ABSTRACT
We present data from animal studies showing that the pedunculopontine tegmental nucleus—conserved through evolution, compartmentalized, and with a complex pattern of inputs and outputs—has functions that involve formation and updates of action–outcome associations, attention, and rapid decision making. This is in contrast to previous hypotheses about pedunculopontine function, which has served as a basis for clinical interest in the pedunculopontine in movement disorders. Current animal literature points to it being neither a specifically motor structure nor a master switch for sleep regulation. The pedunculopontine is connected to basal ganglia circuitry but also has primary sensory input across modalities and descending connections to pontomedullary, cerebellar, and spinal motor and autonomic control systems. Functional and anatomical studies in animals suggest strongly that, in addition to the pedunculopontine being an input and output station for the basal ganglia and key regulator of thalamic (and consequently cortical) activity, an additional major function is participation in the generation of actions on the basis of a first‐pass analysis of incoming sensory data. Such a function—rapid decision making—has very high adaptive value for any vertebrate. We argue that in developing clinical strategies for treating basal ganglia disorders, it is necessary to take an account of the normal functions of the pedunculopontine. We believe that it is possible to use our hypothesis to explain why pedunculopontine deep brain stimulation used clinically has had variable outcomes in the treatment of parkinsonism motor symptoms and effects on cognitive processing. © 2016 International Parkinson and Movement Disorder Society
Keywords: Acetylcholine, Basal ganglia, Cognition, Dopamine, Freezing of gait
The pedunculopontine tegmental nucleus (which will be referred to as the pedunculopontine) has been the focus of much clinical interest in the past few years, notably with regard to the possibility that it could be a target for deep brain stimulation in parkinsonism and related disorders. This interest is predicated on particular views about the functions of the pedunculopontine, largely bound up in the idea that it is a motor structure. In this review, we present data from animal studies showing that the pedunculopontine—conserved through evolution, compartmentalized, and with a complex pattern of inputs and outputs—has functions that go considerably beyond this. It does have motor functions, but as with the basal ganglia (to which it is intimately connected), these appear to do with the formation and updating of action–outcome associations and decision making rather than just the control of coordinated stepping. Moreover, the pedunculopontine has sensory and attentional functions that enable it to take part in making very rapid action selection when needed.
Anatomical Connectivity and Species Differences
Comparative studies of the anatomy of the pedunculopontine show that it has a broadly similar construction and pattern of connections in all vertebrate species: (i) internal segregation into a pars dissipatus and pars compactus (corresponding to the terms anterior and posterior pedunculopontine used by our lab in rodent studies) (The pars dissipatus is the bulk of what in the past has been called the midbrain extrapyramidal area,1 reflecting the fact that it receives considerable output from the basal ganglia.); (ii) a significant population of large cholinergic neurons interdigitated with smaller noncholinergic neurons (containing primarily gamma aminobutyric acid [GABA] and glutamate) distributed differentially through anterior/posterior and mediolateral gradients; (iii) extensive connections with the brain stem (both motor and autonomic systems), spinal cord, and cerebellum; (iv) sensory input from visual, auditory, and tactile systems as well as elements of the ascending reticular activating system; (v) significant inputs to the thalamus, giving rise to the ability to effect cortical activity; and (vi) reciprocal connections with the basal ganglia and associated limbic structures (see Table 1 and Fig. 1).
Table 1.
Principal connections of the pedunculopontine tegmental nucleus; representative references are given for each cluster
| Midbrain, brain stem, cerebellum, and spinal cord | |
| Inferior and superior colliculus (reciprocal) | 96, 97, 98 |
| Pontine and medial reticular formation; nucleus pontis oralis | 99, 100, 101, 102 |
| Motor trigeminal | 103, 104, 105 |
| Medulla | 99, 106 |
| Spinal cord (reciprocal) | 107, 108, 109 |
| Ascending reticular activating system | |
| Dorsal raphe, locus coeruleus, laterodorsal tegmental nucleus | 70, 107, 110, 111 |
| Forebrain | |
| Thalamus | 112, 113, 114, 125 |
| Basal ganglia—striatum; globus pallidus (internal and external); subthalamic nucleus; substantia nigra pars reticulata; and projections to midbrain dopamine‐containing neurons | 47, 107, 115, 116, 117, 118, 119, 120 |
| Extended amygdala, basal forebrain, lateral hypothalamus | 113, 118, 121, 122 |
Figure 1.
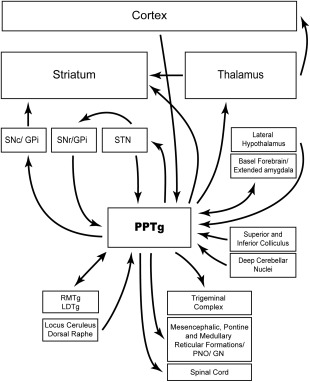
Schematic illustration of pedunculopontine connectivity. Distinct functional types of pedunculopontine subpopulations innervate basal ganglia and in turn basal ganglia structures project back to different neuronal populations in the pedunculopontine. It is important to note that projections form the pedunculopontine to the structures illustrated here are not wholly independent: cholinergic and noncholinergic neurons from topographically distributed populations send collaterals to several structures (eg, to thalamus and basal ganglia). Likewise, descending collaterals of ascending axons contribute to a dense innervation of structures in the lower brainstem, pons, medulla, and spinal cord.125
The connections of the pontomedullary tegmentum in a variety of species were first investigated by Elizabeth Crosby and her colleagues in the 1930s. Those studies showed strong cross‐species similarities in connectivity, which were confirmed in later experiments. It has been shown in various species that the pedunculopontine is highly conserved through evolution, with a similar structure and pattern of connections in teleost fish, amphibians, birds, mammals, and primates (including human).1, 2, 3, 4, 5, 6, 7, 8 Anatomically, the differences between species are a matter of degrees rather than being fundamentally different. For example, the relative strength of afferent and efferent connections of pedunculopontine with the substantia nigra pars reticulata and medal segment of the globus pallidus (which in rodents is the entopeduncular nucleus) differ between species.9 It has been argued that evolution from quadrupedal to bipedal gait has been a cause of differences between species.9 Significant species differentiation might be suggested by the observation of one significant disparity in outcome after pedunculopontine lesions in primates when compared with all other species. In nonprimate species, repeated studies have shown that there are no gross motor deficits after a bilateral loss of the pedunculopontine,10, 11, 12, 13, 14, 15, 16, 17, 18 but akinesia or gait disturbances appear in primates after lesions.19, 20, 21, 22 However, important points in methodology are as (or more) likely to be the cause of this single point of differentiation as is anatomical structure. In particular, the lesions producing frank motor impairments in primates were made using radiofrequency ablation (which destroys fibers as well as neurons and glia) or kainate, a highly potent neurotoxin needing concentration orders of magnitude lower than those used with other excitotoxins and that typically spreads well beyond the intended target. Comparable doses in rats produce very large lesions, which is why kainate stopped being used as a toxin of choice for making excitotoxic lesions in rodent studies more than 20 years ago.23 It is also important to note that electrophysiological studies of single unit activity in primate pedunculopontine have shown patterns of activity consistent with interpretations of pedunculopontine function that are based on data from rodent studies, indicating integrative rather than purely motor functions for the pedunculopontine.24
The Functions of the Pedunculopontine
The animal literature has seen considerable changes in thinking regarding the pedunculopontine during the past decade. The extent of its connectivity and structural heterogeneity underpins the fact that it is involved in diverse processes: autonomic functions, movement and sensorimotor coordination, sleep–wake regulation, attention, and learning. The traditional account of pedunculopontine as part of the ascending reticular activation system involved with sleep regulation25 remains viable. However, firing pattern changes in pedunculopontine that relate to different brain states are complex and do not reflect a role for the pedunculopontine as a “master switch” for behavioral state control.26 Many brain structures have a role in sleep regulation27 with no single structure in overall control. Neurotoxic destruction of the pedunculopontine leaves sleep patterning intact, but changes the ability of animals to respond to challenges such as deprivation of rapid eye movement sleep.25 Recent studies28 have demonstrated that different populations of neurons in the pedunculopontine—cholinergic and noncholinergic—have different roles in behavioral state control. During cortical slow wave states, pedunculopontine neurons are synchronized locally and to cortical oscillatory activity, but during activated states noncholinergic neurons show tonic discharge with little responsiveness to transitions across states. Cholinergic neurons show phasic short latency responses to sensory stimulation—their responding in the activated state is uncoordinated and does not appear to be involved in the maintenance of wakefulness.28 Rather, these neurons appear to be involved in the processing of sensory information. This involvement in the control of thalamocortical activity in the activated (waking) state gives the pedunculopontine very significant potential in regulating cortical processing across a variety of domains. Functional29 and anatomical studies demonstrate pedunculopontine control over thalamic nuclei, including reticular and intralaminar nuclei (see Table 1). Connections through the thalamus with thalamic nuclei give access, for example, to diverse cortical regions, including prefrontal and motor cortex, cingulate, and entorhinal cortices.
The other traditional view of pedunculopontine function—that it is critical for locomotion as part of the so‐called mesencephalic locomotor region (a functionally rather than anatomically defined area)—appears to be no longer tenable based on the bulk of animal studies. The association of the pedunculopontine (and the immediately adjacent cuneiform nucleus) with locomotion came from experiments involving electrical stimulation in the area of the pedunculopontine that elicited coordinated locomotion, sometimes described as machine like. An examination of the mesencephalic locomotor region was typically done in mesencephalic preparations (ie, animals in which descending control of the pedunculopontine and cuneiform nucleus had been severed).30 However, serial studies in rat, mouse, and cat have shown that pedunculopontine loss does not impair movement per se as tested in photocell cages, the home cage, circular corridor, or in open fields10, 11, 12, 13, 14, 15, 16, 17 (and it is worth noting that excitotoxic lesions of the cuneiform nucleus do not impair locomotion either).31 Likewise, pedunculopontine loss does not affect drug‐induced locomotion.11, 12, 13, 14, 15 At a more subtle level of function, our recent data shows that neither full nor partial pedunculopontine lesions affect gait parameters such as stride length, base of support, and swing speed.18 However, performance does decline when excitotoxic lesioned rats are faced with tasks that demand forced acceleration, and grasping tasks are similarly affected.32 Overall, recent literature suggests that there is no gross motor dysfunction after pedunculopontine destruction, but that subtle motor deficits are present and related to task demand. Why should electrical stimulation have such potent effects? A key to understanding this is the fact that the clearest demonstrations of a mesencephalic locomotor region came from local stimulation in transected animals where descending control of the pedunculopontine and cuneiform nucleus had been lost. The descending fibers from the basal ganglia primarily contain the neurotransmitter GABA and work to inhibit activity; that is, it stops the pedunculopontine from taking control of descending motor output (from the pontine and medullary reticular formations). Losing inhibitory control and then stimulating inevitably drives descending pedunculopontine/cuneiform nucleus output and produces locomotion. Similarly, we have seen in unpublished studies that local inhibition of GABA activity in the pedunculopontine produces explosive motor behavior. The functional effects that led to the idea of a mesencephalic locomotor region are perfectly sound and have been easily replicable. What is at issue is interpretation. The data do not imply a purely locomotor role for the pedunculopontine.
An increasing amount of animal literature indicates that the pedunculopontine has a role in cognitive functions, that is, learning and reinforcement processes, the updating of action–outcome associations, and decision making.17, 24, 32, 33, 34, 35, 36, 37, 38, 39, 40 Deficits in cognitive performance following lesions cannot simply be ascribed to poor motor performance. For example, on the 8‐arm radial maze, lesioned rats are quicker than controls moving from arm to arm. What is defective is their decision making about which arm to enter.37 Again, task demand might be an important consideration. When required only to associate one of two places with reward (sucrose‐motivated conditioned place preference), pedunculopontine lesioned rats perform successfully41 but behave at chance levels in the more complex 8‐arm radial maze.37, 42 What appears to be impaired is the ability to form associations. An example of this is the work of Alderson and colleagues, who showed that pedunculopontine lesioned rats could not learn to lever press for drug reward unless they had been pretrained. If they had learned lever press–reward association prior to surgery, their subsequent performance was unimpaired.33 Lesioned rats can do the task, but cannot learn it. That this represents action–outcome association specifically was confirmed later by MacLaren and colleagues43 using a contingency degradation test in which rats with the pedunculopontine inactivated by local injection of muscimol failed to respond properly to changed contingencies in a learning task. They did not stop responding (as the controls did) when action–outcome contingencies shifted.
Differentiation in the Pedunculopontine: Which Neurons Do What?
The pedunculopontine contains a population of large cholinergic neurons (Mesulam's Ch5 group44) as well as GABA and glutamate neurons in addition to peptidergic neurons containing, for example, substance P or atrial natriuretic peptide.44, 46 Cholinergic neurons also possess nicotinamide adenine dinucleotide phosphate diaphorase (the synthetic enzyme for nitric oxide) and have been seen to coexpress glutamate, GABA, and substance P.47, 48 The posterior part of the pedunculopontine—the pars compactus—contains most (although not all) of the cholinergic neurons. Analogies with the structure of substantia nigra have previously been drawn 49—in both cases there is a compact portion containing either cholinergic or dopaminergic neurons with long and widespread projections and a less cell dense portion containing other neurons with more restricted projections and regulated by basal ganglia outflow. Although the size and electrophysiological activity of cholinergic neurons clearly differentiate them from noncholinergic neurons—and although the actions of acetylcholine in target structures such as the thalamus, substantia nigra/ventral tegmental area, and medulla have been described—until recently there has been no opportunity to examine the behavioral functions of these cholinergic neurons selectively. The development of a fusion toxin (urotensin II/diphtheria toxin)50 made examinations of the specific functions of these neurons possible. Excitotoxic lesions of the pedunculopontine destroy all local neurons (and not fibers of passage) and have been seen to impact reward‐related responding and learning, as noted previously. Comprehensive and selective lesions of cholinergic neurons do not impact responding and learning. There is no effect on reward processing,32, 51 locomotion, or learning.52 Reported gait deficits in primates after urotensin II/diphtheria toxin lesions were compromised because the movement problems only became apparent with doses of urotensin II/diphtheria toxin that produced nonspecific lesions.53 Unlike the effects of excitotoxic lesions, skilled motor performance is similarly unaffected by urotensin II/diphtheria toxin lesions. Rats handle small edible objects without difficulty, but deficits emerge when pedunculopontine urotensin II/diphtheria toxin lesioned rats are challenged to maintain stability on an accelerating rotarod treadmill.32 However, whether this is a motor deficit or an attentional problem is uncertain. In rodents, excitotoxic54 as well as selective urotensin II/diphtheria toxin pedunculopontine cholinergic lesions lead to deficits in sustained attention55 and acoustic startle responding. A selective loss of pedunculopontine cholinergic neurons reduced this to the point where it was not detectable, but when intertrial intervals were long enough appropriate responding was seen.56 This returns the cholinergic neurons to a more prominent ascending reticular activing system function, maintaining vigilance and alertness (compatible with a role in behavioral state control) leaving the noncholinergic neurons apparently with greater responsibility for the more basal ganglia–like problems of action selection and learning (although these of course will also benefit from appropriately targeted attention mediated by pedunculopontine cholinergic neurons). Further dissection of the behavioral functions of particular neuronal populations remains a high priority for understanding pedunculopontine functions.
A Functional Hypothesis
The control of action by the CNS is a complex process involving an analysis of sensory data, comparison with past experience, and a selection of one among many competing potential actions—and the need to refresh, switch, or stop the selected action when appropriate. The basal ganglia are thought to provide a central selection mechanism for choosing competing alternative actions.57 The pedunculopontine can be understood as a part of this action selection process but at a lower level of the neuraxis, providing input to thalamo‐cortico‐striatal circuity (through connections with midbrain dopamine [DA] neurons, elements of the basal ganglia, and the thalamus) and receiving output from the basal ganglia. The bulk of this basal ganglia input is inhibitory (the exception being glutamatergic input from the subthalamic nucleus) and appears to control efferents descending from the anterior pedunculopontine, preventing impulsive responding to sensory input in circumstances when it would be inappropriate to do so. This inhibitory basal ganglia output to pedunculopontine is evidently involved in the production of parkinsonian symptomatology. Evidence for this inhibition comes from, for example, the fact that metabolic mapping using 2‐deoxyglucose in primates which had been made hemiparkinsonian by 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) injection highlighted overactivity in the pedunculopontine.58 In addition stimulation or local injections of the GABA antagonist bicucculine attenuated akinesia in primates similarly made parkinsonian with MPTP.59, 60 Like others, we are confident that the pedunculopontine is involved in movement, but we stress that the nature of this involvement is not simply with the control of coordinated locomotor activity but with action selection. The pedunculopontine is in a position to make immediate decisions when there is an imperative need to do so and at moments when comprehensive processing through forebrain circuitry would be too slow. Critical to this is the availability of short latency sensory data, descending connections to sites of motor and autonomic control, and prevention of impulsive responding by inhibitory outflow from the basal ganglia.
To unpack this idea, we first note that the function of the pedunculopontine must reflect the fact that it is differentiated internally (see Fig. 2). Short latency sensory data appears to be received in the posterior part of the pedunculopontine (pars compactus). Electrophysiological recordings in cats and rats show that the neurons have exceptionally short activation latencies to sensory stimuli,61, 62, 63 and in primates pedunculopontine neurons analyze sensory information regarding the salient aspects of a stimulus, that is, the association of a stimulus with a reward, the prediction of the reward value, the reward itself, and the actual value of the reward.24, 64, 65 Similar data have been shown in mice,40 indicating commonality of function across species. Kobayashi and colleagues24, 64, 65 have shown repeatedly that patterns and rates of pedunculopontine neuron firing in primates are not just related to movements (visual saccades) and that the subjects—macaques—had to perform a reward task. Different populations of pedunculopontine neurons responded to reward‐predicting stimuli and their delivery, mirroring the expected reward value.24, 64, 65 Tonic increases and decreases during task execution correlated with response magnitude to larger or smaller reward cues.64 Likewise, when the mice had to make a left‐ or right‐orientating movement following an odor cue to receive a reward, pedunculopontine activity was related to direction selection. Overlapping populations were firing in relation to movement direction and reward outcome.40 Pedunculopontine neurons have an ability to pass information on to basal ganglia systems involved in the considered selection of one among many possible actions. For example, they do this by providing midbrain DA neurons with information about incoming sensory stimuli. This happens fast enough (pedunculopontine sensory response latencies are between 4–80 milliseconds61, 62 to lead us to assume that the pedunculopontine informs DA neurons (which have a response latency of 70–100 milliseconds after stimulus presentation) about external sensory events.66 By influencing midbrain DA neurons67 and activity in the thalamus, the pedunculopontine can assume a role in the construction of goal‐directed actions and movements. It has the capacity to deliver fast sensory data with value already partly assessed to effect corticostriatal processing. In parallel, it is likely that pedunculopontine cholinergic neurons have a simultaneous role in focusing attention and initiating a rapid response to imperative signals.55, 56 The pedunculopontine clearly directs output into forebrain systems, but in addition it projects to motor and associated autonomic control sites lower in the brain stem,8, 68, 69 allowing regulation of key motor, respiratory, and cardiovascular sites that are involved in the production of rapid responses to imperative stimuli. (See Table 1 for details of pedunculopontine connections with the pontine reticular formation, medulla, and spinal cord.) This possession of ascending and descending output is what gives the pedunculopontine the ability to engage in action selection processes through thalamo‐cortico‐striatal systems while influencing behavior more immediately through direct descending connections.
Figure 2.
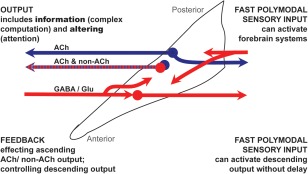
Schematic illustrating the functional inputs and outputs of the pedunculopontine. The dotted line outline of pedunculopontine is taken from the atlas of Paxinos and Watson.95 The colored representation of cholinergic (blue) and noncholinergic neurons (red) is illustrative only; discussion of the topological organization of these can be found elsewhere.7, 126 Sensory input arrives predominantly in the posterior pedunculopontine (pars compactus) and influences ascending activity (which delivers information and guides attention) and has the capacity to stimulate immediate responses to stimuli, likely in advance of any forebrain processing (the acoustic startle response for example56. Input from the forebrain (much of it from the basal ganglia) is largely (but not exclusively) inhibitory, regulating response generation from within the pedunculopontine. It is worth noting that in 1955, building on the analysis of the ascending reticular activating system made by Moruzzi and Magoun127 as well as psychological drive theories, Hebb128 observed that “in general terms, psychologically, we can now distinguish two quite different effects of a sensory event. One is the cue function, guiding behavior; the other, less obvious but no less important, is the arousal or vigilance function. Without a foundation of arousal, the cue function cannot exist” (p. 249). What we are proposing here is highly reminiscent of this—that the pedunculopontine has the capability to (i) deliver information to forebrain systems24, 64, 65, 70; (ii) change the electrophysiological activity of thalamocortical and brainstem circuitry26, 28, 102, 125; (iii) maintain attention54, 55; and (iv) initiate rapid responding when required, further developing on Hebb's idea.56, 72
The pedunculopontine is a deep part of basal ganglia outflow circuitry. Corticostriatal outflow predominantly arrives in the anterior pedunculopontine. Much of it comes from the basal ganglia output nuclei (substantia nigra pars reticulata and the internal segment of the globus pallidus) and is GABA mediated (the exception being glutamatergic input from the subthalamic nucleus), which holds descending pedunculopontine outflow in check. It receives processed information from basal ganglia output nuclei, relating it and comparing it to immediate ongoing sensory input before the information either continues to leave the basal ganglia, is blocked, or reentered to the corticostriatal system for further processing. Behavioral findings support this distinction. Lesions of posterior pedunculopontine impair instrumental learning, presumably interfering with the necessary input to midbrain DA neurons. Anterior pedunculopontine lesions have no effect on learning rate, but cause deficits that can be described as behavioral disinhibition or disorganization, likely the result of a disturbance of basal ganglia outflow.17, 69, 70
Therefore, a distinction is made between the anterior pedunculopontine (a basal ganglia output station) and the posterior pedunculopontine, which makes a first pass analysis of sensory data and, if required, initiates a rapid response before the data are fully processed by forebrain systems. The short response latency to stimuli, involvement in learning, and its role in startle responses and prepulse inhibition36, 56, 71, 72 support the idea that the pedunculopontine has the capacity to act on the brain stem without involving the basal ganglia and that it is involved in learning when not to respond.43 It is imperative that animals have the capacity to make such immediate judgments about the need to act in particular situations (as well as being able to make more deliberate and considered decisions when time permits). Critically, there must be a mechanism to prevent such a system from making impulsive responses: it must be appropriately braked, which is the pedunculopontine. The pedunculopontine is not the only structure involved in this. Similar arguments have been made for the superior colliculus. What perhaps makes the pedunculopontine unique is its capacity for assessing the motivational value of inputs, which necessarily has to involve learning, its polymodal sensory input, and its ability simultaneously to effect lower brain action systems (such as the pontine reticular formation) as well as thalamo‐cortico‐striatal processing.
What Does This Mean for the Neurological Literature in Humans?
Clinical trials of deep brain stimulation (DBS) in the pedunculopontine show heterogeneous results and an overall lack of consistent improvement. The first studies were encouraging.73, 74, 75 The authors reported safe surgical implants and subjective reports from patients of an improved feeling of well‐being and improved motor symptoms. Subsequent studies did not deliver the same results and early improvements could possibly be attributed to placebo effects—follow‐up studies reported only transient gait amelioration.76 The first double‐blind trials proved the importance of careful assessment of stimulation. These studies did not show motor improvements with pedunculopontine‐DBS as measured by the UPDRS77, 78, 79 or by objective freezing measures.77 The freezing of gait and/or falls were reported to improve with pedunculopontine‐DBS, subjectively77 and objectively,80 but not gait parameters, such as stride length. StartReact—an acceleration of a response after a loud auditory stimulus—was shown to be deficient in patients with freezing problems and could be restored by pedunculopontine‐DBS.81 Reaction time in various attentional82 and working memory tasks83, 84 improved, but not the accuracy of responses; improvement was also found in verbal fluency, executive functioning, and delayed recall.83, 85 Furthermore, it was reported that an increase in the duration of rapid eye movement sleep could be achieved with pedunculopontine‐DBS.86, 87 Improvements in attention, memory, and executive function are all consistent with what has been described in experimental studies of nonprimate species.
The pedunculopontine is undoubtedly involved in Parkinson's disease through a loss of neurons and an altered state consequent upon basal ganglia dysfunction—excessive GABA‐mediated inhibition of the anterior parts of the pedunculopontine. What the animal literature predicts—and has been picked up by the literature on humans88—is that the effects of interference with the pedunculopontine through DBS will be critically dependent on the location of the stimulation and its parameters. Specific targeting within pedunculopontine of parkinsonian patients has varied. An Italian group implanted electrodes in caudal pedunculopontine,89, 90 but others were placed rather rostrally83, 91 (if the pedunculopontine was targeted at all92). Prompting the activity of pedunculopontine neurons themselves or their inputs will have profoundly different effects. Stimulation in the posterior part will affect thalamocortical and thalamostriatal processing. In a parkinsonian rodent model, the relation between cortical and pedunculopontine activity was shown to be altered,93 but given that the essential pathology of the basal ganglia will not have been affected by the DBS, this might be no more than a marginal benefit. The same stimulation in the anterior part of the pedunculopontine we would expect, on the basis of animal studies, to be more difficult. The need here is both to eliminate the chronic dysfunction produced by changed basal ganglia input and to restore a normal level of braking of pedunculopontine activity that can be released on demand. It would be preferable to have an action on the inputs to the anterior pedunculopontine rather than the neurons themselves; normalizing the inputs would be better than attempting to activate anterior pedunculopontine neurons themselves while still in receipt of disordered input. We have recently shown that this functional distinction between anterior and posterior pedunculopontine is significant in a rodent model of parkinsonism. We assessed how DBS targeted to either the anterior or posterior pedunculopontine affects gait and postural disturbances in rats bearing extensive loss of striatal DA and in rats with the same DA depletion combined with partial lesions of the pedunculopontine itself, which better mimics the condition that applies in parkinsonism.18 Anterior pedunculopontine stimulation increased gait freezing, but posterior stimulation produced mild gait improvement.
In Summary
Slightly more than 30 years ago David Marsden94 wrote about the mysterious motor functions of the basal ganglia. At that time there was considerable debate about whether the basal ganglia had motor or cognitive functions. What the debate reflected was a relatively poor understanding of what is meant by motor functions. Likewise, previous thinking about the pedunculopontine has occurred within a framework that regarded brain stem function largely as automatic—certainly not cognitive—and that, for the pedunculopontine, focused on the production of locomotion and sleep. We believe that the new conceptual framework that has been developed is a significant advance on this and that studies of a variety of animal species have helped achieve this because the function of pedunculopontine is a very fundamental one, highly conserved through evolution. As well as being a key part of basal ganglia outflow, the pedunculopontine is a critical mechanism for making swift responses essential for survival, which is evident by its compartmentalized nature, its ability to effect thalamo‐cortico‐striatal processing and be regulated by the basal ganglia, its capacity to assess incoming sensory data, and its connections with motor and autonomic control systems in the lower brain stem and spinal cord. Its demonstrated role in action–outcome association learning, attention, and decision making and the complexity of its information processing at a single unit level are evidence of this. Understanding the pedunculopontine in these terms should enable better clinical appreciation of its value as a target for therapeutic interventions.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
N.K.G.: 3A, 3B
P.W.: 3A, 3B
Full financial disclosures of all authors for the past year, regardless of relationship to current manuscript
This research was supported by Medical Research Council grant G0901332 (PW) as part of a European Research Area Network grant. N.K.G. was also supported by a studentship from the School of Psychology, University of St. Andrews and by an award from a Scottish Universities Life Sciences Alliance Strategic Research Development Grant to the University of Strathclyde. P.W. is currently in receipt of a Biotechnology and Biological Sciences Research Council Strategic Skills award (BB/JO13854/1). Previous support to P.W. from the Wellcome Trust and the Biotechnology and Biological Sciences Research Council is gratefully acknowledged. N.K.G. is currently supported by the Swiss National Science Foundation.
Acknowledgments
We are grateful for the support and advice of Professor Jens Volkmann (University of Würzburg) and Professor Peter Redgrave (University of Sheffield).
Funding agencies: P.W. and N.K.G. were supported by the UK Medical Research Council (grant no. G0901332) as part of a European Research Area Network grant. N.K.G. was also supported by a studentship from the School of Psychology, University of St. Andrews and by an award from a Scottish Universities Life Sciences Alliance Strategic Research Development Grant to the University of Strathclyde.
Relevant conflicts of interests/financial disclosures: The authors report no conflict of interest.
References
- 1. Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture cytochemistry and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 1987;259:483‐528. [DOI] [PubMed] [Google Scholar]
- 2. Brantley RK, Bass AH. Cholinergic neurons in the brain of a teleost fish (Porichthys notatus) located with a monoclonal antibody to choline acetyltransferase. J Comp Neurol 1998;275:87‐105. [DOI] [PubMed] [Google Scholar]
- 3. Medina L, Reiner A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol 1994;342:497‐537. [DOI] [PubMed] [Google Scholar]
- 4. Manaye KF, Zweig R, Wu D, et al. Quantification of cholinergic and select non‐cholinergic mesopontine neuronal populations in the human brain. Neuroscience 1999;89:759‐770. [DOI] [PubMed] [Google Scholar]
- 5. Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol 1989;283:611‐633. [DOI] [PubMed] [Google Scholar]
- 6. Marin O, Smeets WJ, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of anuran (Rana perezi Xenopus laevis) and urodele (Pleurodeles waltl) amphibians. J Comp Neurol 1997;382:499‐534. [DOI] [PubMed] [Google Scholar]
- 7. Martinez‐Gonzalez C, Bolam JP, Mena‐Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat 2011;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winn P. Experimental studies of pedunculopontine functions: are they motor sensory or integrative? Parkinsonism Relat Disord 2008;14(suppl 2):S194‐S198. [DOI] [PubMed] [Google Scholar]
- 9. Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 2011;134:11‐23. [DOI] [PubMed] [Google Scholar]
- 10. Inglis WL, Allen LF, Whitelaw RB, Latimer MP, Brace HM, Winn P. An investigation into the role of the pedunculopontine tegmental nucleus in the mediation of locomotion and orofacial stereotypy induced by d‐amphetamine and apomorphine in the rat. Neuroscience 1994;58;817‐833. [DOI] [PubMed] [Google Scholar]
- 11. Alderson HL, Faulconbridge LF, Gregory LP, Latimer MP, Winn P. Behavioural sensitisation to repeated d‐amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience 2003;118:311‐315. [DOI] [PubMed] [Google Scholar]
- 12. Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. Learning disturbances following excitotoxic lesion of cholinergic pedunculo‐pontine nucleus in the rat. Brain Res 1991;544:126‐132. [DOI] [PubMed] [Google Scholar]
- 13. Inglis WL, Dunbar JS, Winn P. Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d‐amphetamine. Neuroscience 1994;62:51‐64. [DOI] [PubMed] [Google Scholar]
- 14. Olmstead MC, Franklin KB. Lesions of the pedunculopontine tegmental nucleus block drug‐induced reinforcement but not amphetamine‐induced locomotion. Brain Res 1994;638:29‐35. [DOI] [PubMed] [Google Scholar]
- 15. Steiniger B, Kretschmer BD. Effects of ibotenate pedunculopontine tegmental nucleus lesions on exploratory behaviour in the open field. Behav Brain Res 2004;151:17‐23. [DOI] [PubMed] [Google Scholar]
- 16. Swerdlow NR, Koob GF. Lesions of the dorsomedial nucleus of the thalamus medial prefrontal cortex and pedunculopontine nucleus: effects on locomotor activity mediated by nucleus accumbens‐ventral pallidal circuitry. Brain Res 1987;412:233‐243. [DOI] [PubMed] [Google Scholar]
- 17. Wilson DI, MacLaren DA, Winn P. Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine, Eur J Neurosci 2009;30:504‐513. [DOI] [PubMed] [Google Scholar]
- 18. Gut NK, Winn P. Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J Neurosci 2015;35:4792‐4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kojima J, Yamaji Y, Matsumura M, et al. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett 1997;226:111‐114. [DOI] [PubMed] [Google Scholar]
- 20. Aziz TZ, Davies L, Stein J, France S. The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br J Neurosurg 1998;12:245‐249. [DOI] [PubMed] [Google Scholar]
- 21. Munro‐Davies LE, Winter J, Aziz TZ, Stein JF. The role of the pedunculopontine region in basal‐ganglia mechanisms of akinesia. Exp Brain Res 1999;129:511‐517. [DOI] [PubMed] [Google Scholar]
- 22. Matsumura M, Kojima J. The role of the pedunculopontine tegmental nucleus in experimental parkinsonism in primates. Stereotact Funct Neurosurg 2001;77:108‐115. [DOI] [PubMed] [Google Scholar]
- 23. Peterson GM, Moore RY. Selective effects of kainic acid on diencephalic neurons Brain Res 1980;202:165‐182. [PubMed] [Google Scholar]
- 24. Kobayashi Y, Okada K. Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Ann N Y Acad Sci 2007;1104:310‐323. [DOI] [PubMed] [Google Scholar]
- 25. Deurveilher S, Hennevin E. Lesions of the pedunculopontine tegmental nucleus reduce paradoxical sleep (PS) propensity: evidence from a short‐term PS deprivation study in rats. Eur J Neurosci 2001;13:1963‐1976. [DOI] [PubMed] [Google Scholar]
- 26. Mena‐Segovia J, Bolam JP. Phasic modulation of cortical high‐frequency oscillations by pedunculopontine neurons. Prog Brain Res 2011;193:85‐92. [DOI] [PubMed] [Google Scholar]
- 27. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 2010;68:1023‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petzold A, Valencia M, Pal B, Mena‐Segovia J. Decoding brain state transitions in the pedunculopontine nucleus: cooperative phasic and tonic mechanisms. Front Neural Circuits 2015;9:article 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ainge JA, Jenkins TA, Winn P. Induction of c‐fos in specific thalamic nuclei following stimulation of the pedunculopontine tegmental nucleus. Eur J Neurosci 2004;20:1827‐1837. [DOI] [PubMed] [Google Scholar]
- 30. Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev 1976;56:465‐501. [DOI] [PubMed] [Google Scholar]
- 31. Allan LF, Inglis WL, Winn P. Is the cuneiform nucleus a critical component of the mesencephalic locomotor region?. An examination of the effects of excitotoxic lesions of the cuneiform nucleus on spontaneous and nucleus accumbens induced locomotion. Brain Res Bull 1996;41:201‐210. [DOI] [PubMed] [Google Scholar]
- 32. MacLaren DAA, Santini JA, Russell AL, Markovic T, Clark SD. Deficits in motor performance after pedunculopontine lesions in rats—impairment depends on demands of task. Eur J Neurosci 2014;40:3224–3236. [DOI] [PubMed] [Google Scholar]
- 33. Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d‐amphetamine self‐administration in pedunculopontine tegmental nucleus‐lesioned rats. Neuroscience 2004;125:349‐358. [DOI] [PubMed] [Google Scholar]
- 34. Alderson HL Latimer MP, Winn P. Intravenous self‐administration of nicotine is altered by lesions of the posterior but not anterior pedunculopontine tegmental nucleus. Eur J Neurosci 2006;23:2169‐2175. [DOI] [PubMed] [Google Scholar]
- 35. Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self‐administration selectively in the rat. Psychopharmacology 2001;158:190‐197. [DOI] [PubMed] [Google Scholar]
- 36. Diederich K, Koch M. Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward‐related behavior in rats. Psychopharmacology 2005;179:402‐408. [DOI] [PubMed] [Google Scholar]
- 37. Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience 2002;112:687‐696. [DOI] [PubMed] [Google Scholar]
- 38. Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci 1998;18:5035‐5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Samson HH, Chappell A. Injected muscimol in pedunculopontine tegmental nucleus alters ethanol self‐administration. Alcohol 2001;23:41‐48. [DOI] [PubMed] [Google Scholar]
- 40. Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision‐making task. J Neurophysiol 2013;110:2817‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alderson HL, Jenkins TA, Kozak R, Latimer MP, Winn P. The effects of excitotoxic lesions of the pedunculopontine tegmental nucleus on conditioned place preference to 4% 12% and 20% sucrose solutions. Brain Res Bull 2001;56:599‐605. [DOI] [PubMed] [Google Scholar]
- 42. Taylor CL, Kozak R, Latimer MP, Winn P. Effects of changing reward on performance of the delayed spatial win‐shift radial maze task in pedunculopontine tegmental nucleus lesioned rats. Behav Brain Res 2004;153:431‐438. [DOI] [PubMed] [Google Scholar]
- 43. MacLaren DA, Wilson DI, Winn P. Updating of action‐outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiol Learn Mem 2013;102:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1‐Ch6). Neuroscience 1983;10:1185‐1201. [DOI] [PubMed] [Google Scholar]
- 45. Ryan MC, Gundlach AL. Anatomical localisation of preproatrial natriuretic peptide mRNA in the rat brain by in situ hybridisation histochemistry: novel identification in olfactory regions. J Comp Neurol 1995;356:168‐182. [DOI] [PubMed] [Google Scholar]
- 46. Vincent SR, Satoh K, Armstrong D, Panula P, Vale W, Fibiger HC. Neuropeptides and NADPH‐diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience 1986;17:167‐182. [DOI] [PubMed] [Google Scholar]
- 47. Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris‐leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol 1996;364:254‐266. [DOI] [PubMed] [Google Scholar]
- 48. Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma‐aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study Brain Res 2003;992:205‐219. [DOI] [PubMed] [Google Scholar]
- 49. Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 1995;47:1‐29. [DOI] [PubMed] [Google Scholar]
- 50. Clark SD, Alderson HL, Winn P, Latimer MP, Nothacker HP, Civelli O. Fusion of diphtheria toxin and urotensin II produces a neurotoxin selective for cholinergic neurons in the rat mesopontine tegmentum. J Neurochem 2007;102:112‐120. [DOI] [PubMed] [Google Scholar]
- 51. Steidl S, Wang H, Wise RA. Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self‐administration or conditioned place preference in rats. PLOS One 2014;9(1): e84412. doi: 10.1371/journal.pone.0084412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. MacLaren DAA, Wilson DIG, Winn P. Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization [published online ahead of print 2015]. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- 53. Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 2010;120:2745‐2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kozak R, Bowman EM, Latimer MP, Rostron CL, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus in rats impair performance on a test of sustained attention. Exp Brain Res 2005;162:257‐264. [DOI] [PubMed] [Google Scholar]
- 55. Cyr M, Parent MJ, Mechawar N, et al. Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post‐mortem immunocytochemistry and in vivo PET imaging with [18F]fluoroethoxybenzovesamicol. Behav Brain Res 2015;278:107‐114. [DOI] [PubMed] [Google Scholar]
- 56. MacLaren DAA, Markovic T, Clark SD. Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons. Eur J Neurosci 2014;40:3526–3537. [DOI] [PubMed] [Google Scholar]
- 57. Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 1999;89:1009‐1023. [DOI] [PubMed] [Google Scholar]
- 58. Mitchell IJ, Clarke CE, Boyce S, et al. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2‐deoxyglucose in monkeys exposed to 1‐methyl‐4‐phenyl‐1236‐tetrahydropyridine. Neuroscience 1989;32:213‐226. [DOI] [PubMed] [Google Scholar]
- 59. Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain 2002;125:2418‐2430. [DOI] [PubMed] [Google Scholar]
- 60. Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey Neuroreport 2004;15:2621‐2624. [DOI] [PubMed] [Google Scholar]
- 61. Dormont JF, Conde H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat I Context‐dependent and reinforcement‐related single unit activity. Exp Brain Res 1998;121:401‐410. [DOI] [PubMed] [Google Scholar]
- 62. Reese NB, Garcia‐Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: I Evoked potentials. Brain Res Bull 1995;37:257‐264. [DOI] [PubMed] [Google Scholar]
- 63. Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci 2005;25:4725‐4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okada K, Kobayashi Y. Reward prediction‐related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci 2013;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci 2009;29:4858‐4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev 2008;58:322‐339. [DOI] [PubMed] [Google Scholar]
- 67. Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci 2003;17:751‐762. [DOI] [PubMed] [Google Scholar]
- 68. Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol 1993;264:R544‐R554. [DOI] [PubMed] [Google Scholar]
- 69. Wilson DIG, MacLaren DAA, Winn P. On the relationships between the pedunculopontine tegmental nucleus corticostriatal architecture and the medial reticular formation In: Groenewegen HJ, Voorn P, Berendse HW, Mulder AB, Cools AR, eds. The Basal Ganglia IX. New York: Springer; 2009:143‐157. [Google Scholar]
- 70. Winn P, Wilson DIG, Redgrave P. Subcortical connections of the basal ganglia In: Heinz S, Kuei YT, eds. Handbook of Basal Ganglia Structure and Function. Cambridge: Academic Press; 2010:397‐408. [Google Scholar]
- 71. Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology 2001;156:216‐224. [DOI] [PubMed] [Google Scholar]
- 72. Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res 1993;97:71‐82. [DOI] [PubMed] [Google Scholar]
- 73. Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport 2005;16:1877‐1881. [DOI] [PubMed] [Google Scholar]
- 74. Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport 2005;16:1883‐1887. [DOI] [PubMed] [Google Scholar]
- 75. Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 2007;130:1596‐1607. [DOI] [PubMed] [Google Scholar]
- 76. Stefani A, Peppe A, Galati S, Bassi MS, D'Angelo V, Pierantozzi M. The serendipity case of the pedunculopontine nucleus low‐frequency brain stimulation: chasing a gait response finding sleep and cognition improvement. Front Neurol 2013;4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ferraye MU, Debu B, Fraix V, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain 2010;133:205‐214. [DOI] [PubMed] [Google Scholar]
- 78. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): process format and clinimetric testing plan. Mov Disord 2007;22:41‐47. [DOI] [PubMed] [Google Scholar]
- 79. Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 2010;133:215‐224. [DOI] [PubMed] [Google Scholar]
- 80. Thevathasan W, Cole MH, Graepel CL, et al. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 2012;135:1446‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thevathasan W, Pogosyan A, Hyam JA, et al. A block to pre‐prepared movement in gait freezing relieved by pedunculopontine nucleus stimulation. Brain 2011;134:2085‐2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thevathasan W, Silburn PA, Brooker H, et al. The impact of low‐frequency stimulation of the pedunculopontine nucleus region on reaction time in parkinsonism. J Neurol Neurosurg Psychiatry 2010;81:1099‐1104. [DOI] [PubMed] [Google Scholar]
- 83. Alessandro S, Ceravolo R, Brusa L, et al. Non‐motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci 2010;289:44‐48. [DOI] [PubMed] [Google Scholar]
- 84. Costa A, Carlesimo GA, Caltagirone C, et al. Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson's disease. Parkinsonism Relat Disord 2010;16:64‐67. [DOI] [PubMed] [Google Scholar]
- 85. Ceravolo R, Brusa L, Galati S, et al. Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol 2011;18:842‐849. [DOI] [PubMed] [Google Scholar]
- 86. Lim AS, Moro E, Lozano AM, et al. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol 209;66:110‐114. [DOI] [PubMed] [Google Scholar]
- 87. Romigi A, Placidi F, Peppe A, et al. Pedunculopontine nucleus stimulation influences REM sleep in Parkinson's disease. Eur J Neurol 2008;15:e64‐e65. [DOI] [PubMed] [Google Scholar]
- 88. Thevathasan W, Pogosyan A, Hyam JA, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012;135:148‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Insola A, Valeriani M, Mazzone P. Targeting the pedunculopontine nucleus: a new neurophysiological method based on somatosensory evoked potentials to calculate the distance of the deep brain stimulation lead from the Obex. Neurosurgery 2012;71:96‐103. [DOI] [PubMed] [Google Scholar]
- 90. Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E. Stereotactic surgery of nucleus tegmenti pedunculopontine [corrected]. Br J Neurosurg 2008;22(suppl 1):S33‐S40. [DOI] [PubMed] [Google Scholar]
- 91. Moreau C, Defebvre L, Devos D, et al. STN versus PPN‐DBS for alleviating freezing of gait: toward a frequency modulation approach? Mov Disord 2009;24:2164‐2166. [DOI] [PubMed] [Google Scholar]
- 92. Zrinzo l, Zrinzo LV, Hariz M. The pedunculopontine and peripeduncular nuclei: a tale of two structures. Brain 2007;130(pt 6):e73. [DOI] [PubMed] [Google Scholar]
- 93. Aravamuthan BR, Bergstrom DA, French RA, Taylor JJ, Parr‐Brownlie LC, Walters JR. Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson's disease. Exp Neurol 2008;213:268‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg lecture. Neurology 1982;32(5):514‐539. [DOI] [PubMed] [Google Scholar]
- 95. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 96. Redgrave P, Mitchell IJ, Dean P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm‐agglutinin conjugated horseradish peroxidase. Exp Brain Res 1987;68:147‐167. [DOI] [PubMed] [Google Scholar]
- 97. Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol 1986;253:525‐538. [DOI] [PubMed] [Google Scholar]
- 98. Motts SD, Schofield BR. Sources of cholinergic input to the inferior colliculus Neuroscience 2009;160:103‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nakamura Y, Tokuno H, Moriizumi T, Kitao Y, Kudo M. Monosynaptic nigral inputs to the pedunculopontine tegmental nucleus neurons which send their axons to the medial reticular formation in the medulla oblongata An electron microscopic study in the cat. Neurosci Lett 1989;103:145‐150. [DOI] [PubMed] [Google Scholar]
- 100. Semba K, Reiner PB, Fibiger HC. Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 1990;38:643‐654. [DOI] [PubMed] [Google Scholar]
- 101. Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol 1996;371:345‐361. [DOI] [PubMed] [Google Scholar]
- 102. Garcia‐Rill E, Skinner RD, Miyazato H, Homma Y. Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons Neuroscience 2001;104:455‐465. [DOI] [PubMed] [Google Scholar]
- 103. Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus II Facial muscle motor systems. Brain Res Brain Res Rev 1997;25:276‐290. [DOI] [PubMed] [Google Scholar]
- 104. Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus I Masticatory muscle motor systems. Brain Res Brain Res Brain Res Rev 1997;25:255‐275. [DOI] [PubMed] [Google Scholar]
- 105. Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus III Lingual muscle motor systems. Brain Res Brain Res Rev 1997;25:291‐311. [DOI] [PubMed] [Google Scholar]
- 106. Skinner RD, Kinjo N, Ishikawa Y, Biedermann JA, Garcia‐Rill E. Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuroreport 1990;1:207‐210. [DOI] [PubMed] [Google Scholar]
- 107. Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro‐ and antero‐grade transport and immunohistochemical study. J Comp Neurol 1992;323:387‐410. [DOI] [PubMed] [Google Scholar]
- 108. Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 1988;269:315‐341. [DOI] [PubMed] [Google Scholar]
- 109. Skinner RD, Kinjo N, Henderson V, Garcia‐Rill E. Locomotor projections from the pedunculopontine nucleus to the spinal cord. Neuroreport 1990;1:183‐186. [DOI] [PubMed] [Google Scholar]
- 110. Steininger TL, Wainer BH, Blakely RD, Rye DB. Serotonergic dorsal raphe nucleus projections to the cholinergic and noncholinergic neurons of the pedunculopontine tegmental region: a light and electron microscopic anterograde tracing and immunohistochemical study. J Comp Neurol 1997;382:302‐322. [DOI] [PubMed] [Google Scholar]
- 111. Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat J Comp Neurol 1985;242:56‐92. [DOI] [PubMed] [Google Scholar]
- 112. Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat J Comp Neurol 1987;262:105‐124. [DOI] [PubMed] [Google Scholar]
- 113. Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol 1988;274:483‐515. [DOI] [PubMed] [Google Scholar]
- 114. Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 2002;39:107–140. [DOI] [PubMed] [Google Scholar]
- 115. Charara A, Parent A. Brainstem dopaminergic cholinergic and serotoninergic afferents to the pallidum in the squirrel monkey. Brain Res 1994;640:155‐170. [DOI] [PubMed] [Google Scholar]
- 116. Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons J Comp Neurol 1994;344:190‐209. [DOI] [PubMed] [Google Scholar]
- 117. Kita T, Kita H. Cholinergic and non‐cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur J Neurosci 2011;33:433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Saper CB, Loewy AD. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res 1982;252:367‐372. [DOI] [PubMed] [Google Scholar]
- 119. Dautan D, Huerta‐Ocampo I, Witten IB, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 2014;34:4509‐4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single‐axon tracing and three‐dimensional reconstruction. J Comp Neurol 2001;439:162‐175. [DOI] [PubMed] [Google Scholar]
- 121. Zahm DS, Williams EA, Latimer MP, Winn P. Ventral mesopontine projections of the caudomedial shell of the nucleus accumbens and extended amygdala in the rat: double dissociation by organization and development. J Comp Neurol 2001;436:111‐125. [PubMed] [Google Scholar]
- 122. Takakusaki K, Takahashi K, Saitoh K, et al. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol 2005;568:1003–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schofield BR. Projections from auditory cortex to midbrain cholinergic neurons that project to the inferior colliculus. Neuroscience 2010;166:231‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Matsumura M, Nambu A, Yamaji Y, et al. Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience 2000;98:97‐110. [DOI] [PubMed] [Google Scholar]
- 125. Ros H, Magill PJ, Moss J, Bolam JP, Mena‐Segovia J. Distinct types of non‐cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience 2010;170:78‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 2009;29:340‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. EEC din. Neurophysiol 1949;1:455‐473. [PubMed] [Google Scholar]
- 128. Hebb DO. Drives and the CNS (conceptual nervous system). Psychol Rev 1955;62:243‐253. [DOI] [PubMed] [Google Scholar]


