Abstract
Thyroid hormone (TH) is essential for adult brain function and its actions include several key roles in the hypothalamus. Although TH controls gene expression via specific TH receptors of the nuclear receptor class, surprisingly few genes have been demonstrated to be directly regulated by TH in the hypothalamus, or the adult brain as a whole. This study explored the rapid induction by TH of retinaldehyde dehydrogenase 1 (Raldh1), encoding a retinoic acid (RA)‐synthesizing enzyme, as a gene specifically expressed in hypothalamic tanycytes, cells that mediate a number of actions of TH in the hypothalamus. The resulting increase in RA may then regulate gene expression via the RA receptors, also of the nuclear receptor class. In vivo exposure of the rat to TH led to a significant and rapid increase in hypothalamic Raldh1 within 4 hours. That this may lead to an in vivo increase in RA is suggested by the later induction by TH of the RA‐responsive gene Cyp26b1. To explore the actions of RA in the hypothalamus as a potential mediator of TH control of gene regulation, an ex vivo hypothalamic rat slice culture method was developed in which the Raldh1‐expressing tanycytes were maintained. These slice cultures confirmed that TH did not act on genes regulating energy balance but could induce Raldh1. RA has the potential to upregulate expression of genes involved in growth and appetite, Ghrh and Agrp. This regulation is acutely sensitive to epigenetic changes, as has been shown for TH action in vivo. These results indicate that sequential triggering of two nuclear receptor signalling systems has the capability to mediate some of the functions of TH in the hypothalamus. GLIA 2016;64:425–439
Keywords: thyroid hormone receptor, thyroid‐stimulating hormone, deiodinase, retinoic acid receptor, growth
Abbreviations
- Agrp
Agouti‐related protein
- ARC
Arcuate
- DMN
Dorsomedial
- HDAC
Histone deacetylase
- PFA
Paraformaldehyde
- PVN
Paraventricular
- Pomc
Proopiomelanocortin
- Raldh1
Retinaldehyde dehydrogenase 1
- RARs
Retinoic acid receptors
- TH
Thyroid hormone
- TSH
Thyroid‐stimulating hormone
- VMN
Ventromedial
Introduction
Thyroid hormones (TH) act to control gene expression by binding and activating specific nuclear receptor family transcription factors. TH is known to regulate energy balance and metabolism, with actions in both peripheral tissues and in the brain (reviewed by Lechan and Fekete, 2006; Lopez et al., 2013). Increased food intake (hyperphagia) is a characteristic symptom of hyperthyroidism, the overproduction of TH. This is generally believed to result from increased energy expenditure in peripheral tissues, but some hyperthyroid patients increase their food intake enough to gain weight (Gurney et al., 1970), suggesting that it is not simply a compensatory change. TH signalling may be important to initiate the drive to feed as deiodinase 2, the enzyme that activates TH signalling by converting thyroxine (T4) into the transcriptionally more active triiodothyronine (T3), is upregulated by fasting in the hypothalamus (Diano et al., 1998), the region of the brain controlling feeding, growth, and reproductive status. Moreover, acute and chronic administration of low doses of T3 has been shown to increase feeding without affecting energy expenditure (Kong et al., 2004). These observations suggest that TH has central, as well as peripheral effects on the regulation of feeding behavior and metabolic states. Hyperthyroid rats show an upregulation of the orexigenic gene agouti‐related protein (Agrp) in the hypothalamus accompanied by a decrease in proopiomelanocortin (Pomc), the precursor of the anorexigen alpha‐melanocyte‐stimulating hormone (α‐MSH; Varela et al., 2012). In addition, the importance of TH signalling in the hypothalamus is highlighted in the regulation of energy balance of seasonal animals (Barrett et al., 2007). This highly conserved pathway is now considered to be the basis of long‐term changes in energy balance, growth and reproduction, in birds and mammals. In Siberian hamsters, hypothalamic implants releasing T3 promote long day‐like (i.e. summer‐like) reproductive and body weight responses (Murphy et al., 2012) and conversely local delivery of T3 is able to block short day (winter‐like)‐induced weight loss (Barrett et al., 2007). From these findings it is now generally concluded that local hypothalamic T3 availability is responsible for long‐term seasonal changes in energy metabolism.
The observations described above suggest that TH signalling is an important regulator of hypothalamic function. TH is thought to control genes affecting growth and energy balance in seasonal mammals including Agrp, Ghrh, and Pomc (Ross et al., 2009), although this control may be indirect. However despite considerable investigation, the genes directly regulated by TH in the hypothalamus are largely unknown; indeed, the genes regulated by TH in much of the brain remain undiscovered. Retinaldehyde dehydrogenase 1 (Raldh1, also known as Aldh1a1) is regulated by a number of hormone activators of nuclear receptors, such as estrogen (Fujiwara et al., 2009) and androgen (MacLean et al., 2008), as well as metabolite‐regulated nuclear receptors such as LXR (Huq et al., 2006). Via its synthesis of retinoic acid (RA) from retinaldehyde, Raldh1 itself then controls the activity of further nuclear receptors, the retinoic acid receptors (RARs). In the hypothalamus, Raldh1 is specifically localized to tanycytes, radial glia‐like cells lining the third ventricle, potentially acting to communicate between the cerebrospinal fluid, circulation, and hypothalamic neurons (Shearer et al., 2010). They have an intermediary role to play in the control of appetite and energy balance (Bolborea and Dale, 2013) and help to mediate molecular exchange between blood, brain, and cerebrospinal fluid (Langlet et al., 2013), including transport of leptin (Balland et al., 2014). Raldh1 was explored in vivo as a TH‐regulated gene in the rat hypothalamus and was found to be rapidly induced and potentially under direct TH control. Gene regulation by RA was studied in an organotypic slice culture system developed for this study. This system was free of secondary in vivo influences but maintained the structures necessary for hypothalamic function. It was shown that RA regulates the expression of hypothalamic genes known to affect energy balance and growth and may act as an intermediary in the action of TH in the hypothalamus. Further, it was demonstrated that Rarb and Ghrh were epigenetically repressed and the RA signalling pathway may be a means of epigenetic control of gene expression in the hypothalamus.
Materials and Methods
Animals
Sprague Dawley rats were bred in the University of Aberdeen animal facility. Fischer F344/N male rats were supplied by Harlan Sprague Dawley Inc. (Indianapolis, USA). All animals were kept in a 12h:12h light:dark cycle with unlimited access to food and water. All procedures conformed to Home Office regulations and local ethics committee guidelines.
T3 Administration
T3 (Sigma Aldrich) was dissolved in 1 N NaOH at 1 mg/ml and then diluted to 40 µM in phosphate‐buffered saline (PBS), pH 7.4. Eight‐week‐old male Sprague Dawley rats were injected subcutaneously with 65 µg/kg (100 nmol/kg) T3. Control animals were injected with an equivalent volume of 1 N NaOH diluted in PBS. 4 hours post‐injection, the animals were killed and the hypothalami were dissected and rapidly frozen on dry ice.
Nissl Staining
Male rat pups were transcardially perfused with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer. The brains were removed and incubated overnight at 4°C in 4% PFA, washed in PBS and transferred to 30% sucrose in PBS. 40 µm‐thick coronal brain sections were cut using a cryostat, mounted on polylysine‐coated slides, and allowed to dry. Tissue sections were stained with cresyl violet, dehydrated through an ethanol series into xylene, and mounted with DPX (Fisher Scientific).
Hypothalamic Organotypic Slice Cultures
To exclude circadian‐driven changes in gene expression, all hypothalamic slice cultures were set up at the same time of day (commencing at ZT07‐8). P10‐12 male rat pups were euthanized with Euthatal. The brains were removed under sterile conditions and placed in ice‐cold slice culture medium consisting of 50% minimal essential medium, 25% Hank's buffered salt solution, 25% heat‐inactivated horse serum, containing penicillin‐streptomycin and Glutamax, supplemented with 5 mg/ml additional glucose and buffered with 25 mM HEPES. The cortices were removed and the brains were sliced into 400 µm coronal sections using a McIlwain tissue chopper. Slices were transferred into cold medium, separated using forceps under a dissection microscope and slices containing the third ventricle were isolated. Each hypothalamus yielded five to six slices. The slices were trimmed and cut in half along the midline, giving two sets of slices per animal, with each containing the same (but alternate) regions. Each sample consisted of one set of slices. After incubation in ice‐cold culture medium for 1 to 2 hours, each set of slices was transferred onto a Millicell‐CM cell culture insert (Millipore) in a six‐well plate using a sterile glass pipette. Excess medium was removed from the tissue and 1 ml of fresh slice culture medium was added below the insert. Slices were transferred to 35°C, 5% CO2. After 24 hours, the medium was removed and replaced with serum‐free, vitamin A‐deficient medium consisting of Neurobasal medium containing B27 supplement without vitamin A, penicillin‐streptomycin and Glutamax (all reagents from Invitrogen) and 5 mg/ml additional glucose. Slices were incubated at 35°C, 5% CO2 for a further 3 days. Slices were maintained in vitamin A‐deficient medium to deplete the tissue of retinol and minimize retinoic acid synthesis by endogenous Raldh1.
The medium was replaced with fresh serum‐free, vitamin A‐free medium and slices were treated with 10 mIU bovine thyroid‐stimulating hormone (TSH), 50 nM T3, 10 nM, or 1 µM all‐trans‐RA (all from Sigma Aldrich), 50 ng/ml trichostatin A (Cayman Chemical Co.) or 100 µM sirtinol (Tocris). Sirtinol, retinol and RA were dissolved in DMSO and therefore an equivalent volume of DMSO (0.1%) was added to wells containing control slices. One set of slices from each animal was treated with the other set being used as control. Treatment times are indicated in figure legends. After treatment, slices were fixed in 4% paraformaldehyde for 2 hours at room temperature for immunohistochemistry or excised from the culture inserts, transferred to microcentrifuge tubes and frozen rapidly on dry ice for RNA extraction.
Slice Immunohistochemistry
After 6 days ex vivo, hypothalamic slices were washed with PBS and fixed by immersion in 4% PFA for 2 hours at room temperature. Slices were labeled using antibodies against vimentin (V9, Sigma Aldrich) and Darpp‐32 (19A3, Cell Signaling) and appropriate fluorescent secondary antibodies (Jackson ImmunoResearch). The membranes carrying the slices were transferred onto microscope slides and mounted using mounting medium containing Hoechst. Labelling was visualized by fluorescence microscopy.
Primary Tanycyte Culture
Primary tanycyte cultures were prepared from 10‐day‐old male Sprague Dawley rat pups as previously described (Bolborea et al., 2015; De Seranno et al., 2004; Prevot et al., 2003). Brains were removed under sterile conditions, placed in ice‐cold DMEM/F‐12 medium containing penicillin/streptomycin and 25 mM HEPES and the median eminence dissected. Median eminences from 8–10 pups were pooled together, dissociated and plated out in DMEM/F‐12 containing 10% foetal calf serum and penicillin/streptomycin in a 25 cm2 cell culture flask. Medium was replaced every 3 days. The composition of primary tanycyte cultures was assessed after 8 to 10 days in vitro by immunohistochemistry for the tanycyte marker vimentin and the astrocyte marker GFAP; all cells expressed vimentin, but only a small number expressed GFAP. RNA was also extracted from cultured cells and tested by PCR for expression of the tanycyte markers Dio2, Darpp32, Rax, Tshr, and Gpr50.
Primary cultured tanycytes were transferred to 12‐well plates for experiments after 8‐10 days in vitro. The day after plating out, tanycytes were treated with 50 nM T3 or vehicle for 24 hours. RNA was extracted from treated cells for qPCR analysis.
Quantitative Polymerase Chain Reaction
Total RNA was extracted from slices using a Qiagen RNeasy RNA purification kit. cDNA was synthesized from 500 ng total RNA using High Capacity RNA‐to‐cDNA Master Mix (Applied Biosystems Ltd). Dio2 and Dio3 primers were obtained from Qiagen (QuantiTect Primer Assays Rn_Dio2_2_SG and Rn_Dio3_1_SG, respectively); other primers (Table 1) were designed using PrimerBLAST (Ye et al., 2012). qPCR reactions were set up using SensiMix SYBR master mix (Bioline) and were run on a Roche LightCycler 480 and analysed using LightCycler 480 1.5 software. Expression of genes of interest was normalized to Actb levels. Standard curves and blank controls were run for all sets of primers. Results shown are from a minimum of two independent experiments per condition. Gene expression in T3‐injected rats was compared to that of vehicle‐injected controls using unpaired Student's t‐tests. Expression in treated hypothalamic slices was compared to control slices from the same individuals by paired Student's t‐tests or ANOVA.
Table 1.
Sequences of primers used for RT‐PCR and qPCR
| Gene | RefSeq code | Product size (bp) | Primer sequences | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Actb | NM_031144.3 | 112 | CCACACCCGCCACCAGTTCG | GACGGCCCGGGGAGCATCGT |
| Agrp | NM_033650.1 | 149 | GGGCGTGGCACCACTGAAGG | GTGGATCTAGCACCTCTGCCAAAGC |
| Cart | NM_017110.1 | 83 | AAGTCCCCATGTGTGACGCTGGA | TCCTCGGGGACAGTCACACAGC |
| Cyp26b1 | NM_181087.2 | 127 | TCCATTGGCGACATCCACCGC | GGCTGCTCCAGGCTCGAAGTG |
| Darpp32 | NM_138521.1 | 127 | ATGGACCCCAAGGACCGCAAGAA | CTGAGACCCGGAACAGCAAGGC |
| Ghrh | NM_031577.1 | 147 | GGCAGCAAGGGGAGAGGAACCA | CGAGGGCTCAAGCCTCCGC |
| Gpr50 | NM_001191915.1 | 147 | GCGCAATGGTCATCACTGTCGTC | ACGGGTAGATGGCCACGAGCA |
| Npy | NM_012614.1 | 142 | GCCAGATACTACTCCGCTCTGCGA | CTTCAAGCCTTGTTCTGGGGGCA |
| Pcsk2 | NM_012746.1 | 222 | CGTGGGGGCAAAGGCAGCAT | TGTGGTAGCCACACCGGCCT |
| Pomc | NM_139326.2 | 225 | TGCCTTTCCGCGACAGAGCC | TGCCTGGAAACACGGGCGTC |
| Raldh1 | NM_022407.3 | 196 | ACGTGGAAGAAGGGGACAAGGCTG | GCAAAGACTTTCCCACCATTGAGTGCC |
| Rarb | NM_031529.1 | 134 | ACACCACGAATTCCAGCGCTGAC | CAGACCTGTGAAGCCCGGCA |
| Rax | NM_053678.1 | 98 | CGACGTGTACAGCCGCGAAGA | GGCGTCTCCACTTGGCTCGAC |
| Tshr | NM_012888.1 | 118 | GGGTGTACTTCTCCACCCTGCGA | TCTCGATGAGCTTCAGAGTCTGGGTG |
Results
Peripheral Administration of T3 Upregulates Hypothalamic Raldh1 Expression
Thyroid hormone signalling in the hypothalamus is thought to play a crucial role in the control of energy balance, but little is known about its direct targets in the hypothalamus. To investigate potential targets of TH signalling in the hypothalamus, male Sprague Dawley rats were given a single subcutaneous injection of 100 nmol/kg T3 at 8 weeks of age. Treated rats were killed 4 hours post‐injection and the hypothalami were rapidly removed for qPCR analysis of hypothalamic genes known to affect energy balance.
Deiodinase 3 (Dio3) has previously been shown to be directly induced by T3 (Barca‐Mayo et al., 2011; Bianco et al., 2002) and was used as a positive control. Dio3 was strongly upregulated in the hypothalamus of T3‐treated rats (Fig. 1A), confirming that peripheral administration of T3 increased hypothalamic T3 and that the time between administration and dissection was sufficient for alterations in hypothalamic gene expression. Despite the presence of T3 in the hypothalamus, no significant changes in the expression of agouti‐related protein (Agrp), growth hormone‐releasing hormone (Ghrh), neuropeptide Y (Npy), cocaine‐ and amphetamine‐regulated transcript (Cart) or proopiomelanocortin (Pomc) were observed in T3‐treated animals after 4 hours (Fig. 1A).
Figure 1.
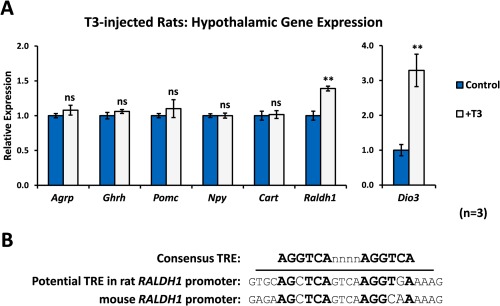
T3 upregulates Raldh1 expression in the rat hypothalamus in vivo. A: Eight‐week old male Sprague Dawley rats were injected subcutaneously with 100 nmol/kg T3. The hypothalamus was removed 4 hours after T3 injection for qPCR analysis. Expression of Agrp, Pomc, Npy, Cart, and Ghrh was unaffected by short‐term T3 treatment, but hypothalamic Raldh1 was significantly upregulated 4 hours after T3 injection, compared with vehicle‐injected animals. Dio3 was used as a positive control for the activity of T3. N = 3 animals per treatment group. B: A sequence closely matching the consensus sequence of a DR4‐type thyroid hormone response element (TRE) was identified in the rat Raldh1 promoter, suggesting direct regulation of Raldh1 expression by T3. Statistical significance was assessed using Student's t‐test. ** P < 0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Raldh1, which encodes the RA synthetic enzyme retinaldehyde dehydrogenase 1 present in tanycytes (Shearer et al., 2010), was investigated as a gene regulated by several nuclear receptor family members (Fujiwara et al., 2009; Huq et al., 2006; MacLean et al., 2008). This gene was significantly upregulated in the hypothalamus of T3‐treated rats (Fig. 1A). In addition, a sequence with high similarity to a DR4‐type TH response element (TRE; AGCTCAgtcaAGGTGA; Fig. 1B) was identified in the promoter of the rat Raldh1 gene, close to the transcription start site. The upregulation of Raldh1 just 4 hours after T3 administration and the presence of a potential TRE in the promoter of the rat Raldh1 gene suggest that Raldh1, and so RA synthesis, may be a direct target of TH signalling in the hypothalamus.
Cultured Hypothalamic Slices with Tanycytes Are Responsive to Thyroid Stimulating Hormone
The function of T3 and RA in the hypothalamus was further explored using an ex vivo organotypic culture system. This technique was modified from that of House et al. (1998) and allowed T3 and RA to be studied independently of feedback from the rest of brain or body. The brain was removed from P10‐12 male Sprague Dawley rat pups and cut into 400 µm‐thick coronal slices. Slices were trimmed dorsally at the level of the mammillothalamic tract and laterally at the fornix, both of which were visible in the slices (Fig. 2A, B). These slices contained the ventricular region and median eminence (ME) in addition to the arcuate (ARC), ventromedial (VMN), dorsomedial (DMN), and paraventricular (PVN) hypothalamic nuclei. Trimming the slices using visible anatomical cues minimized variation but allowed for differences in brain size between individual animals. The slices were standardly cultured in serum‐free vitamin A‐free medium for 72 hours before treatment, depleting the tissue of vitamin A and thereby preventing endogenous synthesis of retinoic acid.
Figure 2.
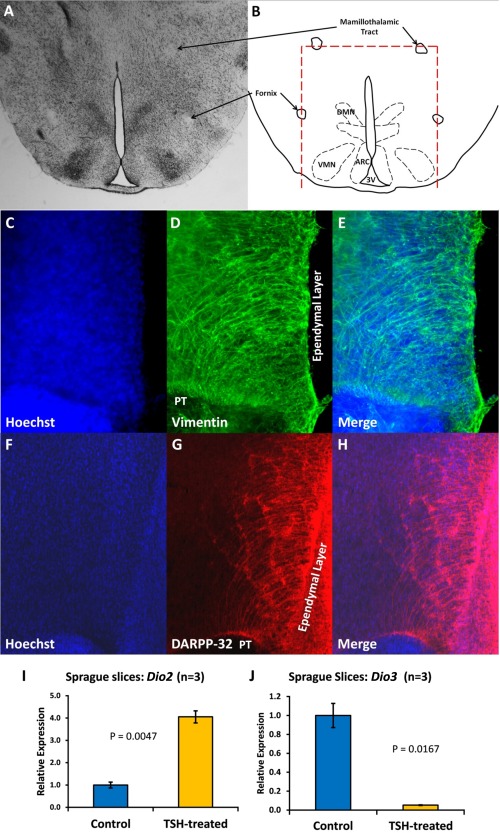
Hormone‐responsive tanycytes are maintained in hypothalamic organotypic culture. 400 μm coronal slices were cut through the hypothalamus of P10‐12 Sprague Dawley rat pups. The mammillothalamic tract and fornix (A, B) were used as anatomical landmarks to trim the slices. Nissl staining of P10 rat sections (A) shows the location of the DMN, VMN and arcuate (ARC) nuclei in relation to these landmarks. Slices fixed after 6 days of culture in vitamin A‐deficient medium and labelled using antibodies against the tanycyte markers vimentin (C–E) and dopamine‐ and cAMP‐regulated phosphoprotein (DARPP‐32; F–H) show that tanycytes are present in cultured slices. To test the response of tanycytes in cultured hypothalamus to hormonal signals, slices were treated with 10 mIU thyroid‐stimulating hormone (TSH) for 48 hours before qPCR analysis of gene expression. TSH upregulated Dio2 (I) and downregulated Dio3 (J), demonstrating that tanycytes are not only present after 6 days ex vivo, but respond as expected to TSH in terms of gene expression. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Crucially for these studies, these slices could be cultured in a way that maintained health of the neurons, the integrity of the hypothalamic nuclei and also the viability of cells vital to TH and RA signalling, the tanycytes. The tanycytes are specialized radial glia‐like cells in the cell layer lining the third ventricle thought to be critical for the transduction of extrahypothalamic signals into the parenchyma. Tanycytes are the cells in which both RA is synthesized (Shearer et al., 2010; Shearer et al., 2012b) and thyroxine (T4) is converted to the more active T3 (Yasuo et al., 2007). To confirm the presence of tanycytes in cultured rat hypothalami, slices were maintained ex vivo for 6 days and then fixed in 4% PFA. Immunohistochemistry using antibodies against the tanycyte markers vimentin (Fig. 2C‐E; Leonhardt et al., 1987) and dopamine‐ and cAMP‐regulated phosphoprotein (DARPP‐32, Fig. 2F‐H; Meister et al., 1988) demonstrated that tanycytes were present after 6 days of ex vivo culture, with processes projecting into the parenchyma of the hypothalamus.
Short‐term slice cultures of adult mouse hypothalamus have been shown to be responsive to thyroid‐stimulating hormone (TSH) after 4 hours ex vivo, upregulating Dio2 expression and downregulating Dio3 in the ependymal cell layer of the ventromedial hypothalamus (Unfried et al., 2009). TSH was used as a positive control to test the response of tanycytes in cultured rat slices to a hormonal signal. Slices were maintained in culture for 4 days, then treated for 48 hours with 10 mIU bovine TSH before qPCR analysis. TSH treatment of cultured slices induced a 4‐fold increase in Dio2 expression (P=0.005; Fig. 2I) and reduced Dio3 by 95% (P = 0.02; (Fig. 2J). These data demonstrate that tanycytes are maintained in cultured rat hypothalamus for up to 6 days and that cultured tissue can behave like the in vivo hypothalamus in terms of hormonal control of gene expression.
T3 Upregulates Raldh1 Expression in Cultured Hypothalamus
A key function for TSH in the hypothalamus is to increase deiodinase 2 and decrease deiodinase 3 in cells, including tanycytes, of the ependymal layer of the hypothalamus (Helfer et al., 2013) and so locally increase T3 levels, the most transcriptionally active form of TH. T3 is known to signal within the hypothalamus and is essential as a mediator for environmental regulators of growth and energy balance (Mullur et al., 2014). However, the genes on which T3 directly acts upon to bring about these changes in the hypothalamus are poorly understood. Given Raldh1's induction by T3 in vivo (Fig. 1A), this was tested in the ex vivo slice culture assay. Slices were treated with 50 nM T3 for 48 hours before analysis of gene expression by qPCR.
Raldh1 was potently induced by T3, with a 4‐fold increase in expression in T3‐treated hypothalamic slices (P < 0.001; Fig. 3A), indicating that activation of thyroid hormone signalling in the hypothalamus itself is sufficient to upregulate Raldh1 expression and therefore regulate synthesis of RA in the hypothalamus. Thus, regulation of Raldh1 expression, and therefore potentially the rate of RA synthesis, in the hypothalamus may provide one route by which T3 can bring about changes in hypothalamic gene expression.
Figure 3.
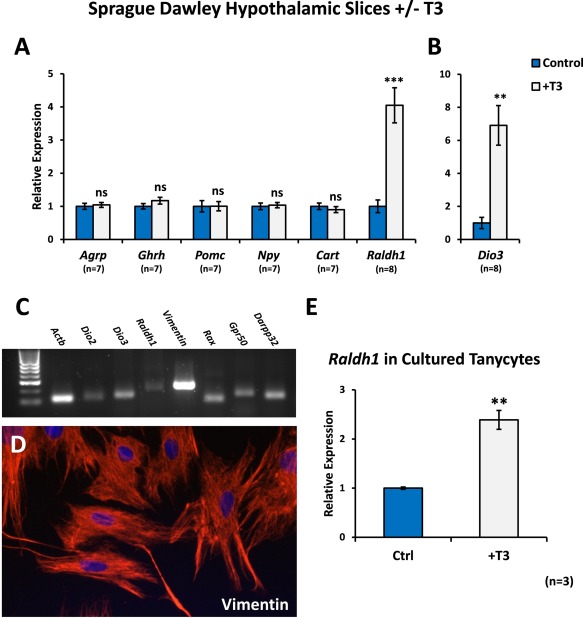
T3 upregulates Raldh1 in organotypic cultures of rat hypothalamus. A: Hypothalamic slices from P10 male Sprague Dawley rats were cultured for 4 days and then treated for 48 hours with 50 nM T3 before qPCR analysis. As in vivo, Raldh1 was upregulated in T3‐treated hypothalamic slices. Agrp, Ghrh, Pomc, Npy and Cart were unaffected by T3. B: Dio3 was used as a positive control for T3 activity. Numbers of samples are shown. C: Primary cultures of tanycytes expressed tanycyte markers Dio3, Vim, Rax, Gpr50 and Darpp‐32. Some markers such as Dio2, were only weakly expressed and this was also the case for Raldh1. D: The cultured tanycytes strongly expressed vimentin by immunohistochemistry and several of the cells extended long processes. E: Addition of 50nM of T3 to cultured tanycytes significantly induced their expression of Raldh1. Statistical significance was assessed using paired Student's t‐test. ** P < 0.01; *** P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Induction of Raldh1 was similar to that of the T3 catabolic enzyme Dio3, previously shown to be upregulated by T3 (Bianco et al., 2002) and with a TRE in its promoter (Barca‐Mayo et al., 2011) and which was increased over six‐fold in T3‐treated slices compared to controls (Fig. 3B; P = 0.0003). Both the rat and mouse Raldh1 promoters contain a potential TRE (Fig. 1B). In contrast, T3 did not significantly alter expression of Agrp, Ghrh or Pomc, genes thought to be regulated by TH in vivo in F344 rats (Ross et al., 2009) as part of its action to control weight and energy balance (Fig. 3A). Similarly, other genes known to be involved in hypothalamic regulation of these processes, Npy and Cart, were unaffected by T3 (Fig. 3A).
In vivo, the only cells of the hypothalamus that normally express Raldh1 are the tanycytes (Shearer et al., 2012b), the same cells in which T4 is converted to T3 by deiodinase 2 (Dio2). To confirm whether Raldh1 can be induced in tanycytes by T3, primary tanycyte cultures were established following previously described protocols (Bolborea et al., 2015; De Seranno et al., 2004; Prevot et al., 2003) which are approximately 95% pure. These cells expressed the typical markers of tanycytes including Dio3, Vim, Rax, Gpr50 and Darpp‐32 (and weakly Dio2 and Raldh1) by PCR (Fig 3C) and vimentin by immunochemistry (Fig 3D) and T3 was found to significantly induce Raldh1 by 2.5 fold (P = 0.0020; Fig. 3E).
To investigate whether RA may be synthesized locally in the hypothalamus by TH‐induced Raldh1 the expression of a gene regulated by RA, and not by TH in the hypothalamus, was investigated. Cyp26b1 is highly RA‐inducible in hypothalamic slices (P = 0.0006; Fig. 4A) but was not induced by 50 nM T3 after 4 or 48 hours (Fig. 4B, D). Weak, although significant induction is seen after incubation with T3 for 24 hours (Fig. 4C) which may represent a weak response to T3 or possibly may result from low amounts of RA generated from endogenous retinol in the slices, mediated by T3‐induced Raldh1. In contrast to Cyp26b1, treatment of hypothalamic slices with T3 resulted in robust induction of both Dio3 (as a positive control; P = 0.025) and Raldh1 (P < 0.0001) in hypothalamic slices after 24 hours (Fig. 4C) which was maintained at 48 hours (Fig. 4D).
Figure 4.
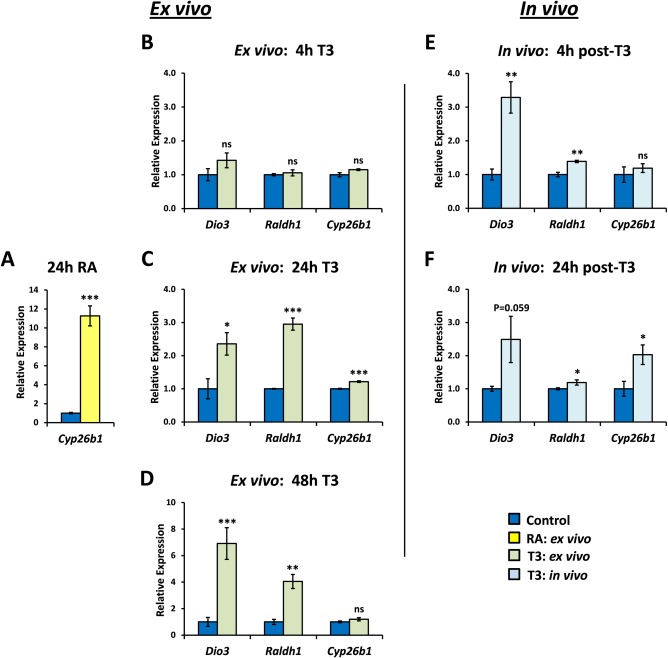
Time course of ex vivo and in vivo induction of genes in the hypothalamus by thyroid hormone. The relative timing of gene induction by T3 was examined for Dio3, as a positive control Raldh1, the RA synthetic enzyme of the hypothalamus and Cyp26b1, a RA‐responsive gene. Cyp26b1 was demonstrated to respond to RA by direct addition of RA to hypothalamic slices (A). None of the three genes examined were responsive within 4 hours of addition of T3 to hypothalamic slices (B) but significant increases of both Dio3 and Raldh1 were evident after 24 (C) or 48 hours T3 treatment (D). Cyp26b1 was only weakly (but significantly) induced at 24 hours (C). In vivo, Dio3 and Raldh1 were both significantly and rapidly induced by 4 hours (E) and, at least for Raldh1, maintained for 24 hours (F). Cyp26b1 did not respond as rapidly in vivo but was induced two‐fold by 24 hours (F) and thus follows the expression of the Raldh1 gene necessary for RA synthesis. Statistical significance was assessed using unpaired Student's t‐test. * P < 0.05; ** P < 0.01; *** P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The influence of TH on the same set of genes was then investigated in vivo. TH rapidly induced both Dio3 and Raldh1 (P = 0.0097 and P = 0.006, respectively; Fig. 4E). This was faster in vivo than ex vivo, suggesting that culture of slices possibly results in a decline in the speed of tissue responsiveness or that in vivo TH is perhaps transported or concentrated more effectively in the hypothalamus, or possibly that extrahypothalamic effects on tanycytes may potentiate Raldh1 expression in vivo. Cyp26b1 was not induced so rapidly by TH (Fig. 4E) but significant 2‐fold induction was evident by 24 hours (P = 0.0192; Fig. 4F) following sequentially from the early increase in Raldh1. This temporal delay in Cyp26b1 induction would be expected if Cyp26b1 was responding not to exogenous T3, but to an increase in RA synthesis by Raldh1.
RA Upregulates Growth‐Associated Genes in Cultured Hypothalamus
If RA may act as a downstream mediator of TH's actions within the hypothalamus then it would be presumed that RA may induce some of the growth‐associated genes which TH does not directly control. Hypothalamic slice cultures were treated with 1 µM RA for 48 hours before qPCR analysis. Rarb was used as a positive control for RA activity, as its promoter contains a well‐characterized RA response element (RARE; Leid et al., 1992). Rarb expression was 7.8‐fold higher in RA‐treated slices than controls (P = 0.004; Fig. 5).
Figure 5.
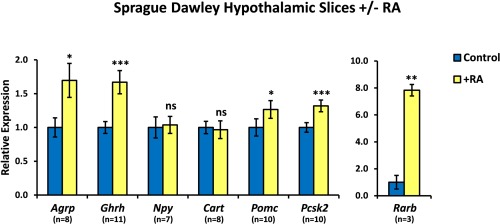
Retinoic acid regulates expression of Agrp and Ghrh in the rat hypothalamus. Hypothalamic slices from male Sprague Dawley rats were cultured for 4 days and then treated for 48 hours with 1 μM RA before qPCR analysis. Agrp and Ghrh expression was significantly upregulated in RA‐treated slices. Pomc and Pcsk2 showed smaller, but still significant increases in expression in RA‐treated cultures. Npy and Cart were unaffected by RA treatment. Rarb was used as a positive control for RA activity. Numbers of samples are shown. Statistical significance was assessed using paired Student's t‐test. * P < 0.05; ** P < 0.01; *** P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In cultured slices, RA significantly upregulated expression of the orexigenic gene Agrp (70% increase relative to control; P = 0.012; Fig. 5) as well as Ghrh (67% increase; P < 0.001). Npy and Cart expression was unaffected by RA treatment (Fig. 5). Pomc showed a small but significant increase in expression in RA‐treated slices (27% increase; P = 0.040). The gene encoding PC2, a prohormone convertase involved in the processing of POMC into hormones including ACTH and α‐MSH (Pritchard et al., 2002), was also upregulated by RA treatment (Pcsk2; 32% increase; P < 0.001).
The experiments described thus far were performed in hypothalamic slices from outbred Sprague Dawley rats. To determine if the effects of RA are applicable to multiple strains of rat, hypothalamic slice cultures were established using tissue from another commonly used rat strain, the photosensitive inbred Fischer F344/N. Slices from F344/N rat pups were treated with 1 µM RA after 4 days ex vivo and harvested after 48 hours for qPCR analysis (Fig. 6). The response of F344/N hypothalamic slices to RA was similar to that of the Sprague Dawley hypothalamus (Fig. 6). As was observed in Sprague Dawley slices, RA induced significant upregulation of Rarb (5.96‐fold, P < 0.001) and Ghrh expression (1.80‐fold, P = 0.004) in the F344/N hypothalamus. Smaller but significant increases in the expression of Pomc (1.40‐fold, P = 0.022) were seen with RA. RA treatment of F344/N hypothalamus increased Agrp expression by a similar amount to that seen in Sprague Dawley slices (F344/N: 1.68‐fold increase, P = 0.051; Sprague Dawley: 1.70‐fold increase, P = 0.012), although this increase was not quite significant. Npy expression was unaffected by RA. Together, these data suggest that the hypothalamic response to RA treatment is similar between the two rat strains.
Figure 6.
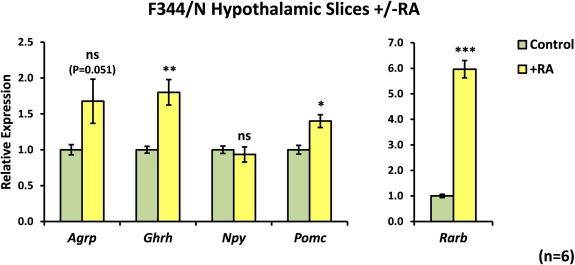
The F344/N rat hypothalamus is also responsive to RA. Hypothalamic slices from male F344/N rats were cultured for 4 days and then treated for 48 hours with 1 μM RA before qPCR analysis. Responses to RA in slices from F344/N rat pups were very similar to those in Sprague Dawley slices. Ghrh was significantly upregulated in RA‐treated slices, with smaller but significant increases in Pomc. Agrp appeared upregulated to a similar extent to that observed in Sprague Dawley slices (Fig. 4), although this did not reach statistical significance in this case. Rarb was used as a positive control for the activity of RA. N = 6 per group for all genes. Statistical significance was assessed using paired Student's t‐test. * P < 0.05; ** P < 0.01; *** P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Hypothalamic RA Signalling Is under Epigenetic Control
Epigenetic regulation of gene expression via DNA/histone modifications is known to control aspects of hypothalamic function, such as hormone synthesis (Miller et al., 2011) and sexual maturation of the brain (Matsuda et al., 2011). In some cases, this is believed to be a behavioural control mechanism that, for instance, contributes to imprinting action of the environment on the young animal that results in altered behaviour in the mature animal. Epigenetic control via histone acetylation is particularly active in promoting RA signalling, and derepression of RA signalling may be a major rate‐limiting target of histone deacetylase (HDAC) inhibitors (Epping et al., 2007). To explore whether the RA signalling system in the hypothalamus is acted upon by such an epigenetic mechanism, cultured hypothalamic slices were treated with a low concentration of RA (10 nM) which results in weaker induction of gene expression. This was combined with trichostatin A (TSA), a class I/II HDAC inhibitor (HDACI), or sirtinol, an inhibitor of the sirtuin (class III) family of HDACs.
In cultured slices from F344/N rats, 10 nM RA was sufficient to increase expression of Rarb (2.5‐fold increase relative to controls; Fig. 7A), but not Ghrh (Fig. 7B). TSA alone did not affect Rarb expression, but potentiated the response to RA (4.1‐fold increase relative to control; 64% higher than RA alone). Ghrh expression was not altered by 10 nM RA or TSA alone, but RA and TSA in combination induced a 2.25‐fold increase in Ghrh (Fig. 7B). Sirtinol had no effect on Ghrh expression and although the response of Rarb to RA was higher in the presence of sirtinol (3.3‐fold increase relative to controls) than with RA alone (2.5‐fold; Fig. 7A), the difference was not significant. Expression of Agrp, Pomc and Npy was not significantly altered by 10 nM RA even in the presence of TSA or sirtinol (Fig. 7C‐E). These data suggest that the expression of some RA‐responsive genes in the hypothalamus may be further regulated as a result of epigenetic chromatin modifications by class I/II HDACs.
Figure 7.
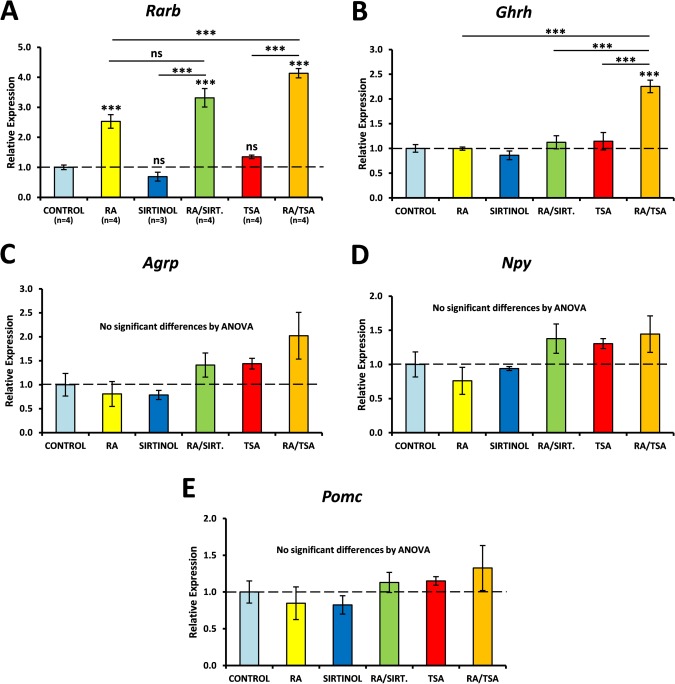
RA regulation of gene expression in the hypothalamus is subject to epigenetic control by class I/II histone deacetylases. After 4 days ex vivo, F344/N hypothalamic slices were treated for 48 hours with trichostatin A (TSA), a class I/II histone deacetylase inhibitor (HDACI), or sirtinol, a class III HDACI, in the presence or absence of 10 nM RA. qPCR analysis was performed using primers for Rarb, Ghrh, Agrp, Npy and Pomc. Rarb was upregulated by 10 nM RA and TSA, but not sirtinol, enhanced the effect of RA (A). Ghrh expression was significantly upregulated by 10 nM RA only in the presence of TSA (B). Agrp, Npy and Pomc were unaffected by HDACIs (D–E). Numbers of samples per condition are shown in (A) and were the same for each gene examined. Data were analysed by ANOVA followed by Tukey's post hoc tests. ** P < 0.01; *** P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
TH controls growth‐associated physiology but T3, the most active TH metabolite, does not directly regulate genes involved in this process and gene targets of TH in the hypothalamus have proved enigmatic (Barrett et al., 2007). This study shows that, in vivo, T3 very rapidly induces expression of a key enzyme, Raldh1, required for RA synthesis by tanycytes, the source of RA for the hypothalamus (Shearer et al., 2010). That RA levels rise in the hypothalamus on addition of TH is suggested by the induction of a RA reporter gene, Cyp26b1, a gene relatively refractory to TH. An organotypic culture system was then developed as a simple system to identify genes immediately downstream of RA and demonstrated that RA (but not T3) has the potential to induce several growth‐associated genes. Thus, the capacity of TH to increase RA synthesis provides a putative mechanism by which TH may control RA‐inducible genes which may include those that regulate appetite and growth. Further, it was shown that the RA signalling system in the hypothalamus is itself partially repressed by an epigenetic mechanism (histone deacetylation) and can be stimulated by inhibition of histone deacetylases.
RA has only recently been recognized to be active in the hypothalamus as a regulatory factor and the hypothalamus is one of the few brain regions in which RA functions, alongside regions such as the hippocampus and olfactory system (Goodman et al., 2012; Hagglund et al., 2006; Shearer et al., 2012a). However the importance of RA and its precursor, vitamin A, to control feeding and weight, was recognized much earlier. The sudden removal of vitamin A from the diet of rats causes rapid weight loss and a reduction in food intake which can be countered by administration of RA (Anzano et al., 1979). Weight loss induced by vitamin A deficiency (VAD) persists even in force‐fed animals, indicating that VAD affects the fundamental mechanisms of weight control in addition to regulating feeding behaviour. Further evidence for the involvement of vitamin A and RA in growth and energy balance comes from the study of photoperiodic animals such as hamsters which display increased growth and feeding under long day (summer‐like) conditions compared to short (winter‐like) daylength. Many components of the RA signalling pathway are upregulated in the hypothalamus of long day‐acclimatized rodents including the retinoic X receptor (RXR) which can heterodimerize with either the TH receptor or the RA related receptor (Helfer et al., 2012; Ross et al., 2005; Ross et al., 2004; Shearer et al., 2010; Shearer et al., 2012b). Finally, the importance of Raldh1 in energy balance is highlighted in the finding that Raldh1 −/− mice are highly resistant to diet‐induced obesity (Ziouzenkova et al., 2007). This phenotype was ascribed to an excess of retinaldehyde in adipose tissue due to a lack of Raldh1. However, more recent studies have identified Raldh1 as the only RA‐synthesizing enzyme in the mouse hypothalamus (Helfer et al., 2012; Shearer et al., 2010) and therefore the downregulation of hypothalamic RA signalling in Raldh1 −/− mice may also play a role in prevention of obesity.
The data presented in this study suggest that TH signalling has the potential to lie upstream of RA in the hypothalamus, as T3 upregulated Raldh1 expression both in vivo and ex vivo. This is consistent with previous observations in VAD rats. Expression of both TRs and RARs is suppressed in the brain of VAD rats (Husson et al., 2003). Injecting VAD rats with RA only reactivated RA signalling, but T3 administration reactivates RAR and TR expression, suggesting that RA signalling can lie downstream of TH signalling in the brain. In the hypothalamus, RA is synthesized by tanycytes, the only cells in the hypothalamus that express both Dio2 and Raldh1, with Dio2 synthesizing T3, which can then act to induce Raldh1 and increase levels of RA potentially to act on both tanycytes and neurons. This proposed pathway is illustrated in Figure 8. This system is notable for the very rapid induction of Raldh1 transcript. Raldh1 protein is transported along the length of the tanycyte fibres (Shearer et al., 2010), which have been shown to contact Agrp/Npy neurons in the hypothalamus (Coppola et al., 2007), potentially releasing RA immediately adjacent to the target cells. This novel pathway provides a new route by which TH may promote expression of neuronal Agrp to increase appetite (Varela et al., 2012) and may also provide a mechanism by which TH could increase Ghrh to promote growth (Ross et al., 2011). The potential also exists for this to be a mechanism by which TH controls neurogenesis given the recent finding of RA's control of cell proliferation in the neurogenic regions of the hypothalamus (Shearer et al., 2012b). β2‐tanycytes have been proposed as a neural stem cell population (Lee et al., 2012) and express Fgf10 (Haan et al., 2013). This potentially influences the birth of new neurons that modulate hypothalamic control of energy balance (Kokoeva et al., 2005; Lee et al., 2012; McNay et al., 2012).
Figure 8.
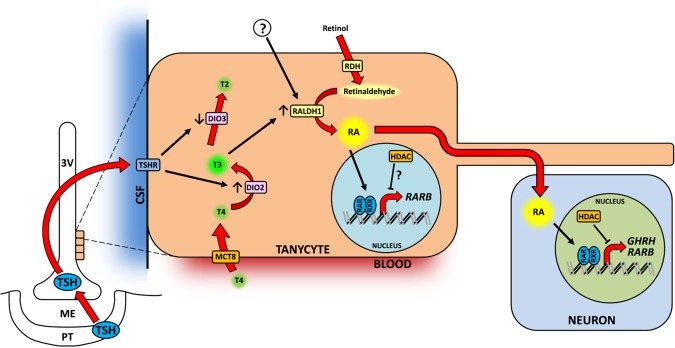
A proposed model of retinoic acid function in the hypothalamus. Thyroid hormone circulates in the blood in the form of thyroxine (T4) and is taken into hypothalamic tanycytes via transporters such as monocarboxylate transporter 8 (MCT8). T4 is converted by type II deiodinase (DIO2) to the more active triiodothyronine (T3), which is inactivated by DIO3. Stimuli such as fasting can result in a local increase in T3 in the tanycytes, upregulating Raldh1 expression. RALDH1 synthesizes retinoic acid (RA), which enters the nucleus and upregulates expression of target genes via binding to retinoic acid receptors (RAR). Tanycytes project long processes into the parenchyma which contact hypothalamic neurons and RA may be released immediately adjacent to target cells. Histone deacetylases (HDAC) may further modulate the action of RA on its target genes. In seasonal animals, the release of thyroid‐stimulating hormone (TSH) from the pars tuberalis of the pituitary (PT) is increased in summer‐like, long‐day conditions. TSH binds to its receptor (TSHR) on the surface of tanycytes and increases DIO2 expression while supressing DIO3, and leading to an increase in T3 in the tanycytes. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The action of Raldh1 as a downstream effector of TH was recently described in the developing mouse cerebral cortex (Gil‐Ibanez et al., 2014). Our own analysis of the mouse Raldh1 promoter identified a putative TRE, similar to the rat. Further, non‐verified gene array analysis of hypothyroid rats, 24 hours after TH treatment, identified Raldh1 as a putative TH‐inducible gene in the hypothalamus (Barrett et al., 2014). This same gene array analysis also identified two other unverified retinoid‐related genes potentially downstream of TH; the first Dhrs7c (dehydrogenase/reductase SDR family member 7C, SRP‐35) which synthesizes retinaldehyde from vitamin A, the substrate of Raldh1 (Treves et al., 2012). Also putatively identified to be strongly induced by TH was Rpe65, a retinoid isomerase (Kiser and Palczewski, 2010).
This study did not show an effect of T3 or RA on Npy/Cart expression. However Npy is presumed to be downstream of TH given that it is upregulated in hyperthyroid animals (Lopez et al., 2010). Hence, there must be alternative regulatory pathways downstream of TH signalling that control NPY, either in the hypothalamus, or extrahypothalamic. Other signalling routes may also exist for TH regulation of Agrp, as recently suggested to involve mTOR (Varela et al., 2012), but such pathways may set up long‐term changes in contrast to the rapid action of TH to induce Raldh1. That RA was found to result in a slight increase in Pomc expression was unexpected given that Agrp was increased and these factors usually display reciprocal changes. Pomc expression is known to be regulated by RA in the pituitary (Paez‐Pereda et al., 2001) and protein expression of adrenocorticotropic hormone (ACTH), a product of POMC, appeared increased by RA in organotypic cultures of mouse hypothalamus (Shearer et al., 2010). There are instances in which Pomc and Agrp are simultaneously increased in the hypothalamus, in conditions where both TH and RA signalling are increased (Ross et al., 2009). Further, there is some evidence that NPY suppresses Pomc expression via the Y2 NPY receptor (Garcia de Yebenes et al., 1995) and in vivo NPY may be capable of opposing the transcriptional activation of Pomc by RA.
Some, but not all, of the RA‐regulated genes in the hypothalamus were subject to regulation by epigenetic modifications. Epigenetic control of gene expression via histone acetylation has been previously shown to mediate, in part, some of the effects of environmental influences on the brain (reviewed by Fagiolini et al., 2009), including alteration of energy balance. For example, changes in HDAC expression and histone acetylation have been observed in the ventromedial and paraventricular hypothalamic nuclei of mice that were either fasted or fed a high‐fat diet (Funato et al., 2011). In addition, epigenetic changes in the foetal hypothalamus caused by maternal stress have been linked to long‐term alterations in energy balance, including susceptibility to diet‐induced obesity (Paternain et al., 2012; Stevens et al., 2010).
These observations suggest that epigenetic modifiers play an important role in the regulation of metabolic states, particularly in the case of persistent changes over longer timescales. There is some evidence that the RA signalling pathway is a major target of HDACIs (Epping et al., 2007). Unliganded RARα is known to suppress transcription of RA target genes by recruiting components of the corepressor complex (Hauksdottir et al., 2003), including HDACs, and HDACIs act partly via derepression of RA signalling (Epping et al., 2007). The data presented in this study, in which induction of Rarb and Ghrh by RA was potentiated in the presence of a class I/II HDACI, suggests that epigenetic regulation of hypothalamic function can, in part, act via the RA signalling pathway.
In summary, this study has demonstrated an extra step of regulatory control in the hypothalamic tanycytes which provides a mechanism by which TH has the possibility to control gene expression through sequential nuclear receptor steps, first the TH receptor followed by the RA receptor. Such a route may provide an amplification step for TH signalling. This pathway also provides a point of epigenetic regulation of hypothalamic function. In the hypothalamus, HDACs are involved in masculinization of the brain during the early postnatal period potentially through the nuclear receptor estrogen receptor α and aromatase (Matsuda et al., 2011). Control of corticotropin‐releasing hormone in the hypothalamus by the glucocorticoid receptor, another member of the nuclear receptor superfamily, is potentially mediated by HDACI (Miller et al., 2011). Epigenetic changes in the DNA methylation and histone acetylation states of the promoters of hypothalamic genes involved in energy balance, such as Pomc and Npy, have been found to result from maternal undernutrition or stress (Paternain et al., 2012; Stevens et al., 2010). This represents a mechanism by which hypothalamic plasticity may be moulded.
Acknowledgment
Grant sponsor: Biotechnology and Biological Sciences Research Council; Grant number: BB/K001043/1.
References
- Anzano MA, Lamb AJ, Olson JA. 1979. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr 109:1419–31. [DOI] [PubMed] [Google Scholar]
- Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel‐Morel A, Anouar Y, Dehouck B, E Trinquet, R Jockers, SG Bouret, V Prevot. 2014. Hypothalamic tanycytes are an ERK‐gated conduit for leptin into the brain. Cell Metab 19:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca‐Mayo O, Liao XH, Alonso M, Di Cosmo C, Hernandez A, Refetoff S, Weiss RE. 2011. Thyroid hormone receptor alpha and regulation of type 3 deiodinase. Mol Endocrinol 25:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, ZA Archer, JG Mercer, PJ Morgan. 2007. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology 148:3608–17. [DOI] [PubMed] [Google Scholar]
- Barrett P, Herwig A, Campbell G, Mayer CD, Boelen A, Anderson R, Ross A, Mercer J. 2014. A thyroid hormone challenge in hypothyroid rats identifies T3 regulated genes in the hypothalamus and in models with altered energy balance and glucose homeostasis. Thyroid 24:1575–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89. [DOI] [PubMed] [Google Scholar]
- Bolborea M, Dale N. 2013. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neurosci 36:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolborea M, Helfer G, Ebling FJ, Barrett P. 2015. Dual signal transduction pathways activated by TSH receptors in rat primary tanycyte cultures. J Mol Endocrinol 54:241–50. [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, S Diano. 2007. A central thermogenic‐like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab 5:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V. 2004. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci 24:10353–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Goglia F, Horvath TL. 1998. Fasting‐induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology 139:2879–2884. [DOI] [PubMed] [Google Scholar]
- Epping MT, Wang L, Plumb JA, Lieb M, Gronemeyer H, Brown R, Bernards R. 2007. A functional genetic screen identifies retinoic acid signaling as a target of histone deacetylase inhibitors. Proc Natl Acad Sci U S A 104:17777–17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. 2009. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol 19:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Kikuchi M, Horiguchi K, Kusumoto K, Kouki T, Kawanishi K, Yashiro T. 2009. Estrogen receptor alpha regulates retinaldehyde dehydrogenase 1 expression in rat anterior pituitary cells. Endocr J 56:963–73. [DOI] [PubMed] [Google Scholar]
- Funato H, Oda S, Yokofujita J, Igarashi H, Kuroda M. 2011. Fasting and high‐fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One 6:e18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Yebenes E, Li S, Fournier A, St‐Pierre S, Pelletier G. 1995. Regulation of proopiomelanocortin gene expression by neuropeptide Y in the rat arcuate nucleus. Brain Res 674:112–116. [DOI] [PubMed] [Google Scholar]
- Gil‐Ibanez P, Bernal J, Morte B. 2014. Thyroid hormone regulation of gene expression in primary cerebrocortical cells: Role of thyroid hormone receptor subtypes and interactions with retinoic acid and glucocorticoids. PLoS One 9:e91692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Crandall JE, Nanescu SE, Quadro L, Shearer K, Ross A, McCaffery P. 2012. Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus 22:2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney C, Hall R, Harper M, Owen SG, Roth M, Smart GA. 1970. Newcastle thyrotoxicosis index. Lancet 2:1275–1278. [DOI] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi‐Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. 2013. Fgf10‐expressing tanycytes add new neurons to the appetite/energy‐balance regulating centers of the postnatal and adult hypothalamus. J Neurosci 33:6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund M, Berghard A, Strotmann J, Bohm S. 2006. Retinoic acid receptor‐dependent survival of olfactory sensory neurons in postnatal and adult mice. J Neurosci 26:3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauksdottir H, Farboud B, Privalsky ML. 2003. Retinoic acid receptors beta and gamma do not repress, but instead activate target gene transcription in both the absence and presence of hormone ligand. Mol Endocrinol 17:373–385. [DOI] [PubMed] [Google Scholar]
- Helfer G, Ross AW, Morgan PJ. 2013. Neuromedin U partly mimics thyroid‐stimulating hormone and triggers Wnt/beta‐catenin signalling in the photoperiodic response of F344 rats. J Neuroendocrinol 25:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer G, Ross AW, Russell L, Thomson LM, Shearer KD, Goodman TH, McCaffery PJ, Morgan PJ. 2012. Photoperiod regulates vitamin A and Wnt/beta‐catenin signaling in F344 rats. Endocrinology 153:815–824. [DOI] [PubMed] [Google Scholar]
- House SB, Thomas A, Kusano K, Gainer H. 1998. Stationary organotypic cultures of oxytocin and vasopressin magnocellular neurones from rat and mouse hypothalamus. J Neuroendocrinol 10:849–861. [DOI] [PubMed] [Google Scholar]
- Huq MD, Tsai NP, Gupta P, Wei LN. 2006. Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. Embo J 25:3203–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson M, Enderlin V, Alfos S, Féart C, Higueret P, Pallet V. 2003. Triiodothyronine administration reverses vitamin A deficiency‐related hypo‐expression of retinoic acid and triiodothyronine nuclear receptors and of neurogranin in rat brain. Br J Nutr 90:191–198. [DOI] [PubMed] [Google Scholar]
- Kiser PD, Palczewski K. 2010. Membrane‐binding and enzymatic properties of RPE65. Prog Retin Eye Res 29:428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. 2005. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 310:679–683. [DOI] [PubMed] [Google Scholar]
- Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR. 2004. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology 145:5252–5258. [DOI] [PubMed] [Google Scholar]
- Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn‐Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, SG Bouret, V Prevot, B Dehouck. 2013. Tanycytic VEGF‐A boosts blood‐hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. 2006. The TRH neuron: A hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda‐Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, S Aja, E Ford, G Fishell, S Blackshaw. 2012. Tanycytes of the hypothalamic median eminence form a diet‐responsive neurogenic niche. Nat Neurosci 15:700–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M, Kastner P, Chambon P. 1992. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci 17:427–433. [DOI] [PubMed] [Google Scholar]
- Leonhardt, Krisch, Erhardt. 1987. Organization of the neuroglia in the midsagittal plane of the central nervous system: A speculative report In: Scharrer B, Korf HW, Hartwig HG, editors. Functional morphology of neuroendocrine systems: Berlin: Springer‐Verlag; pp 175–187. [Google Scholar]
- Lopez M, Alvarez CV, Nogueiras R, Dieguez C. 2013. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 19:418–27. [DOI] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez‐Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, PB Martinez de Morentin, S Tovar, R Nogueiras, D Carling, C Lelliott, R Gallego, M Oresic, K Chatterjee, AK Saha, K Rahmouni, C Dieguez, A Vidal‐Puig. 2010. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. 2008. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. Faseb J 22:2676–2689. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. 2011. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152:2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay DE, Briancon N, Kokoeva MV, Maratos‐Flier E, Flier JS. 2012. Remodeling of the arcuate nucleus energy‐balance circuit is inhibited in obese mice. J Clin Invest 122:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Hokfelt T, Tsuruo Y, Hemmings H, Ouimet C, Greengard P, Goldstein M. 1988. DARPP‐32, a dopamine‐ and cyclic AMP‐regulated phosphoprotein in tanycytes of the mediobasal hypothalamus: distribution and relation to dopamine and luteinizing hormone‐releasing hormone neurons and other glial elements. Neuroscience 27:607–622. [DOI] [PubMed] [Google Scholar]
- Miller L, Foradori CD, Lalmansingh AS, Sharma D, Handa RJ, Uht RM. 2011. Histone deacetylase 1 (HDAC1) participates in the down‐regulation of corticotropin releasing hormone gene (crh) expression. Physiol Behav 104:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullur R, Liu YY, Brent GA. 2014. Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Jethwa PH, Warner A, Barrett P, Nilaweera KN, Brameld JM, Ebling FJ. 2012. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology 153:101–112. [DOI] [PubMed] [Google Scholar]
- Paez‐Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E, Losa M, Stalla J, Grubler Y, Missale C, E Arzt, GK Stalla. 2001. Retinoic acid prevents experimental Cushing syndrome. J Clin Invest 108:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J. 2012. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high‐fat‐sucrose diet in prenatally stressed female rats. Neuroendocrinology 96:249–260. [DOI] [PubMed] [Google Scholar]
- Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. 2003. Activation of erbB‐1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity. J Neurosci 23:10622–10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LE, Turnbull AV, White A. 2002. Pro‐opiomelanocortin processing in the hypothalamus: Impact on melanocortin signalling and obesity. J Endocrinol 172:411–21. [DOI] [PubMed] [Google Scholar]
- Ross AW, Bell LM, Littlewood PA, Mercer JG, Barrett P, Morgan PJ. 2005. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinology 146:1940–1947. [DOI] [PubMed] [Google Scholar]
- Ross AW, Helfer G, Russell L, Darras VM, Morgan PJ. 2011. Thyroid hormone signalling genes are regulated by photoperiod in the hypothalamus of F344 rats. PLoS One 6:e21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AW, Johnson CE, Bell LM, Reilly L, Duncan JS, Barrett P, Heideman PD, Morgan PJ. 2009. Divergent regulation of hypothalamic neuropeptide Y and agouti‐related protein by photoperiod in F344 rats with differential food intake and growth. J Neuroendocrinol 21:610–619. [DOI] [PubMed] [Google Scholar]
- Ross AW, Webster CA, Mercer JG, Moar KM, Ebling FJ, Schuhler S, Barrett P, Morgan PJ. 2004. Photoperiodic regulation of hypothalamic retinoid signaling: association of retinoid X receptor gamma with body weight. Endocrinology 145:13–20. [DOI] [PubMed] [Google Scholar]
- Shearer KD, Goodman TH, Ross AW, Reilly L, Morgan PJ, McCaffery PJ. 2010. Photoperiodic regulation of retinoic acid signaling in the hypothalamus. J Neurochem 112:246–257. [DOI] [PubMed] [Google Scholar]
- Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ. 2012a. A vitamin for the brain. Trends Neurosci 35:733–741. [DOI] [PubMed] [Google Scholar]
- Shearer KD, Stoney PN, Nanescu SE, Helfer G, Barrett P, Ross AW, Morgan PJ, McCaffery P. 2012b. Photoperiodic expression of two RALDH enzymes and the regulation of cell proliferation by retinoic acid in the rat hypothalamus. J Neurochem 122:789–799. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. 2010. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151:3652–3664. [DOI] [PubMed] [Google Scholar]
- Treves S, Thurnheer R, Mosca B, Vukcevic M, Bergamelli L, Voltan R, Oberhauser V, Ronjat M, Csernoch L, Szentesi P, F Zorzato. 2012. SRP‐35, a newly identified protein of the skeletal muscle sarcoplasmic reticulum, is a retinol dehydrogenase. Biochem J 441:731–741. [DOI] [PubMed] [Google Scholar]
- Unfried C, Ansari N, Yasuo S, Korf HW, von Gall C. 2009. Impact of melatonin and molecular clockwork components on the expression of thyrotropin beta‐chain (Tshb) and the Tsh receptor in the mouse pars tuberalis. Endocrinology 150:4653–4662. [DOI] [PubMed] [Google Scholar]
- Varela L, Martinez‐Sanchez N, Gallego R, Vazquez MJ, Roa J, Gandara M, Schoenmakers E, Nogueiras R, Chatterjee K, Tena‐Sempere M, C Dieguez, M Lopez. 2012. Hypothalamic mTOR pathway mediates thyroid hormone‐induced hyperphagia in hyperthyroidism. J Pathol 227:209–222. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Iigo M, Nakamura TJ, Watanabe T, Takagi T, Ono H, Ebihara S, Yoshimura T. 2007. Differential response of type 2 deiodinase gene expression to photoperiod between photoperiodic Fischer 344 and nonphotoperiodic Wistar rats. Am J Physiol Regul Integr Comp Physiol 292:R1315–R1319. [DOI] [PubMed] [Google Scholar]
- J Ye, G Coulouris, I Zaretskaya, I Cutcutache, S Rozen, TL Madden. 2012. Primer‐BLAST: A tool to design target‐specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, G Duester, J Plutzky. 2007. Retinaldehyde represses adipogenesis and diet‐induced obesity. Nat Med 13:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]


