Abstract
Owners and their domestic animals via skin shedding and secretions, mutually exchange microbiomes, potential pathogens and innate immune molecules. Among the latter especially lipocalins are multifaceted: they may have an immunomodulatory function and, furthermore, they represent one of the most important animal allergen families. The amino acid identities, as well as their structures by superposition modeling were compared among human lipocalins, hLCN1 and hLCN2, and most important animal lipocalin allergens, such as Can f 1, Can f 2 and Can f 4 from dog, Fel d 4 from cats, Bos d 5 from cow's milk, Equ c 1 from horses, and Mus m 1 from mice, all of them representing major allergens. The β‐barrel fold with a central molecular pocket is similar among human and animal lipocalins. Thereby, lipocalins are able to transport a variety of biological ligands in their highly conserved calyx‐like cavity, among them siderophores with the strongest known capability to complex iron (Fe3+). Levels of human lipocalins are elevated in nonallergic inflammation and cancer, associated with innate immunoregulatory functions that critically depend on ligand load. Accordingly, deficient loading of lipocalin allergens establishes their capacity to induce Th2 hypersensitivity. Our similarity analysis of human and mammalian lipocalins highlights their function in innate immunity and allergy.
Keywords: allergen, animal, canine, iron, lipocalin
The current view on immune tolerance vs Th2 skewing of allergens
IgE‐mediated allergy is characterized by a Th2‐dominant immune response. The formed IgE may be directed against the genuine allergen or secondarily bind to molecules with similar epitopes on their surface, causing the phenomenon of cross‐reactivity. A limited number of allergen families can initiate Th2 responses strong enough to result in IgE formation in spite of sometimes having limited amino acid homologies. In these settings, functional similarity may be a dominant feature for their Th2 induction capacity.
Consequently, it is essential to understand the very first events that actually skew the immune response to Th2. Immune cells like NKT, eosinophils (that especially in the small intestine via CD103 DC activation control Th2 priming 1), basophils 2, nuocytes, and NKT cells 3, but also macrophages 4 are known sources of early IL‐4 and IL‐13. These cytokines are released quickly upon stimulating the cells under involvement of a Notch‐regulated CNS‐2 enhancer 5 and the events are connected to memory in CD4 T cells 2. Disturbances of the skin and mucosal barriers and subsequent TSLP release may be crucial upstream events 6. One explanation could be that some allergens have enzymatic functions and thereby produce an epithelial danger signal 7, but many allergens have no proteolytic activity and do still exert ‘adjuvant‐like activation’ 8. It is thus not entirely clear which danger signals go along with allergens that initiate priming or triggering of these cells to release Th2 cytokines, and would thereby mark allergens as special. It is noteworthy that among the known protein families in the 2009 Pfam database only 1.4% are considered allergens 9. In the case of respiratory mammalian allergens a few protein families are absolutely dominant: lipocalins, secretoglobins and kallikreins 10, and IgE levels against them correlate with the severity of asthma 11.
Animal keeping: who is at risk?
The question of whether interactions with animals provide protection or increase risk is hotly debated, with particular points of discussion being the subject's age at time of exposure, and the exact animal species or breed. In German‐speaking Europe between 16% and 25% of households own cats, and 12% dogs (Table 1), with an estimated dark number of plus 30%. The interrelation between people and domestic animals has thus considerably changed in the last decades. Humans and pets in Western societies are cohabitating more close and in a cleaner way than ever before, a development which is associated with a good economy. Simultaneously, the rates of allergies and asthma in human patients caused by animal dander are increasing 12. In a cohort study of 696 school children 259 were sensitized to animals, and the majority of them to multiple species 13. The higher awareness today of allergy risk associated with pet ownership and the desire for nonfurry pet animals go hand in hand, introducing novel, alternative allergen threats into homes such as those presented from feed insects for reptiles 14. However, pets may also shuttle traditional allergens into homes: house dust mites can be identified in bedding, skin and hair coats of dogs 15. Interestingly, also pet animals and horses increasingly suffer from atopic eczema and other forms of allergies 16, 17. Therefore, it might be important to look at the problem bi‐directionally and watch for systematic problems in our society and environment.
Table 1.
Statistical comparison of percentage and numbers of animals in German‐speaking households (Statistics Germany http://www.wissenswertes.at/index.php?id=haustiere-statistik)
| Species | Austria (%) | Switzerland (%) | Germany (%) | Numbers in Germany (Mio.) |
|---|---|---|---|---|
| Cats | 25.6 | 25 | 16.5 | 8.2 |
| Dogs | 30 | 12 | 13.3 | 5.4 |
| Rodents | 11 | 6.4 | 5.6 | |
| Birds | 7 | 4.9 | 3.4 | |
| Aquaria | 6 | 4.4 | 2.0 | |
| Ponds | 4.0 | 2.1 | ||
| Terraria | 1.2 | 0.4 |
In contrast to the earlier trend to minimize allergen release by washing pets 18, most recent work suggests that the introduction of pets into homes early in life is protective 19. It has further been shown that lowering pet exposure may reduce the IgE and symptom levels if allergy is established 20. The interplay of allergens and the microbiome may be decisive for inducing hypersensitivity: Low levels of Firmicutes and Bacteriodetes in the skin flora concomitant with high allergen exposure enhance the risk for atopy and asthma 21. Needless to say that forced hygiene, washing and disturbance of the skin pH, enzymatic shampooing 7, and even the water quality 22, may impact the physiologic skin flora 23. In some studies cat and dog ownership did not elevate the risk of asthma 24, which might be correlated with exposure to the associated ‘good’ microbiomes of the animals. However, people with dog allergy more seldom keep dogs 25.
The observation that there are breed‐specific differences in eliciting symptoms has initiated the search for ‘hypoallergenic’ cats, dogs, and horses and stimulates breeders to single out mutants. The experimental evidence, however, is sparse and this topic needs to be addressed in its whole complexity regarding the countless breeds of domestic animals. The situation gets even more complicated as an animal's age and gender also play a role in allergies to them 26. Here, molecular diagnosis has significantly contributed to important practical recommendations 19, including the neutering, or avoidance of male animals 27, 28. Whereas the expectations were that hypoallergenic animals would shed less allergens, the contrary turned out to be the case: in a recent study putative hypoallergenic dogs produced higher levels of Can f 1, a lipocalin allergen 29.
Comparing animal, human, and other lipocalins
What is not yet understood is why allergens in particular provoke a Th2 shift in the immune response and lead to IgE production. Why exactly, are lipocalins so important elicitors of allergies to animals even though humans also do express lipocalins, a fact that is maybe less known to the allergist?
Lipocalins owe their name to the fact that they usually carry lipids (or other hydrophobic compounds) accommodated in a large, calyx‐like cavity formed by their characteristic β‐barrel fold (Fig. 1). Ligand specificity of lipocalins is regulated by the amino acid residues that line their binding pocket. A large variety of ligands, most of them hydrophobic or amphiphilic, binds to lipocalins that include lipids, steroids, hormones as well as metabolites such as vitamins, cofactors, and odorants. In some cases, binding of the ligand is critical for the biological functions of the lipocalin.
Figure 1.
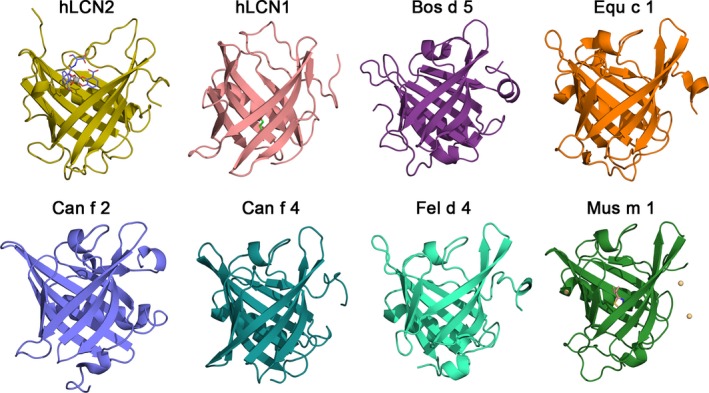
Gallery of human and animal lipocalins. PDB codes for experimental structures are given in parentheses. hLCN2: human LCN2 in complex with the siderophore enterobactin shown as sticks chelating the central Fe3+ ion shown as a gray sphere (1L6M 82). hLCN1: human tear lipocalin in complex with 1,4‐butanediol (3EYC 83). Bos d 5: bovine β‐lactoglobulin unliganded form (3NPO 84). Equ c 1: major horse allergen (1EW3 85). Can f 2: dog allergen (3L4R 45) with IgE cross‐reactivity with the cat allergen Fel d 4. Can f 4: dog dander allergen (4ODD 86). Fel d 4: cat allergen, homology model structure based on template Equ c 1. Mus m 1: pheromone binding rodent major urinary protein in complex with 2‐(sec‐butyl)‐thiazole and Cd2+ ions shown as yellow spheres (1MUP 87).
Exploring the PDB by entering the query ‘lipocalin’, renders nearly 300 experimental structures available for lipocalins belonging to 24 eukaryotic species and 10 bacteria species of which 79 structures correspond to humans (Table 2).
Table 2.
Organisms with experimental structure of lipocalins available in the Protein Data Bank
| Organism | Organism | ||
|---|---|---|---|
| Eukaryota | Number of entries | Bacteria | Number of entries |
| Anguilla japonica | 3 | Bacillus pumilus | 1 |
| Arabidopsis thaliana | 2 | Bacillus subtilis | 1 |
| Argas monolakensis | 4 | Bacteroides eggerthii | 1 |
| Argas reflexus | 2 | Bacteroides ovatus | 1 |
| Bos taurus | 37 | Bacteroides thetaiotaomicron | 2 |
| Canis lupus familiaris | 2 | Bacteroides uniformis | 3 |
| Capra hircus | 3 | Bradyrhizobium diazoefficiens | 3 |
| Coturnix coturnix | 1 | Enterococcus faecalis | 1 |
| Coturnix japonica | 2 | Escherichia coli | 6 |
| Diploptera punctata | 3 | Escherichia coli K‐12 | 2 |
| Equus caballus | 1 | Helicobacter pylori | 1 |
| Homarus gammarus | 4 | Total | 22 |
| Homo sapiens | 79 | ||
| Lingulodinium polyedrum | 1 | ||
| Mesocricetus auratus | 1 | ||
| Mus musculus | 34 | ||
| Ornithodoros moubata | 4 | ||
| Ovis aries | 2 | ||
| Pieris brassicae | 5 | ||
| Rattus norvegicus | 4 | ||
| Rhipicephalus appendic | 6 | ||
| Rhodnius prolixus | 68 | ||
| Sus scrofa | 6 | ||
| Trichosurus vulpécula | 3 | ||
| Total | 277 |
As of June 22, 2015, the search for ‘lipocalin’ in the PDB yielded 299 entries distributed between eukaryota and bacteria.
However, these numbers include mutants, engineered variants and the same protein in complex with different ligands, particularly for the most studied cases such as bovine, murine, and human lipocalins.
Allergenic lipocalins have been especially identified in furry animals 19, for instance in dogs: Can f 1, ‐2, ‐4, ‐6, cats: Fel d 4, ‐7, mice: Mus m 1, horses: Equ c 1, guinea pigs: Cav p 2, ‐3 30, and hamsters 31. Lipocalins are also found in tick saliva 32 where they sequester histamine and thereby interfere with its signaling via H1 receptor 32, and contribute to camouflaging the bite. Importantly, humans also express ‘a menagerie of lipocalins’ 33, such as LCN2 (alternatively called siderocalin/SCN; neutrophil gelatinase‐associated lipocalin/NGAL, 24p3), or human LCN1 (alias tear lipocalin, TLC). A gallery of human and animal lipocalins for which experimental structures exist is represented in Fig. 1. Their well‐recognized characteristic β‐barrel fold has emerged in the last few years as a successful molecular scaffold to engineer novel binding proteins, so called anticalins, with human lipocalins playing a particularly relevant role in clinical applications 33. In an innovative approach four different canine lipocalins were recently linked into one multimeric molecule which indeed induced IgG upon vaccinating mice and which therefore may be used for vaccination against dog allergy in the future 34.
Lipocalins occur in secretions such as saliva, in urine (besides kallikreins) 28, and shed skin, which are all important sources for the release of allergens into our environment 35. Taken the intense interactions between owner and animal it is plausible that there is a bidirectional exchange of allergens, as it is the case with phylotypes of the microbiome of human dog owners and dogs 36.
Given their importance, many lipocalins of domestic animals and pets have been cloned and their production improved (such as Can f 1, ‐2, or ‐4 37, 38, 39, 40), but methods were also developed for the quantification of the natural allergens to be able to evaluate the exposure levels in different environments, e.g., for Can f 4 41 which is important for over 80% of people with dog allergies for IgE binding. Such measurements have recently brought up the fact that so‐called hypoallergenic dogs shed even higher levels of Can f 1 into homes 29.
Comparing structural features of lipocalins
Lipocalins display a well‐known overall similarity in their fold in spite of the relatively low degree of amino acid sequence identities of below 25% 42. This fold is characterized by a calyx‐ shaped cavity within the central β‐barrel formed by eight antiparallel β‐strands βA‐βH (in what follows, labels refer to Fig. S1). Loops around the cavity regulate ligand access to the calyx binding site. The extended loop L1 which comprises a 310(2) helix together with hairpin loops L3, L5, and L7 define the top wider end of the barrel, whereas hairpin loops L2, L4, and L6 make the bottom narrower end. This arrangement settles a topology of the β‐barrel which is one of the distinctive features of lipocalins. This fold also presents a conserved N‐terminal 310(1) helix as well as a medium‐size (about 10 residues) conserved α helix H1 and an additional β‐strand, βI, near the C‐terminal segment. Some lipocalins (e.g., bovine lactoglobulin) display additional secondary structure elements such a small N‐terminal β‐strand (β) and a C‐terminal 310(3) helix. The presence of one to three structurally conserved regions (SCR1–SCR3) associated to particular local arrangements of strands and helices have been used to divide lipocalins into two structural subfamilies termed kernel lipocalins (those having the three SCRs) and outlier lipocalins (those having a maximum of two SCRs). SCR1 comprises 310(1) and βA in the N‐terminal region, SCR2 is composed of sections of βF and βG together with loop L6, and SCR3 is defined by the end of βH together with adjacent residues of H1 in the C‐terminal region (Fig. S1). Obviously, amino acid residues in loops L1, L3, L5, and L7 lining the binding site regulate the ligand specificity of lipocalins. Although the name of this protein family makes explicit mention of lipophilic (hydrophobic) compounds, there is a large variety of ligands including amphiphilic molecules or compounds with hydrophobic segments also bearing polar groups that bind to lipocalins.
All these structural similarities observed in these proteins could help to understand IgE cross‐reactivities 43 between animal and human lipocalins, and between lipocalins from different animals 44, 45, 46, although not necessarily correlating with the symptoms.
For instance, in the current view at the amino acid level the dog allergens Can f 1 and Can f 2 correspond to human LCN1 and ‐2, respectively, whereas the four molecules only share 25% amino acid similarities between them. The amino acid sequences of various animal and human lipocalins LCN1 and ‐2 are aligned in Fig. 2. Identities above 60% occur between Fel d 4 and Equ c 1, Fel d 4 and Can f 6, Ory c 4, Rat n 1 and Mus m 1, and Can f 1 and Fel d 7.
Figure 2.
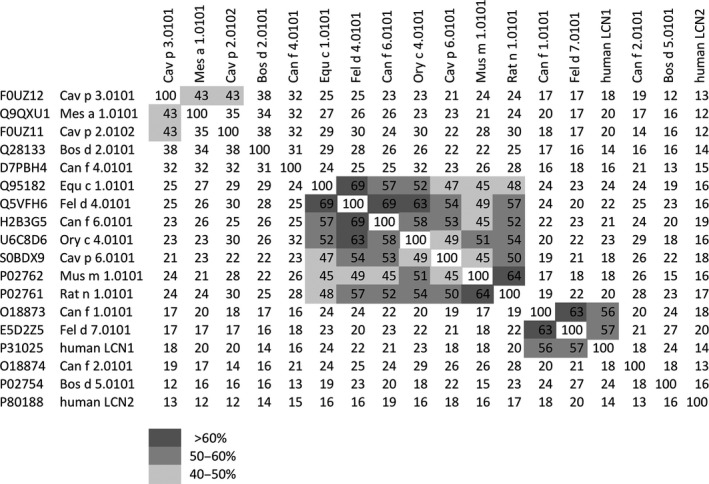
Comparison of human lipocalins and major animal lipocalin allergens. Amino acid identities (%) between mammalian lipocalin allergens and human lipocalin 1 and 2 were determined with Clustal Omega via http://www.ebi.ac.uk/Tools/msa/clustalo/. Highlighted regions show identities higher than 40%. The different gray shades illustrate the levels of amino acid identities. Amino acid sequences were retrieved from UniProtKB database (http://www.uniprot.org/uniprot/).
In contrast, there was no amino acid homology among human LCN2 and any of the analyzed animal lipocalins, only 57% among human LCN1 and Fel d 7, and 56% among LCN1 and Can f 1.
T‐cell responses have been found to be ‘suboptimal’ for Can f 1 47, although resembling those to human LCN1 48. Can f 4 T‐cell responses were shown to be a Th2‐deviated memory response, in part to peptides with promiscuous HLA‐binding capacity 49, whereas the DR4–DQ8 haplotype and T‐cell receptor Vbeta T‐cell subset were indicative for the absence of Can f 1 allergy 50.
The above implies that amino acid sequence identities alone do not sufficiently explain why all lipocalins are important in innate immune regulation, and why they are typically allergenic. The known anti‐bacterial activity of the human neutrophil lipocalin LCN2 (NGAL) is caused by the high affinity of this lipocalin for the siderophore‐Fe3+ complex and it should be investigated systematically whether from this it can be extrapolated to animal lipocalins. In fact, the molecular ligand transport function of lipocalins and of lipocalin‐like proteins could be particularly significant for the Th2 skewing capacity, independent of their mammalian 51 or plant 52 origin. We propose that this feature is likely connected with the similar β‐barrel fold in human and animal lipocalins (Fig. 3) 33, as well as with the binding plasticity of their calyx‐like cavity able to accommodate a great disparity of substances involved in metabolism and communication 33, 39, 53. Major sources of (at least the human) LCN2 are the liver 54 and macrophages 55, with sphingosin‐1‐phosphate inducing its production. Lipocalins are elevated and released during inflammation 56, infections 57 and sepsis 58, 59, 60, especially when the urinary tract is affected in humans and dogs 61, 62; in cardiovascular inflammation they get overexpressed in the brain and linked with depression 63; in cancer LCN2 is overexpressed 11, 64 prompting interaction with metalloproteinase‐9 (MMP‐9) 65, but only LCN2 is prognostic 66. In the immunosuppressive environment of the tumor stroma LCN2 cooperates with CCL2 to induce immunoregulatory DCs and subsequently CD4(+)FoxP3(+) Treg cells and supports metastasis 67. Moreover, local IL‐10 stimulates tumor infiltrating M2 macrophages to release LCN2 and iron 68, 69. Hence, in cancer the opposite of what is needed in allergy is the problem 70. It is further known that macrophages convert to an anti‐inflammatory type by IL10 and glucocorticoid stimulation 71. Alternatively activated macrophages further regulate the immune response by secreting LCN2, which is directly linked with IL10 formation (and in our own hands glucocorticoid release). Taken together, lipocalins are regarded as biomarkers for several diseases 72 and are at the crossing point between immunity and tolerance that is of particular interest for allergen immunotherapy.
Figure 3.
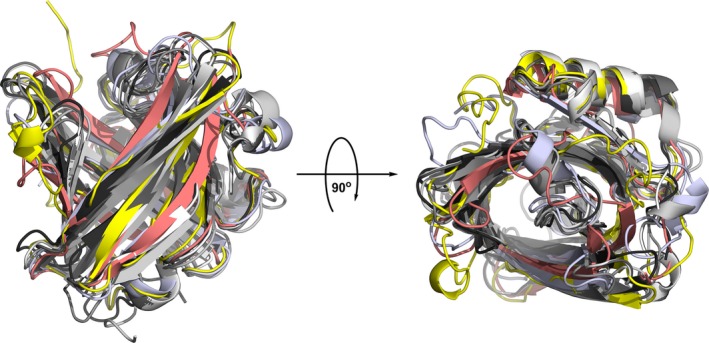
Symphony of human and animal lipocalins by structural superimposition. Human LCN2 and LCN1 lipocalins are colored yellow and salmon, respectively, Bos d 5 is colored blue white and the remaining lipocalins in Fig. 1 are colored in different shades of gray from white (Equ c 1) to black (Mus m 1). The rotated image on the right gives an upper view of the calyx‐like cavity.
The secret function of lipocalins determined by their pocket load
Some important human lipocalins are considered acute phase proteins with innate immune function, especially against gram‐negative bacteria. Their growth depends on iron, which they secure via the secretion of a bacterial siderophore, [= a high‐affinity iron acquisition molecule], like enterobactin, an iron‐specific chelating agent closely related to the ligand in the crystal structure of human LCN2 displayed in Fig. 1. We speculate that, like LCN2 which withdraws iron from bacteria by accepting it into their own pocket, also some of the other mammalian lipocalins may act bacteriostatically (Fig. 3) 73. On the other side of this evolutionary interplay, bacterial siderophores can inhibit the function of neutrophil myeloperoxidase and enhance the bacterial survival 74, or simply overwhelm the lipocalin defense system 75. A lipocalin‐2 receptor (termed 24p3/NGAL) and Lipoprotein Receptor‐Related Protein have been described binding LCN in humans and rodents 65, 76, possibly also involving MMP9 in a triple interaction.
Less attention has been given to a potentially differential immune function of lipocalins depending on the load state of their pocket, either holo – full, or apo – empty. Interestingly, the major birch pollen allergen Bet v 1 has a relation to bacterial proteins 77 by its old scaffold and a central pocket, which was predicted to bind quercetin‐3‐O‐sophoroside 78. However, the flavonoid quercetin also behaves as a siderophore 52. By means of an in silico approach using flexible docking calculations and geometry optimizations we were able to analyze the optimal binding of human LCN2 to several siderophore‐iron complexes with 1, 2, or 3 catechol chelating moieties 79. This study allowed us to estimate protein‐ligand dissociation constants in the micro‐ to nanomolar range indicative of a high affinity that arises to a great extent from the electrostatic interactions between amino acid residues surrounding the outer part of the cup‐like cavity and polar groups of the siderophore 79. A further illustration of this optimal binding is depicted in Fig. 4 where the model complex between hLCN2 and a hexadentate iron chelator, Fe(DHBA)3, is shown. Note that the presence of hydrophobic moieties and polar groups in the ligand is consistent with the presence of hydrophobic residues together with charged (mainly basic) side chains in the protein that produce the electrostatic effects largely responsible for this efficient protein‐ligand binding. However, we emphasize that more experimental studies have to be undertaken not only to approve our estimation with certainty in vitro, but also to be able to extrapolate to other lipocalin allergens.
Figure 4.
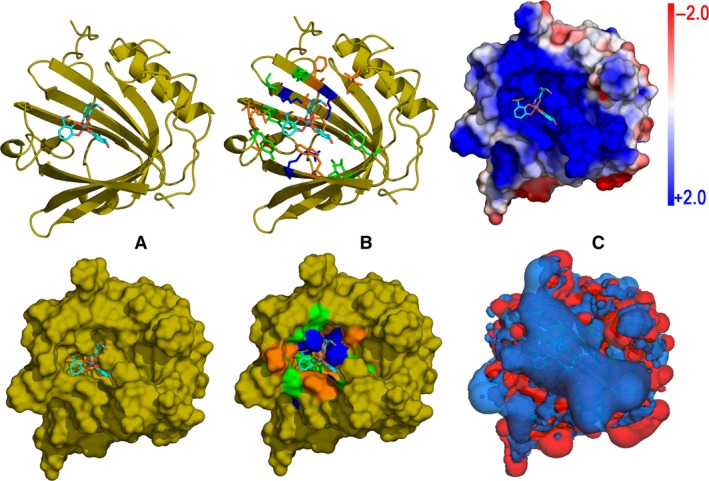
Prototype of lipocalin‐siderophore binding: model complex of human LCN2 and Fe(DHBA)3, a hexadentate iron‐chelator. The siderophore (DHBA = 2,3‐dihydroxybenzoic acid) is depicted as sticks with carbons in cyan, oxygens in red, and Fe3+ ion as a gray sphere. (A) Geometry of the siderophore in the cup‐like cavity (top) and the corresponding protein surface showing the topography of the pocket (bottom). (B) Residues within a 5 Å distance from the ligand color‐coded as follows: basic = blue, polar = green, and nonpolar = orange. Top: ribbon and sticks diagram. Bottom: the corresponding color‐coded surface. (C) Poisson–Boltzmann (PB) electrostatic potential of hLCN2. Top: PB potential mapped onto the protein surface according to the scale indicated in the bar. The strongly positive electrostatic nature of the pocket area stabilizes the electrically negative oxygenated groups in the siderophore. Bottom: +2 (blue) and ‐2 (red) 3D isosurfaces of the PB electrostatic field created by hLCN2. A large positive isosurface covers the cavity area in which the siderophore is accommodated.
More importantly, the load of this binding pocket is decisive for the immunomodulatory function of lipocalins. Only the apo‐form of lipocalin Bos d 5 and lipocalin‐like allergen Bet v 1 51, 52 promoted the survival of CD4+ T cells and IL‐13 production in human PBMCs, whereas the holo‐form was nonallergenic, so far at least in vitro. Consistent with this function, the loading state of brain lipocalin is decisive for the survival of neuronal cells 80. Furthermore, in a dose‐dependent loading approach, holo‐LCN2 had the highest capacity to induce HLA‐G(+)/FoxP3(+) T‐regulatory cells 81. Therefore, it may be anticipated that exogenous lipocalins, animal allergens, may be tolerated rather than inducing allergy, when complexed with ligands.
Synopsis
Humans and their domestic animals share molecules that play a role both physiologically in innate immunity, and pathologically as allergens. Whereas many important animal allergens relevant for humans belong to the lipocalin family, it is not known yet whether vice versa human lipocalins may act as allergens for pet animals. It is not by accident that the lipocalin fold is highly conserved in the animal kingdom. It allows lipocalins to withdraw iron from microbes into their molecular pocket and thereby act bacteriostatic.
In conclusion lipocalins may, independent of their mammalian other animal, or plant origin, house siderophore ligands, which critically determine their innate immunomodulatory as well as allergenic characteristics. It has been shown that in loaded state human lipocalins induce regulatory T cells, whereas empty lipocalins rather promote Th2 responses and inflammation. To this end, the interplay between exogenous lipocalins and human LCNs is not resolved, but it is tempting to speculate that they interfere with each other either via receptor binding or via capturing each other's iron ligand.
Author contributions
Jensen‐Jarolim E responsible for scientific concept, writing of the manuscript, and literature search. Pacios Luis F performed calculations, computer modeling, and structural alignments of all structures of this study (Figs 1, 3 and 4). Bianchini R and Hofstetter G involved in amino acid sequence searches and comparisons and contributed to the writing of the manuscript; Hofstetter G also contributed to the design of Fig. 2. Pacios, LF, and Roth‐Walter, F contributed to the overall scientific concept and writing of the manuscript.
Conflicts of interest
Erika Jensen‐Jarolim, Luis F. Pacios and Franziska Roth‐Walter declare that they are inventors on PCT/EP2015/050126. Rodolfo Bianchini and Gerlinde Hofstetter declare no conflict of interest.
Supporting information
Figure S1. 3D structure and topology of a prototypical lipocalin.
Acknowledgment
We thank Amelia Wein for proofreading. The study was supported by the Austrian Science Fund FWF, grant SFB F4606‐B19.
Jensen‐Jarolim E, Pacios Luis F, Bianchini R, Hofstetter G, Roth‐Walter F. Structural similarities of human and mammalian lipocalins, and their function in innate immunity and allergy. Allergy 2016; 71: 286–294.
Edited by: Reto Crameri
References
- 1. Chu DK, Jimenez‐Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop‐Guevara A et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med 2014;211:1657–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL‐4 production during a memory T‐dependent response. J Exp Med 2004;200:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH et al. Blocking IL‐25 signalling protects against gut inflammation in a type‐2 model of colitis by suppressing nuocyte and NKT derived IL‐13. J Gastroenterol 2012;47:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest 2011;140:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M et al. The interleukin‐4 enhancer CNS‐2 is regulated by Notch signals and controls initial expression in NKT cells and memory‐type CD4 T cells. Immunity 2006;24:689–701. [DOI] [PubMed] [Google Scholar]
- 6. Salter B, Oliveria J, Nusca G, Smith S, Watson R, Comeau M et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL‐3 dependent. J Allergy Clin Immunol 2015 ;136:1636–1644. [DOI] [PubMed] [Google Scholar]
- 7. Stremnitzer C, Manzano‐Szalai K, Willensdorfer A, Starkl P, Pieper M, Konig P et al. Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57BL/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J Invest Dermatol 2015;135:1790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osterlund C, Gronlund H, Gafvelin G, Bucht A. Non‐proteolytic aeroallergens from mites, cat and dog exert adjuvant‐like activation of bronchial epithelial cells. Int Arch Allergy Immunol 2011;155:111–118. [DOI] [PubMed] [Google Scholar]
- 9. Breiteneder H. Protein families: implications for allergen nomenclature, standardisation and specific immunotherapy. Arb Paul Ehrlich Inst Bundesinstitut Impfstoffe Biomed Arzneim Langen Hess 2009;96:249–254. [PubMed] [Google Scholar]
- 10. Aalberse RC. Mammalian airborne allergens. Chem Immunol Allergy 2014;100:243–247. [DOI] [PubMed] [Google Scholar]
- 11. Wu B, Li C, Du Z, Yao Q, Wu J, Feng L et al. Network based analyses of gene expression profile of LCN2 overexpression in esophageal squamous cell carcinoma. Sci Rep 2014;4:5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris DO. Human allergy to environmental pet danders: a public health perspective. Vet Dermatol 2010;21:441–449. [DOI] [PubMed] [Google Scholar]
- 13. Bjerg A, Winberg A, Berthold M, Mattsson L, Borres MP, Ronmark E. A population‐based study of animal component sensitization, asthma and rhinitis in schoolchildren. Pediatr Allergy Immunol 2015;26(6):557–63. [DOI] [PubMed] [Google Scholar]
- 14. Jensen‐Jarolim E, Pali‐Schöll I, Jensen S, Robibaro B, Kinaciyan T. Caution: reptile pets shuttle grasshopper allergy and asthma into homes. World Allergy Organ J 2015;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson A, Foster A, Hart B, Helps C, Shaw S. Prevalence of house dust mites and dermatophagoides group 1 antigens collected from bedding, skin and hair coat of dogs in south‐west England. Vet Dermatol 2005;16:32–38. [DOI] [PubMed] [Google Scholar]
- 16. Jensen‐Jarolim E, Einhorn L, Herrmann I, Thalhammer JG, Panakova L. Pollen allergies in humans and their dogs, cats and horses: differences and similarities. Clin Transl Allergy 2015;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mueller R, Janda J, Jensen‐Jarolim E, Rhyner C, Marti E. Allergens in veterinary medicine. Allergy 2015 Aug 17. doi: 10.1111/all.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avner DB, Perzanowski MS, Platts‐Mills TA, Woodfolk JA. Evaluation of different techniques for washing cats: quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J Allergy Clin Immunol 1997;100:307–312. [DOI] [PubMed] [Google Scholar]
- 19. Konradsen JR, Fujisawa T, van Hage M, Hedlin G, Hilger C, Kleine‐Tebbe J et al. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol 2015;135:616–625. [DOI] [PubMed] [Google Scholar]
- 20. Erwin EA, Woodfolk JA, James HR, Satinover SM, Platts‐Mills TA. Changes in cat specific IgE and IgG antibodies with decreased cat exposure. Ann Allergy Asthma Immunol 2014;112:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M et al. Effects of early‐life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014;134:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka A, Matsuda A, Jung K, Jang H, Ahn G, Ishizaka S et al. Ultra‐pure soft water ameliorates atopic skin disease by preventing metallic soap deposition in NC/Tnd mice and reduces skin dryness in humans. Acta Derm Venereol 2015;95:787–91. [DOI] [PubMed] [Google Scholar]
- 23. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science 2014;346:954–959. [DOI] [PubMed] [Google Scholar]
- 24. Ezell JM, Wegienka G, Havstad S, Ownby DR, Johnson CC, Zoratti EM. A cross‐sectional analysis of pet‐specific immunoglobulin E sensitization and allergic symptomatology and household pet keeping in a birth cohort population. Allergy Asthma Proc 2013;34:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Medjo B, Atanaskovic‐Markovic M, Nikolic D, Spasojevic‐Dimitrijeva B, Ivanovski P, Djukic S. Association between pet‐keeping and asthma in school children. Pediatr Int 2013;55:133–137. [DOI] [PubMed] [Google Scholar]
- 26. Smallwood J, Ownby D. Exposure to dog allergens and subsequent allergic sensitization: an updated review. Curr Allergy Asthma Rep 2012;12:424–428. [DOI] [PubMed] [Google Scholar]
- 27. Basagana M, Bartolome B, Pastor‐Vargas C, Mattsson L, Lidholm J, Labrador‐Horrillo M. Involvement of Can f 5 in a case of human seminal plasma allergy. Int Arch Allergy Immunol 2012;159:143–146. [DOI] [PubMed] [Google Scholar]
- 28. Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol 2009;123:362–368. [DOI] [PubMed] [Google Scholar]
- 29. Vredegoor DW, Willemse T, Chapman MD, Heederik DJ, Krop EJ. Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. J Allergy Clin Immunol 2012;130:904–909. [DOI] [PubMed] [Google Scholar]
- 30. Hilger C, Swiontek K, Kler S, Diederich C, Lehners C, Vogel L et al. Evaluation of two new recombinant guinea‐pig lipocalins, Cav p 2 and Cav p 3, in the diagnosis of guinea‐pig allergy. Clin Exp Allergy 2011;41:899–908. [DOI] [PubMed] [Google Scholar]
- 31. Torres JA, de Las Heras M, Maroto AS, Vivanco F, Sastre J, Pastor‐Vargas C. Molecular and immunological characterization of the first allergenic lipocalin in hamster: the major allergen from Siberian hamster (Phodopus sungorus). J Biol Chem 2014;289:23382–23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valdes JJ. Antihistamine response: a dynamically refined function at the host‐tick interface. Parasit Vectors 2014;7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiefner A, Skerra A. The menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc Chem Res 2015;48:976–985. [DOI] [PubMed] [Google Scholar]
- 34. Nilsson OB, Neimert‐Andersson T, Bronge M, Grundstrom J, Sarma R, Uchtenhagen H et al. Designing a multimer allergen for diagnosis and immunotherapy of dog allergic patients. PLoS One 2014;9:e111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polovic N, Waden K, Binnmyr J, Hamsten C, Gronneberg R, Palmberg C et al. Dog saliva – an important source of dog allergens. Allergy 2013;68:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song S, Lauber C, Costello E, Lozupone C, Humphrey G, Berg‐Lyons D et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013;2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamata Y, Miyanomae A, Nakayama E, Miyanomae T, Tajima T, Nishimura K et al. Characterization of dog allergens Can f 1 and Can f 2. 2. A comparison of Can f 1 with Can f 2 regarding their biochemical and immunological properties. Int Arch Allergy Immunol 2007;142:301–308. [DOI] [PubMed] [Google Scholar]
- 38. Konieczny A, Morgenstern JP, Bizinkauskas CB, Lilley CH, Brauer AW, Bond JF et al. The major dog allergens, Can f 1 and Can f 2, are salivary lipocalin proteins: cloning and immunological characterization of the recombinant forms. Immunology 1997;92:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mattsson L, Lundgren T, Olsson P, Sundberg M, Lidholm J. Molecular and immunological characterization of Can f 4: a dog dander allergen cross‐reactive with a 23 kDa odorant‐binding protein in cow dander. Clin Exp Allergy 2010;40:1276–1287. [DOI] [PubMed] [Google Scholar]
- 40. Saarelainen S, Taivainen A, Rytkonen‐Nissinen M, Auriola S, Immonen A, Mantyjarvi R et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy 2004;34:1576–1582. [DOI] [PubMed] [Google Scholar]
- 41. Rytkonen‐Nissinen M, Saarelainen S, Randell J, Hayrinen J, Kalkkinen N, Virtanen T. IgE reactivity of the dog lipocalin allergen Can f 4 and the development of a sandwich ELISA for its quantification. Allergy Asthma Immunol Res 2015;7:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hilger C, Kuehn A, Hentges F. Animal lipocalin allergens. Curr Allergy Asthma Rep 2012;12:438–447. [DOI] [PubMed] [Google Scholar]
- 43. Saarelainen S, Rytkonen‐Nissinen M, Rouvinen J, Taivainen A, Auriola S, Kauppinen A et al. Animal‐derived lipocalin allergens exhibit immunoglobulin E cross‐reactivity. Clin Exp Allergy 2008;38:374–381. [DOI] [PubMed] [Google Scholar]
- 44. Kamata Y, Miyanomae A, Nakayama E, Miyanomae T, Tajima T, Hoshi H. Characterization of dog allergens Can f 1 and Can f 2. 1. Preparation of their recombinant proteins and antibodies. Int Arch Allergy Immunol 2007;142:291–300. [DOI] [PubMed] [Google Scholar]
- 45. Madhurantakam C, Nilsson OB, Uchtenhagen H, Konradsen J, Saarne T, Hogbom E et al. Crystal structure of the dog lipocalin allergen Can f 2: implications for cross‐reactivity to the cat allergen Fel d 4. J Mol Biol 2010;401:68–83. [DOI] [PubMed] [Google Scholar]
- 46. Nilsson OB, Binnmyr J, Zoltowska A, Saarne T, van Hage M, Gronlund H. Characterization of the dog lipocalin allergen Can f 6: the role in cross‐reactivity with cat and horse. Allergy 2012;67:751–757. [DOI] [PubMed] [Google Scholar]
- 47. Juntunen R, Liukko A, Taivainen A, Narvanen A, Durand G, Kauppinen A et al. Suboptimal recognition of a T cell epitope of the major dog allergen Can f 1 by human T cells. Mol Immunol 2009;46:3320–3327. [DOI] [PubMed] [Google Scholar]
- 48. Liukko AL, Kinnunen TT, Rytkonen‐Nissinen MA, Kailaanmaki AH, Randell JT, Maillere B et al. Human CD4+ T cell responses to the dog major allergen Can f 1 and its human homologue tear lipocalin resemble each other. PLoS One 2014;9:e98461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ronka AL, Kinnunen TT, Goudet A, Rytkonen‐Nissinen MA, Sairanen J, Kailaanmaki AH et al. Characterization of human memory CD4 T‐cell responses to the dog allergen Can f 4. J Allergy Clin Immunol 2015;136:1047–1054. [DOI] [PubMed] [Google Scholar]
- 50. Kinnunen T, Taivainen A, Partanen J, Immonen A, Saarelainen S, Rytkonen‐Nissinen M et al. The DR4‐DQ8 haplotype and a specific T cell receptor Vbeta T cell subset are associated with absence of allergy to Can f 1. Clin Exp Allergy 2005;35:797–803. [DOI] [PubMed] [Google Scholar]
- 51. Roth‐Walter F, Pacios LF, Gomez‐Casado C, Hofstetter G, Roth GA, Singer J et al. The major cow milk allergen Bos d 5 manipulates T‐helper cells depending on its load with siderophore‐bound iron. PLoS One 2014;9:e104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roth‐Walter F, Gomez‐Casado C, Pacios LF, Mothes‐Luksch N, Roth GA, Singer J et al. Bet v 1 from birch pollen is a lipocalin‐like protein acting as allergen only when devoid of iron by promoting Th2 lymphocytes. J Biol Chem 2014;289:17416–17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stopkova R, Dudkova B, Hajkova P, Stopka P. Complementary roles of mouse lipocalins in chemical communication and immunity. Biochem Soc Trans 2014;42:893–898. [DOI] [PubMed] [Google Scholar]
- 54. Xu MJ, Feng D, Wu H, Wang H, Chan Y, Kolls J et al. Liver is the major source of elevated serum lipocalin‐2 levels after bacterial infection or partial hepatectomy: a critical role for IL‐6/STAT3. Hepatology 2015;61:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sola A, Weigert A, Jung M, Vinuesa E, Brecht K, Weis N et al. Sphingosine‐1‐phosphate signalling induces the production of Lcn‐2 by macrophages to promote kidney regeneration. J Pathol 2011;225:597–608. [DOI] [PubMed] [Google Scholar]
- 56. Asimakopoulou A, Borkham‐Kamphorst E, Tacke F, Weiskirchen R. Lipocalin‐2 (NGAL/LCN2), a “HELP‐ME” signal in organ inflammation. Hepatology 2015 Jun 5. doi: 10.1002/hep.27930. [DOI] [PubMed] [Google Scholar]
- 57. Alpizar‐Alpizar W, Laerum OD, Illemann M, Ramirez JA, Arias A, Malespin‐Bendana W et al. Neutrophil gelatinase‐associated lipocalin (NGAL/Lcn2) is upregulated in gastric mucosa infected with Helicobacter pylori . Virchows Arch 2009;455:225–233. [DOI] [PubMed] [Google Scholar]
- 58. Jha MK, Jeon S, Jin M, Lee WH, Suk K. Acute phase protein Lipocalin‐2 is associated with formalin‐induced nociception and pathological pain. Immune Netw 2013;13:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Otto GP, Hurtado‐Oliveros J, Chung HY, Knoll K, Neumann T, Muller HJ et al. Plasma neutrophil gelatinase‐associated lipocalin is primarily related to inflammation during sepsis: a translational approach. PLoS One 2015;10:e0124429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shields‐Cutler RR, Crowley JR, Hung CS, Stapleton AE, Aldrich CC, Marschall J et al. Human urinary composition controls siderocalin's antibacterial activity. J Biol Chem 2015;290:15949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daure E, Belanger MC, Beauchamp G, Lapointe C. Elevation of neutrophil gelatinase‐associated lipocalin (NGAL) in non‐azotemic dogs with urinary tract infection. Res Vet Sci 2013;95:1181–1185. [DOI] [PubMed] [Google Scholar]
- 62. Hsu WL, Lin YS, Hu YY, Wong ML, Lin FY, Lee YJ. Neutrophil gelatinase‐associated lipocalin in dogs with naturally occurring renal diseases. J Vet Intern Med 2014;28:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gouweleeuw L, Naude PJ, Rots M, DeJongste MJ, Eisel UL, Schoemaker RG. The role of neutrophil gelatinase associated lipocalin (NGAL) as biological constituent linking depression and cardiovascular disease. Brain Behav Immun 2015;46:23–32. [DOI] [PubMed] [Google Scholar]
- 64. Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS et al. Roles of neutrophil gelatinase‐associated lipocalin (NGAL) in human cancer. Oncotarget 2014;5:1576–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ghosh M, Sodhi SS, Kim JH, Kim NE, Mongre RK, Sharma N et al. An integrated in silico approach for the structural and functional exploration of Lipocalin 2 and its functional insights with metalloproteinase 9 and lipoprotein receptor‐related protein 2. Appl Biochem Biotechnol 2015;176:712–729. [DOI] [PubMed] [Google Scholar]
- 66. Ruiz‐Morales JM, Dorantes‐Heredia R, Arrieta O, Chavez‐Tapia NC, Motola‐Kuba D. Neutrophil gelatinase‐associated lipocalin (NGAL) and matrix metalloproteinase‐9 (MMP‐9) prognostic value in lung adenocarcinoma. Tumour Biol 2015;36:3601–3610. [DOI] [PubMed] [Google Scholar]
- 67. Kudo‐Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis 2013;30:393–405. [DOI] [PubMed] [Google Scholar]
- 68. Jung M, Mertens C, Brune B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology 2015;220:295–304. [DOI] [PubMed] [Google Scholar]
- 69. Jung M, Weigert A, Tausendschon M, Mora J, Oren B, Sola A et al. Interleukin‐10‐induced neutrophil gelatinase‐associated lipocalin production in macrophages with consequences for tumor growth. Mol Cell Biol 2012;32:3938–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jensen‐Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M et al. AllergoOncology: the role of IgE‐mediated allergy in cancer. Allergy 2008;63:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest 2013;123:3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Makris K, Kafkas N. Neutrophil gelatinase‐associated lipocalin in acute kidney injury. Adv Clin Chem 2012;58:141–191. [DOI] [PubMed] [Google Scholar]
- 73. Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A et al. Iron traffics in circulation bound to a siderocalin (Ngal)‐catechol complex. Nat Chem Biol 2010;6:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N et al. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun 2015;6:7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Holden VI, Lenio S, Kuick R, Ramakrishnan SK, Shah YM, Bachman MA. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1alpha and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect Immun 2014;82:3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Langelueddecke C, Roussa E, Fenton RA, Thevenod F. Expression and function of the lipocalin‐2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS One 2013;8:e71586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Radauer C, Lackner P, Breiteneder H. The Bet v 1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol Biol 2008;8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seutter von Loetzen C, Hoffmann T, Hartl MJ, Schweimer K, Schwab W, Rosch P et al. Secret of the major birch pollen allergen Bet v 1: identification of the physiological ligand. Biochem J 2014;457:379–390. [DOI] [PubMed] [Google Scholar]
- 79. Gomez‐Casado C, Roth‐Walter F, Jensen‐Jarolim E, Diaz‐Perales A, Pacios LF. Modeling iron‐catecholates binding to NGAL protein. J Mol Graph Model 2013;45:111–121. [DOI] [PubMed] [Google Scholar]
- 80. Chia WJ, Tan FC, Ong WY, Dawe GS. Expression and localisation of brain‐type organic cation transporter (BOCT/24p3R/LCN2R) in the normal rat hippocampus and after kainate‐induced excitotoxicity. Neurochem Int 2015;87:43–59. [DOI] [PubMed] [Google Scholar]
- 81. La Manna G, Ghinatti G, Tazzari PL, Alviano F, Ricci F, Capelli I et al. Neutrophil gelatinase‐associated lipocalin increases HLA‐G(+)/FoxP3(+) T‐regulatory cell population in an in vitro model of PBMC. PLoS One 2014;9:e89497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore‐mediated iron acquisition. Mol Cell 2002;10:1033–1043. [DOI] [PubMed] [Google Scholar]
- 83. Breustedt DA, Chatwell L, Skerra A. A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr 2009;65:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Loch J, Polit A, Gorecki A, Bonarek P, Kurpiewska K, Dziedzicka‐Wasylewska M et al. Two modes of fatty acid binding to bovine beta‐lactoglobulin–crystallographic and spectroscopic studies. J Mol Recognit 2011;24:341–349. [DOI] [PubMed] [Google Scholar]
- 85. Lascombe MB, Gregoire C, Poncet P, Tavares GA, Rosinski‐Chupin I, Rabillon J et al. Crystal structure of the allergen Equ c 1. A dimeric lipocalin with restricted IgE‐reactive epitopes. J Biol Chem 2000;275:21572–21577. [DOI] [PubMed] [Google Scholar]
- 86. Niemi MH, Rytkonen‐Nissinen M, Janis J, Virtanen T, Rouvinen J. Structural aspects of dog allergies: the crystal structure of a dog dander allergen Can f 4. Mol Immunol 2014;61:7–15. [DOI] [PubMed] [Google Scholar]
- 87. Bocskei Z, Groom CR, Flower DR, Wright CE, Phillips SE, Cavaggioni A et al. Pheromone binding to two rodent urinary proteins revealed by X‐ray crystallography. Nature 1992;360:186–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 3D structure and topology of a prototypical lipocalin.


