Abstract
Purpose of review
Metals play major roles in children's health and are associated with negative health outcomes via deficiency, overload or toxicity. Constantly evolving analytical technology can provide new insight into how metal metabolism and exposure biology are intertwined in a range of biological matrices.
Recent findings
Exposure can occur prenatally as many metals cross the placental barrier. The placenta is permeable to many metal species, some through tightly regulated transporters, and others because of a limited capacity for detoxification. Post-birth, metal exposure continues to exert long-term health effects, ranging from exposure to exogenous heavy metals, such as lead, to overload of otherwise essential metals, including manganese. Increasing evidence supports the existence of critical developmental windows when susceptibility to toxicants and nutritional deficiencies is highest. Elemental imaging technology provides micro-spatial information on metal uptake and retention across tissue architecture, which provides important insights into exposure and biologic response.
Summary
Imaging the spatial distribution of elements, both essential and toxic, provides information that bulk measures cannot, including cell-specific distributions and timing of exposure.
Keywords: elemental imaging, metals, environmental exposure, placenta
Introduction
Many metals are essential for life, due primarily to their capacity to mediate electron transfer in a multitude of biochemical reactions, from oxygen transport to primary mitochondrial function. Evolutionary processes have resulted in elegant metal chaperoning mechanisms to restrict unwanted reactions that can precipitate cell damage, such as iron (Fe)-mediated oxidative stress [1]. However, the industrial revolution introduced the release of toxic heavy metals and otherwise essential metals, such as zinc (Zn), into the environment at a rate unparalleled in human history [2,3]. These excessive environmental exposures have the potential to overwhelm the finite capacity to which the human body has evolved to compensate, which may result in significant risk of toxicity to the human body, and particularly to the developing child.
Both animal studies and epidemiological surveys have demonstrated that the early-life period, spanning from conception through childhood, represents a critical window of heightened susceptibility to metal exposures [4]. The precise reasons why certain metal exposures can cause significant long-term negative health outcomes are often unclear, which is something that contemporary analytical chemistry is now in a prime position to address. Elemental bio-imaging, wherein a range of imaging modalities can be used to generate low-to-sub micron scale, quantitative images of the spatial distributions of metal species, is an emerging technology with the potential to revolutionise the study of metal exposure biology. This review will briefly discuss the techniques available and how they can be implemented into wider-scale epidemiological studies of early-life metal exposures. We will also discuss the diversity of information that imaging provides, including the capacity to retrospectively assess metal exposures using novel biomarkers and investigate maternal-fetal transfer through placental tissues, and finally, we will speculate on the potential of immunohistochemical methods to study both metal exposures and associated biological response factors.
The placenta as a window into fetal exposure
Here, we focus on the placenta as a useful matrix for monitoring metal exposures in children that are particularly suited for bio-imaging [5]. The placenta has a great potential for exposure assessment as an indicator of maternal-fetal transfer of metals and the resultant biological responses.
As the sole barrier between mother and fetus, the placenta regulates the transfer of nutrients, essential biomolecules, and wastes between the mother and child through passive and active transport mechanisms, whilst providing some degree of protection against toxins. It is not impervious to all harmful chemical species, and it is particularly permeable to industrial toxins and chemicals [6], including heavy metals. Although the precise mechanisms through which heavy metals actively cross the placental barrier remain unclear, it is likely that these otherwise foreign trace metals usurp the roles of evolutionarily conserved methods for the transfer of essential elements to the developing fetus. Lead (Pb), for instance, is able to utilize existing mechanisms for Fe homeostasis to disperse throughout the human body, including across the placenta [7,8]. Even excessive exposure to otherwise essential metals can compromise the placental barrier; for example, high manganese (Mn) concentrations have been observed in placental tissues from children with neural tube defects [9]. In addition, the placenta itself exhibits immune and endocrine functions that are critical for a healthy pregnancy, which can be perturbed by adverse environmental exposures [10], including possibly heavy metals [11,12]. Given the accessibility and ease of placental tissue sample collection following birth, the placenta is a valuable yet underutilized target tissue to monitor in utero metal exposures at the maternal/fetal interface, though ample consideration must be given to the effects of long-term formalin storage on metal content and distribution [13], and, where possible, age-matched (in terms of storage) samples should be used.
Imaging biological exposures
A number of techniques are available for imaging metals in biological matrices, ranging from traditional histochemistry to synchrotron-based microprobes (see reviews by McRae et al. [14] and Hare et al. [15] for comprehensive overviews of technologies available). Here, we discuss three of the most widely used techniques, and we provide a brief overview of additional techniques that have not yet been used to image metals in matrices relevant to children's health, but have the potential to provide additional resources.
Laser ablation-inductively coupled plasma-mass spectrometry
Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) was first used to image metals in biological samples by Wang et al. [16] in 1994. This technique uses a focused laser beam that is rastered across a sample surface, generating a stream of ablated particles that are detected in real-time by an ICP-MS and used to generate spatial maps of metal distributions [17]. LA-ICP-MS has achievable detection limits for some elements as low as 10 ng g−1 [14], and it is particularly sensitive to heavy metals due to their high atomic masses and low environmental background levels. LA-ICP-MS instruments are now capable of producing 1-μm spatial resolution images [18], though sensitivity for ultra-trace elements is restricted when the volume of the ablated sample is reduced to this level.
LA-ICP-MS has been used extensively to study the distribution of metals in various biological tissues to assess both concentrations and distributions [19], though few applications have directly addressed exposure assessment. LA-ICP-MS was used to study uranium (U), plutonium (Pu) and thorium (Th) exposure in lymphatic tissue from ex-nuclear workers years after suffering acute occupational exposures [20], and background levels of Th and U in human hippocampus tissue was proposed as a possible internal standard for measuring endogenous metals in tissue [21], though this approach fails to meet contemporary guidelines for internal standardization for LA-ICP-MS due to each element's extremely low abundance [17,22].
X-ray fluorescence microscopy
X-ray fluorescence microscopy (XFM) uses energetic hard X-ray beams (> 10 keV) generated by the brilliant light sources obtained from synchrotrons for the high-resolution mapping of element concentrations in biological samples that takes advantage of the characteristic electronic properties of each element. A detailed description of the principles behind XFM and a selection of biological applications can be found in the review by Pushie et al [23]. The major advantage of XFM is the high spatial resolution, which is now well below subcellular resolution due to recent upgrades at the Advanced Photon Source in the USA, the European Synchrotron Radiation Facility in France, and the SPRing-8 synchrotron in Japan. Like LA-ICP-MS, the potential of this technique for imaging exposure biology in children's tissue has not been fully exploited, though a range of applications to exposure biology in both environmental and adult health applications is summarised in the review by Paunesku et al. [24].
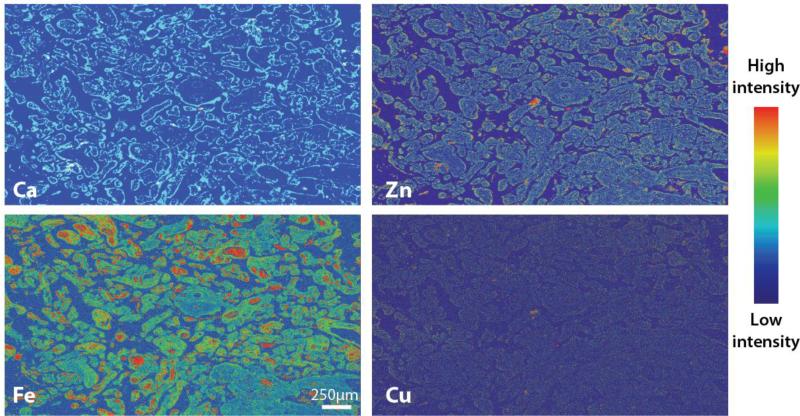
In the first step toward the integration of XFM imaging in epidemiologic birth cohort studies, Punchon and colleagues recently demonstrated for the first time the feasibility of XFM imaging in human placental chorionic villi specimens [25]. An example of the application of XFM imaging in formalin-fixed, paraffin-embedded (FFPE) human placental tissue is shown in Figure 1. Here, we used traditional immunohistochemistry to identify localized areas of inflammation (CD68+ and CD66b+ cells) in chorionic villi, and we mapped element distributions in one of these regions in an adjacent tissue section using XFM.
Figure 1.
XFM imaging in human chorionic villi.
The potential of XFM is limited by access: only around 40 synchrotron facilities have been constructed worldwide, each with differing capabilities, and beamlines are often oversubscribed with high access costs and highly-competitive application procedures. Regardless, the continuing improvements to XFM technology enables the probing of metal distributions at nanoscale resolution whilst maintaining sensitivity [26], which is paramount for assessing the toxicity of metals that occur at extremely low concentrations.
Particle-induced X-ray emission
Particle (or proton) induced X-ray emission spectroscopy (PIXE) operates according to similar principles as XFM, though rather than using a hard X-ray source, highly-energetic particles are directed onto a sample in a vacuum, resulting in the same inner-shell electron ejection and emission of characteristic fluorescence observed in XFM. Like XFM, PIXE has superior resolution to LA-ICP-MS, but is also less accessible. PIXE has, however, long been used for assessing heavy metal concentrations following environmental exposure in relevant tissues, such as bone [27]. Again, few examples in the literature specifically describe the use of PIXE in the analysis of tissue relevant to children's health, though there are abundant studies demonstrating the capacity of this technique for imaging metals.
Beyond metals: imaging protein-metal associations
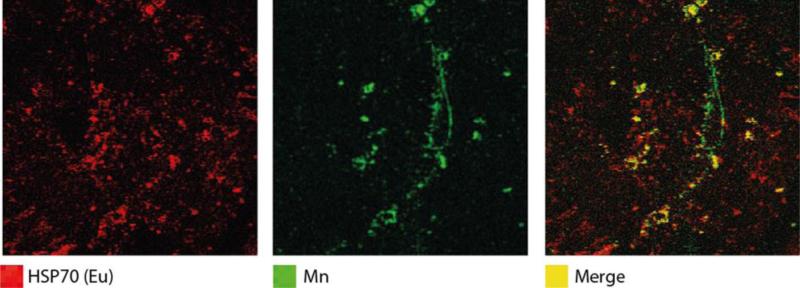
Imaging metals tells but part of the story in exposure biology. Other factors, including transport proteins and biologic effect markers, are equally as important for understanding metal toxicity as the metal itself. Several recent studies have demonstrated the utility of immunohistochemical methods employing antibodies labelled with exogenous metal tags, combined with elemental imaging, as a novel approach to associate specific metal distributions with a protein, peptide or enzyme of interest [28,29]. In Figure 2, we show how labelling of heat shock protein 70 (Hsp70), a marker of stress, in the placenta with europium-tagged antibodies shows the spatial association of Hsp70 with areas of high Mn concentration, a known redox-active metal and source of increased oxidative stress at high concentrations.
Figure 2.
Co-localization of Hsp70 and Mn in human placental tissue. A 5-μm-thick placental FFPE tissue section was stained with anti-Hsp70 primary antibody (1:1000) and europium (Eu)-tagged IgG secondary antibody (1:100), and element concentrations were imaged using LA-ICP-MS (40-μm spot size). Ion intensities for Hsp70 and Mn are represented in red and green, respectively, and pixels with co-occurrence of Hsp70 and Mn are represented in the merged image in yellow.
Not only can metal-tagged antibodies be used to identify associations between metals and biologically-relevant proteins, they can also be used to identify rare yet important cell types in a number of chronic diseases [30], which may have implications for rare diseases in children. While most immunohistochemical approaches rely on secondary antibodies to amplify the signal for target detection, the sensitive nature of elemental imaging allows the direct use of metal-tagged primary antibodies, thus enabling the development of highly-multiplexed imaging methods. Giesen et al. [31] used a particularly elegant method employing fast-scanning and high-resolution laser ablation coupled to a CyTOF (a modified ICP-MS typically used for flow, or in this case ‘mass’, cytometry) to simultaneously image 32 individual antigens in human breast cancer samples, each labelled with a monoisotopic rare earth element, which delineated unique cell subpopulation phenotypes associated with patient classifications. Continued development of this technology, in line with integration of the validated standard operating procedures typical of clinical biochemistry presents a promising future for elemental imaging into the coming decades.
Conclusion
The technological capacity has been in place for some time, but only now is the medical research community embracing the potential of tissue imaging for uncovering the roles of metals in disease processes. Nowhere is this more relevant than exposure biology; considering the expanding knowledge regarding critical windows of susceptibility to environmental exposures and chronic disease risk later in life, elemental imaging of placentas and other relevant tissues has the potential to make a substantial impact on children's environmental health research. However, increased interdisciplinary collaboration between the chemists and physicists who develop these methods and the biologists and clinicians who can most benefit from them, the use of this technology into epidemiologic and clinical research will be increased. This opinion piece is intended to make the contemporary paediatrician and paediatric researcher aware of how these technologies may be integrated into their field, in the hope that greater communication between disciplines may facilitate the transition of metal imaging into population health research and, ultimately, into the clinic.
Key points.
Imaging the spatial distribution of exogenous heavy metals in the placenta can provide important insight into the mechanisms by which these toxic species are transferred to the fetus.
Spatial resolution using advanced analytical techniques permits subcellular mapping.
Correlating metal distribution with the expression of specific antigens labelled with exotic metal tags and other endogenous metals provides further information on the body's response to heavy metal exposure
Acknowledgements
We would like to thank the X-ray Fluorescence Microscopy Beamline at the Australian Synchrotron.
Financial Support and Sponsorship:
This work was supported by the Department of Preventive Medicine at the Ichan School of Medicine at Mount Sinai, USA; the University of Technology Sydney, Australia; The Florey Institute for Neuroscience and Mental Health at the University of Melbourne, Australia; and the Victorian Government's Operational Infrastructure Support Program, Australia.
A/Prof Arora is currently receiving grants (R00ES019597 and DP2ES025453) from the NIEHS.
Footnotes
Conflicts of Interest:
The other authors have no conflicts of interest.
References
- 1.Meneghini R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free Radic Biol Med. 1997;23(5):783–92. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- 2.Ayres RU. Toxic heavy metals: materials cycle optimization. Proc Natl Acad Sci U S A. 1992;89(3):815–20. doi: 10.1073/pnas.89.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candelone JP, Hong S, Pellone C, et al. Post - Industrial Revolution changes in large - scale atmospheric pollution of the northern hemisphere by heavy metals as documented in central Greenland snow and ice. J Geophys Res-Atmos. 1995;100(D8):16605–16. [Google Scholar]
- 4.Dietert RR, Etzel RA, Chen D, et al. Workshop to identify critical windows of exposure for children's health: immune and respiratory systems work group summary. Environ Health Perspect. 2000;108(Suppl 3)(Suppl 3):483–90. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roverso M, Berté C, Di Marco V, et al. The metallome of the human placenta in gestational diabetes mellitus. Metallomics. 2015 doi: 10.1039/c5mt00050e. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gundacker C, Hengstschläger M. The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr. 2012;162(9-10):201–6. doi: 10.1007/s10354-012-0074-3. [DOI] [PubMed] [Google Scholar]
- 8.Kayaalti Z, Kaya-Akyüzlü D, Söylemez E, et al. Maternal hemochromatosis gene H63D single-nucleotide polymorphism and lead levels of placental tissue, maternal and umbilical cord blood. Environ Res. 2015;140:456–61. doi: 10.1016/j.envres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Jin L, Zhang L, et al. Placental concentrations of manganese and the risk of fetal neural tube defects. J Trace Elem Med Biol. 2013;27(4):322–5. doi: 10.1016/j.jtemb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 10.LaRocca J, Binder AM, McElrath TF, et al. First-Trimester Urine Concentrations of Phthalate Metabolites and Phenols and Placenta miRNA Expression in a Cohort of US Women. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xenofon M, Chrisostomos S, Antonios K, et al. Toxicological impact of heavy metals on the placenta: A literature review. Hellenic Journal of Obstetrics and Gynecology. 2014;13:115–9. [Google Scholar]
- 12.Caserta D, Graziano A, Lo Monte G, et al. Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci. 2013;17(16):2198–206. [PubMed] [Google Scholar]
- 13*.Schrag M, Dickson A, Jiffry A, et al. The effect of formalin fixation on the levels of brain transition metals in archived samples. BioMetals. 2010;23(6):1123–7. doi: 10.1007/s10534-010-9359-4. [This paper highlights considerations that must be taken to preseve endogenous metal distribution in post-mortem samples.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRae R, Bagchi P, Sumalekshmy S, et al. In situ imaging of metals in cells and tissues. Chem Rev. 2009;109(10):4780–827. doi: 10.1021/cr900223a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Hare DJ, New EJ, de Jonge MD, et al. Imaging metals in biology: balancing sensitivity, selectivity and spatial resolution. Chem Soc Rev. 2015;44(17):5941–58. doi: 10.1039/c5cs00055f. [This tutorial review describes in detail the wide range of techniques available for bio-imaging of elements.] [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Brown R, Gray DJ. Application of laser ablation-ICPMS to the spatially resolved micro-analysis of biological tissue. Appl Spectrosc. 1994;48(11):1321–5. [Google Scholar]
- 17.Hare D, Austin C, Doble P. Quantification strategies for elemental imaging of biological samples using laser ablation-inductively coupled plasma-mass spectrometry. Analyst. 2012;137:1527–37. doi: 10.1039/c2an15792f. [DOI] [PubMed] [Google Scholar]
- 18.Wang HAO, Grolimund D, Giesen C, et al. Fast Chemical Imaging at High Spatial Resolution by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Anal Chem. 2013;85(21):10107–16. doi: 10.1021/ac400996x. [DOI] [PubMed] [Google Scholar]
- 19*.Pozebon D, Scheffler GL, Dressler VL, et al. Review of the applications of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to the analysis of biological samples. J Anal At Spectrom. 2014;29(12):2204–28. [This review paper describes the most relevant applications of LA-ICP-MS imaging to date.] [Google Scholar]
- 20.Hare D, Tolmachev S, James A, et al. Elemental bio-imaging of thorium, uranium, and plutonium in tissues from occupationally exposed former nuclear workers. Anal Chem. 2010;82(8):3176–82. doi: 10.1021/ac902650w. [DOI] [PubMed] [Google Scholar]
- 21.Becker JS, Zoriy MV, Pickhardt C, et al. Imaging of Copper, Zinc, and Other Elements in Thin Section of Human Brain Samples (Hippocampus) by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Anal Chem. 2005;77(10):3208–16. doi: 10.1021/ac040184q. [DOI] [PubMed] [Google Scholar]
- 22.Austin C, Fryer F, Lear J, et al. Factors affecting internal standard selection for quantitative elemental bio-imaging of soft tissues by LA-ICP-MS. J Anal At Spectrom. 2011;26(7):1494–501. [Google Scholar]
- 23.Pushie MJ, Pickering IJ, Korbas M, et al. Elemental and Chemically Specific X-ray Fluorescence Imaging of Biological Systems. Chem Rev. 2014;114(17):8499–541. doi: 10.1021/cr4007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paunesku T, Vogt S, Maser J, et al. X - ray fluorescence microprobe imaging in biology and medicine. J Cell Biochem. 2006;99(6):1489–502. doi: 10.1002/jcb.21047. [DOI] [PubMed] [Google Scholar]
- 25**.Punshon T, Chen S, Finney L, et al. High-resolution elemental mapping of human placental chorionic villi using synchrotron X-ray fluorescence spectroscopy. Anal Bioanal Chem. 2015;407(22):6839–50. doi: 10.1007/s00216-015-8861-5. [This study shows the potential of XFM imaging for assessing micro-scale distribution of elements in human placenta tissue.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohic S, Cotte M, Salomé M, et al. Biomedical applications of the ESRF synchrotron- based microspectroscopy platform. J Struct Biol. 2012;177(2):248–58. doi: 10.1016/j.jsb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Lindh U, Brune D, Nordberg G, et al. Levels of antimony, arsenic, cadmium, copper, lead, mercury, selenium, silver, tin and zinc in bone tissue of industrially exposed workers. The Science of the total environment. 1980;16(2):109–16. doi: 10.1016/0048-9697(80)90018-2. [DOI] [PubMed] [Google Scholar]
- 28.Hare DJ, Lei P, Ayton S, et al. An iron–dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chem Sci. 2014;5(6):2160–9. [Google Scholar]
- 29.Paul B, Hare DJ, Bishop DP, et al. Visualising mouse neuroanatomy and function by metal distribution using laser ablation-inductively coupled plasma-mass spectrometry imaging. Chem Sci. 2015;6(10):5383–93. doi: 10.1039/c5sc02231b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Managh AJ, Managh AJ, Hutchinson RW, et al. Laser ablation-inductively coupled plasma mass spectrometry: an emerging technology for detecting rare cells in tissue sections. J Immunol. 2014;193(5):2600–8. doi: 10.4049/jimmunol.1400869. [DOI] [PubMed] [Google Scholar]
- 31**.Giesen C, Wang HAO, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Meth. 2014;11(4):417–22. doi: 10.1038/nmeth.2869. [This recent study demonstrates the capacity of specialized laser ablation-CyTOF to perform highly multiplexed imaging of over 30 antigens simultaneously.] [DOI] [PubMed] [Google Scholar]