstruction, mobility, and the analysis of ancient skeletal tissues: Expanding the prospects of stable isotope research in archaeology. Journal of Archaeological Science. 2015;56:146–158. [Google Scholar]
- 65.Reynard B, Balter V. Trace elements and their isotopes in bones and teeth: Diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeography, Palaeoclimatology, Palaeoecology. 2014;416:4–16. [Google Scholar]
- 66.Balter V. Allometric constraints on Sr/Ca and Ba/Ca partitioning in terrestrial mammalian trophic chains. Oecologia. 2004;139:83–88. doi: 10.1007/s00442-003-1476-0. [DOI] [PubMed] [Google Scholar]
- 67.Sponheimer M, et al. Sr/Ca and early hominin diets revisited: new data from modern and fossil tooth enamel. Journal of Human Evolution. 2005;48(2):147–156. doi: 10.1016/j.jhevol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Lee-Thorp J, Sponheimer M. Contributions of biogeochemistry to understanding hominin dietary ecology. American Journal of Physical Anthropology. 2006;(Suppl 43):131–48. doi: 10.1002/ajpa.20519. [DOI] [PubMed] [Google Scholar]
- 69.Chenery C, et al. Strontium and stable isotope evidence for diet and mobility in Roman Gloucester, UK. Journal of Archaeological Science. 2010;37(1):150–163. [Google Scholar]
- 70.Lamb AL, et al. Multi-isotope analysis demonstrates significant lifestyle changes in King Richard III. Journal of Archaeological Science. 2014;50:559–565. [Google Scholar]
- 71.Dupras TL, Tocheri MW. Reconstructing infant weaning histories at Roman period Kellis, Egypt using stable isotope analysis of dentition. American Journal of Physical Anthropology. 2007;134(1):63–74. doi: 10.1002/ajpa.20639. [DOI] [PubMed] [Google Scholar]
- 72.Eerkens JW, Berget AG, Bartelink EJ. Estimating weaning and early childhood diet from serial micro-samples of dentin collagen. Journal of Archaeological Science. 2011;38(11):31013111. [Google Scholar]
- 73.Fahy GE, et al. Stable nitrogen isotope analysis of dentine serial sections elucidate sex differences in weaning patterns of wild chimpanzees (Pan troglodytes) American Journal of Physical Anthropology. 2014;153(4):635–642. doi: 10.1002/ajpa.22464. [DOI] [PubMed] [Google Scholar]
- 74.Humphrey LT. Isotopic and trace element evidence of dietary transitions in early life. Annals of Human Biology. 2014;41(4):348–57. doi: 10.3109/03014460.2014.923939. [DOI] [PubMed] [Google Scholar]
- 75.Reynard LM, Tuross N. The known, the unknown and the unknowable: weaning times from archaeological bones using nitrogen isotope ratios. Journal of Archaeological Science. 2014;53:618–625. [Google Scholar]
- 76**.Austin C, et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498(7453):216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphrey LT, et al. Tracking Dietary Transitions in Weanling Baboons (Papio hamadryas anubis) Using Strontium/Calcium Ratios in Enamel. Folia Primatologica. 2008;79(4):197–212. doi: 10.1159/000113457. [DOI] [PubMed] [Google Scholar]
- 78.Mays S. Bone strontium: calcium ratios and duration of breastfeeding in a Mediaeval skeletal population. Journal of Archaeological Science. 2003;30(6):731–741. [Google Scholar]
- 79.Kohn MJ, Morris J, Olin P. Trace element concentrations in teeth – a modern Idaho baseline with implications for archeometry, forensics, and palaeontology. Journal of Archaeological Science. 2013;40(4):1689–1699. [Google Scholar]
- 80.Whittaker DK, Richards D. Scanning electron microscopy of neonatal line in human enamel. Archives of Oral Biology. 1978;23(1):45–50. doi: 10.1016/0003-9969(78)90052-3. [DOI] [PubMed] [Google Scholar]
- 81.Eli I, Sarnat H, Talmi E. Effect of the birth process on the neonatal line in primary tooth enamel. Pediatric Dentistry. 1989;11(3):220–223. [PubMed] [Google Scholar]
- 82.Fitzgerald CM, Saunders SR. Test of histological methods of determining chronology of accentuated striae in deciduous teeth. American Journal of Physical Anthropology. 2005;127(3):277–290. doi: 10.1002/ajpa.10442. [DOI] [PubMed] [Google Scholar]
- 83.Goodman AH, Rose JC. Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. American Journal of Physical Anthropology. 1990;33(S11):59–110. [Google Scholar]
- 84.Guatelli-Steinberg D. What can developmental defects of enamel reveal about physiological stress in nonhuman primates? Evolutionary Anthropology: Issues, News, and Reviews. 2001;10(4):138–151. [Google Scholar]
- 85.Schwartz G, et al. A faithful record of stressful life events recorded in the dental developmental record of a juvenile gorilla. International Journal of Primatology. 2006;27(4):1201–1219. [Google Scholar]
- 86.Smith TM. Teeth and human life-history evolution. Annual Review of Anthropology. 2013;42:191–208. [Google Scholar]
- 87.Birch W, Dean MC. A method of calculating human deciduous crown formation times and of estimating the chronological ages of stressful events occurring during deciduous enamel formation. Journal of Forensic & Legal Medicine. 2014;22:127–44. doi: 10.1016/j.jflm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Dirks W, et al. The Relationship of Accentuated Lines in Enamel to Weaning Stress in Juvenile Baboons (Papio hamadryas anubis) Folia Primatologica. 2010;81:207–223. doi: 10.1159/000321707. [DOI] [PubMed] [Google Scholar]
- 89.Dirks W, et al. Out of the mouths of baboons: Stress, life history, and dental development in the Awash National Park hybrid zone, Ethiopia. American Journal of Physical Anthropology. 2002;118(3):239–252. doi: 10.1002/ajpa.10089. [DOI] [PubMed] [Google Scholar]
- 90.Austin C, et al. Uncovering system-specific stress signatures in primate teeth with multimodal imaging. Scientific Reports. 2015 doi: 10.1038/srep18802. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


