Abstract
Background
The case‐crossover (CXO) and self‐controlled case series (SCCS) designs are increasingly used in pharmacoepidemiology. In both, relative risk estimates are obtained within persons, implicitly controlling for time‐fixed confounding variables.
Objectives
To examine the consistency of relative risk estimates of hip/femur fractures (HFF) associated with the use of benzodiazepines (BZD) across case‐only designs in two databases (DBs), when a common protocol was applied.
Methods
CXO and SCCS studies were conducted in BIFAP (Spain) and CPRD (UK). Exposure to BZD was divided into non‐use, current, recent and past use. For CXO, odds ratios (OR; 95%CI) of current use versus non‐use/past were estimated using conditional logistic regression adjusted for co‐medications (AOR). For the SCCS, conditional Poisson regression was used to estimate incidence rate ratios (IRR; 95%CI) of current use versus non/past‐use, adjusted for age. To investigate possible event‐exposure dependence the relative risk in the 30 days prior to first BZD exposure was also evaluated.
Results
In the CXO current use of BZD was associated with an increased risk of HFF in both DBs, AORBIFAP = 1.47 (1.29–1.67) and AORCPRD = 1.55 (1.41–1.70). In the SCCS, IRRs for current exposure was 0.79 (0.72–0.86) in BIFAP and 1.21 (1.13–1.30) in CPRD. However, when we considered separately the 30‐day pre‐exposure period, the IRR for current period was 1.43 (1.31–1.57) in BIFAP and 1.37 (1.27–1.47) in CPRD.
Conclusions
CXO designs yielded consistent results across DBs, while initial SCCS analyses did not. Accounting for event‐exposure dependence, estimates derived from SCCS were more consistent across DBs and designs. © 2015 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Keywords: case crossover (CXO), self‐controlled case series (SCCS), benzodiazepines, hip fractures, electronic healthcare records databases (DBs), pharmacoepidemiology
Introduction
Case‐only designs overcome some key confounding issues such as lack of information on potential confounders, and difficulties in selecting appropriate controls in numerous settings.1 One common characteristic of those designs is that comparisons are within‐person not between‐persons, thereby controlling implicitly for all intrinsic factors, both measured and unmeasured, that remain constant over the study period. For these reasons they are increasingly being used in pharmacoepidemiology.2
The case‐crossover (CXO) method was developed by Maclure (1991), to investigate the risk of transient and immediate acute events.3 The particularity is that ‘controls’ come from the person‐time of the case. It uses the difference in exposure rates just before the event (the ‘case moment’) with those at other times (‘controls moments’) to estimate an odds ratio (OR) of the outcome associated with exposure. It depends on strong assumptions, being suitable for transient exposures with short term effects.4 Hence, the intermittency of drug use and the length of the exposure time window may have an impact on the estimates obtained.2 Also, as a conditional logistic regression model is employed with more than one control moment, distribution of exposures must be exchangeable between those periods to emulate a case–control design where the order of controls is irrelevant.5
The self‐controlled case series (SCCS) method was developed by Farrington (1995) to study the association between vaccination and adverse events.6 The SCCS follows the cohort design approach; it is derived from a Poisson distribution model by conditioning on an individual's total number of events and their exposure history.7
The SCCS is based on several assumptions; one is that the occurrence of the event of interest does not influence the chance of subsequent exposure. However, in situations where the event could temporarily increase or decrease the likelihood of exposure, a valid approach is to separately categorise a short period of time before exposure, thereby removing this time from the reference category (baseline or period of no exposure) to avoid a biassed event rate in that period.
Because the designs share some features but follow a different approach, we assessed whether they reached similar results across electronic healthcare records databases (DBs) from the United Kingdom (UK) and Spain following the same protocol and methodology. As a case study we used the well‐established association8, 9 of benzodiazepines and related drugs (BZD) with hip/femur fractures (HFF). BZD are often used intermittently, and with these case‐only designs some confounding such as frailty could be addressed.
The present research was undertaken within the frame of the Pharmacoepidemiological Research on Outcomes of Therapeutics by a European Consortium (IMI‐PROTECT) project (http://www.imi‐protect.eu/).
Patients and Methods
The study was performed in two primary care DBs: The UK Clinical Practice Research Datalink (CPRD GOLD),10 and ‘Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria’ (BIFAP) from Spain. These DBs have been described in detail elsewhere.11 The protocol was registered in The European Network of Centres for Pharmacoepidemiolgy and Pharmacovigilance, ENCePP.12 A blinding procedure was maintained until results were made available to the coordinating centre at Utrecht University, the Netherlands.
Study population
The study period was considered from the 1 January 2001 until 31 December 2009. All data were used when the practices were considered ‘Up to (research) Standard’ (a marker of data quality). Patients who had at least one year of registration with the general practitioner (GP), were ≥18 years old, and were 12 months free of HFF were included in the study population. All patients were required to have a recorded diagnosis of HFF during the study period, i.e. they were all ‘cases’. For the SCCS, patients were required to have 6 months free of BZD prescriptions before entering the study to restrict the population to new users. This criterion was not applied for the CXO to ensure that all case or control moments had the opportunity to be exposed to BZD. Patients could enter at any time they fulfilled the criteria above. The start date was the date patients met the cited criteria.
Case definition
HFF was searched in the BIFAP database using the International Classification of Primary Care: ICPC‐2, code L75 and in CPRD using READ codes (Table S1 online). In BIFAP, cases were identified through free‐text (in addition to codes). For that reason, a review of all cases was carried out for validation. As a result, similar to the companion Cohort/NCC paper,13 30% of cases were excluded (of them, about 15% because of high‐energy trauma, 60% because of other fractures (i.e. pelvis), and the remaining patients did not have a clear date of the event). Such a revision was not feasible in the CPRD, but previous validation confirmed 91% of recorded hip fractures in the CPRD.14 In patients with a history of past HFF, a minimum of 12 months must have elapsed between the current episode and any previous fracture to ensure these represented separate events.
Exposure definition
BZD was the exposure of interest, comprising all those classified as anxiolytics, hypnotics and related drugs in the Anatomical Therapeutic Chemical (ATC) classification.15 (Supplementary Table S2 online). Related drugs (Z‐drugs and clomethiazole) were included in this research because their therapeutic actions are similar to benzodiazepines.16
Duration of each prescription was estimated based on the prescribed amount and daily dose. The expected duration of use was calculated following the methods of Gardarsdottir et al.17 When a gap of more than 30 days occurred between the theoretical end date of a prescription and the date of the subsequent prescription, exposure was considered to be discontinuous, and a new treatment episode was considered.
The person‐time of each patient was divided according to their exposure into periods of current, recent, past and non‐use. Thus, current use was the period from the start of a BZD prescription until 30 days after the estimated end date of the supply; recent use was the period up to 60 days after current use; past use was the period after recent use until the patient became exposed again or the end of follow‐up; non‐use was the period between the start date and the first BZD prescription within the study period. Combined non‐use and past use was considered the reference category or baseline (Figure 1). For the SCCS, current use was further divided into five risk time windows: 1–30, 31–60, 61–182, 183–365 and >365 days. BZD are thought to increase the risk of fractures during the early stages of treatment,18, 19 and this was taken into account when defining exposure time windows.
Figure 1.
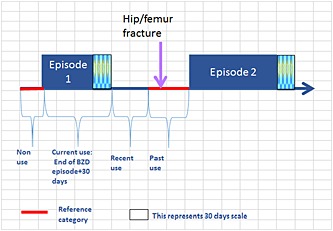
Structure of exposure definition. The follow‐up continued beyond the date of hip/femur fracture only for the SCCS
For the CXO, each case serves as its own control, and up to four control moments were defined at 91, 182, 273 and 365 days prior to the HFF (case moment). This method assumes that the baseline risk for an exposure is constant, and this assumption was tested using up to four control periods per case, improving the precision of the effect size, and the efficiency by using the whole year prior to the event.20
For each patient, exposure at the case moment was compared to exposure at control moments. In addition for this design, the current use period was further categorised as single use of anxiolytics, single use of hypnotics and use of both.
Potential confounders
These studies are part of a common protocol where four analytical study designs were performed to investigate the same study question (http://www.encepp.eu/encepp/viewResource.htm?id=6179).
In the CXO, all medications mentioned in the protocol (see Table S3 online) were considered as potential confounders. Indicator terms for medication use were added to the model denoting the absence or presence of prescriptions of each separate type of medication listed in Table S3, within the 91 days prior to the case or control moments.
In the SCCS, age was considered as the most important potential confounder, given its strong association with fracture risk21 and given that many relevant unmeasured factors are likely to be age‐related (e.g. frailty or increase in severity of underlying diseases) as well as related to BZD pattern of use.
Analysis
Analyses in BIFAP were performed using Stata®‐11; in CPRD analyses were performed using SAS v9.2 for the CXO and Stata®‐10 for the SCCS.
In the CXO, conditional logistic regression was used to estimate the relative risk in terms of ORs with corresponding 95% confidence intervals (CI).
In the SCCS, conditional Poisson regression was used to estimate the relative risk in terms of incidence rate ratios (IRRs) with corresponding 95% CI.22
To examine potential event‐exposure dependence, a pre‐exposure time risk window was created in the SCCS with a length of 30 days, allowing us to examine whether an incident HFF has a short‐term impact on the likelihood of being prescribed a BZD. IRR were estimated excluding this pre‐exposure time of 30 days from the reference category in a sensitivity analysis.
Results
Case crossover
Characteristics of study populations (BIFAP and CPRD)
In BIFAP, 5412 cases were included, with a similar mean age of 78 (±13) years old. From these, 85% contributed four control moments (Table 1). In CPRD, a total of 12 853 cases of HFF were included as the study population, with a mean age (±SD) of 79 (±13) years old. From these, 88% were also registered in the DB during the four control moments. Distribution by sex was similar in both DBs, 78% females and 22% males. The characteristics of patients are described in Table S4 online.
Table 1.
Number of cases and its control moments participating in case‐crossover study in BIFAP and CPRD
| Number of cases with M controls (N%) | Number of cases with at least M controls (N%) | |||
|---|---|---|---|---|
| Control moments (M) | CPRD | BIFAP | CPRD | BIFAP |
| 1 | 530 (4.1) | 267 (4.7) | 12 853 (100) | 5412 (100) |
| 2 | 474 (3.7) | 272 (4.8) | 12 323 (95.9) | 5145 (95.1) |
| 3 | 492 (3.8) | 274 (4.8) | 11 849 (92.2) | 4873 (90.0) |
| 4 | 11 357 (88.4) | 4599 (80.6) | 11 357 (88.4) | 4599 (85.0) |
Effect of BZD (BIFAP and CPRD)
Crude ORs (95%CI) and adjusted OR (AORs) (95%CI) for current use of BZD, compared to past/non‐use, were similar between DBs: OR = 1.70 (1.50–1.92), AOR = 1.47 (1.29–1.67) in BIFAP and OR = 1.75 (1.60–1.92), AOR = 1.55 (1.41–1.70) in CPRD (Figure 2).
Figure 2.
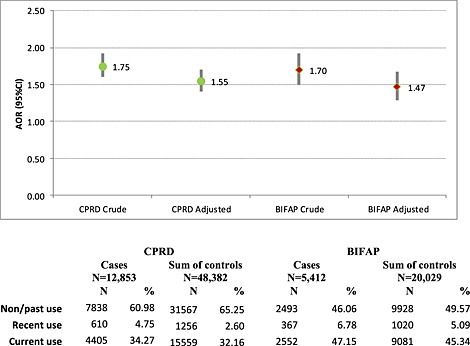
Crude and co‐medication adjusted risk of hip/femur fracture associated to current use of BZD. Case‐crossover study
Effect of BZD treatment duration in CPRD
In CPRD, although the highest relative risk was observed within the first 30 days of treatment AOR (95%CI): 1.70 (1.49–1.94), a model accounting for duration class did not provide a significantly better fit to the data than one considering presence/absence of BZD exposure alone (chi‐square for comparison of −2log L scores = 6.82, DF = 4, p = 0.15) (Figure 3).
Figure 3.
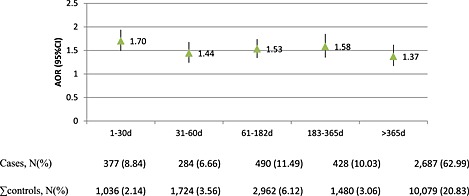
Co‐medication adjusted risk of hip/femur fracture associated to duration of current use of BZD in CPRD. Case‐crossover study
Self‐controlled case series
Characteristics of study populations (BIFAP and CPRD)
In CPRD, a total of 8333 cases were included as the study population, and a total of 4450 cases were included in BIFAP. In both populations the age and gender distribution was similar; 77% and 74% were females in BIFAP and CPRD respectively. The mean age at first exposure to BZD was about 76 years old in both DBs. The percentage of HFF occurring during the exposure to BZD (current use) was higher in BIFAP (35%) than in CPRD (22%), and the median duration of the observation period was shorter in BIFAP (5.4 years) than in CPRD (7.0 years). Of 8333 patients exposed to BZD in CPRD, 4790 had only a single continuous period of BZD exposure compared to 3543 patients (42.52%) who had intermittent BZD use. In the same way, of 4450 patients exposed to BZD in BIFAP, 1782 had only a single continuous period of BZD exposure compared to 2668 (59.96%) who had intermittent BZD use.
Effect of BZD and related drugs
Adjusted IRR of HFF associated with current use was 1.21 (1.13–1.30) in CPRD and 0.79 (0.72–0.86) in BIFAP in analyses ignoring the potential for event‐exposure dependence. In CPRD, an apparent decreasing trend of risk with duration of treatment was observed, ranging from 1.42 (1.27–1.59) in the first 30 days of use to 0.89 (0.79–1.02) with >365 days of use. In BIFAP, no increased risk was observed in any time window category (Table 2).
Table 2.
Risk of hip/femur fracture associated with current use of BZD from SCCS studies in BIFAP and CPRD without adjustment for event‐exposure dependence
| BIFAP (N = 4450) | CPRD (N = 8333) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model crude | Model adjusted by age | Model crude | Model adjusted by age | |||||||||||||
| Cases | Follow‐up days | IRR | IC (95%) | IRR | IC (95%) | Cases | Follow‐up days | IRR | IC (95%) | IRR | IC (95%) | |||||
| Past/non‐use | 2615 | 5 169 915 | 1.00 | — | — | 1.00 | — | — | 6060 | 15 769 427 | 1.00 | — | — | 1.00 | — | — |
| Recent use | 292 | 476 872 | 1.14 | 1.00 | 1.29 | 0.97 | 0.85 | 1.10 | 439 | 792 974 | 1.42 | 1.28 | 1.57 | 1.26 | 1.14 | 1.39 |
| Current use | 1543 | 2 945 532 | 1.02 | 0.94 | 1.11 | 0.79 | 0.72 | 0.86 | 1834 | 3 274 091 | 1.55 | 1.45 | 1.66 | 1.21 | 1.13 | 1.30 |
| 1–30 days | 213 | 409 985 | 0.92 | 0.80 | 1.07 | 0.79 | 0.68 | 0.92 | 342 | 510 624 | 1.59 | 1.42 | 1.78 | 1.42 | 1.27 | 1.59 |
| 31–60 days | 201 | 362 943 | 0.99 | 0.85 | 1.15 | 0.85 | 0.73 | 0.99 | 253 | 425 445 | 1.44 | 1.27 | 1.64 | 1.27 | 1.11 | 1.44 |
| 61–182 days | 314 | 614 880 | 0.93 | 0.82 | 1.06 | 0.75 | 0.66 | 0.86 | 383 | 647 956 | 1.48 | 1.33 | 1.66 | 1.19 | 1.06 | 1.33 |
| 183–365 days | 246 | 437 601 | 1.11 | 0.95 | 1.29 | 0.83 | 0.71 | 0.96 | 300 | 498 354 | 1.66 | 1.46 | 1.89 | 1.20 | 1.05 | 1.37 |
| >365 days | 569 | 1 120 123 | 1.28 | 1.12 | 1.47 | 0.73 | 0.63 | 0.84 | 556 | 1 191 712 | 1.62 | 1.43 | 1.82 | 0.89 | 0.79 | 1.02 |
Sensitivity analysis of event‐exposure dependence
When a 30‐day pre‐exposure window was removed from the reference period, an increased risk was observed across all exposure period time windows. The highest risk was exhibited by the pre‐exposure time window, with 2.52 (95%CI: 2.30–2.76) and 6.47 (95%CI: 5.91–7.09) adjusted for age, in CPRD and BIFAP, respectively. Excluding this pre‐exposure time from the reference category we observed in BIFAP a marked change in the magnitude of the estimates, over all exposure time windows, reaching an IRR of 1.43 (1.31–1.57) when current use was aggregated in just one category. Such increment was less for CPRD varying from 1.21 (1.13–1.30) to 1.37 (1.27–1.47) in the current use category (Table 3).
Table 3.
Risk of hip/femur fracture associated with current use of BZD from SCCS studies in BIFAP and CPRD including adjustment for event‐exposure dependence
| BIFAP (N = 4450) | CPRD (N = 8333) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model crude | Model adjusted by age | Model crude | Model adjusted by age | |||||||||||||
| Cases | Follow‐up days | IRR | IC (95%) | IRR | IC (95%) | Cases | Follow‐up days | IRR | IC (95%) | IRR | IC (95%) | |||||
| Past/non‐use | 1898 | 4 941 912 | 1.00 | — | — | 1.00 | — | — | 5549 | 15 344 912 | 1 | — | — | 1 | — | — |
| Recent use | 172 | 361 218 | 1.37 | 1.16 | 1.60 | 1.21 | 1.03 | 1.42 | 401 | 712 289 | 1.58 | 1.43 | 1.76 | 1.41 | 1.27 | 1.57 |
| Current use | 1543 | 2 945 532 | 1.77 | 1.62 | 1.93 | 1.43 | 1.31 | 1.57 | 1834 | 3 274 091 | 1.75 | 1.63 | 1.87 | 1.37 | 1.27 | 1.47 |
| 1–30 days | 213 | 409 985 | 1.58 | 1.36 | 1.83 | 1.40 | 1.21 | 1.62 | 342 | 510 624 | 1.79 | 1.60 | 2.00 | 1.59 | 1.42 | 1.78 |
| 31–60 days | 201 | 362 943 | 1.69 | 1.45 | 1.97 | 1.49 | 1.28 | 1.74 | 253 | 425 445 | 1.62 | 1.43 | 1.85 | 1.42 | 1.25 | 1.62 |
| 61–182 days | 314 | 614 880 | 1.63 | 1.43 | 1.86 | 1.37 | 1.20 | 1.57 | 383 | 647 956 | 1.68 | 1.50 | 1.89 | 1.35 | 1.20 | 1.51 |
| 183–365 days | 246 | 437 601 | 1.95 | 1.68 | 2.27 | 1.53 | 1.32 | 1.79 | 300 | 498 354 | 1.88 | 1.65 | 2.14 | 1.36 | 1.19 | 1.55 |
| >365 days | 569 | 1 120 123 | 2.27 | 1.97 | 2.60 | 1.42 | 1.22 | 1.65 | 556 | 1 191 712 | 1.83 | 1.62 | 2.06 | 1.02 | 0.90 | 1.16 |
| Pre‐exposure period | 837 | 343 657 | 7.17 | 6.55 | 7.84 | 6.47 | 5.91 | 7.09 | 549 | 505,200 | 2.83 | 2.59 | 3.11 | 2.52 | 2.30 | 2.76 |
Discussion
Under PROTECT's framework of Pharmacoepidemiology studies, four analytical designs were performed in different databases focusing on the methodological aspects of the studies rather than the clinical consequences of the association under investigation. Two case‐only designs, studying the association of BZD with HFF, are presented here. The results of the cohort and nested case–control (NCC) studies for the same association are presented elsewhere.13
CXO study
Crude and AORs were similar between databases. Other CXO studies showed similar associations. Neutel et al.,23 for example, found a crude OR = 1.7, 95%CI: 1.0–2.9, for exposure to BZD and Berry et al.24 found an AOR = 1.66, 95%CI: 1.45–1.90 associated with the use of non‐BZD hypnotics.
Concerning the effect of BZD subgroup, in both DBs the highest risk was observed in patients taking both anxiolytics and hypnotics, similar to the results seen with other study designs evaluated in PROTECT.13 As falls and fractures are dose‐related adverse effects,25 the use of several drugs could be seen as equivalent to the use of a higher dose. Alternatively, this could partly be related to the higher severity of the underlying conditions of these patients.
Regarding the effect of BZD treatment duration in CPRD, although the highest relative risk was observed within the first 30 days of treatment AOR (95%CI): 1.70 (1.49–1.94), a duration effect was not supported by formal test (p = 0.15). There is a lack of published articles exploring this short‐term effect of BZD and related drugs with this design. This method assumes immediate and transient effect as well as intermittent exposures and the power to detect the effect of continuous treatment may be limited. Possible explanations for increasing or decreasing the risk with duration of treatment have been discussed previously.13
In BIFAP, it was not possible to examine duration of use in this design because data available before the study period were insufficient for assessing all duration categories.
SCCS study
An increased risk of HFF with the use of BZD was observed in CPRD but not in any exposure category in initial analyses after adjusting for age in BIFAP (Table 2).
However, within these analyses, we found evidence of a strong but temporary dependence of event and exposure implying that some patients who sustained a HFF were prescribed a BZD shortly after the event. This dependence violates one of the key assumptions of this design. Separating a 30‐day period from the reference category, the results in the SCCS were similar to the CXO in both BIFAP and CPRD, again suggesting an increased fracture risk associated with exposure to BZD in all current use windows.
Gibson et al.22 also used a pre‐exposure time to assess the risk of motor vehicle crashes with BZD, and found elevated risks (IRR = 1.94, 99% CI: 1.62, 2. 32). A similar situation was observed by Lai C et al.14 studying the risk of HFF associated with alpha blockers using a SCCS design.
Comparison across all designs
Both traditional (cohort and NCC) and case‐only designs suggested an increased risk of HFF associated with current use of BZD. However, designs differed in the magnitude of risk with traditional designs showing slightly lower relative risks (RR) than case‐only designs. Differences observed between designs might be because of the fact that chronic users of BZD with no unexposed observation time are excluded from the estimated RR in the case‐only designs, but can contribute in cohort and NCC analyses. Results must be interpreted in the light of these differences and in some instances, case‐only results may be more accurately generalised to intermittent users. If chronic users of BZD had a lower risk than short term users, explained by a better adaptation of regular users to those drugs or by the consequence of ‘healthy user effect’,26 traditional designs would be expected to yield lower RR estimates than case‐only designs. Conversely, traditional designs estimate between‐person RR, while case‐only estimate within‐person RR,27, 28 which may not be necessarily of the same magnitude because of unmeasured factors difficult to adjust for such as severity of underlying diseases, or frailty. Such factors may increase the risk of fall and fractures and may make physicians reluctant to prescribe BZD (e.g. confounding by contraindication). This confounding would lead to an underestimate of the relative risk of HFF associated with BZD use. In case‐only designs, time invariant confounding factors are implicitly controlled for by design, although a confounding by transient changes in other factors cannot be excluded.
The fact that the cohort/NCC analyses were restricted to BZD users with a reference category of past‐use, and therefore, all patients were exposed at least once to the drug of interest, made the comparison between cohort members more similar than if an external cohort of non‐users had been used. In contrast, a reference category of non‐use was appropriate for the case‐only analyses, as differences between persons are removed by design.
Results obtained with both case‐only designs were similar, although the precision of the estimates was higher in the SCCS than in the CXO which may be considered as potential advantage of the former.
The experience of comparing different designs using the same source population is limited. The study of Madigan et al.,29 part of the Observational Medical Outcomes Partnership (OMOP) project, studied this drug‐event pair employing a SCCS and a cohort design within the same data source, and compared the results across ten DBs. Seven out of ten DBs found no increased risk of HFF associated with BZD use, when a SCCS was employed. However, this study was not specific to BZD‐HFF, and explored 53 drug‐event pairs under a surveillance perspective rather than specifically addressing this pair in a formal hypothesis testing study with a pair specific protocol.
There are some publications comparing designs yielding different results, although studying different associations.30, 31 In most articles, relative risk estimates with case‐only designs were lower than those obtained with cohort or NCC designs, with authors generally concluding that the higher estimates obtained using these designs may be because of between‐person confounding.
Strengths and limitations
We only had access to prescribing data, rather than the precise dates on which medication was actually taken. It is therefore possible that exposure periods are misclassified to some extent, with both exposed and unexposed periods affected to some degree. The effect of this would tend to bias results towards the null, and so it is possible that we have underestimated any real effect of treatment with BZD. A major strength of this research is the use of a common protocol allowing the use of harmonised methods and definitions across DBs, aiding the direct comparison of results.
In general, case‐only designs are limited by their underlying assumptions. In this study, the assumption of independence between event and exposure in the SCCS design was not met but was subsequently corrected for by the use of a pre‐exposure risk period.22
In our case‐only two DBs have been employed with just one drug‐event association, so results might not be extrapolated to other settings and certainly not be generalised to other drug‐event pairs.
Conclusions
CXO designs yielded consistent results across DBs. Once we accounted for the event‐exposure dependence, estimates derived from SCCS were also consistent across DBs and across designs. Case‐only designs may offer better control for time invariant confounding factors than traditional designs and are a useful choice when intrinsic factors may represent relevant confounding, and when the effects of transient exposures are to be measured. Care is needed to ensure the underlying assumptions of these designs are met and to interpret the results obtained as they may not always generalise to patients receiving continuous treatment with the medication being assessed.
These studies together with the cohort and case–control analyses have shown that performing multi‐site studies using a common protocol provides useful comparisons across countries and across designs, contributing to a better understanding of potential differences between pharmacoepidemiological studies used to assess drug safety.
Conflict of Interest
OK and MdG have received unrestricted funding for pharmacoepidemiological research from the Dutch private‐public funded Top Institute Pharma.
JL, DW and NB are employees and stockholders of GlaxoSmithKline.
RS is a Novartis employee and owns Novartis shares.
AB and RR are employees and stockholders of Pfizer, Inc.
IJD consults for and holds stock in GSK, and consults for Gilead.
Key Points.
Case‐only designs may offer better control for time invariant confounding factors than traditional designs and may be a useful choice when intrinsic factors may represent relevant confounding.
Care is needed to ensure that the underlying assumptions of these designs are met and to interpret the results obtained as they may not always generalise to patients receiving continuous treatment with the medication being assessed.
In the SCCS design, it is important to explore the potential event‐exposure dependence as even temporary effects can have a large impact on results.
This research has shown that performing multi‐site studies, using a common protocol provides useful comparisons across countries and across designs.
Ethics Statement
Study protocols were approved by institutional review boards responsible for each individual database.
Author Contributions
All authors contributed to the study conception and design. The corresponding authors responsible per DB performed data extraction and raw data analysis. GR, JL and IJD wrote the first draft, and all authors contributed with critical comments to the final version.
Supporting information
Supporting info item
Acknowledgements
The research leading to these results was conducted as part of the PROTECT consortium (Pharmacoepidemiological Research on Outcomes of Therapeutics by a European ConsorTium, www.imi‐protect.eu) which is a public–private partnership coordinated by the European Medicines Agency. The paper is on behalf of WP2 (members can be found at introduction of the special issue).
The authors would like to thank the excellent collaboration of physicians in the participating countries, whose contribution in recording their professional practice with high quality standards makes possible the availability of DBs used in this research.
The PROTECT project has received support from the Innovative Medicines Initiative Joint Undertaking (IMI JU) (www.imi.europa.eu) under Grant Agreement no 115004, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in‐kind contribution. As a special form of the IMI JU grant, Utrecht University and University of Alcalá received a direct financial contribution from Pfizer and Astra‐Zeneca, respectively. JL, NB, DW, AB, RS and RR belong to EFPIA (European Federation of Pharmaceutical Industries and Association) member companies in the IMI JU, and costs related to their part in the research were carried by the respective company as in‐kind contribution under the IMI JU scheme. The views expressed are those of the authors only and not of their respective institution or company. IJD is funded by a Medical Research Council Methodology Fellowship.
Requena, G. , Logie, J. , Martin, E. , Boudiaf, N. , González González, R. , Huerta, C. , Alvarez, A. , Webb, D. , Bate, A. , García Rodríguez, L. A. , Reynolds, R. , Schlienger, R. , Gardarsdottir, H. , de Groot, M. , Klungel, O. H. , de Abajo, F. , and Douglas, I. J. (2016) Do case‐only designs yield consistent results across design and different databases? A case study of hip fractures and benzodiazepines. Pharmacoepidemiol Drug Saf, 25: 79–87. doi: 10.1002/pds.3822.
An abstract of this paper has been submitted to participate as a poster in the ICPE 2014, 30th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, with the abstract number #433.
References
- 1. Smeeth L, Donnan PT, Cook DG. The use of primary care databases: case–control and case‐only designs. Fam Pract 2006; 23(5): 597–604. [DOI] [PubMed] [Google Scholar]
- 2. Delaney JA, Suissa S. The case‐crossover study design in pharmacoepidemiology. Stat Methods Med Res 2009; 18(1): 53–65. [DOI] [PubMed] [Google Scholar]
- 3. Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133(2): 144–153. [DOI] [PubMed] [Google Scholar]
- 4. Hebert C, Delaney JA, Hemmelgarn B, Levesque LE, Suissa S. Benzodiazepines and elderly drivers: a comparison of pharmacoepidemiological study designs. Pharmacoepidemiol Drug Saf 2007; 16(8): 845–849. [DOI] [PubMed] [Google Scholar]
- 5. Farrington CP. Control without separate controls: evaluation of vaccine safety using case‐only methods. Vaccine 2004; 22(15–16): 2064–2070. [DOI] [PubMed] [Google Scholar]
- 6. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med 2006; 25(10): 1768–1797. [DOI] [PubMed] [Google Scholar]
- 7. Farrington CP, Whitaker HJ. Semiparametric analysis of case series data. J Roy Stat Soc 2006; 55(5): 553–594. [Google Scholar]
- 8. Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half‐life and the risk of hip fracture. JAMA 1989; 262(23): 3303–3307. [PubMed] [Google Scholar]
- 9. Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs 2003; 17(11): 825–837. [DOI] [PubMed] [Google Scholar]
- 10. Williams T, Van Staa T, Puri S, et al. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012; 3(2): 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbing‐Karahagopian V, Kurz X, de Vries F, et al. Bridging differences in outcomes of pharmacoepidemiological studies: design and first results of the protect project. Curr Clin Pharmacol 2014; 9: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ENCePP ENoCfPaP . ENCePP (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance). Available at: http://www.encepp.eu/encepp/studiesDatabase.jsp [October 2012].
- 13. Requena G, Huerta C, Gardarsdottir H, et al. Hip/femur fractures associated with the use of BZDs (benzodiazepines anxiolytics, hypnotics and related drugs): a methodological approach to assess consistencies across databases. Pharmacoepidemiol Drug Saf 2015; (Supplement). DOI: 0.1002/pds.3816. [DOI] [PubMed] [Google Scholar]
- 14. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000; 15(6): 993–1000. [DOI] [PubMed] [Google Scholar]
- 15. WHO CCfDSM . Anatomical Therapeutic Chemical (ATC) Classification system, 2013. Available at: http://www.whocc.no/atc_ddd_index/ [September 2014].
- 16. Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf 2003; 26(4): 261–282. [DOI] [PubMed] [Google Scholar]
- 17. Gardarsdottir H, Souverein PC, Egberts TC, Heerdink ER. Construction of drug treatment episodes from drug‐dispensing histories is influenced by the gap length. J Clin Epidemiol 2010; 63(4): 422–427. [DOI] [PubMed] [Google Scholar]
- 18. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Sturmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19(12): 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half‐life, dosage, and duration on risk of hip fracture. Am J Psychiatry 2001; 158(6): 892–898. [DOI] [PubMed] [Google Scholar]
- 20. Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case‐crossover studies: an assessment of relative efficiency. Am J Epidemiol 1995; 142(1): 91–98. [DOI] [PubMed] [Google Scholar]
- 21. van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001; 29(6): 517–522. [DOI] [PubMed] [Google Scholar]
- 22. Gibson JE, Hubbard RB, Smith CJ, Tata LJ, Britton JR, Fogarty AW. Use of self‐controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol 2009; 169(6): 761–768. [DOI] [PubMed] [Google Scholar]
- 23. Neutel CI, Perry S, Maxwell C. Medication use and risk of falls. Pharmacoepidemiol Drug Saf 2002; 11(2): 97–104. [DOI] [PubMed] [Google Scholar]
- 24. Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173(9): 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. File SE. Behavioural pharmacology of benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry 1984; 8(1): 19–31. [DOI] [PubMed] [Google Scholar]
- 26. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011; 26(5): 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maclure M, Fireman B, Nelson JC, et al. When should case‐only designs be used for safety monitoring of medical products? Pharmacoepidemiol Drug Saf 2012; 21(Suppl 1): 50–61. [DOI] [PubMed] [Google Scholar]
- 28. Maclure M. ‘Why me?’ versus ‘why now?’—differences between operational hypotheses in case‐control versus case‐crossover studies. Pharmacoepidemiol Drug Saf 2007; 16(8): 850–853. [DOI] [PubMed] [Google Scholar]
- 29. Madigan D, Ryan PB, Schuemie M, et al. Evaluating the impact of database heterogeneity on observational study results. Am J Epidemiol 2013; 178(4): 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douglas IJ, Evans SJ, Hingorani AD, et al. Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs. BMJ 2012; 345: e4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravera S, van Rein N, de Gier JJ, de Jong‐van den Berg LT. A comparison of pharmacoepidemiological study designs in medication use and traffic safety research. Eur J Epidemiol 2012; 27(6): 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


