Abstract
Memory functioning in Autism Spectrum Disorder (ASD) is characterized by impairments in the encoding of relational but not item information and difficulties in the recollection of contextually rich episodic memories but not in the retrieval of relatively context‐free memories through processes of familiarity. The neural underpinnings of this profile and the extent to which encoding difficulties contribute to retrieval difficulties in ASD remain unclear. Using a paradigm developed by Addis and McAndrews [2006; Neuroimage, 33, 1194–1206] we asked adults with and without a diagnosis of ASD to study word‐triplets during functional Magnetic Resonance Imaging (fMRI) scanning that varied in the number of category relations amongst component words. Performance at test confirmed attenuated recollection in the context of preserved familiarity based retrieval in ASD. The results also showed that recollection but not familiarity based retrieval increases as a function of category relations in word triads for both groups, indicating a close link between the encoding of relational information and recollection. This link was further supported by the imaging results, where blood oxygen level dependent (BOLD) signal responses in overlapping regions of the inferior prefrontal cortex were sensitive to the relational encoding manipulation as well as the contrast between recollection versus familiarity based retrieval. Interestingly, however, there was no evidence of prefrontal signal differentiation for this latter contrast in the ASD group for whom signal changes in a left hippocampal region were also marginally attenuated. Together, these observations suggest that attenuated levels of episodic recollection in ASD are, at least in part, attributable to anomalies in relational encoding processes. Autism Res 2015, 8: 317–327. © 2015 International Society for Autism Research, Wiley Periodicals, Inc.
Keywords: autism, relational memory, item memory, recollection, familiarity
Introduction
The broader cognitive profile of Autism Spectrum Disorder (ASD) is now well known to include a pattern of memory difficulties that holds clues to the neuropathology underlying the disorder and has important implications for the design of effective educational programs [see Boucher & Bowler, 2008; Bowler, Gaigg, & Lind, 2011; Boucher, Mayes, & Bigham, 2012; Gaigg & Bowler, 2012 for comprehensive reviews]. Briefly, working memory poses some difficulties for individuals with ASD when tasks probe the maintenance of progressively more numerous spatial locations [Morris, Rowe, Fox, Feigenbaum, Miotto, & Howlin, 1999; Steele, Minshew, Luna, & Sweeney, 2007; Williams, Goldstein, Carpenter, & Minshew, 2005] or when the unaided retrieval of the precise order of events is required [Poirier, Martin, Gaigg, & Bowler, 2011; Gaigg, Bowler, & Gardiner, 2013]. When demands go beyond the limited capacity of working memory, unaided free recall tends to be compromised, particularly for material that can be organized conceptually [Bowler, Matthews, & Gardiner, 1997; Bowler, Gaigg, & Gardiner, 2009, 2010; Cheung, Chan, Sze, Leung, & To, 2010; Gaigg, Gardiner, & Bowler, 2008; Tager‐Flusberg, 1991], or when learning is assessed over multiple trials [Bennetto, Pennington, & Rogers, 1996; Bowler, Gaigg, & Gardiner, 2008a; Minshew & Goldstein, 2001]. When individuals with ASD do recall previously encountered material they frequently fail to retrieve contextual details associated with the study episode such as where, when, how or from whom they have learned a particular fact [Bowler, Gardiner, & Berthollier, 2004; Hala, Rasmussen, & Henderson, 2005; Lind & Bowler, 2009; O'Shea, Fein, Cillessen, Klin, & Schultz, 2005; Russell & Jarrold, 1999]. Their recall of autobiographical memories also tends to be relatively void of contextual details that characterizes the personally experienced past [Crane & Goddard, 2008; Crane, Goddard, & Pring, 2009; Goddard, Howlin, Dritschel, & Patel, 2007; Lind & Bowler, 2010; Millward, Powell, Messer, & Jordan, 2000]. Contrasting these difficulties on tests of unaided recall, the performance of individuals with ASD on supported test procedures tends to be generally unaffected. Thus, tasks using rhymes [Tager‐Flusberg, 1991], word fragments [Boucher & Warrington, 1976; Bowler, Matthews, & Gardiner, 1997; Gardiner, Bowler, & Grice, 2003], category labels [Bowler, et al., 2009; Mottron, Morasse, & Belleville, 2001] or paired associates [Gardiner, et al., 2003; Minshew & Goldstein, 2001] as cues to previously studied material generally yield preserved levels of performance in ASD. Similarly, tests of recognition memory that require participants to discriminate studied from novel stimuli pose relatively few difficulties [Barth, Fein, & Waterhouse, 1995; Beversdorf, et al., 2000; Boucher, Cowell, Howard, Broks, Mayes, & Roberts, 2005; Bowler, Gaigg, & Gardiner, 2008b; Bowler, Gardiner, & Grice, 2000; Bowler, Gardiner, & Gaigg, 2007; Salmond, Ashburner, Connelly, Friston, Gadian, & Vargha‐Khadem, 2005].
The pattern of performance across supported and unsupported test procedures is indicative of relatively greater impairments in the retrieval than the encoding of information and has led Bowler et al. [2014, 2004] to formulate the “Task Support Hypothesis” according to which performance decrements in ASD can be alleviated by procedures that scaffold particularly memory retrieval. There are, however, important exceptions in the relevant literature. First, some studies report attenuated performance on cued recall and recognition tests by individuals with ASD [Bowler et al.2014, 2004; Chen et al.,2014, 2009; Scherf, Behrmann, Minshew, & Luna, 2008]. Second, when overall recognition performance is preserved, individuals with ASD consistently report fewer experiences of recollecting contextual information associated with the items they recognize, reporting a sense of familiarity that is contextually relatively void instead [Bowler et al.2014, 2000; Bowler et al.2014,2014, 2007]. Finally, when recognition is tested for specific combinations of items or item features, individuals with ASD perform significantly worse [Bowler, Gaigg, & Gardiner, 2014]. These exceptions indicate that certain encoding processes may also be compromised in ASD, which is further supported by studies that manipulate encoding conditions whilst holding retrieval conditions relatively constant [e.g., Gaigg et al., 2008; Mottron, et al., 2001; Toichi & Kamio, 2002]. In particular, the encoding of relations between items and between items and their contexts (relational information) appears to be compromised in ASD while the encoding of item‐specific information, including physical as well as conceptual features of items (e.g., that a banana is a curved, yellow fruit) is relatively preserved [Bowler, et al., 2011; Gaigg et al., 2008].
Disentangling encoding from retrieval processes is notoriously difficult because we inevitably retrieve information about the material we encode and we (re)encode material when we retrieve it. Nevertheless, elegant behavioral experimentation and advances in neuroimaging methods have led to a relatively detailed understanding of the functional organization of the human declarative memory system including the contributions of encoding and retrieval processes to performance on various memory tasks. Briefly, during encoding enthorhinal (ErC) and perirhinal (PrC) cortices of the medial temporal lobe (MTL) are thought to process information specific to individual elements of experience (item‐specific information) whereas the hippocampus establishes relations between them to bring about unique event representations [e.g., Mayes, Montaldi, & Migo, 2007]. Regions in the prefrontal cortex (PFC) modulate these encoding processes as a function of stimulus properties and task demands and during retrieval they orchestrate retrieval strategies and monitor their success. Contextually rich recollection is thought to ensue when the hippocampus (under the influence of PFC) successfully re‐establishes the spatial‐temporal relations that uniquely define a specific prior event, whilst a sense of familiarity prevails when information is retrieved through ErC and PrC processes that do not yield sufficient relational context to support the reconstruction of unique episodes [see Brown & Aggleton, 2001; Eichenbaum, 2004; Eichenbaum, Yonelinas, & Ranganath, 2007; Fletcher & Henson, 2001; Henson, 2005; Simons & Pierce, 2003; Spaniol, Davidson, Kim, Han, Moscovitch, & Grady, 2009, Squire, Wixted, & Clark, 2007 for reviews].
To date, relatively few studies have examined the neural underpinnings of memory decrements in ASD through imaging methods, with those that have focusing primarily on the domain of working memory. The evidence in this context suggests reduced involvement of prefrontal regions in the online maintenance of information over short (a few seconds) periods of time [Luna, et al., 2002; Koshino, Carpenter, Minshew, Cherkassky, Keller, & Just, 2005; see Brandse, et al., 2013 for a review]. Such abnormalities may contribute to difficulties over longer delays by hampering the generation of relations between elements of an episode, thus attenuating the tendency for contextually rich recollection at retrieval [Bigham, Boucher, Mayes, & Anns, 2010; Boucher, 2007; Bowler et al.2014, 2007]. A recent EEG experiment lends some support to this possibility by demonstrating that event related potentials (ERPs) associated with recollection are relatively undifferentiated from those associated with familiarity based retrieval in ASD [Massand, Bowler, Mottron, Hosein, & Jemel, 2013]. It remains unclear, however, to what extent these anomalies might reflect differences already at the stage of encoding. The present study examines this issue, by drawing on a paradigm by Addis and McAndrews [2006] who asked participants to study word‐triplets during fMRI scanning that varied in the number of conceptual relations between component words for a later recognition task. Their results suggested that the inferior frontal gyrus (IFG) of the PFC is involved in generating relational information when it is not obviously given by the stimulus, whilst the hippocampus binds available relations in the service of later retrieval [see also Lepage, Habib, Cormier, Houle, & McIntosh, 2000].
If difficulties in contextually rich recollection in ASD are, at least in part, mediated by difficulties in the encoding of relational information we would expect the following pattern of results on a task such as that by Addis & McAndrews [2006]. First, based on the view that relational encoding fosters subsequent contextually rich recollection we would expect that experiences of recollection but not familiarity would increase as a function of the number of conceptual relations in the to‐be‐remembered word triplets. Second, individuals with ASD would be expected to report fewer experiences of recollecting studied word triplets despite overall preserved levels of recognition memory. Third, the IFG encoding processes that have been linked to the generation of relational information should be attenuated in ASD. And fourth, the MTL processes typically associated with the binding of available relational information should also be attenuated in ASD.
Materials and Methods
Participants
Fourteen individuals with a diagnosis of ASD and fourteen typically developing (TD) comparison adults served as participants. Three individuals (1 ASD, 2 TD) were excluded from subsequent analyses because of inattention during encoding, failure to follow task instructions, or identification of neuropathology on a routine inspection of structural scans. All remaining individuals were free of medication and reported no family history of psychiatric or neurological disorders other than ASD. The experimental procedures were prospectively reviewed and approved by the National Research Ethics Service (Essex 2 Research Ethics Committee).
The final ASD group comprised 12 males and 1 female (all right handed) who were all diagnosed by local health professionals according to the 4th edition of the diagnostic and statistical manual of mental disorders (DSM‐IV) criteria (American Psychiatric Association, 2000]. Assessment with the Autism Diagnostic Observation Schedule [ADOS; Lord, et al., 1989) further supported these diagnoses. TD participants (11 males, 1 female; 1 left handed male) were matched to ASD participants on the basis of chronological age and Wechsler IQ [WAIS‐IIIUK; The Psychological Corporation, 2000] and were screened for characteristics that may be commensurate with a diagnosis of ASD using the Autism Spectrum Quotient questionnaire [ASQ; Baron‐Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001]. Descriptive statistics for the two groups are summarized in Table 1.
Table 1.
Descriptive Statistics for Participant Groups
| Measure | ASD (n = 13) | TD (n = 12) | Cohen's d | ||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | ||
| Age (years) | 35.6 | 10.3 | 22.6–55.5 | 35.5 | 10.5 | 22.9–54.5 | 0.01 |
| VIQ | 106.4 | 12.4 | 81–128 | 113.1 | 15.2 | 86–134 | 0.48 |
| PIQ | 107.3 | 17.6 | 84–136 | 108.0 | 13.8 | 81–125 | 0.04 |
| FIQ | 106.2 | 16.3 | 81–127 | 110.2 | 14.8 | 83–127 | 0.26 |
| ASQ | 34.5a | 7.1 | 22–45 | 15.8a | 4.9 | 8–22 | 3.07a |
| ADOS Com. | 3.2 | 1.3 | 1–5 | — | — | — | — |
| ADOS RSI. | 7.2 | 2.5 | 3–12 | — | — | — | — |
| ADOS Total | 10.3 | 3.2 | 5–17 | — | — | — | — |
(t = 7.07, df = 23, P < 0.001).
ASD and TD groups were well matched in terms of Age (in years), Verbal (VIQ), Performance (PIQ) and Full‐scale (FIQ) Wechsler intelligence quotients. The ASD group scored significantly (t = 7.07, df = 23, P < 0.001) higher on the Autism Spectrum Questionnaire (ASQ). Autism Spectrum Diagnostic Observation Schedule (ADOS) Communication (Com.), Reciprocal Social Interaction (RSI) and Total algorithm scores supported the diagnosis for ASD participants.
Materials
With the exception of minor amendments during test, the materials and procedures of this experiment were identical to those used by Addis and McAndrews [2006].1 Briefly, 108 word triads were constructed, each comprising a capitalized category label and two words in lower‐case font. Either none, one or both of these words were legitimate examples of the named category (36 triads each); hereafter, “0‐link,” “1‐link,” and “2‐link” triads, respectively. During the encoding scan 36 control triads, comprising the words “None,” “One,” or “All,” were randomly interspersed with target triads. For the two‐alternative forced choice recognition test that participants performed outside the scanner, encoded word triads were presented alongside lure triads on the top and bottom half of a laptop monitor. Lure triads differed from encoded triads only with respect to one of the lower‐case exemplar words, which was substituted with a conceptually related item. The position of target and lure triads on the screen and the left right position of substituted items in lure triads was counterbalanced across items. For half of the “1‐link” triads the lure triads substituted the legitimate category exemplar while for the remaining half the unrelated exemplar word was substituted. Figure 1 provides examples of the experimental materials.
Figure 1.
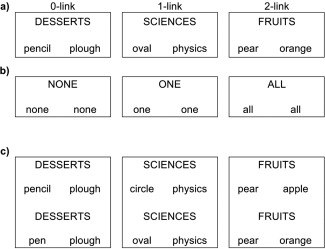
Examples of a) to‐be‐remembered encoding triads, b) control triads, and c) two‐alternative forced‐choice recognition items.
Procedure
Participants were familiarized with the encoding task outside the scanner through a series of 18 practice trials (12 encoding triads and 6 control triads). They were asked to indicate, via a keyboard response, whether “none,” “one,” or “all” of the exemplar words were legitimate examples of the named category (or to press the key corresponding to the words shown on the screen in the case of control triads). The timing of practice trials was identical to that to be used in the scanner with 6 sec per triad followed by a central fixation cross varying in duration between 4 and 8 sec to provide jitter. Response keys on the keyboard (“J,” “K,” and “L”) during practice were chosen to mimic the relative positions of the buttons on the response box in the scanner (index finger = “none”; middle finger = “one”; ring finger = “all”). Instructions clarified that memory for the triads would be tested afterwards although the nature of the memory test was not disclosed. Once participants indicated that they understood what was required, their written informed consent was obtained and they were prepared for scanning.
The scanning session lasted approximately 1 hr and began with a series of structural scans for an unrelated project. Experimental trials were presented in three 9.6 min functional runs comprising 36 encoding (12 of each link type) and 12 control triads each. The order of trials was random with respect to triad type but fixed across subjects. Stimuli were presented in black Arial font on a light‐grey background and back‐projected onto a white screen that participants viewed through a mirror mounted on the head‐coil. Responses were made through an MR‐compatible four‐button response box. Stimulus presentation and recording of participants' responses were controlled by in‐house software. Immediately after the final run, participants were taken to a quiet room where they first completed a 7 min nonverbal distracter task (mental rotation). Participants were then told that they would see all of the word triads they had studied in the scanner once more alongside similar triads they had not seen. The order of test trials was randomized for each participant, whose principal task was to decide which of the two triads they had seen earlier. Unlike Addis and McAndrews [2006] we gave participants unlimited time to make their decisions during this test and we also asked participants to qualify their choices using the “Remember/Know/Guess” procedure [Gardiner, Ramponi, & Richardson‐Klavehn, 2002]. Thus, participants indicated whether they recollected the study episode (“Remember”) for a particular triad, whether they were simply familiar with one of the triads (“Know”) or whether they were purely guessing (“Guess”).
fMRI Acquisition, Processing, and Analysis
Data were acquired on a 3.0T GE Signa system (General Electric Medical Systems) at the Institute of Psychiatry, King's College London. fMRI data were acquired through T2* weighted Gradient Echo sequences (TE = 30 ms, TR = 2000 ms, FOV = 240 mm) during which 38 slices (3 mm thick, 0.3mm gap), horizontally aligned to the AC‐PC line and covering the entire brain, were collected. All preprocessing and analyses were performed in SPM5 (Wellcome Department of Cognitive Neurology, UK) unless otherwise specified. Functional images were realigned for motion correction, slice‐time corrected, spatially normalized to an MNI template, and smoothed using a Gaussian kernel of 8 mm full‐width half maximum. Recognition performance during test was used to retrospectively classify each stimulus event during scanning as either a subsequently “Remembered,” “Known,” “Guessed,” or “Forgotten” (i.e., a triplet for which the participant chose the incorrect option during the forced‐choice test) word triad. These events, together with control triads and the participant's key‐presses, were modeled as fixed effects at the individual level using the canonical hemodynamic response function in SPM5 (head‐movement parameters were also included as regressors). Statistical parametric maps of the t‐statistic (SPM{t}) were generated for each subject and the contrast images were stored for further random‐effects analyses at the second level (see results for details). Only trials were modeled for which participants gave a correct response during the encoding task, to ensure that temporary lapses of concentration did not contaminate the analyses. To identify regions sensitive to the number of relational links in encoded triads, additional models were estimated that included linear parametric predictors [0 1 2]. Within and between‐group effects of interest were examined at the second level within full‐factorial random‐effects models.
Similar to Addis and McAndrews [2006] we focused our analyses primarily on anatomical regions of interest within bilateral IFG and MTL after confirming, at the whole brain level using stringent thresholds (P ≤ 0.005, whole‐brain FDR corrected, minimum extent threshold of 10 voxels) that these regions were indeed involved in the successful encoding of stimuli. To test for the specific within and between group effects of interest we used an uncorrected threshold of P ≤ 0.005 with a minimum extent threshold of 10 contiguously activated voxels. For these analyses a single ROI mask was generated using MARINA (Bender Institute of Neuroimaging; University of Giessen, Germany), comprising the hippocampi and parahippocampal gyri of the MTL and the opercular as well as triangular parts of the IFG bilaterally. Anatomical locations of observed signal contrasts are reported using the Talairach coordinate system and anatomical labels were obtained with the aid of the Talairach client [Lancaster, et al., 2000]. Percent signal changes were extracted and averaged from all the suprathreshold voxels of first‐level contrasts that fell within the region of suprathreshold voxels at the second level using the rfxplot toolbox for SPM5 [Gläscher, 2009]. Similar to the MarsBaR toolbox [Brett, Anton, Valabregue, & Poline, 2002], the rfxplot toolbox computes percent signal changes relative to the voxel‐wise baseline (i.e., the mean signal within the selected voxels) rather than a whole‐brain baseline.
Results
Behavioral Data
Table 2 summarizes the reaction time and accuracy data for participants' responses during the encoding runs. Reaction time data for 1 individual in the TD group were not available due to a misunderstanding of the instructions (a response was given after rather than during triad presentation). A 2 (Group: ASD vs. TD) × 3 (Triad Type: 0‐link vs. 1‐link vs. 2‐link) analysis of variance (ANOVA) of reaction times yielded a main effect of Triad Type (F(2,44) = 9.55, P < 0.001) that was due to slower responses during 0‐link than 1‐link (t = 2.79, df = 24, P < 0.05) or 2‐link triads (t = 3.84, df = 24, P < 0.01), which in turn did not differ significantly from one another (t = 1.54, df = 24, P = 0.14). Although there was no overall group effect (F(1,22) =.15, P = 0.69), there was a Group × Triad Type interaction (F(2,44) = 3.31, P < 0.05), whereby TD participants responded fastest during 1‐link triads while ASD participants responded fastest during 2‐link triads. Response accuracy was also characterized by a main effect of Triad Type (F(2,44) = 15.24, P < 0.001) and a Group × Triad Type interaction (F(2,44) = 5.71, P < 0.01) in the absence of a main effect of Group (F(1,22) = 2.17, P = 0.16). This pattern was the result of participants generally responding most accurately to 0‐link triads and least accurately to 2‐link triads with the ASD group performing worse than the TD group on 2‐link triads (t = 2.89, df = 22, P < 0.01) but not 0‐link (t = 1.00, df = 22, P = 0.33) or 1‐link triads (t = .28, df = 22, P = 0.78). Considered together, these results do not suggest gross differences in encoding performance between groups.
Table 2.
Reaction Time and Accuracy During the Encoding Task in the Scanner
| ASD | TD | Cohen's d | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Reaction Time (ms) | |||||
| 0‐link | 2880 | 532 | 2795 | 508 | 0.16 |
| 1‐link | 2789 | 599 | 2563 | 443 | 0.43 |
| 2‐link | 2567 | 618 | 2628 | 501 | 0.13 |
| Accuracy | |||||
| 0‐link | 0.95 | 0.06 | 0.97 | 0.03 | 0.42 |
| 1‐link | 0.93 | 0.08 | 0.94 | 0.07 | 0.13 |
| 2‐link | 0.87 | 0.06 | 0.94 | 0.05 | 1.27 |
Both groups performed the encoding task (i.e., deciding how many exemplar words were valid members of the named category) at near ceiling levels of accuracy with ASD participants committing somewhat more errors for 2‐link triads.
Performance on the forced‐choice recognition test following the scan is set out in Figure 2. A 2 (Group: ASD vs. TD) × 3 (Triad Type: 0‐link vs. 1‐link vs. 2‐link) × 3 (Recognition Judgment: Remember vs. Know vs. Guess) mixed ANOVA of these data revealed main effects for Trial Type (F(2,46) = 26.40, P < 0.001) and Recognition Judgment (F(2,46) = 5.06, P < 0.05), with better performance on 1‐link (t = 5.69, df = 25, P < 0.001) and 2‐link (t = 6.30, df = 25, P < 0.001) as compared to 0‐link trials and overall more Remember than Know (t = 4.44, df = 25, P < 0.001) and more Know than Guess responses (t = 8.62, df = 25, P < 0.001). More importantly, we observed the predicted interaction between Group and Recognition Judgment (F(2,46) = 6.10, P < 0.01), which replicates earlier demonstrations of attenuated “Remembering” in ASD (t = 2.95, df = 23, P < 0.01) despite overall preserved levels of recognition memory [e.g. Bowler, et al., 2007]. In addition, the data were characterized by the expected Recognition Judgment × Triad Type (F(4,92) = 26.40, P < 0.001) interaction whereby “Remember” responses increased as a function of the number of category relations in word triads (F(2,50) = 49.68, P < 0.001) while “Know” responses were unaffected (F(2,50) = 0.10, P = 0.90) and “Guess” responses decreased (F(2,50) = 21.04, P < 0.001). This interaction confirms that recollection, as indexed by “Remembering” at retrieval, is strongly associated with the processing of relational information during encoding.
Figure 2.
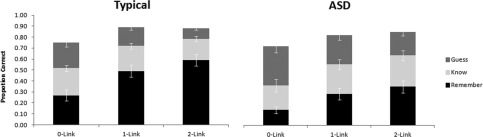
Average proportion of “Remember” (black), “Know” (light grey), and “Guess” (dark grey) responses that make up the correct choices during the 2 alternative‐forced‐choice recognition test for ASD and TD groups as a function of triad type (Error bars represent 1 standard error). Despite overall equivalent correct recognition performance in the two groups, the data replicate previous observations of selectively attenuated Remembering in the ASD group. It is also evident that only Remember responses increase as a function of the number of relational links.
fMRI Results
Successful encoding effects
To identify regions involved in the successful encoding of stimuli in ASD and TD participants, the fixed‐effects models generated for each participant at the first level of the SPM analysis were contrasted as random‐effects at the second level using a 2 (ASD vs. TD) × 2 (Combined Remember and Know vs. Baseline Triads) whole‐brain ANOVA (P ≤ 0.005, whole‐brain FDR corrected, minimum extent threshold of 10 voxels). In line with Addis and McAndrews [2006] there was evidence of robust bilateral activation of PFC and MTL regions during successfully encoded (Remembered and Known) as compared to baseline triads. This observation held for both groups individually and a conjunction analysis showed considerable group overlap, including in clusters of the left IFG and the middle segment of the left hippocampus (see Table 3 for details). Somewhat unexpectedly, a group comparison of this contrast within our anatomical ROIs revealed a more pronounced successful encoding contrast in ASD as compared to TD individuals in left IFG (BA45; x = −51, y = 24, z = 8; z‐score = 2.90; P < 0.005). No group differences were apparent in the MTL, unless the statistical criterion was relaxed to P < 0.01 (maintaining a minimum extent threshold of 10 contiguous voxels). At this threshold, and in line with predictions, the successful encoding signal in a posterior region of the left hippocampus (x = −31, y = −38, z = −5; z‐score = 2.57) was enhanced in TD as compared to ASD participants.
Table 3.
Brain Regions Associated with Successful Encoding Processes in Both ASD and TD Groups
| Brain Regions | Talairarch | z‐score | ||
|---|---|---|---|---|
| x | y | z | ||
| L Lingual Gyrus (BA 18) | −18 | −94 | −14 | 6.24 |
| L Middle/IFG (BA 46/47) | −44 | 15 | 23 | 5.96 |
| L Cerebellum | −42 | −61 | −24 | 5.79 |
| L Parahippocampal/Hippocampus | −30 | −17 | −14 | 4.53 |
| L Precentral Gyrus (BA 4) | −42 | −10 | 53 | 4.27 |
| L Fusiform Gyrus (BA 37) | −47 | −40 | −8 | 3.93 |
| R Inferior Occipital Gyrus (BA 17) | 21 | −93 | −6 | 6.67 |
| R Cerebellum | 34 | −67 | −23 | 4.34 |
| R Insula (BA13) | 31 | 25 | 0 | 3.68 |
A conjunction analysis of ASD and TD groups identified the tabulated regions as significantly involved in successful encoding processes (i.e., combined Remember & Know versus Baseline triad contrast) in both participant groups (P < 0.005, whole‐brain FDR corrected; minimum extent 10 contiguous voxels).
Remember/know effects
To examine the above group differences in encoding processes more closely, and to begin to establish how they might underpin diminished recollection in ASD at retrieval, we first modeled the main effect of recognition judgment (Remember > Know) across both groups, followed by the interaction between recognition judgment and group in a 2 (Group) × 2 (Remember vs. Know) full‐factorial ANOVA. In line with previous observations [e.g., Ranganath, Yonelinas, Cohen, Dy, Tom, & D'Esposito, 2003; see Kim, 2011 for a review] robust clusters extending over large areas of the middle and inferior frontal gyri exhibited increased signal changes during the encoding of items subsequently “Remembered” as opposed to “Known” (BA46; x = −44, y = 16, z = 19; z‐score = 3.34 and x = −40, y = 30, z =10; z‐score = 2.87; P < 0.005). When examining the groups separately this pattern was reliable only for TD participants and when the Group × recognition judgment interaction was modeled directly, two clusters in the left (BA6; x = −40, y = −2, z = 27; z‐score = 3.67; P < 0.005) and right (BA6; x = 36, y = 5, z = 31; z‐score = 3.61; P < 0.005) IFG were identified. As Figure 3 illustrates, this interaction is the result of robust signal differentiation between subsequently Remembered versus Known word triads in TD but not ASD participants, which, incidentally, helps to explain why the successful encoding contrast in the ASD group in the analysis above was enhanced overall. In other words, because groups did not differ in relation to signal changes related to baseline triads (shown for comparison in Fig. 3), the reduced signal differentiation between “Remembered” versus “Known” triads in ASD participants essentially augments the overall successful encoding contrast in comparison to TD participants. Turning to the MTL regions of interest, no reliable Remember versus Know signal contrasts or group differences in such contrasts were observed and no regions in either IFG or MTL demonstrated enhanced signal changes for subsequently “Known” over “Remembered” word triads.
Figure 3.
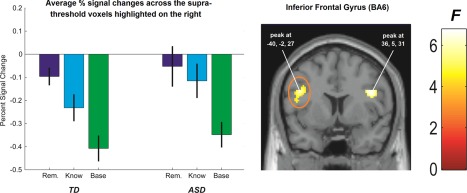
Voxels in left and right inferior prefrontal cortex that are sensitive to a Group × Recognition Judgement interaction (P < 0.005; uncorrected with a minimum extent threshold of 10 contiguous voxels). Average percent signal changes (relative to the voxel‐wise baseline) are shown across all voxels of the left IFG region that are sensitive to this interaction as a function of group (Left set of bars = TD; Right set of bars = ASD) and subsequent recognition judgement (Remembered vs. Known; baseline trials are shown for comparison)—Error Bars represent 1 standard error. A successful encoding effect (i.e., combined Remember & Know > Baseline) is evident in both groups but significant differences between subsequently recollected versus familiar word triads are aparent only in the TD but not the ASD group.
Parametric effects of the number of categorical links in word triads
The evidence above suggests that the encoding of subsequently “Remembered” versus “Known” word triads is subserved by distinguishable neural processes in TD but not ASD participants in regions of the prefrontal cortex. To shed further light on this pattern, we next examined the parametric modulation of encoding related signal changes in this area as a function of the number of relational links in word triads. For this analysis, we subjected the linear parametric predictors (with three levels identifying 0‐link, 1‐link, and 2‐link triads) entered at the first level to a 2 (Group) × 2 (predictor associated with Remembered vs. Known triads) full‐factorial ANOVA at the second level. In line with Addis and McAndrews [2006], this analysis confirmed, irrespective of recognition judgment and across both groups, a negative association between relational links and signal changes in the left IFG (BA 45/9; x = −45, y = 23, z = 4; z‐score = 3.90; P < 0.005). When examining this association for the two groups separately, it was found to be reliable only for ASD but not TD participants. The source of this somewhat surprising observation became apparent when we extracted % signal changes, which are set out in Figure 4 as a function of group, recognition judgment (Remember vs. Know) and relational links (zero, one, or two). As the data illustrate, in the ASD group signal changes decreased as a function of relational links irrespective of whether triads were subsequently “Remembered” or “Known.” In the TD group by contrast, the negative association between signal changes and relational links was apparent only for subsequently “Known” but not “Remembered” triads. Unlike Addis & McAndrews [2006], we did not observe the predicted positive association between signal changes in the hippocampus and the number of relational links in word triads.
Figure 4.
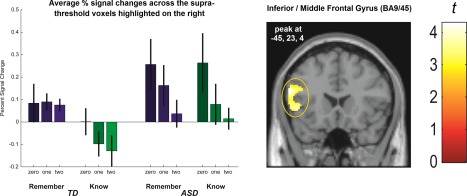
Parametric modulation of percent signal changes (relative to voxel‐wise baseline) in Inferior prefrontal cortex as a function of participant group (Left set of bars = TD; Right set of bars = ASD), recognition judgement (Remembered triads purple; Known triads green) and the number of relational links in word triads (zero link, one link, and two link triads). The coronal section illustrates the voxel cluster that is sensitive to the inverse association between prefrontal signal changes and relational links in word triads across both groups of participants (P < 0.005; uncorrected with a minimum extent threshold of 10 contiguous voxels). As the barchart shows, however, this inverse relationship in TD participants was only observed for subsequently known but not remembered word triads, whereas in the ASD group the liniar decrease was robust irrespective of subsequent recognition judgement. Error bars represent 1 standard error.
Discussion
Based on existing behavioral evidence concerning memory functioning in ASD it remains unclear whether difficulties in this domain stem from atypicalities at the stage of encoding, retrieval or both. We tested the prediction that difficulties in recollection in ASD are, at least in part, attributable to anomalies in the encoding of relational information, and the behavioral data provided clear support for this prediction. Specifically, recollection but not familiarity based recognition was compromised in ASD [e.g., Bowler et al2014, 2007] and only recollection but not familiarity increased as a function of the number of relational links in the studied word‐triads. Together, these observations confirm that recollection at retrieval is closely linked to the processing of relational information at encoding and they lend support to the suggestion that attenuated recollection in ASD is in part attributed to anomalies in relational encoding processes.
At the neural level, the present results offer an independent replication of Addis & McAndrews [2006] observation that signal changes in the inferior frontal region of the left PFC generally increase as a function of decreasing category relations in to‐be‐remembered word‐triads, supporting the notion that this region is important for the generation of relational information when this is not immediately given by the stimulus environment. The observations also confirm previous demonstrations of robust signal differentiation in the IFG for subsequently recollected versus familiar stimuli [e.g., Ranganath, et al., 2003]. Although hippocampal activity was related to later retrieval success, the results did not replicate Addis & McAndrews [2006] observations of a positive association between hippocampal signal changes and the number of category relations in to‐be‐remembered triplets that would underscore the role of the hippocampus in relational binding processes [Mayes et al., 2007]. Since the sample size, and scanning parameters are very comparable between the current and Addis & McAndrews [2006] original study, the most likely source for these discrepancies are the changes we implemented to the recognition procedure. Specifically, participants had unlimited time to respond during the recognition test (compared to the original 6s time limit) and they were required to qualify their choices as either “Remembered,” “Known,” or “Guessed.” This may have led participants to engage more elaborate and varied recognition strategies that potentially obscured some of the hippocampal effects that would otherwise be driven by stimulus characteristics.
In ASD, we expected the prefrontal and medial‐temporal processes mediating the generation and binding of relational information to be attenuated. In line with predictions, the data indicated somewhat reduced engagement of a left posterior hippocampal region in the ASD group, which suggests anomalies in relational binding processes. This observation, however, merits replication in larger samples. In relation to prefrontal processes the prediction of attenuated or atypically modulated encoding processes in ASD was clearly not confirmed. Instead the successful encoding contrasts were overall enhanced in the PFC in the ASD group, and signal changes in this region demonstrated a robust inverse relation with the number of category relations in to‐be‐remembered word triads. Increases in prefrontal activity during memory formation have also been observed in the elderly [Miller et al., 2008; Presson et al., 2006], where they are thought to reflect the engagement of more effortful encoding processes that compensate for age‐related structural and/or functional declines in memory networks. Structural and functional PFC abnormalities are widely reported in the ASD literature [e.g., Duerden, Mak‐Fan, Taylor, & Roberts, 2012] and behaviorally some parallels have been noted between the memory profile seen in ASD and that seen in older adults [see Bowler & Gaigg, 2008] and patients with frontal lobe pathology [e.g., Bowler et al.2010, 2014; Steele, et al.2014, 2007]. Thus, it seems highly likely, that the enhanced successful encoding contrast observed in the current study, is a reflection of the engagement of more effortful encoding strategies, possibly to compensate for attenuated hippocampal binding processes.
Besides the overall enhanced encoding related PFC activation in ASD, there was also a relative lack of signal differentiation between subsequently recollected versus familiar word triads in this group. This may simply be a corollary of more effortful encoding processes in ASD, which could result in a ceiling‐type effect within the PFC whereby each triplet is processed with the maximum resources available. This seems unlikely, however, since PFC activation is clearly sensitive to the number of conceptual relations available for processing, and ceiling effects should attenuate also these effects. Another possibility is that ASD constitutes an example of a single dissociation of functions where one of two processes is either absent or so significantly compromised that the other process dominates behavior. This suggestion was first put forward by Massand et al., [2013] who observed that in TD participants, temporally and topographically distinct ERP components are associated with item (line drawings) versus associative (line drawing—color association) recognition judgments during retrieval whereas in an ASD group the same ERP components were associated with both types of recognition judgments. In previous behavioral studies, we have shown that memory encoding processes in ASD tend to be biased to the processing of item‐specific information, whereas TD participants tend to process relational as well as item‐specific information in parallel [Gaigg et al., 2008; Bowler et al.2009, 2014]. Thus, the neural observations in the current study and in Massand et al. [2013] may be a reflection of a processing bias for item‐specific information in ASD. Because such a bias would be less optimal, it is likely to require greater effort. In other words, a processing bias could account for both the overall greater PFC engagement during encoding as well as the relative lack of signal differentiation as a function of subsequent recognition judgment.
Importantly, and in line with the “Task Support Hypothesis” [Bowler et al.2014, 2004], previous behavioral work suggests that certain encoding conditions that promote the explicit processing of relational information can ameliorate memory difficulties in ASD [Gaigg et al., 2008; Bowler et al., 2010] similar to how retrieval support does. It would be of interest for future studies to establish in how far such encoding conditions also “normalise” neural encoding processes, since any conditions that do may be utilized fruitfully by educators and practitioners to ameliorate behavioral difficulties in the domain of learning and memory in ASD and also promote the development of neural circuitry that may not mature typically without targeted support.
Acknowledgments
This work was supported by a City University Annual Research Prize, by the Autism Imaging Multicentre Study Consortium, Medical Research Council UK Grant G0400061, and by European Autism Interventions—A Multicentre Study for Developing New Medications (EU‐AIMS), which receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115300. The latter includes financial contributions from the European Union Seventh Framework Programme (FP7/2007–2013), the European Federation of Pharmaceutical Industries and Associations companies (in kind), and from Autism Speaks. Calvo‐Merino was furthermore supported by the grants RYC‐2008‐3090 and PSI2012‐34558 from the Spanish Ministry of Economy and Comptetitiveness. We thank the National Institute for Health Research Biomedical Research Centre for Mental Health, and the Dr Mortimer and Theresa Sackler Foundation for their financial support as well as the physicists and radiographers at the Centre for Neuroimaging Sciences at the Institute of Psychiatry, King's College London for their invaluable help with this work.
Footnotes
We thank the authors for kindly providing copies of their materials.
References
- Addis, D.R. , & McAndrews, M.P. (2006). Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. Neuroimage, 33, 1194–1206. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2000). Diagnostic and statistical manual of mental disorders (DSM‐IV‐TR). Washington, DC: Author. [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Skinner, R. , Martin, J. , & Clubley, E. (2001). The autism‐spectrum Quotient (AQ): Evidence from asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Barth, C. , Fein, D. , & Waterhouse, L. (1995). Delayed match‐to‐sample performance in autistic children. Developmental Neuropsychology, 11, 53–69. [Google Scholar]
- Bennetto, L. , Pennington, B. , & Rogers, S. (1996). Intact and impaired memory functions in autism. Child Development, 67, 1816–1835. [PubMed] [Google Scholar]
- Beversdorf, D.Q. , Smith, B.W. , Crucian, G.P. , Anderson, J.M. , Keillor, J.M. , Barrett, A.M. , et al. (2000). Increased discrimination of “false memories” in autism spectrum disorder. Proceedings of the National Academy of Sciences, 97, 8734–8737. [DOI] [PMC free article] [PubMed]
- Bigham, S. , Boucher, J. , Mayes, A. , & Anns, S. (2010). Assessing recollection and familiarity in autistic spectrum disorders: Methods and findings. Journal of Autism and Developmental Disorders, 40, 878–889. [DOI] [PubMed] [Google Scholar]
- Boucher, J. (2007). Memory and generativity in very high functioning autism: A firsthand account, and an interpretation. Autism, 11, 277–286. [DOI] [PubMed] [Google Scholar]
- Boucher, J. , Cowell, P. , Howard, M. , Broks, P. , Mayes, A. , & Roberts, N. (2005). A combined clinical neuropsychological and neuroanatomical study of adults with high‐functioning autism. Cognitive Neuropsychiatry, 10, 165–214. [DOI] [PubMed] [Google Scholar]
- Boucher, J. , Mayes, A. , & Bigham, S. (2012). Memory in autistic spectrum disorder. Psychological Bulletin, 138, 458–496. [DOI] [PubMed] [Google Scholar]
- Boucher, J. , & Bowler, D.M. (2008). Memory in autism: theory and evidence. Cambridge: Cambridge University Press. [Google Scholar]
- Boucher, J. , & Warrington, E.K. (1976). Memory deficits in early infantile autism: Some similarities to the amnesic syndrome. British Journal of Psychology, 67, 73–87. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , & Gaigg, S.B. (2008). Memory in ASD: Emerging themes and future prospects In Boucher J. & Bowler D.M. (Eds.), Memory in autism: Theory and evidence. Cambridge: Cambridge University Press. [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Gardiner, J.M. (2008a). Subjective organisation in the free recall of adults with Asperger's syndrome. Journal of Autism and Developmental Disorders, 38, 104–113. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Gardiner, J.M. (2008b). Effects of related and unrelated context on recall and recognition by adults with high‐functioning autism spectrum disorder. Neuropsychologia, 46, 993–999. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Gardiner, J.M. (2009). Free recall learning of hierarchically organized lists by adults with Asperger's syndrome: Additional evidence for diminished relational processing. Journal of Autism and Developmental Disorders, 39, 589–595. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Gardiner, J.M. (2010). Multiple list learning in adults with autistic spectrum disorder: Parallels with frontal lobe damage or further evidence of impaired relational encoding? Journal of Autism and Developmental Disorders, 40, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Gardiner, J.M. (2014). Binding of multiple features in memory by high‐functioning adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44, 2355–2362. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gaigg, S.B. , & Lind, S. (2011). Memory in autism: Binding, self, and brain In Roth I. & Rezaie P. (Eds.), Researching the autistic spectrum: Contemporary perspectives (pp. 316 −347). Cambridge, England: Cambridge University Press. [Google Scholar]
- Bowler, D.M. , Gardiner, J.M. , & Berthollier, N. (2004). Source memory in adolescents and adults with Asperger syndrome. Journal of Autism and Developmental Disorders, 34, 533–542. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gardiner, J.M. , & Gaigg, S.B. (2007). Factors affecting conscious awareness in the recollective experience of adults with Asperger's syndrome. Consciousness and Cognition, 16, 124–143. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Gardiner, J.M. , & Grice, S. (2000). Episodic memory and remembering in adults with Asperger syndrome. Journal of Autism and Developmental Disorders, 30, 295–304. [DOI] [PubMed] [Google Scholar]
- Bowler, D.M. , Matthews, N.J. , & Gardiner, J.M. (1997). Asperger's syndrome and memory: Similarity to autism but not amnesia. Neuropsychologia, 35, 65–70. [DOI] [PubMed] [Google Scholar]
- Brandse, E.M. , Hendriks, M.P.H. , Jansen, J.F.A. , Backes, W.H. , Hofman, P.A.M. , Thoonen, G ., et al. (2013). Working memory deficits in high‐functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. Journal of Neurodevelopmental Disorders, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett, M. , Anton, J.‐L. , Valabregue, R. , & Poline, J.‐B. (2002). Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. Neuroimage, Vol 16, No 2, abstract 497. [Google Scholar]
- Brown, M.W. , & Aggleton, J.P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus. Nature Reviews Neuroscience, 2, 51–61. [DOI] [PubMed] [Google Scholar]
- Chan, A.S. , Cheung, M.‐C. , Han, Y.M.Y. , Sze, S.L. , Leung, W.W. , Man, H.S. , & To, C.Y. (2009). Executive function deficits and neural discordance in children with autism spectrum disorders. Clinical Neuropsychology, 120(6), 1107–1115. [DOI] [PubMed] [Google Scholar]
- Cheung, M.‐C. , Chan, A. , Sze, S.L. , Leung, W.W. , & To, C.Y. (2010). Verbal memory deficits in relation to organization strategy in high‐ and low‐functioning autistic children. Research in Autism Spectrum Disorders, 4, 764–771. [Google Scholar]
- Crane, L. , & Goddard, L. (2008). Episodic and semantic autobiographical memory in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 38, 498–506. [DOI] [PubMed] [Google Scholar]
- Crane, L. , Goddard, L. , & Pring, L. (2009). Specific and general autobiographical knowledge in adults with autism spectrum disorders: The role of personal goals. Memory, 17, 557–576. [DOI] [PubMed] [Google Scholar]
- Duerden, E.G. , Mak‐Fan, K.M. , Taylor, M.J. , & Roberts, S.W. (2012). Regional differences in grey and white matter in children and adults with autism spectrum disorders: An activation likelihood estimate (ALE) meta‐analysis. Autism Research, 5, 49–66. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44, 109–120. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. , Yonelinas, A.R. , & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Reviews of Neuroscience, 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, P.C. , & Henson, R.N.A. (2001). Frontal lobes and human memory: Insights from functional neuroimaging. Brain, 124, 849–881. [DOI] [PubMed] [Google Scholar]
- Gaigg, S.B. , & Bowler, D.M. (2012). Relational memory difficulties in Autism Spectrum Disorder: Implications for education. British Journal of Psychological Society, Monograph Series II, 9, 34–52. [Google Scholar]
- Gaigg, S.B. , Bowler, D.M. , & Gardiner, J.M. (2013). Episodic but not semantic order memory difficulties in autism spectrum disorder: Evidence from the historical figures task. Memory, 22, 669−978. [DOI] [PubMed] [Google Scholar]
- Gaigg, S.B. , Gardiner, J.M. , & Bowler, D.M. (2008). Free recall in autism spectrum disorder: The role of relational and item‐specific encoding. Neuropsychologia, 46, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, J.M. , Bowler, D.M. , & Grice, S. (2003). Perceptual and conceptual priming in autism: An extension and replication. Journal of Autism and Developmental Disorders, 33, 259–269. [DOI] [PubMed] [Google Scholar]
- Gardiner, J.M. , Ramponi, C. , & Richardson‐Klavehn, A. (2002). Recognition memory and decision processes: a meta‐analysis of remember, know, and guess responses. Memory, 10, 83–89. [DOI] [PubMed] [Google Scholar]
- Gläscher (2009). Visualization of group inference data in functional neuroimaging. Neuroinformatics, 7, 73–82. [DOI] [PubMed] [Google Scholar]
- Goddard, L. , Howlin, P. , Dritschel, B. , & Patel., T. (2007). Autobiographical memory and social problem solving in Asperger Syndrom. Journal of Autism and Developmental Disorders, 37, 291–300. [DOI] [PubMed] [Google Scholar]
- Hala, S. , Rasmussen, C. , & Henderson, A. (2005). Three types of source monitoring in children with and without autism: The role of executive function. Journal of Autism and Developmental Disorders, 35, 75–89. [DOI] [PubMed] [Google Scholar]
- Henson, R. (2005). A mini‐review of fMRI studies of human medial temporal lobe activity associated with recognition memory. The Quarterly Journal of Experimental Psychology, 5813, 340–360. [DOI] [PubMed] [Google Scholar]
- Kim, H. (2011). Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. Neuroimage, 54, 2446–2461. [DOI] [PubMed] [Google Scholar]
- Koshino, H. , Carpenter, P.A. , Minshew, N.J. , Cherkassky, V.L. , Keller, T.A. , & Just, M.A. (2005). Functional connectivity in an fMRI working memory task in high‐functioning autism. Neuroimage, 24, 810–821. [DOI] [PubMed] [Google Scholar]
- Lancaster, J.L. , Woldorff, M.G. , Parsons, L.M. , Liotti, M. , Freitas, C.S. , Rainey, L. , et al. (2000). Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage, M. , Habib, R. , Cormier, H. , Houle, S. , & McIntosh, A.R. (2000). Neural correlates of semantic associative encoding in episodic memory. Cognitive Brain Research, 9, 271–280. [DOI] [PubMed] [Google Scholar]
- Lind, S. , & Bowler, D. M. (2009). Recognition memory, self– other source memory, and theory‐of‐mind in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 39, 1231–1239. [DOI] [PubMed] [Google Scholar]
- Lind, S.E. , & Bowler, D.M. (2010). Episodic memory and episodic future thinking in adults with autism. Journal of Abnormal Psychology, 119, 896–905. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , Goode, S. , Heemsbergen, J. , Jordan, H. , Mawhood, L. , & Schopler, E. (1989). Autism diagnostic observation schedule: A standardized observation of communicative and social behaviour. Journal of Autism and Developmental Disorders, 19, 185–212. [DOI] [PubMed] [Google Scholar]
- Luna, B. , Minshew, N.J. , Garver, K.E. , Lazar, N.A. , Thurborn, K.R. , Eddy, W.F. , et al. (2002). Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology, 24, 834–840. [DOI] [PubMed] [Google Scholar]
- Massand, E. , Bowler, D.M. , Mottron, L. , Hosein, A. , & Jemel, B. (2013). ERP correlates of recognition memory in autism spectrum disorder. Journal of Autism and Developmental Disorders, 43, 2038–2047. [DOI] [PubMed] [Google Scholar]
- Mayes, A. , Montaldi, D. , & Migo, E. (2007). Associative memory and the medial temporal lobes. Trends in Cognitive Sciences, 11, 126–135. [DOI] [PubMed] [Google Scholar]
- Miller, S.L. , Celone, K. , De Peau, K. , Diamond, E. , Dicherson, B.C. , Rentz, D. , et al. (2008). Age‐related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences, 105, 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward, C. , Powell, S. , Messer, D. , & Jordan, R. (2000). Recall for self and other in autism: Children's memory for events experienced by themselves and their peers. Journal of Autism and Developmental Disorders, 30, 15–28. [DOI] [PubMed] [Google Scholar]
- Minshew, N. , & Goldstein, G. (2001). The pattern of intact and impaired memory functions in autism. Journal of Child Psychology and Psychiatry, 42, 1095–1101. [DOI] [PubMed] [Google Scholar]
- Morris, R. , Rowe, A. , Fox, N. , Feigenbaum, J. , Miotto, E. , & Howlin, P. (1999). Spatial working memory in Asperger's syndrome and in patients with focal frontal and temporal lob lesions. Brain and Cognition, 41, 9–26. [DOI] [PubMed] [Google Scholar]
- Mottron, L. , Morasse, K. , & Belleville, S. (2001). A study of memory functioning in individuals with autism. Journal of Child Psychology and Psychiatry, 42, 253–260. [PubMed] [Google Scholar]
- O'Shea, A. , Fein, D. , Cillessen, A. , Klin, A. , & Schultz. R. (2005). Source memory in children with autism spectrum disorders. Developmental Neuropsychology, 27, 337–360. [DOI] [PubMed] [Google Scholar]
- Poirier, M. , Martin, J.S. , Gaigg, S. , & Bowler, D.M. (2011). Short‐term memory in autism spectrum disorder. Journal of Abnormal Psychology, 120, 247–252. [DOI] [PubMed] [Google Scholar]
- Presson, J. , Nyberg, L. , Lind, J. , Larsson, A. , Nilsson, L.‐G. , Ingvar, M. , et al. (2006). Structure‐Function correlates of cognitive decline in aging. Cerebral Cortex, 16, 907–915. [DOI] [PubMed] [Google Scholar]
- Ranganath, C. , Yonelinas, A. , Cohen, M.X. , Dy, C.J. , Tom, S.M. , & D'Esposito, M. (2003). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia, 42, 2–13. [DOI] [PubMed] [Google Scholar]
- Russell, J. , & Jarrold, C. (1999). Memory for actions in children with autism: Self versus other. Cognitive Neuropsychiatry, 4, 303–331. [DOI] [PubMed] [Google Scholar]
- Salmond, C. , Ashburner, J. , Connelly, A. , Friston, K. , Gadian, D. , & Vargha‐Khadem, F. (2005). The role of the medial temporal lobe in autistic spectrum disorders. The European Journal of Neuroscience, 22, 764–772. [DOI] [PubMed] [Google Scholar]
- Scherf, K.S. , Behrmann, M. , Minshew, N. , & Luna, B. (2008). Atypical development of face and greeble recognition in autism. The Journal of Child Psychology and Psychiatry, 49(8), 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, J.S. , & Spiers, H.J. (2003). Prefrontal and medial temporal lobe interaction in long‐term memory. Nature Reviews Neuroscience, 4, 637–648. [DOI] [PubMed] [Google Scholar]
- Spaniol, J. , Davidson, P.S.R. , Kim, A.S.N. , Han, H. , Moscovitch, M. , & Grady, C.L. (2009). Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia, 47, 1765–1779. [DOI] [PubMed] [Google Scholar]
- Squire, L.R. , Wixted, J.T. , & Clark, R.E. (2007). Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews, 8, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, S. , Minshew, N. , Luna, B. , & Sweeney, J. (2007). Spatial working memory deficits in autism. Journal of Autism and Developmental Disorders, 37, 605–612. [DOI] [PubMed] [Google Scholar]
- Tager‐Flusberg, H. (1991). Semantic processing in the free recall of autistic children. British Journal of Developmental Psychology, 9, 417–430. [Google Scholar]
- The Psychological Corporation . (2000). Wechsler adult intelligence scale III UK edition (WAIS IIIUK). 3rd ed. London: The Psychological Corporation. [Google Scholar]
- Toichi, M. , & Kamio, Y. (2002). Long‐term memory and levels‐of processing in autism. Neuropsychologia, 40, 964–969. [DOI] [PubMed] [Google Scholar]
- Williams, D.L. , Goldstein, G. , Carpenter, P. , & Minshew, N. (2005). Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders, 35, 747–756. [DOI] [PubMed] [Google Scholar]


